Simulating Atmospheric Organic Aerosol in the Boreal Forest Using Its Volatility-Oxygen Content Distribution
Abstract
1. Introduction
2. Materials and Method
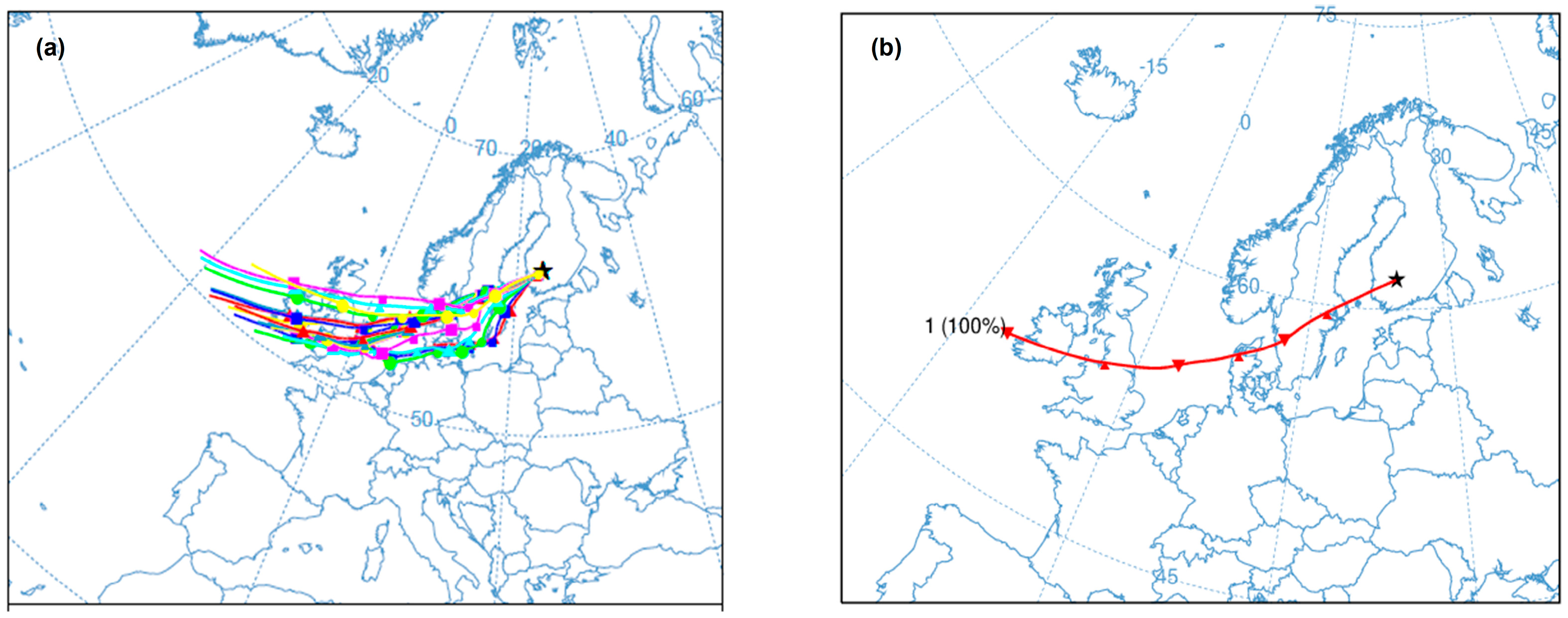
2.1. Site Description and Measurement Period
2.2. PMCAMx-Trj Model
2.3. Simulated Periods
2.4. The Seven Aging Schemes
2.5. Performance Evaluation Metrics of Parameterizations
3. Results
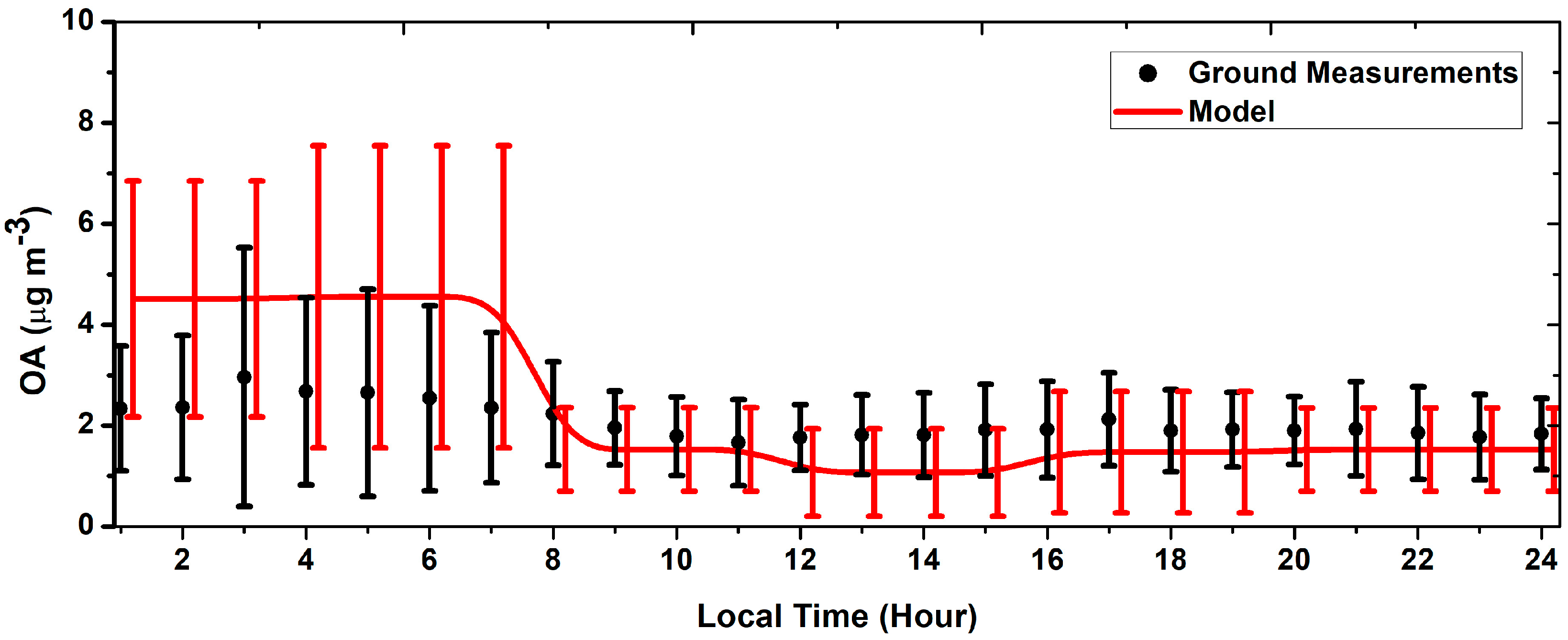
3.1. Simple Functionalization Scheme (1-Bin Case)
3.2. Evaluation of Aging Schemes
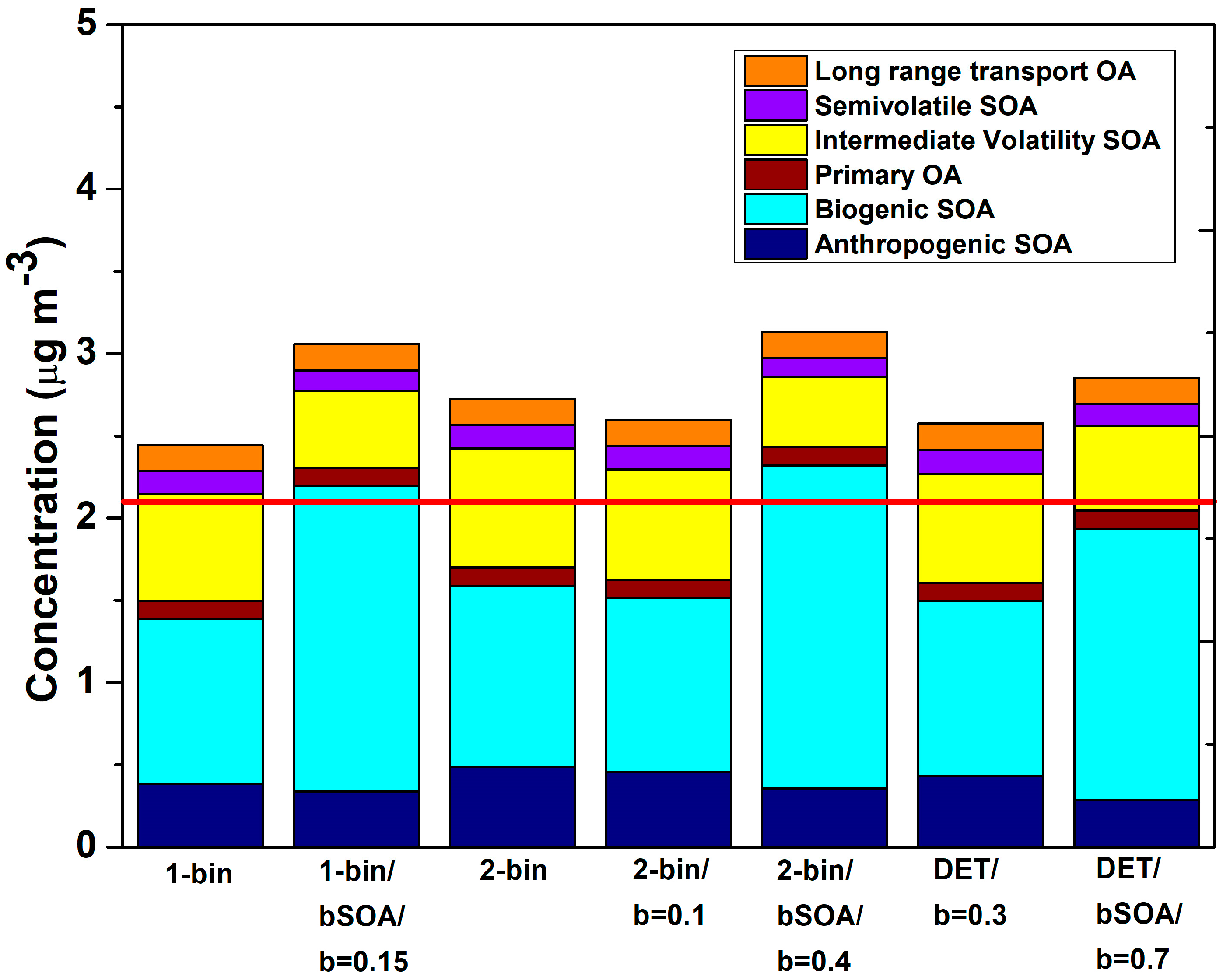
3.3. Predicted OA Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC (Intergovernmental Panel on Climate Change). Climate Change 2014: Mitigation of Climate Change; Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Nel, A. Air pollution-related illness: Effects of particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Ezzati, M.; Dockery, D.W. Fine-particulate air pollution and life expectancy in the United States. N. Engl. J. Med. 2009, 360, 376–386. [Google Scholar] [CrossRef]
- Caiazzo, F.; Ashok, A.; Waitz, I.A.; Yim, S.H.L.; Barrett, S.R. Air pollution and early deaths in the United States. Part I: Quantifying the impact of major sectors in 2005. Atmos. Environ. 2013, 79, 198–208. [Google Scholar] [CrossRef]
- Zhang, Q.; Jimenez, J.L.; Canagaratna, M.R.; Allan, J.D.; Coe, H.; Ulbrich, I.; Alfarra, M.R.; Takami, A.; Middlebrook, A.M.; Sun, Y.L.; et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys. Res. Lett. 2007, 34, L13801. