Abstract
Background: Biological components of atmospheric aerosol affect the quality of atmospheric air. Long-term trends in changes of the concentrations of total protein (a universal marker of the biogenic component of atmospheric aerosol) and culturable microorganisms in the air are studied. Methods: Atmospheric air samples are taken at two locations in the south of Western Siberia and during airborne sounding of the atmosphere. Sample analysis is carried out in the laboratory using standard culture methods (culturable microorganisms) and the fluorescence method (total protein). Results: Negative trends in the average annual concentration of total protein and culturable microorganisms in the air are revealed over more than 20 years of observations. For the concentration of total protein and culturable microorganisms in the air, intra-annual dynamics is revealed. The ratio of the maximum and minimum values of these concentrations reaches an order of magnitude. The variability of concentrations does not exceed, as a rule, two times for total protein and three times for culturable microorganisms. At the same time, for the data obtained in the course of airborne sounding of the atmosphere, a high temporal stability of the vertical profiles of the studied concentrations was found. The detected biodiversity of culturable microorganisms in atmospheric air samples demonstrates a very high variability at all observation sites. Conclusions: The revealed long-term changes in the biological components of atmospheric aerosol result in a decrease in their contribution to the atmospheric air quality index.
1. Introduction
The quality of atmospheric air is mainly determined by the concentrations of classical atmospheric pollutants—nitrogen dioxide, sulfur dioxide, ozone and other photooxidants, and aerosol particles [1,2,3,4]. The composition of many aerosol particles includes organic and biological molecules, as well as various microorganisms, that affect the air quality index [4,5,6,7,8,9,10,11,12,13]. A wide variety of biogenic molecules included in the composition of aerosol particles was observed in publications [4,5,6,7,8,9,10,11,12,14,15,16,17,18,19,20]. A universal marker for molecules of biological origin in an atmospheric aerosol sample was previously proposed: total protein [21]. This marker is more versatile than those used in [22,23,24,25,26,27], since (1,3)-β-d-glucan, cellulose, levoglucosan, various lipids, sugars, etc. belong only to certain classes of biological compounds, while protein molecules are present in almost all aerosol particles of biogenic origin, including all microorganisms. The use of total organic carbon as a marker, which is often used in the characterization of atmospheric aerosol (see, for example, [28,29,30]), is not appropriate, because a significant part of it is due to low molecular weight organic compounds. According to [31,32], over 70% of organic carbon does not belong to biogenic carbon. Unfortunately, all articles devoted to the bioaerosol markers listed above do not belong to long-term studies and usually cover a time interval of no more than 2–3 years [10,14,15,16,17,18,19,20,23,31,32]. Therefore, one of the main goals of this work is to obtain long-term data on the concentration of total protein in atmospheric aerosols and analyze the obtained data.
Microorganisms are an extremely important component of atmospheric aerosol. Despite the fact that they usually make a small contribution to the air quality index (see, for example, [4], in which studies were carried out for a recreational region in which there is no industry), microorganisms in aerosol particles can cause toxic reactions, allergies, and even infectious diseases in humans, animals, and plants [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51].
Many hundreds of publications are devoted to the study of the concentration and biodiversity of microorganisms in atmospheric aerosol. Some of them will be discussed in Section 4. Quantitative studies of microorganisms in atmospheric aerosols, starting from the pioneering work of Pasteur and his students [52,53] and almost until the end of the last century, were carried out using cultural methods only. That is why in this work, cultural methods are taken as the basis for the study of biological diversity. For culturable bacteria and actinomycetes, a method has been developed for assessing their potential hazard to humans, which does not require experimental determination of this potential hazard using humans or laboratory animals [54]. The development of molecular biological methods has made it possible to significantly expand knowledge about the biological diversity of microorganisms whose genetic material is found in atmospheric aerosols, primarily for the possibility of finding uncultured microorganisms in samples [55,56,57,58,59,60,61,62]. However, there is no certainty that, firstly, the detected genetic material belongs specifically to microorganisms, it could be part of the debris of animals, plants or insects, and, secondly, studies of the biological properties of non-culturable microorganisms are extremely complex. Therefore, from the point of view of the authors, it is the culturable microorganisms that should be included in the priorities for monitoring the biological components of atmospheric aerosol.
