Research Progress on Heterogeneous Reactions of Pollutant Gases on the Surface of Atmospheric Mineral Particulate Matter in China
Abstract
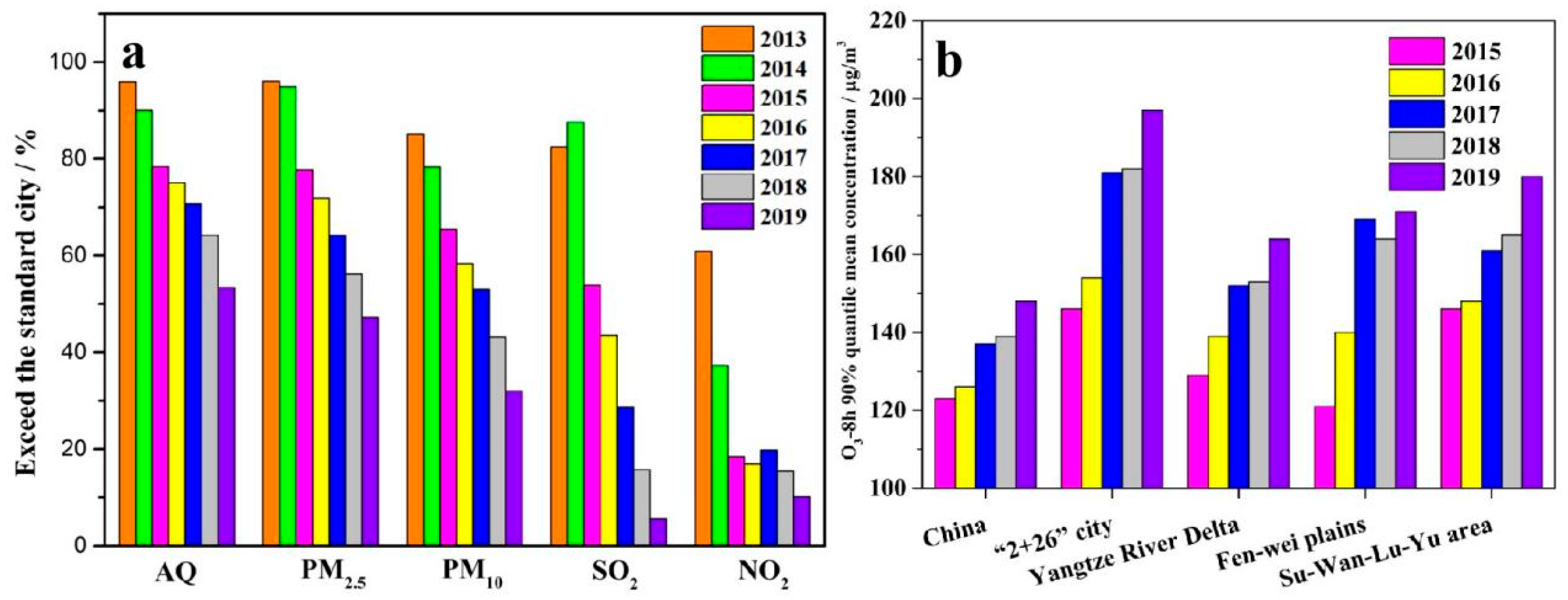
1. Introduction
2. Source and Composition of APM
3. Surface Heterogeneous Reactions of AMPM
3.1. Heterogeneous Reactions of Inorganic Gases on the Surface of AMPM
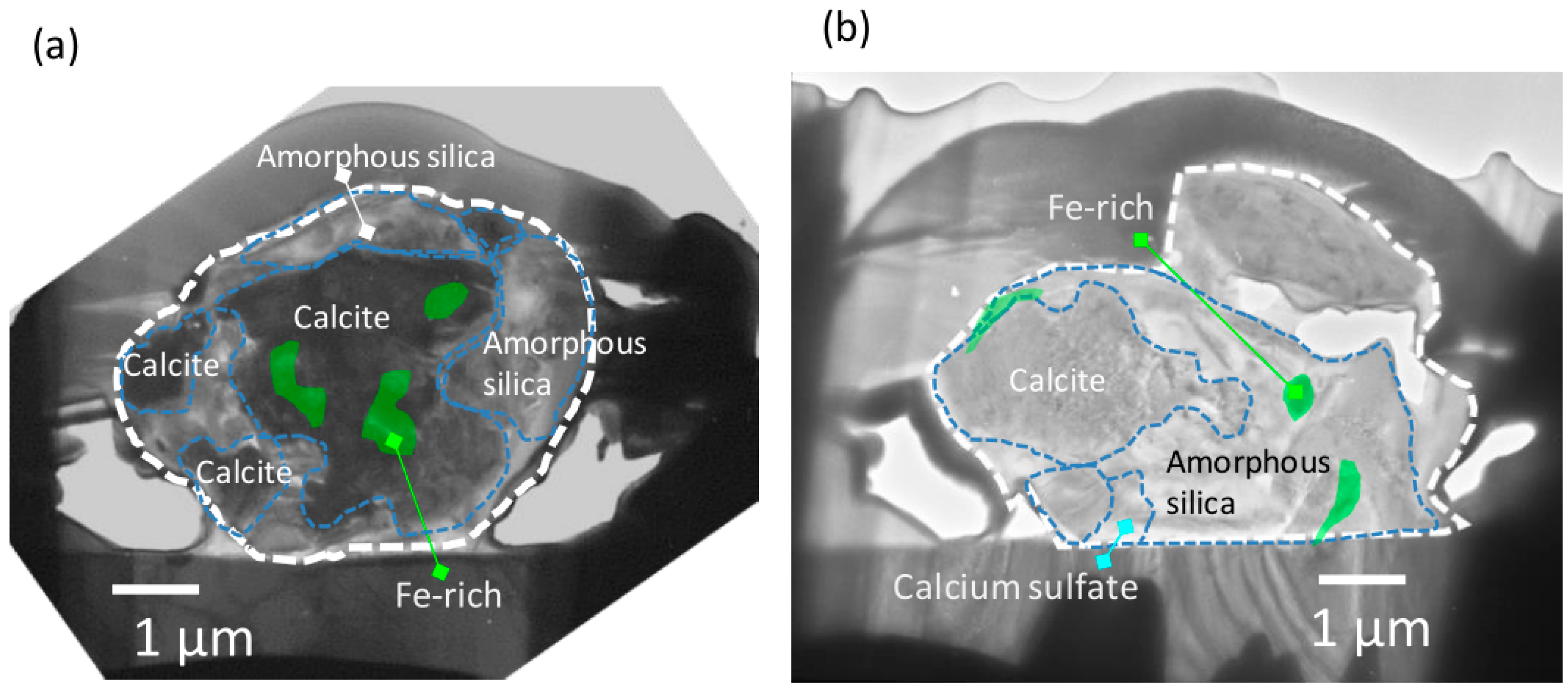
3.1.1. Heterogeneous Oxidation Reaction of SO2 on the Surface of AMPM
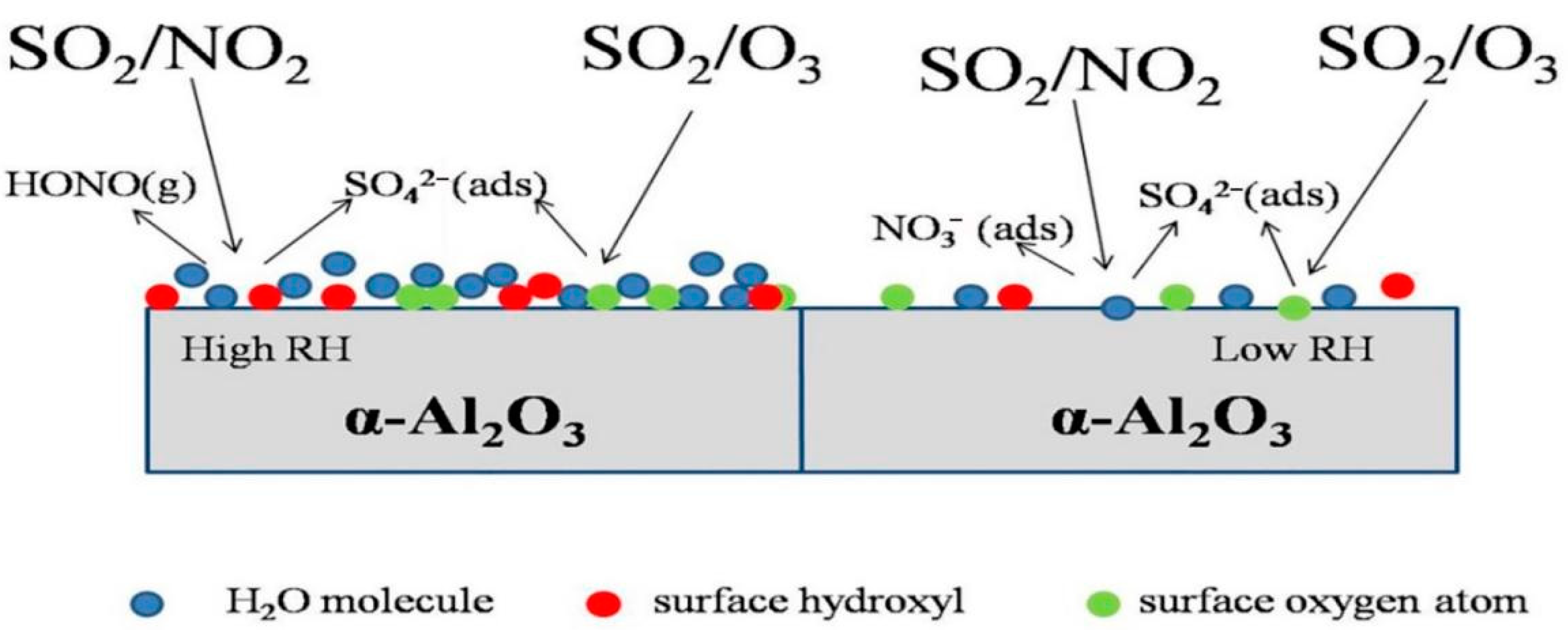
3.1.2. Heterogeneous Reaction of SO2 and O3 on the Surfaces of AMPM
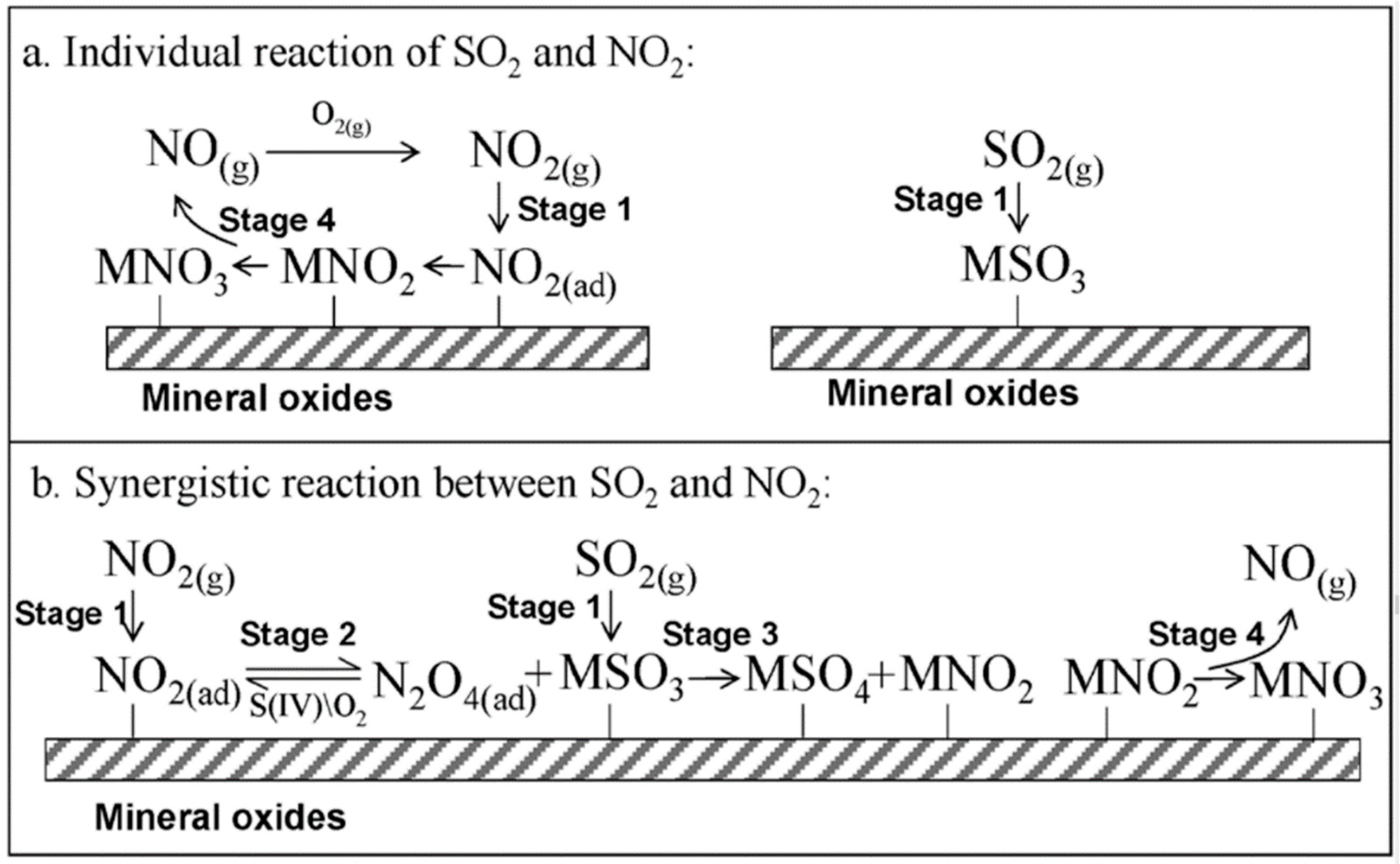
3.1.3. Heterogeneous Reactions of SO2 and NO2 on the Surfaces of AMPM
3.1.4. Heterogeneous Reactions of SO2 and H2O2 on the Surfaces of AMPM
3.1.5. Heterogeneous Reactions of NH3/CO2 and SO2 on the Surfaces of AMPM
3.2. Heterogeneous Reactions of Organic Gases on the Surface of AMPM
3.2.1. Heterogeneous Uptake of VOCs on AMPM
3.2.2. Heterogeneous Catalytic Oxidation of VOCs on AMPM
3.3. Heterogeneous Reactions of Mixed Gas on the Surface of AMPM
3.3.1. Heterogeneous Reaction of VOCs and SO2 on the Surfaces of AMPM
3.3.2. Heterogeneous Reactions of O3 and VOCs on the Surfaces of AMPM
3.3.3. Heterogeneous Reactions of NO2 and VOCs on the Surfaces of AMPM
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, B.; Tong, D.; Li, M.; Liu, F.; Hong, C.; Geng, G.; Li, H.; Li, X.; Peng, L.; Qi, J.; et al. Trends in China’s anthropogenic emissions since 2010 as the consequence of clean air actions. Atmos. Chem. Phys. 2018, 18, 14095–14111. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. China Ecological and Environment Bulletin. Available online: https://www.mee.gov.cn/hjzl/ (accessed on 2 February 2022).