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prévôt, A.S.H.; Zhang, Q.; Kroll, J.H.; Decarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; McKay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Szidat, S.; Ruff, M.; Perron, N.; Wacker, L.; Synal, H.-A.; Hallquist, M.; Shannigrahi, A.S.; Yttri, K.E.; Dye, C.; Simpson, D. Fossil and non-fossil sources of organic carbon (OC) and elemental carbon (EC) in Göteborg, Sweden. Atmos. Chem. Phys. 2009, 9, 1521–1535. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K.; Kanaya, Y.; Wang, Z. Contributions of biogenic volatile organic compounds to the formation of secondary organic aerosols over Mt. Tai, Central East China. Atmos. Environ. 2010, 44, 4817–4826. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Russell, L.M.; Sjostedt, S.J.; Vlasenko, A.; Slowik, J.G.; Abbatt, J.P.D.; Macdonald, A.M.; Li, S.M.; Liggio, J.; Toom-Sauntry, D.; et al. Biogenic oxidized organic functional groups in aerosol particles from a mountain forest site and their similarities to laboratory chamber products. Atmos. Chem. Phys. 2010, 10, 5075–5088. [Google Scholar] [CrossRef]
- Finessi, E.; Decesari, S.; Paglione, M.; Giulianelli, L.; Carbone, C.; Gilardoni, S.; Fuzzi, S.; Saarikoski, S.; Raatikainen, T.; Hillamo, R.; et al. Determination of the biogenic secondary organic aerosol fraction in the boreal forest by NMR spectroscopy. Atmos. Chem. Phys. 2012, 12, 941–959. [Google Scholar] [CrossRef]
- Pandis, S.N.; Harley, R.A.; Cass, G.R.; Seinfeld, J.H. Secondary organic aerosol formation and transport. Atmos. Environ. 1992, 26, 2266–2282. [Google Scholar] [CrossRef]