Thus, the most representative indicators for monitoring the biogenic components of atmospheric aerosol are the concentrations of total protein and culturable microorganisms. Therefore, the goal of this article is to analyze the data of a long-term study of the total protein and culturable microorganisms’ concentrations in the atmospheric aerosol in the south of Western Siberia in order to identify the main patterns of their intra- and interannual behavior and changes in concentrations with sampling height.
2. Materials and Methods
2.1. Air Sampling
Atmospheric air sampling was carried out in three locations of the region: on the territory of the FBSI SSC VB “Vector” of Rospotrebnadzor (Vector) 4 times a day (sampling starts at 10:00, 16:00, 22:00, and 4:00 the next day) in the middle of each month; in the Klyuchi settlement—once a season for 7 consecutive days (usually sampling starts at 8:00–9:00) for the detection of culturable microorganisms and 30 consecutive days of round-the-clock sampling for the detection of total protein; high-altitude sampling—about 50 km south of Novosibirsk using the “Optik” laboratory (see Figures S1 and S2) on one of the days of each month [56]. A map showing the position of two sampling points and a typical laboratory aircraft flight path is shown in Figure S3. The Optic laboratory, installed on Antonov-30 aircraft or later on Tupolev-134 [63,64,65,66], includes a bioaerosol sampling device and additional devices for recording the physicochemical characteristics of the aerosol, meteorological conditions, etc. [63,67]. Isokinetic air sampling was carried out through a special air intake outside the cockpit (inserts in Figures 2 and 3 from [56]) [63,68] at an aircraft cruising speed of approximately 360 km/h. The work of the air intake with reduced dynamic pressure is based on the Venturi tube principle. The outside air with a pressure equal to the air pressure inside the aircraft cabin was supplied to the impingers, where samples were taken to analyze the presence and concentration of culturable microorganisms. Obviously, both the outside air inlet and the pipes leading to the impingers are characterized by a certain percentage of aerosol particle losses during their transportation. However, since the air inlet and air transportation tubes length are the same in all samples, these losses are the same for all samples and were no more than 10%. Samples were taken during the flight (12:00–15:00 local time) over the Karakan pine forest (see Figure S3) successively at eight heights: 7000, 5500, 4000, 3000, 2000, 1500, 1000, and 500 m [54]. To create sterile conditions, before each sampling or each flight, the available air communications were flushed with ethanol.
Air sampling was carried out using stainless steel impingers with a critical nozzle [54] (its analogue is described in [69]). These devices (manufactured by JSC “Experimental-design bureau of biological precision engineering”, Kirishi, Russia) maintain a constant flow rate at an air pressure differential of more than 4 × 104 Pa through the device. An A-D1-04 pump (JSC “Kot”, St. Petersburg, Russia) was used to pump air samples through the impinger. Fifty milliliters of colorless Hanks’ solution (Sigma, St. Louis, MO, USA) was used as an absorbing liquid. Above ground samples were taken at a flow rate of 50 ± 5 L/min for 30 min and samples at height for 5 to 10 min at each height. The retention efficiency of this impinger for aerosols of more than 0.3 μm exceeds 80%, making up a constant value of 90 ± 15% for particles with a diameter of more than 2 μm [21].
2.2. Culturable Microorganisms’ Concentration
The concentrations of culturable microorganisms were determined by standard microbiological methods. Samples were seeded onto Petri dishes containing agarized media. LB medium [70] was used to detect saprophytic bacteria; depleted LB medium (diluted 1:10) was used to isolate microorganisms inhibited by excess organic matter; starch-ammonia medium [71] was used to detect actinomycetes; soil agar was used for soil microorganisms; and Sabouraud medium [71] was used for lower fungi and yeasts. If necessary, serial dilutions of the samples were prepared. The cultures were incubated in a thermostat at 28–30 °C for 3–14 days. Phase-contrast microscopy was used to study the morphological characteristics of living cells of bacteria and fungi. Additionally, details of the fine structure of microorganism cells were studied using light microscopy of stained, fixed preparations (microscope Axioskop 40 Carl Zeiss, Oberkochen, Germany). Colony morphology was studied visually and using a Stemi 2000-C Carl Zeiss stereomicroscope (Germany). The taxonomic groups to which the detected microorganisms belong were determined according to [72,73,74]. The number of culturable microorganisms in the samples was calculated according to the standard method [75]. The number of microorganisms was averaged over 3–4 parallel samples inoculated on 4–5 different media.