- Xing, J.; Wang, J.; Mathu, R.; Wang, S.; Sarwar, G.; Pleim, J.; Hogrefe, C.; Zhang, Y.; Jiang, J.; Wong, D.C.; et al. Impacts of aerosol direct effects on tropospheric ozone through changes in atmospheric dynamics and photolysis rates. Atmos. Chem. Phys. 2017, 17, 9869–9883. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zheng, S.; He, Y. Aerosol effects on ozone concentrations in Beijing: A model sensitivity study. J. Environ. Sci. 2014, 24, 645–656. [Google Scholar] [CrossRef]
- Ding, A.; Fu, C.; Yang, X.; Sun, J.; Zheng, F.; Xie, Y.; Herrmann, E.; Nie, W.; Petaja, T.; Kerminen, V.; et al. Ozone and fine particle in the western Yangtze River Delta: An overview of 1 yr data at the SORPES station. Atmos. Chem. Phys. 2013, 13, 5813–5830. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- George, I.J.; Abbatt, J.P. Heterogeneous oxidation of atmospheric aerosol particles by gas-phase radicals. Nat. Chem. 2010, 2, 713–722. [Google Scholar] [CrossRef]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C.; et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef]
- Ramanathan, V.; Crutzen, P.J.; Kiehl, J.T.; Rosenfeld, D. Aerosols, Climate, and the Hydrological Cycle. Science 2001, 294, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Huang, X.; Lu, K.; Ge, M.; Li, Y.; Cheng, P.; Zhu, T.; Ding, A.; Zhang, Y.; Gligorovski, S.; et al. Heterogeneous reactions of mineral dust aerosol: Implications for tropospheric oxidation capacity. Atmos. Chem. Phys. 2017, 17, 11727–11777. [Google Scholar] [CrossRef]
- George, C.; Ammann, M.; D’Anna, B.; Donaldson, D.J.; Nizkorodov, S.A. Heterogeneous Photochemistry in the Atmosphere. Chem. Rev. 2015, 115, 4218–4258. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Shao, L.; Feng, C.; Ju, Y.; Li, J.; Huo, T.; Zhao, Y. Interfacial Reacyion of Atmospheric Micro/Nano Particles and Significance of Mineral Coevolution. Earth Sci. 2018, 43, 1709–1724, (In Chinese with English abstract). [Google Scholar]
- Ciere, R.; Querol, X. Solid Particulate Matter in the Atmosphere. Elements 2010, 6, 215–222. [Google Scholar] [CrossRef]
- Novak, G.A.; Bertram, T.H. Reactive VOC production from photochemical and heterogeneous reactions occurring at the air–ocean interface. Acc. Chem. Res. 2020, 53, 1014–1023. [Google Scholar] [CrossRef]
- Kolb, C.E.; Cox, R.A.; Abbatt, J.P.; Ammann, M.; Davis, E.J.; Donaldson, D.J.; Garrett, B.C.; George, C.; Griffiths, P.T.; Hanson, D.R.; et al. An overview of current issues in the uptake of atmospheric trace gases by aerosols and clouds. Atmos. Chem. Phys. 2010, 10, 10561–10605. [Google Scholar] [CrossRef]
- Shi, B.; Wang, W.; Zhou, L.; Li, J.; Wang, J.; Chen, Y.; Zhang, W.; Ge, M. Kinetics and mechanisms of the gas-phase reactions of OH radicals with three C15 alkanes. Atmos. Environ. 2019, 207, 75–81. [Google Scholar] [CrossRef]
- Lv, S.; Liu, Q.; Zhao, Y.; Zhang, M.; Jiang, L.; He, S. Formaldehyde Generation in Photooxidation of Isoprene on Iron Oxide Nanoclusters. J. Phys. Chem. C 2019, 123, 5120–5127. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, H.; Li, G.; An, T. Theoretical investigation on the role of mineral dust aerosol in atmospheric reaction: A case of the heterogeneous reaction of formaldehyde with NO2 onto SiO2 dust surface. Atmos. Environ. 2015, 103, 207–214. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Z.; Zhang, W.; Ji, Y.; Li, G.; An, T. Contribution of reaction of atmospheric amine with sulfuric acid to mixing particle formation from clay mineral. Sci. Total Environ. 2022, 821, 153336. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Cheng, J.; Lee, K.; Stocker, R.; He, X.; Yao, M.; Wang, J. Effects of relative humidity on heterogeneous reaction of SO2 with CaCO3 particles and formation of CaSO4·2H2O crystal as secondary aerosol. Atmos. Environ. 2021, 268, 118776. [Google Scholar] [CrossRef]
- Kameda, T.; Azumi, E.; Fukushima, A.; Tang, N.; Matsuki, A.; Kamiya, Y.; Toriba, A.; Hayakawa, K. Mineral dust aerosols promote the formation of toxic nitropolycyclic aromatic compounds. Sci. Rep. 2016, 6, 24427. [Google Scholar] [CrossRef][Green Version]
- Zhu, T. Heterogeneous reactions on the surface of atmospheric particulate matter. Sci. Sin. Chim. 2010, 40, 1729–1730. (In Chinese) [Google Scholar]
- Gentner, D.R.; Jathar, S.H.; Gordon, T.D.; Bahreini, R.; Day, D.A.; Haddad, I.E.; Hayes, P.L.; Pieber, S.M.; Platt, S.M.; Gouw, J.A.; et al. A review of urban secondary organic aerosol formation from gasoline and diesel motor vehicle emissions. Environ. Sci. Technol. 2017, 51, 1074–1093. [Google Scholar] [CrossRef] [PubMed]
- Huneeus, N.; Schulz, M.; Balkanski, Y.; Griesfeller, J.; Prospero, J.; Kinne, S.; Bauer, S.; Boucher, O.; Chin, M.; Dentener, F.; et al. Global dust model intercomparison in AeroCom phase I. Atmos. Chem. Phys. 2011, 11, 7781–7816. [Google Scholar] [CrossRef]
- Ning, C.; Gao, Y.; Zhang, H.; Wang, L.; Yu, H.; Zou, L.; Cao, R.; Chen, J. Molecular chemodiversity of water-soluble organic matter in atmospheric particulate matter and their associations with atmospheric conditions. Sci. Total Environ. 2021, 809, 151171. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, Y.; Bozzetti, C.; Ho, K.; Cao, J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef]