- Donahue, N.M.; Robinson, A.L.; Stanier, C.O.; Pandis, S.N. Coupled partitioning, dilution, and chemical aging of semivolatile organics. Environ. Sci. Technol. 2006, 40, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.E.; Donahue, N.M.; Pandis, S.N. Simulating secondary organic aerosol formation using the volatility basis-set approach in a chemical transport model. Atmos. Environ. 2008, 42, 7439–7451. [Google Scholar] [CrossRef]
- Donahue, N.M.; Kroll, J.H.; Pandis, S.N.; Robinson, A.L. A two-dimensional volatility basis set: 1. Organic-aerosol mixing thermodynamics. Atmos. Chem. Phys. 2011, 11, 3303–3318. [Google Scholar] [CrossRef]
- Donahue, N.M.; Kroll, J.H.; Pandis, S.N.; Robinson, A.L. A two-dimensional volatility basis set—Part 2: Diagnostics of organic-aerosol evolution. Atmos. Chem. Phys. 2012, 12, 615–634. [Google Scholar] [CrossRef]
- Murphy, B.N.; Donahue, N.M.; Fountoukis, C.; Pandis, S.N. Simulating the oxygen content of ambient organic aerosol with the 2D volatility basis set. Atmos. Chem. Phys. 2011, 11, 7859–7873. [Google Scholar] [CrossRef]
- Murphy, B.N.; Donahue, N.M.; Fountoukis, C.; Dall’Osto, M.; O’Dowd, C.; Kiendler-Scharr, A.; Pandis, S.N. Functionalization and fragmentation during ambient organic aerosol aging: Application of the 2-D volatility basis set to field studies. Atmos. Chem. Phys. 2012, 12, 10797–10816. [Google Scholar] [CrossRef]
- Karnezi, E.; Murphy, B.N.; Poulain, L.; Herrmann, H.; Wiedensohler, A.; Rubach, F.; Kiendler-Scharr, A.; Mentel, T.F.; Pandis, S.N. Simulation of atmospheric organic aerosol using its volatility–oxygen-content distribution during the PEGASOS 2012 campaign. Atmos. Chem. Phys. 2018, 18, 10759–10772. [Google Scholar] [CrossRef]