2.3. Total Protein Concentration
A fluorimetric method based on the acquisition of intense fluorescence by a protein after its modification with a fluorogenic reagent was used to determine the concentration of the total protein in the samples. The 3,4-carboxybenzoylquinoline-2-carboxyaldehyde (CBQCA) reagent was used as a modifying reagent, which upon interaction with proteins forms fluorescent derivatives with a higher fluorescence quantum yield than other dyes [76]. Proteins can be determined in the presence of lipids, detergents, and surfactants. The detection threshold of the Shimadzu RF-520 spectrofluorimeter for total protein using CBQCA was 0.5 ng/mL of the concentrated sample, and the concentration error did not exceed 20%. Since the fluorescence of some PAHs is in the same wavelength range as the fluorescence of the total protein, the PAH-related value, which was determined independently, was subtracted from the total fluorescence values [77].
2.4. Data Analysis and Statistical Processing
The long-term dynamics of the concentrations of biological components of atmospheric aerosol was determined as follows. First, for all measurements carried out during the calendar year, the mean annual values of the quantity and its standard deviation from the mean were calculated, considering the statistics of the two-tailed Student’s t-distribution [78]. Further, in the Microsoft Excel program, a graph of the dependence of the average annual values on the year of measurements was built, and the values of the trends and their significance at the level of 95% were calculated using Microsoft Excel tools. The equations describing the trends of these concentrations and the significance of the trend difference from zero were calculated using the regression analysis built into Microsoft Excel.
When constructing the intra-annual dynamics of the determined concentrations of the biological components of atmospheric aerosol, we proceeded as follows. The measured values for each month of the year were normalized to the average annual value of the value. Further, for such normalized values, the average of all measurements in a particular month of all years and their root-mean-square deviations from the average were calculated considering the statistics of the two-tailed Student’s t-distribution [78].
Vertical profiles for the quantities measured during airborne sounding of the atmosphere were constructed as follows. The concentration values determined at each of the atmospheric sounding heights were normalized to the average value over all heights. Further, such normalized profiles for each of the altitudes were averaged over a calendar year or for a selected month for all years of the study. Their root-mean-square deviations from the mean were also calculated considering the statistics of the two-tailed Student’s t-distribution [78].
Since the results of the study of the diversity of cultivated microorganisms in samples demonstrate, as will be shown below, a very large variability, the microorganisms were grouped according to morphological features. Morphological features of culturable microorganisms are used at the initial stage of their identification. Further refinement of taxonomic affiliation is carried out in the study of the fine structure of cellular structures, biochemical characteristics, and growth properties of microorganisms in various environmental conditions, using the methods of genomic analysis. Such studies are labor intensive and time consuming. Since the ongoing monitoring studies involve the sampling and study of more than 250 samples per year, in-line and in-depth studies of the biochemistry and growth properties of grown isolates were not possible. Therefore, it was decided to confine the study to the morphological characteristics of the isolates. The following morphological groups were formed: cocci (spherical bacteria); bacilli (rod-shaped bacteria that form endospores); non-spore-bearing bacteria (bacteria that do not form spores); actinomycetes; and fungi (and a separate group—yeast). In additional studies, certain microbial isolates of particular interest in biotechnology have been identified as genus or species using standard identification methods. Proposed morpho-groups do not coincide with the classical taxonomic classification of microorganisms; however, the variability of these groups is somewhat lower than that of standard taxonomic groups.
3. Results
3.1. Long Term Trends
Long-term trends in the average annual concentrations of total protein and culturable microorganisms in the atmospheric aerosol of the south of Western Siberia are shown in Figure 1 and Figure 2 for land-based sites since 2001 and for airborne atmospheric sounding since 1999.
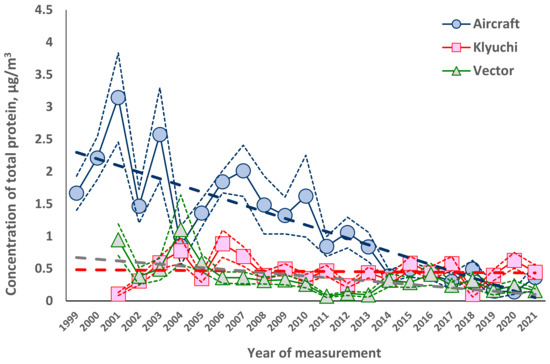
Figure 1.