- Liu, W.; He, X.; Pang, S.; Zhang, Y. Effect of relative humidity on O3 and NO2 oxidation of SO2 on α-Al2O3 particles. Atmos. Environ. 2017, 167, 245–253. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, S.; Wang, X.; Zhang, H.; Yuan, S. Atomistic insights into heterogeneous reaction of hydrogen peroxide on mineral oxide particles. Appl. Surf. Sci. 2021, 556, 149707. [Google Scholar] [CrossRef]
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.; Cadle, S.; Chan, T.; Mulawa, P. The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Cao, G. Aerosol Chemical Compositions of Beijing PM1 and Its Control Countermeasures. J. Appl. Meteorol. Sci. 2012, 23, 257–264, (In Chinese with English abstract). [Google Scholar]
- Shao, L.; Li, W.; Yang, S.; Shi, Z.; Lv, S. Mineralogical characteristics of airborne particles collected in Beijing during a severe Asian dust storm period in spring 2002. Sci. China Ser. D Earth Sci. 2007, 6, 953–959. [Google Scholar] [CrossRef]
- Dong, F.; He, X.; Li, G. Study on the basic characteristics of several atmospheric dusts in the norther China. Miner. Pet. 2005, 25, 114–117, (In Chinese with English abstract). [Google Scholar]
- Wang, W.; Shao, L.; Zhang, D.; Li, Y.; Li, W.; Liu, P.; Xing, J. Mineralogical similarities and differences of dust storm particles at beijing from deserts in the north and northwest. Sci. Total Environ. 2022, 803, 149980. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Cziczo, D.J.; Grassian, V.H. Interactions of Water with Mineral Dust Aerosol: Water Adsorption, Hygroscopicity, Cloud Condensation, and Ice Nucleation. Chem. Rev. 2016, 116, 4205–4259. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Peng, J.; Guo, S.; Hu, M. Secondary aerosol formation in winter haze over the BeijingTianjin-Hebei Region, China. Front. Environ. Sci. Eng. 2021, 15, 34. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, P.; Yang, J.; Zhang, J.; Zhu, G.; Cao, Z.; Fan, J.; Liu, Z.; Wang, Y. Chemical characteristics and source apportionment of PM2.5 in a petrochemical city:implications for primary and secondary carbonaceous component. J. Environ. Sci. 2021, 103, 322–335. [Google Scholar] [CrossRef]
- Gao, X.; Yang, L.; Cheng, S.; Gao, R.; Zhou, Y.; Xue, L.; Shou, Y.; Wang, J.; Wang, X.; Nie, W.; et al. Semi-continuous measurement of water-soluble ions in PM2.5 in Jinan, China: Temporal variations and source apportionments. Atmos. Environ. 2011, 45, 6048–6056. [Google Scholar] [CrossRef]
- Lai, S.; Zou, S.; Cao, J.; Lee, S.; Ho, K. Characterizing ionic species in PM2.5 and PM10 in four Pearl River Delta cities, South China. J. Environ. Sci. 2007, 19, 939–947. [Google Scholar] [CrossRef]
- Wang, W.; Fu, G. The Research of Source and Composition of Dust Haze in Chengdu and its Control Countermeasures. Adv. Mater. Res. 2014, 726–731, 2066–2073. [Google Scholar] [CrossRef]
- Kim, B.M.; Teffera, S.; Zeldin, M.D. Characterization of PM 25 and PM 10 in the South Coast Air Basin of Southern California: Part 1—Spatial Variations. J. Air Waste Manag. Assoc. 2000, 50, 2034–2044. [Google Scholar] [CrossRef]
- Rajeev, P.; Rajput, P.; Gupta, T. Chemical characteristics of aerosol and rain water during an el-nio and pdo influenced indian summer monsoon. Atmos. Environ. 2016, 145, 192–200. [Google Scholar] [CrossRef]
- Weagle, C.L.; Snider, G.; Li, C.; Donkelaar, A.V.; Philip, S.; Bissonnette, P.; Burke, J.; Jackson, J.; Latimer, R. Global Sources of Fine Particulate Matter: Interpretation of PM2.5 Chemical Composition Observed by SPARTAN using a Global Chemical Transport Model. Environ. Sci. Technol. 2018, 52, 11670–11681. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, D.; TSONA, N.; GE, M. Anthropogenic Effects on Biogenic Secondary Organic Aerosol Formation. Adv. Atmos. Sci. 2021, 38, 1053–1084. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, B.; Wang, S.; Yang, S.; Xing, J.; Morawska, L.D.; Ding, A.; Kulmala, M.; Kerminen, V.M.; Kujansuu, J.; et al. Particulate matter pollution over China and the effects of control policies. Sci. Total Environ. 2017, 584–585, 426–447. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Tang, G.; Lv, B.; Li, X.; Wang, Y. Source apportionment of PM2.5 and visibility in Jinan, China. J. Environ. Sci. 2021, 102, 207–215. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cheng, Z.; Qin, X.; Dong, P. Reversible and irreversible gas–particle partitioning of dicarbonyl compounds observed in the real atmosphere. Atmos. Chem. Phys. 2022, 22, 6971–6987. [Google Scholar] [CrossRef]
- Shi, B.; Wang, W.; Zhou, L.; Sun, Z.; Fan, C.; Chen, Y.; Zhang, W.; Qiao, Y.; Qiao, Y.; Ge, M. Atmospheric oxidation of C10~14 n-alkanes initiated by Cl atoms: Kinetics and mechanism. Atmos. Environ. 2020, 222, 117166. [Google Scholar] [CrossRef]
- Zeineddine, M.N.; Romanias, M.N.; Gaudion, V.; Riffault, V.; Thévenet, F. Heterogeneous interaction of isoprene with natural gobi dust. ACS Earth Space Chem. 2017, 1, 236–243. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, Y.; Chen, Z.; Huang, D. Heterogeneous reactions of volatile organic compounds in the atmosphere. Atmos. Environ. 2013, 68, 297–314. [Google Scholar] [CrossRef]
- Tillmann, R.; Hallquist, M.; Jonsson, Å.M.; Kiendler-Scharr, A.; Saathoff, H.; Iinuma, Y.; Mentel, T.F. Influence of relative humidity and temperature on the production of pinonaldehyde and OH radicals from the ozonolysis of α-pinene. Atmos. Chem. Phys. 2010, 10, 7057–7072. [Google Scholar] [CrossRef]