- Murphy, B.N.; Pandis, S. Simulating the formation of semivolatile primary and secondary organic aerosol in a regional chemical transport model. Environ. Sci. Technol. 2009, 43, 4722–4728. [Google Scholar] [CrossRef]
- Murphy, B.N.; Pandis, S.N. Exploring summertime organic aerosol formation in the eastern United States using a regional-scale budget approach and ambient measurements. J. Geophys. Res. 2010, 115, D24216. [Google Scholar] [CrossRef]
- Semeniuk, K.; Dastoor, A. current state of atmospheric aerosol thermodynamics and mass transfer modeling: A review. Atmosphere 2020, 11, 156. [Google Scholar] [CrossRef]
- Ling, Z.; Wu, L.; Wang, Y.; Shao, M.; Wang, X.; Huang, W. Roles of semivolatile and intermediate-volatility organic compounds in secondary organic aerosol formation and its implication: A review. J. Environ. Sci. 2022, 114, 259–285. [Google Scholar] [CrossRef]
- Hari, P.; Kulmala, M. Station for Measuring Ecosystem-Atmosphere Relations (SMEAR II). Boreal Environ. Res. 2005, 10, 315–322. [Google Scholar]
- Ng, N.L.; Herndon, S.C.; Trimborn, A.; Canagaratna, M.R.; Croteau, P.; Onasch, T.M.; Sueper, D.; Worsnop, D.R. An Aerosol Chemical Speciation Monitor (ACSM) for routine monitoring of atmospheric aerosol composition. Aerosol. Sci. Technol. 2011, 45, 770–784. [Google Scholar] [CrossRef]
- Carter, W.P.L. Programs and Files Implementing the SAPRC-99 Mechanism and Its Associated Emissions Processing Procedures for Models-3 and Other Regional Models. Available online: https://intra.cert.ucr.edu/~carter/pubs/s99mod3.pdf (accessed on 23 January 2023).