Trends in the average annual concentration of total protein, measured at three locations near Novosibirsk. The figure shows the equations of the trend lines and the values of the reliability of the constructed approximation.
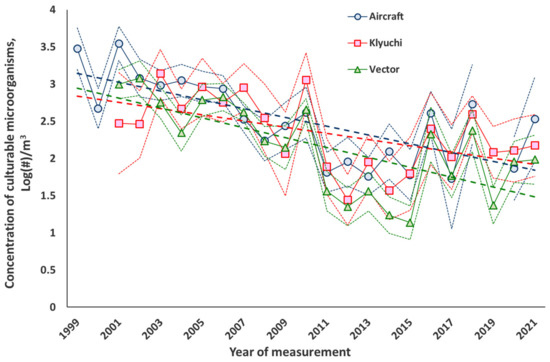
Figure 2.
The same for culturable microorganisms.
Using the Microsoft Excel program, equations were obtained that describe long-term trends in the concentration of total protein Y for all observation sites (mean and standard deviations of the values) and the significance levels of the difference between these trends from zero.
Airplane. Y = 2.400 ± 0.193 − (0.102 ± 0.014) × t, where t is the number of years (it equals 1, 2, 3, etc.). The trend is significant at the 95% reliability level.
Klyuchi. Y = 0.481 ± 0.092 − (0.002 ± 0.007) × t, where t is the number of years. The trend is not significant even at the 50% reliability level.
Vector. Y = 0.646 ± 0.092 − (0.026 ± 0.007) × t, where t is the number of years. The trend is significant at the 50% reliability level.
Similar calculations for the concentrations of culturable microorganisms gave the following results (we remind you that here the Y values are in decimal logarithms of the values):
Airplane. Y = 3.220 ± 0.172 − (0.061 ± 0.013) × t, where t is the number of years.
Keys. Y = 2.794 ± 0.191 − (0.042 ± 0.015) × t, where t is the number of years.
Vector. Y = 2.8866 ± 0.209 − (0.066 ± 0.017) × t, where t is the number of years.
The trends are significant at the 95% reliability level.
3.2. Intra-Annual Dependencies
The intra-annual dynamics of the concentrations of total protein and cultivated microorganisms in the atmospheric aerosol of the south of Western Siberia are shown in Figure 3 and Figure 4. For ground sampling sites, averaging for each month was carried out since 2001 for all years of the study and for airborne atmospheric sounding since 1999.
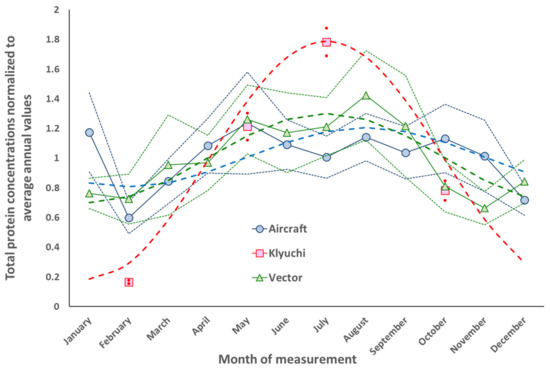
Figure 3.
Dynamics of the normalized intra-annual concentration of total protein, measured at three locations near Novosibirsk. Explanations are in the text.
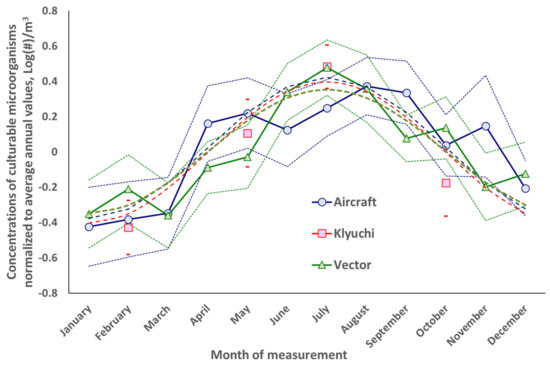
Figure 4.
Dynamics of the normalized intra-annual concentration of culturable microorganisms, measured at three locations near the city of Novosibirsk. Explanations are in the text.