- Mila, A.; Ningbo, G.; Rong, C.; Zhang, H.; Xiuhua, Z.; Jiping, C. Research progress of analytical methods and pollution characteristics of typical organic pollutant in atmospheric particulate matter. Environ. Chem. 2021, 40, 3774–3786, (In Chinese with English abstract). [Google Scholar]
- Kyllnen, K.; Vestenius, M.; Anttila, P.; Makkonen, U.; Aurela, M.; Wangberg, I.; Mastromonaco, M.N.; Hakola, H. Trends and source apportionment of atmospheric heavy metals at a subarctic site during 1996–2018. Atmos. Environ. 2020, 236, 117644. [Google Scholar] [CrossRef]
- He, A.; Xie, J.; Yuan, C. Heavy Metal Speciation Analysis and Distribution Characteristics in Atmospheric Particulate Matters. Prog. Chem. 2021, 33, 1627–1647, (In Chinese with English abstract). [Google Scholar]
- Xu, J.W.; Martin, R.V.; Henderson, B.H.; Meng, J.; Öztanere, Y.B.; Handf, J.L.; Hakamie, A.; Strum, M.; Phillips, S.B. Simulation of airborne trace metals in fine particulate matter over north america. Atmos. Environ. 2019, 214, 116883. [Google Scholar] [CrossRef] [PubMed]
- Rajput, J.S.; Trivedi, M.K. Determination and assessment of elemental concentration in the atmospheric particulate matter: A comprehensive review. Environ. Monit. Assess. 2022, 194, 243. [Google Scholar] [CrossRef]
- Maia, P.D.; Vieira-Filho, M.; Prado, L.F.; Silva, L.C.; Sodré, F.F.; Ribeiro, H.D.; Ventura, R.S. Assessment of atmospheric particulate matter (PM10) in Central Brazil: Chemical and morphological aspects. Atmos. Pollut. Res. 2022, 13, 101362. [Google Scholar] [CrossRef]
- Zhi, M.; Zhang, K.; Lv, W. A review of research advances in the distributions and risk assessments of metal elements in atmospheric particles with different particle sizes. J. Environ. Eng. 2022, 12, 998–1006, (In Chinese with English abstract). [Google Scholar]
- Zou, T.; Kang, W.; Zhang, J.; Wang, M.; Fang, X.; Xue, S.; Zhong, B. Concentrations and Distribution Characteristics of Atmospheric Heavy Metals in Urban Areas of China. Res. Environ. Sci. 2015, 28, 1053–1061, (In Chinese with English abstract). [Google Scholar]
- Tan, J.; Duan, J. Heavy metals in aerosol in China: Pollution, sources, and control strategies. J. Univ. Chin. Acad. Sci. 2013, 30, 145–155, (In Chinese with English abstract). [Google Scholar]
- Wang, M.; Deng, Y.Q.; Dong, F.Q.; He, X.C.; Dai, Q.W.; Sun, S.Y.; Huang, Y.B.; Tang, J.; Chen, W.; Liu, L.Z.; et al. Speciation analysis of cadmium in dust fall about northern China towns. Asian J. Chem. 2013, 25, 7522–7526. [Google Scholar] [CrossRef]
- Iuga, C.; Sainz-Díaz, C.I.; Vivier-Bunge, A. Interaction energies and spectroscopic effects in the adsorption of formic acid on mineral aerosol surface models. J. Phys. Chem. C 2012, 116, 2904–2914. [Google Scholar] [CrossRef]
- Usher, C.R.; Michel, A.E.; Grassian, V.H. Reactions on mineral dust. Chemical reviews. Chem. Rev. 2003, 103, 4883–4939. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Deng, Y.; Fu, H.; Zhang, L.; Chen, J. Adsorption of SO2 on mineral dust particles influenced by atmospheric moisture. Atmos. Environ. 2018, 191, 153–161. [Google Scholar] [CrossRef]
- Goodman, A.L.; Li, P.; Usher, C.R.; Grassian, V.H. Heterogeneous uptake of sulfur dioxide on aluminum and magnesium oxide particles. J. Phys. Chem. A 2001, 105, 6109–6120. [Google Scholar] [CrossRef]
- Usher, C.R.; Al-Hosney, H.; Carlos-Cuellar, S.; Grassian, V.H. A laboratory study of the heterogeneous uptake and oxidation of sulfur dioxide on mineral dust particles. J. Geophys. Res. Atmos. 2002, 107, ACH 16-1–ACH 16-9. [Google Scholar] [CrossRef]
- Ullerstam, M.; Johnson, M.S.; Vogt, R.; Ljungström, E. DRIFTS and Knudsen cell study of the heterogeneous reactivity of SO2 and NO2 on mineral dust. Atmos. Chem. Phys. 2003, 3, 2043–2051. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Q.; Tong, S.; Jing, B.; Wang, W.; Guo, Y.; Ge, M. Mechanism and Kinetics of Heterogeneous Reactions of Unsaturated Organic Acids on α-Al2O3 and CaCO3. ChemPhysChem 2016, 17, 3515–3523. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, Y.; Li, H.; Chen, Z. Kinetics of heterogeneous reaction of sulfur dioxide on authentic mineral dust: Effects of relative humidity and hydrogen peroxide. Environ. Sci. Technol. 2015, 49, 10797–10805. [Google Scholar] [CrossRef] [PubMed]
- Kebede, M.A.; Varner, M.E.; Scharko, N.K.; Gerber, R.B.; Raff, J.D. Photooxidation of Ammonia on TiO2 as a Source of NO and NO2 under Atmospheric Conditions. J. Am. Chem. Soc. 2013, 135, 8606–8615. [Google Scholar] [CrossRef]
- Fu, H.; Wang, X.; Wu, H.; Yin, Y.; Chen, J. Heterogeneous uptake and oxidation of SO2 on iron oxides. J. Phys. Chem. C 2007, 111, 6077–6085. [Google Scholar] [CrossRef]
- Wang, R.; Yang, N.; Li, J.; Xu, L.; Tsona, N.T.; Du, L.; Wang, W. Heterogeneous reaction of SO2 on CaCO3 particles: Different impacts of NO2 and acetic acid on the sulfite and sulfate formation. J. Environ. Sci. 2022, 114, 149–159. [Google Scholar] [CrossRef]