- Fountoukis, C.; Racherla, P.N.; van der Gon, H.A.C.D.; Polymeneas, P.; Charalampidis, P.E.; Pilinis, C.; Wiedensohler, A.; Dall’Osto, M.; O’Dowd, C.; Pandis, S.N. Evaluation of a three-dimensional chemical transport model (PMCAMx) in the European domain during the EUCAARI May 2008 campaign. Atmos. Chem. Phys. 2011, 11, 10331–10347. [Google Scholar] [CrossRef]
- Draxler, R.; Stunder, B.; Rolph, G.; Stein, A.; Taylor, A.; Zinn, S.; Loughner, C.; Crawford, A. HYSPLIT4 User’s Guide; Version 5.2; 2022. Available online: https://www.arl.noaa.gov/documents/reports/hysplit_user_guide.pdf (accessed on 23 January 2023).
- Nieminen, T.; Yli-Juuti, T.; Manninen, H.E.; Petäjä, T.; Kerminen, V.-M.; Kulmala, M. Technical note: New particle formation event forecasts during PEGASOS-Zeppelin Northern mission 2013 in Hyytiälä, Finland. Atmos. Chem. Phys. 2015, 15, 12385–12396. [Google Scholar] [CrossRef]
- Heikkinen, L.; Äijälä, M.; Riva, M.; Luoma, K.; Dällenbach, K.; Aalto, J.; Aalto, P.; Aliaga, D.; Aurela, M.; Keskinen, H.; et al. Long-term sub-micrometer aerosol chemical composition in the boreal forest: Inter- and intra-annual variability. Atmos. Chem. Phys. 2020, 20, 3151–3180. [Google Scholar] [CrossRef]

| Parameterization Name | Functionalization Scheme | bSOA Increase during Aging | Fragmentation Probability (b) |
|---|---|---|---|
| 1-bin | 1-bin | No | 0 |
| 1-bin/bSOA/b = 0.15 | 1-bin | Yes | 0.15 |
| 2-bin | 2-bin | No | 0 |
| 2-bin/b = 0.1 | 2-bin | No | 0.1 |
| 2-bin/bSOA/b = 0.4 | 2-bin | Yes | 0.4 |
| DET/b = 0.3 | DET | No | 0.3 |
| DET/bSOA/b = 0.7 | DET | Yes | 0.7 |
| 2D-VBS Parameterization | Predicted Average (μg m−3) | Fractional Error | Fractional Bias | Absolute Error (μg m−3) | Absolute Bias (μg m−3) | Root Mean Square Error (μg m−3) |
|---|---|---|---|---|---|---|
| 1-bin | 2.32 | 0.38 | −0.05 | 0.92 | 0.23 | 1.16 |
| 1-bin/bSOA/b = 0.15 | 2.91 | 0.34 | 0.17 | 1.08 | 0.82 | 1.69 |
| 2-bin | 2.59 | 0.31 | 0.07 | 0.92 | 0.5 | 1.35 |
| 2-bin/b = 0.1 | 2.46 | 0.34 | 0.02 | 0.91 | 0.38 | 1.25 |
| 2-bin/bSOA/b = 0.4 | 2.98 | 0.31 | 0.22 | 1.06 | 0.90 | 1.67 |
| DET/b = 0.3 | 2.45 | 0.33 | 0.02 | 0.89 | 0.36 | 1.21 |
| DET/bSOA/b = 0.7 | 2.73 | 0.26 | 0.15 | 0.84 | 0.64 | 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karnezi, E.; Heikkinen, L.; Kulmala, M.; Pandis, S.N. Simulating Atmospheric Organic Aerosol in the Boreal Forest Using Its Volatility-Oxygen Content Distribution. Atmosphere 2023, 14, 763. https://doi.org/10.3390/atmos14050763
Karnezi E, Heikkinen L, Kulmala M, Pandis SN. Simulating Atmospheric Organic Aerosol in the Boreal Forest Using Its Volatility-Oxygen Content Distribution. Atmosphere. 2023; 14(5):763. https://doi.org/10.3390/atmos14050763
Chicago/Turabian StyleKarnezi, Eleni, Liine Heikkinen, Markku Kulmala, and Spyros N. Pandis. 2023. "Simulating Atmospheric Organic Aerosol in the Boreal Forest Using Its Volatility-Oxygen Content Distribution" Atmosphere 14, no. 5: 763. https://doi.org/10.3390/atmos14050763
APA StyleKarnezi, E., Heikkinen, L., Kulmala, M., & Pandis, S. N. (2023). Simulating Atmospheric Organic Aerosol in the Boreal Forest Using Its Volatility-Oxygen Content Distribution. Atmosphere, 14(5), 763. https://doi.org/10.3390/atmos14050763