Figure 3 and Figure 4 built in accordance with the procedure described in Section 2, show approximation dependences of the form Y = A × sin(2π × N/12 + φ), where A is the amplitude of the sinusoid, N is the ordinal number of the month, and φ is the phase of the maximum value of this function. These three parameters are optimized by the least square’s method for each of the observation sites. A detailed discussion of these results is given in Section 4, but here we note that for the northern hemisphere such a course of concentrations of total protein and culturable microorganisms in atmospheric aerosol is natural. In the cold season, many sources of bioaerosols “fall asleep” and, accordingly, the concentration of bioaerosols in the atmosphere decreases. On the contrary, in the warm season, these sources “wake up” and the concentrations of bioaerosols in the atmosphere increase.
3.3. Vertical Profiles of Culturable Microorganisms and Total Protein
Vertical profiles for quantities measured during airborne sounding of the atmosphere are shown in Figure 5. These are the concentration profiles of total protein and culturable microorganisms averaged over the entire measurement time, calculated in accordance with the procedure described in Section 2.4. Similar dependences for each year of measurements and for each month of measurements for all years of research are presented in Figures S4–S7 in the Supplementary Materials to the article. It should be noted that in these figures, the solid line connecting points at different heights represents the mean values at that height for each study year or selected month for all study years, and the dotted lines represent the standard deviations from the mean values as described in Section 2.4.
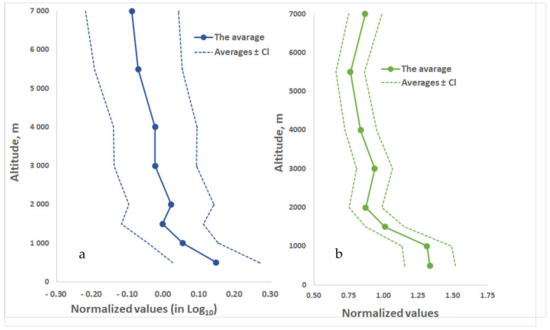
Figure 5.
Averaged over the entire measurement period, vertical profiles of the concentrations of: (a) culturable microorganisms (logarithmic scale) and (b) total protein.
Please note that the weak dependence of the concentrations of culturable microorganisms and total protein on height remains for each year of research (Figures S1 and S2) and for different months (Figures S3 and S4), and this conservation occurs against the background of the fact that the concentrations of culturable microorganisms themselves and total protein in the atmosphere during the year change by about an order of magnitude.
3.4. Observed Biodiversity of Culturable Microorganisms
The observed average annual biodiversity of culturable microorganisms is shown in Figure 6, Figure 7 and Figure 8. These figures show the proportions (as a percentage of the recorded concentration) of various morphogroups of microorganisms detected in all samples taken during one experiment (eight samples at different altitudes, four samples at the site of the FBSI SRC VB “Vector” of Rospotrebnadzor, seven samples on consecutive days in the village of Klyuchi).
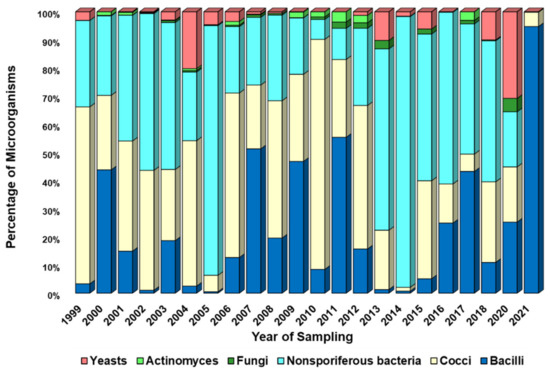
Figure 6.
Average annual variability of the ratio of culturable microorganisms in samples obtained during airborne sounding of the atmosphere. Explanations in the text.
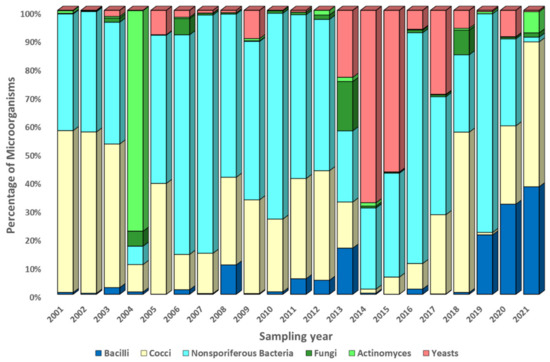
Figure 7.