- Ullerstam, M.; Vogt, R.; Langer, S.; Ljungström, E. The kinetics and mechanism of SO2 oxidation by O3 on mineral dust. Phys. Chem. Chem. Phys. 2002, 4, 4694–4699. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Zhang, Y.; Zhu, T.; Li, J.; Ding, J. Kinetics and mechanism of heterogeneous oxidation of sulfur dioxide by ozone on surface of calcium carbonate. Atmos. Chem. Phys. 2006, 6, 2453–2464. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; He, H. Synergistic Effect between NO2 and SO2 in Their Adsorption and Reaction on γ-Alumina. J. Phys. Chem. A 2008, 112, 6630–6635. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, Q.; Liu, Y.; Ma, J.; He, H. Synergistic reaction between SO2 and NO2 on mineral oxides: A potential formation pathway of sulfate aerosol. Phys. Chem. Chem. Phys. 2012, 14, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, D.; Ge, S.; Liu, S.; Wu, C.; Wang, Y.; Chen, Y.; Lv, S.; Wang, F.; Meng, J.; et al. Rapid sulfate formation from synergetic oxidation of SO2 by O3 and NO2 under ammonia-rich conditions: Implications for the explosive growth of atmospheric PM2.5 during haze events in China. Sci. Total Environ. 2021, 17, 9869–9883. [Google Scholar] [CrossRef]
- Liu, S.; Huang, D.D.; Wang, Y.Q.; Zhang, S.; Liu, X.D.; Wu, C.; Du, W.; Wang, G.H. Synergetic effects of NH3 and NOx on the production and optical absorption of secondary organic aerosol formation from toluene photooxidation. Atmos. Chem. Phys. 2021, 21, 17759–17773. [Google Scholar] [CrossRef]
- Chu, B.; Chen, T.; Liu, Y.; Ma, Q.; Mu, Y.; Wang, Y.; Ma, J.; Zhang, P.; Liu, J.; Liu, C.S.; et al. Application of smog chambers in atmospheric process studies. Natl. Sci. Rev. 2022, 9, 16. [Google Scholar] [CrossRef]
- Ueda, S.; Miki, Y.; Kato, H.; Miura, K.; Uematsu, M. Internal structure of asian dust particles over the western north pacific: Analyses using focused ion beam and transmission electron microscopy. Atmosphere 2020, 11, 78. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, N.; Zhao, D.; Shang, J.; Zhu, T. Using Micro-Raman Spectroscopy to Investigate Chemical Composition, Mixing States, and Heterogeneous Reactions of Individual Atmospheric Particles. Environ. Sci. Technol. 2021, 55, 10243–10254. [Google Scholar] [CrossRef]
- Xu, L.; Lingaswamy, A.P.; Zhang, Y.; Liu, L.; Wang, Y.; Zhang, J.; Ma, Q.; Li, W. Morphology, composition, and sources of individual aerosol particles at a regional background site of the YRD, China. J. Environ. Sci. 2019, 77, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, J.; Zhang, M.; Wang, X.; Li, Y.; Jones, T.; Feng, X.; Silva, L.O.; Li, W. Morphology, composition and mixing state of individual airborne particles: Effects of the 2017 Action Plan in Beijing, China. J. Clean. Prod. 2021, 329, 129748. [Google Scholar] [CrossRef]
- Chen, H.; Duan, F.; Du, J.; Yin, R.; Zhu, L.; Dong, J.; He, K.; Sun, Z.; Wang, S. Surface-enhanced raman scattering for mixing state characterization of individual fine particles during a haze episode in Beijing, China. J. Environ. Sci. 2021, 104, 216–224. [Google Scholar] [CrossRef]
- Yang, W.; He, H.; Ma, Q.; Ma, J.; Liu, Y.; Liu, P.; Mu, Y. Synergistic formation of sulfate and ammonium resulting from reaction between SO2 and NH3 on typical mineral dust. Phys. Chem. Chem. Phys. 2016, 18, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, Q.; Liu, Y.; Ma, J.; Chu, B.; He, H. The effect of water on the heterogeneous reactions of SO2 and NH3 on the surfaces of α-Fe2O3 and γ-Al2O3. Environ. Sci. Nano 2019, 6, 2749–2758. [Google Scholar] [CrossRef]
- Dentener, F.J.; Carmichael, G.R.; Zhang, Y.; Lelieveld, J.; Crutzen, P.J. Role of mineral aerosol as a reactive surface in the global troposphere. J. Geophys. Res. Atmos. 1996, 2, 22869–22889. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, G.; Chen, J.; Wang, Y.; Wang, X.; An, Z.; Zhang, P. Heterogeneous reactions of sulfur dioxide on typical mineral particles. J. Phys. Chem. B 2006, 110, 12588–12596. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, Y.; Li, H.; Chen, Z. Hydrogen peroxide maintains the heterogeneous reaction of sulfur dioxide on mineral dust proxy particles. Atmos. Environ. 2016, 141, 552–559. [Google Scholar] [CrossRef]
- Shang, H.; Liu, X.; Chneg, N.; Li, M.; Chen, Z.; Ai, Z.; Zhang, L. Research progress on heterogeneous reactions of SO2 on mineral particles. J. Huazhong Agric. Univ. 2020, 39, 9–16, (In Chinese with English abstract). [Google Scholar]
- Baltrusaitis, J.; Cwiertny, D.M.; Grassian, V.H. Adsorption of sulfur dioxide on hematite and goethite particle surfaces. Phys. Chem. Chem. Phys. 2007, 9, 5542–5554. [Google Scholar] [CrossRef]
- Dupart, Y.; King, S.M.; Nekat, B.; Nowak, A.; Wiedensohler, A.; Herrmann, H.; David, G.; Thomas, B.; Miffre, A.; Rairoux, P.; et al. Mineral dust photochemistry induces nucleation events in the presence of SO2. Proc. Natl. Acad. Sci. USA 2012, 109, 20842–20847. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jang, M.; Yu, Z. Heterogeneous photo-oxidation of SO2 in the presence of two different mineral dust particles: Gobi and Arizona dust. Environ. Sci. Technol. 2017, 51, 9605–9613. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jang, M.; Park, J. Modeling atmospheric mineral aerosol chemistry to predict heterogeneous photooxidation of SO2. Atmos. Chem. Phys. 2017, 17, 10001–10017. [Google Scholar] [CrossRef]
- Han, L.; Liu, X.; Chen, Y.; Xiang, X.; Wang, H. Key factors influencing the formation of sulfate aerosol on the surface of mineral aerosols: Insights from laboratory simulations and acsm measurements. Atmos. Environ. 2021, 253, 118341. [Google Scholar] [CrossRef]