Average annual variability of the ratio of culturable microorganisms in samples taken at the site in the village of Klyuchi. Explanations in the text.
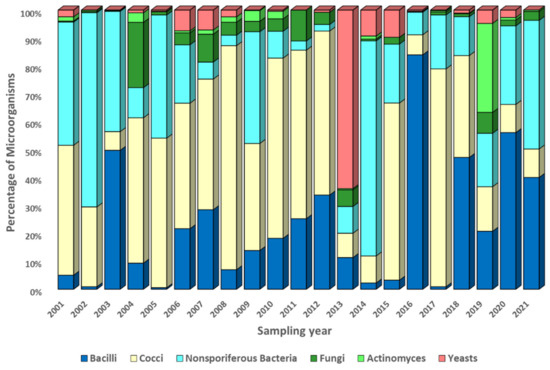
Figure 8.
The average annual variability of the ratio of culturable microorganisms in samples taken on the territory of the Vector’ site. Explanation in the text.
It should be noted that the morphogroups presented in Figure 6, Figure 7, Figure 8 and Figures S8–S10, which unite the studied microorganisms, are rather arbitrary and, as noted in Section 2.4, may belong to different taxonomic units. A high variability of the biological diversity of culturable microorganisms in atmospheric aerosol was found. This variability, driven both by different sources of bioaerosols and by pathways of aerosol particle transport in the atmosphere, is most pronounced in “neighboring” samples (Figures S5–S7) and is not significantly smoothed out when averaging annually (Figure 6, Figure 7 and Figure 8).
4. Discussion
The results obtained show that in the south of Western Siberia, for 20 years or more of observation, there is a decrease in the average annual concentrations of the biological components of atmospheric aerosol. For culturable microorganisms, this decrease reaches about an order of magnitude over the time of observation. For the average annual concentration of total protein, measured at altitudes of 500–7000 m, this decrease is also approximately an order of magnitude; for the site of the Vector the decrease is 2–3 times, while for the site in the village of Klyuchi, there is nearly no decrease. This is probably due to the fact that ongoing climate changes affect, first of all, remote sources of bioaerosols, whose contribution to the observed concentrations of biological components of atmospheric aerosol increases with altitude, where the contribution from local sources of bioaerosols decreases. Moreover, almost the same rates of decline in the average annual concentrations of culturable microorganisms at three observation sites indicate that climate change affects both the location and power of bioaerosol sources and their methods of transport in the atmosphere [21,33,34,79,80,81]. In addition, the difference in local sources of bioaerosols can explain the differences in the observed concentrations of the biological components of atmospheric aerosol at the research and production site of Vector and in the suburbs of Novosibirsk, in the village of Klyuchi. Unfortunately, for other regions in the northern hemisphere, observations lasting more than 10 years are presented only in single publications concerning only one kingdom or even a type of microorganism [34,82,83,84,85], and there are no such data in the literature for the total protein at all.
Analysis of the data in Figure 1 and Figure 2, which show the average annual values of the value and its standard deviation from the mean, considering the statistics of the two-tailed Student’s t-distribution [78], shows that for the concentrations of total protein, the variability is approximately 20% and for culturable microorganisms it is about 30%, which is comparable to the error in determining the concentration of culturable microorganisms. To a much greater extent, the variability of concentrations is manifested in the average annual values calculated for different years. Even for successive years, the difference in average concentrations can exceed two times. There are even more pronounced differences in the composition of culturable microorganisms in samples taken at adjacent altitudes within the same airborne sounding of the atmosphere: in some cases, such samples do not contain the same microorganisms at all.
The intra-annual variability of the concentrations of total protein and culturable microorganisms in atmospheric aerosol, built according to the algorithm described in Section 2.4, has the expected form for all observation sites. These concentrations are at their maximum in the warm season and at their minimum in the cold season. Moreover, according to the constructed approximation, all dependences have a maximum in July but for total protein aircraft samples, the maximum is in August. Only the January average value for the weighted concentration of total protein in high-altitude samples significantly exceeds the sinusoid constructed over the entire data set. For the temperate climatic zone of the northern hemisphere, similar dependences were also observed in other regions [81,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103].