- Yang, N.; Tsona, N.T.; Cheng, S.; Li, S.; Xu, L.; Wang, Y.; Wu, L.; Du, L. Competitive reactions of SO2 and acetic acid on α-Al2O3 and CaCO3 particles. Sci. Total Environ. 2020, 699, 134362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Fang, X.; Deng, Y.; Cheng, H.; Fu, H.; Zhang, L. Impact of greenhouse gas CO2 on the heterogeneous reaction of SO2 on alpha-Al2O3. Chin. Chem. Lett. 2020, 31, 2712–2716. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, A.; Chen, Z.; Jin, X.; Gu, C. Transformation of gaseous 2-bromophenol on clay mineral dust and the potential health effect. Environ. Pollut. 2019, 250, 686–694. [Google Scholar] [CrossRef]
- Xu, J.; Huang, M.; Feng, Z.; Cai, S.; Zhao, W.; Hu, C.; Gu, X.; Zhang, W. Effects of inorganic seed aerosol on the formationof nitrogen-containing organic compoundsfrom reaction of ammonia with photooxidationproducts of toluene. Pol. J. Environ. Stud. 2020, 29, 909–917. [Google Scholar] [CrossRef]
- Hettiarachchi, E.; Grassian, V. Heterogeneous Reactions of α-Pinene on Mineral Surfaces: Formation of Organonitrates and α-Pinene Oxidation Products. J. Phys. Chem. A 2022, 126, 4068–4079. [Google Scholar] [CrossRef]
- Rubasinghege, G.; Ogden, S.; Baltrusaitis, J.; Grassian, V.H. Heterogeneous uptake and adsorption of gas-phase formic acid on oxide and clay particle surfaces: The roles of surface hydroxyl groups and adsorbed water in formic acid adsorption and the impact of formic acid adsorption on water uptake. J. Phys. Chem. A 2013, 117, 11316–11327. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; Liu, C.; He, H. Heterogeneous reaction of acetic acid on MgO, α-Al2O3, and CaCO3 and the effect on the hygroscopic behaviour of these particles. Phys. Chem. Chem. Phys. 2012, 14, 8403–8409. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Wu, L.; Ge, M.; Wang, W.; Pu, Z. Heterogeneous chemistry of monocarboxylic acids on α-Al2O3 at different relative humidities. Atmos. Chem. Phys. 2010, 10, 7561–7574. [Google Scholar] [CrossRef]
- Tang, M.; Larish, W.A.; Fang, Y.; Gankanda, A.; Grassian, V.H. Heterogeneous reactions of acetic acid with oxide surfaces: Effects of mineralogy and relative humidity. J. Phys. Chem. A 2016, 120, 5609–5616. [Google Scholar] [CrossRef]
- Fang, Y.; Tang, M.; Grassian, V.H. Competition between displacement and dissociation of a strong acid compared to a weak acid adsorbed on silica particle surfaces: The role of adsorbed water. J. Phys. Chem. A 2016, 120, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Alstadt, V.J.; Kubicki, J.D.; Freedman, M.A. Competitive Adsorption of Acetic Acid and Water on Kaolinite. J. Phys. Chem. A 2016, 120, 8339–8346. [Google Scholar] [CrossRef] [PubMed]
- Romanias, M.N.; Zeineddine, M.N.; Gaudion, V.; Lun, X.; Thevenet, F.; Riffault, V. Heterogeneous interaction of isopropanol with natural Gobi dust. Environ. Sci. Technol. 2016, 50, 11714–11722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Y.; Zheng, S.; Hashisho, Z. Adsorption of volatile organic compounds onto natural porous minerals. J. Hazard. Mater. 2019, 364, 317–324. [Google Scholar] [CrossRef]
- Romanías, M.N.; Ourrad, H.; Thévenet, F.; Riffault, V. Investigating the heterogeneous interaction of VOCs with natural atmospheric particles: Adsorption of limonene and toluene on saharan mineral dusts. J. Phys. Chem. A 2016, 120, 1197–1212. [Google Scholar] [CrossRef]
- Makarouni, D.; Lycourghiotis, S.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Dourtoglou, V. Transformation of limonene into P-cymene over acid activated natural Mordenite utilizing atmospheric oxygen as a green oxidant: A novel mechanism. Appl. Catal. B Environ. 2018, 224, 740–750. [Google Scholar] [CrossRef]
- Lycourghiotis, S.; Makarouni, D.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Dourtoglou, V. Transformation of Limonene into High Added Value Products over Acid Activated Natural Montmorillonite. Catal. Today 2020, 355, 757–767. [Google Scholar] [CrossRef]
- Niu, H.; Li, K.; Chu, B.; Su, W.; Li, J. Heterogeneous reactions between toluene and NO2 on mineral particles under simulated atmospheric conditions. Environ. Sci. Technol. 2017, 51, 9596–9604. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Wang, W.; Ge, M. Heterogeneous uptake of formic acid and acetic acid on mineral dust and coal fly ash. ACS Earth Space Chem. 2020, 4, 202–210. [Google Scholar] [CrossRef]
- Styler, S.A.; Donaldson, D.J. Heterogeneous photochemistry of oxalic acid on Mauritanian sand and Icelandic volcanic ash. Environ. Sci. Technol. 2012, 46, 8756–8763. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yi, J.; Ji, Y.; Zhao, B.; Ji, Y.; Li, G.; An, T. Enhanced H-abstraction contribution for oxidation of xylenes via mineral particles: Implications for particulate matter formation and human health. Environ. Res. 2020, 186, 109568. [Google Scholar] [CrossRef] [PubMed]
- Ponczek, M.; George, C. Kinetics and product formation during the photooxidation of butanol on atmospheric mineral dust. Environ. Sci. Technol. 2018, 52, 5191–5198. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, D.; Su, S.; Ren, L.; Ren, H.; Wei, L.; Yue, S.; Xie, Q.; Zhang, Z.; Wang, Z.; et al. Photochemical Degradation of Organic Matter in the Atmosphere. Adv. Sustain. Syst. 2021, 51, 2100027. [Google Scholar] [CrossRef]
- Chu, B.; Wang, Y.; Yang, W.; Ma, J.; Ma, Q.; Zhang, P.; Liu, Y.; He, H. Effects of NO2 and C3H6 on the heterogeneous oxidation of SO2 on TiO2 in the presence or absence of UV–Vis irradiation. Atmos. Chem. Phys. 2019, 19, 14777–14790. [Google Scholar] [CrossRef]
- Wu, L.; Tong, S.; Zhou, L.; Wang, W.; Ge, M. Synergistic Effects between SO2 and HCOOH on α-Fe2O3. J. Phys. Chem. A 2013, 117, 3972–3979. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fang, X.; Wang, T.; Gong, K.; Tahir, M.A.; Wang, W.; Han, J.; Cheng, H.Y.; Xu, G.J.; Zhang, L.W. Atmospheric organic complexation enhanced sulfate formation and iron dissolution on nano α-Fe2O3. Environ. Sci. Nano 2021, 8, 698. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, L.; Sun, Z.; Ding, X.; Cheng, T.; Yang, X.; Chen, J. Interactions between heterogeneous uptake and adsorption of sulfur dioxide and acetaldehyde on hematite. J. Phys. Chem. A 2015, 119, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.; Han, D.; Bao, L.; Mei, Q.; Wei, B.; An, Z.; He, M.; Yuan, S.; Xie, J.; et al. Gaseous and heterogeneous reactions of low-molecular-weight (LMW) unsaturated ketones with O3: Mechanisms, kinetics, and effects of mineral dust in tropospheric chemical processes. Chem. Eng. J. 2020, 395, 125083. [Google Scholar] [CrossRef]
- Lian, H.; Pang, S.; He, X.; Yang, M.; Ma, J.; Zhang, Y. Heterogeneous reactions of isoprene and ozone on α-Al2O3: The suppression effect of relative humidity. Chemosphere 2020, 240, 124744. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, Y.; Ma, Q.; Liu, C.; He, H. Heterogeneous photochemical reaction of ozone with anthracene adsorbed on mineral dust. Atmos. Environ. 2013, 72, 165–170. [Google Scholar] [CrossRef]
- Chen, Y.; Tong, S.; Wang, J.; Peng, C.; Ge, M.; Xie, X.; Sun, J. Effect of titanium dioxide on secondary organic aerosol formation. Environ. Sci. Technol. 2018, 52, 11612–11620. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, Y.; He, H. Heterogeneous reactions between NO2 and anthracene adsorbed on SiO2 and MgO. Atmos. Environ. 2011, 45, 917–924. [Google Scholar] [CrossRef]
- Wang, H.; Hasegawa, K.; Kagaya, S. The nitration of pyrene adsorbed on silica particles by nitrogen dioxide. Chemosphere 2000, 41, 1479–1484. [Google Scholar] [CrossRef]
- Chu, B.; Zhang, X.; Liu, Y.; He, H.; Sun, Y.; Jiang, J.; Li, J.; Hao, J. Synergetic formation of secondary inorganic and organic aerosol: Effect of SO2 and NH3 on particle formation and growth. Atmos. Chem. Phys. 2016, 16, 14219–14230. [Google Scholar] [CrossRef]



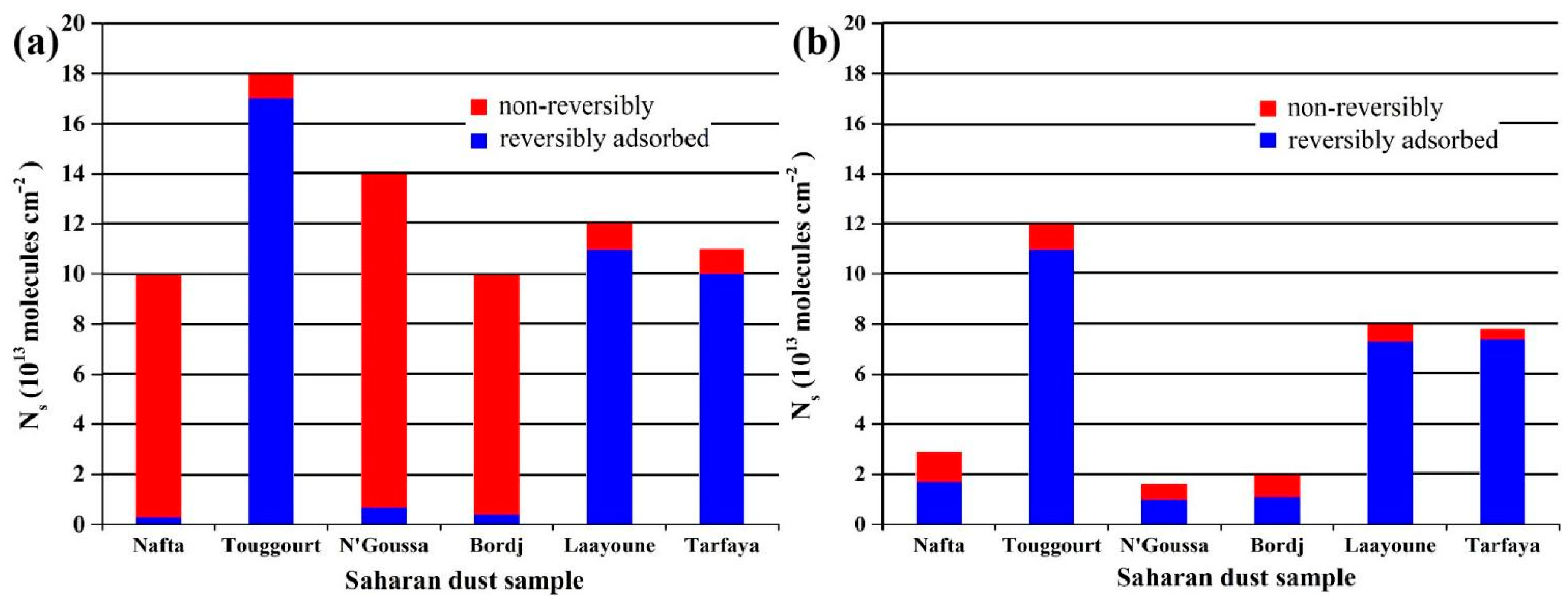

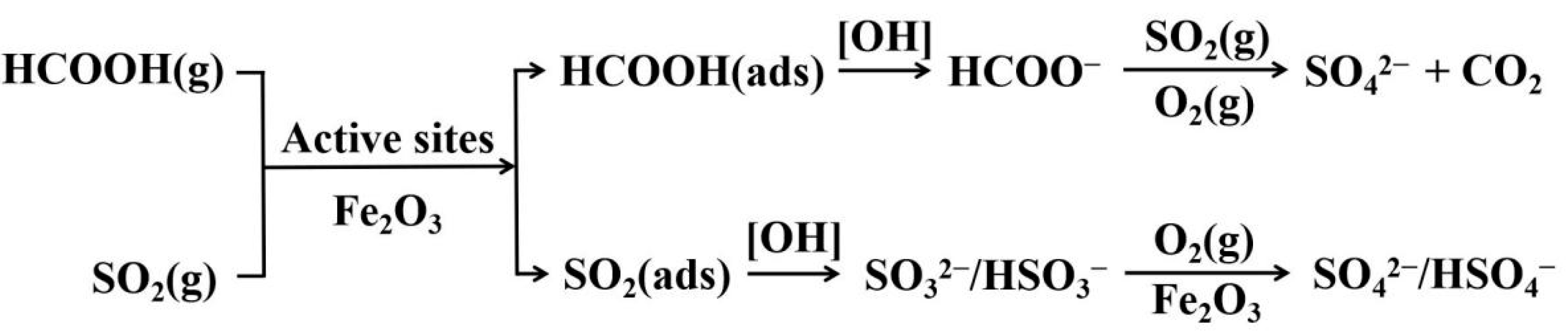
| Site | PM2.5 | SIA | SO42− | NH4+ | NO3− | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| obs | GC | obs | GC | obs | GC | obs | GC | obs | GC | |
| Beijing, China | 67.1 ± 9.9 | 75.0 | 19.7 ± 2.3 | 36.3 | 11.2 ± 1.4 | 13.3 | 3.6 ± 0.6 | 9.0 | 4.9 ± 1.4 | 1.4 |
| Bandung, Indonesia | 30.8 ± 4.5 | 20.0 | 7.6 ± 0.8 | 9.9 | 5.6 ± 0.7 | 7.2 | 1.4 ± 0.3 | 2.6 | 0.6 ± 0.2 | 0.1 |
| Manila, Philippines | 19.2 ± 2.8 | 24.0 | 3.0 ± 0.3 | 12.0 | 2.1 ± 0.3 | 9.1 | 0.5 ± 0.1 | 2.9 | 0.4 ± 0. | 0.0 |
| Rehovot, Israel | 17.5 ± 2.6 | 23.0 | 6.4 ± 0.7 | 7.7 | 4.7 ± 0.6 | 5.6 | 0.9 ± 0.1 | 2.0 | 0.8 ± 0.2 | 0.1 |
| Dhaka, Bangladesh | 49.9 ± 7.3 | 79.0 | 11.3 ± 1.2 | 28.0 | 7.1 ± 0.9 | 15.1 | 2.2 ± 0.4 | 7.2 | 2.0 ± 0.6 | 5.7 |
| Buenos Aires, Argentina | 10.7 ± 1.6 | 15.0 | 2.5 ± 0.3 | 6.2 | 1.3 ± 0.2 | 4.4 | 0.4 ± 0.1 | 1.5 | 0.8 ± 0.2 | 0.3 |
| Ilorin, Nigeria | 15.8 ± 2.3 | 17.5 | 2.4 ± 0.2 | 1.9 | 1.7 ± 0.2 | 1.3 | 0.5 ± 0.1 | 0.5 | 0.2 ± 0.1 | 0.1 |
| Singapore, Vietnam | 15.8 ± 2.4 | 15.6 | 4.0 ± 0.4 | 3.5 | 3.2 ± 0.4 | 2.2 | 0.6 ± 0.1 | 0.9 | 0.2 ± 0.1 | 0.4 |
| Kanpur, India | 71.9 ± 10.6 | 94.0 | 18.6 ± 1.9 | 29.2 | 10.2 ± 1.3 | 16.6 | 4.6 ± 0.1 | 7.6 | 3.8 ± 1.1 | 5.0 |
| Hanoi, Vietnam | 50.9 ± 7.5 | 45.0 | 17.2 ± 1.8 | 17.1 | 10.1 ± 1.3 | 10.0 | 3.4 ± 0.6 | 4.5 | 3.7 ± 1.1 | 2.6 |
| Pretoria, South Africa | 17.5 ± 2.6 | 30.6 | 7.3 ± 0.7 | 15.7 | 5.3 ± 0.7 | 11.3 | 1.4 ± 0.2 | 3.7 | 0.6 ± 0.2 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F.; Dong, F.; Zhou, L.; Chen, Y.; Yu, J.; Luo, X.; Zhang, X.; Lv, Z.; Xia, X.; Xue, J. Research Progress on Heterogeneous Reactions of Pollutant Gases on the Surface of Atmospheric Mineral Particulate Matter in China. Atmosphere 2022, 13, 1283. https://doi.org/10.3390/atmos13081283
Zheng F, Dong F, Zhou L, Chen Y, Yu J, Luo X, Zhang X, Lv Z, Xia X, Xue J. Research Progress on Heterogeneous Reactions of Pollutant Gases on the Surface of Atmospheric Mineral Particulate Matter in China. Atmosphere. 2022; 13(8):1283. https://doi.org/10.3390/atmos13081283
Chicago/Turabian StyleZheng, Fei, Faqin Dong, Lin Zhou, Yunzhu Chen, Jieyu Yu, Xijie Luo, Xingyu Zhang, Zhenzhen Lv, Xue Xia, and Jingyuan Xue. 2022. "Research Progress on Heterogeneous Reactions of Pollutant Gases on the Surface of Atmospheric Mineral Particulate Matter in China" Atmosphere 13, no. 8: 1283. https://doi.org/10.3390/atmos13081283
APA StyleZheng, F., Dong, F., Zhou, L., Chen, Y., Yu, J., Luo, X., Zhang, X., Lv, Z., Xia, X., & Xue, J. (2022). Research Progress on Heterogeneous Reactions of Pollutant Gases on the Surface of Atmospheric Mineral Particulate Matter in China. Atmosphere, 13(8), 1283. https://doi.org/10.3390/atmos13081283