For regions close to the equatorial zone, inverse relationships can also be observed [104,105,106,107], since the rainy seasons of the year, or even a weak dependence on the season in general [107], are likely to have a great influence on local sources of bioaerosols. Inverse relationships were also found in [96] for bacteria of the genus Pseudomonadales in Colorado in 2010–2011 and some other genera of microorganisms (determined using molecular biological methods, [108]). For greater Taipei, the maximum values of the total number of bacteria in the city itself were observed in spring/summer and for the suburbs in winter/spring [109]. For bacterial endotoxin, which is an integral component of some bacteria, a maximum was found in Beijing in the spring [110].
The literature presents intra-annual dynamics also, both of individual representatives of any genera of fungi and their total concentration [89,90,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133]. However, unlike the concentration of bacteria, which mainly reach a maximum in the atmosphere of regions in the warm season, for some genera of fungi, maximum concentrations are reached in other seasons [90,113,114,115,117,118,124,131,134].
For the vertical profiles normalized to average concentrations of total protein and cultivated microorganisms in atmospheric aerosol for each flight (Figure 5), these concentrations decrease with altitude, which is generally consistent with the data of other authors [135,136,137,138,139,140]. However, this decrease is not as large as, for example, a decrease in the number concentration of dust particles [141]. At the same time, the average values of the concentrations of total protein and cultivated microorganisms in atmospheric aerosol for each year or for each month of measurements for all years of research do not show a tendency to decrease in concentrations with height, Figures S4–S7. Furthermore, only on the basis of all the measurements taken, the profiles presented in Figure 5 reveal a decrease in the concentrations of total protein and cultivated microorganisms with height.
Let us now turn to the analysis of the diversity of culturable microorganisms in the samples. Figure 6, Figure 7 and Figure 8 show changes in the annual composition of culturable microorganisms for three sampling sites. As follows from these figures, there is no regularity in the change in the composition of culturable microorganisms over the year, even for one sampling site. Sometimes different bacilli predominate and sometimes different cocci or nonsporiferous bacteria predominate. At ground sampling sites, fungi, actinomycetes, or yeasts sometimes also predominate. Thus, the observed diversity of culturable microorganisms in atmospheric aerosol exhibits very high variability. It is caused both by the variety of sources of microorganisms and their location from which microorganisms enter the sample, by current weather conditions (for example, storms, precipitation, etc.), and by weather conditions in which airborne microorganisms were in the process of transfer. An even greater variability in the composition of culturable microorganisms is revealed in samples that combine only the results of one experiment (for example, airborne sounding of the atmosphere at eight altitudes or a 7-day sampling session in the village of Klyuchi), Figures S8–S10, not to mention huge variability from individual samples.
The concentration of culturable bacteria in the atmospheric air for some observation sites, including Western Siberia, is higher than that for culturable fungi [21,57,89,91,106,112,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156]. For other sites or sampling times, reverse [112,156,157,158,159,160,161,162,163] or approximately equal ratios of their concentrations [95,105,135,142,156,164,165,166,167] were found. In general, the observed concentration ratios largely depend on the proximity of certain local sources of bioaerosols and their power. The article [168] shows that in an area with a predominance of vegetation in the air, the concentration of fungi is higher, and in urban areas the concentration of cultivated bacteria is higher.
An analysis of the literature data shows that the observed dynamics of changes in the concentration of microorganisms in atmospheric aerosols, as noted in [169,170,171], are mainly determined by many external factors (meteorology, including extreme events, season, region, etc.), particle dispersity and chemical composition of particles in which microorganisms are located, and atmospheric pollution and the genus (species, strain) of the microorganism itself. Simple relationships linking the observed concentrations of microorganisms in atmospheric aerosols with these factors cannot currently be identified. In particular, the work [172] gives data according to which even in 1 min the concentration of bacteria in an aerosol in a forest can change by 500 times.
Literature data also indicate a high variability in the diversity of culturable microorganisms in samples (see, for example, [57,58,59,60,103]). It should be noted that modern molecular biological methods reveal a much greater diversity of microorganisms in samples; however, this diversity contains a large proportion of non-culturable microorganisms (see, for example, [57,60,61,62,81]), which we have not detected in our long-term studies.
It is presented in the literature that the proportion of culturable microorganisms in their total number in various samples ranges from 0.001% or less to about 75% [173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193], only in rare cases reaching almost 100% [105,113,194], which significantly narrows the actually detected diversity microorganisms in the aerosol. In addition, for cultural methods for detecting microorganisms in the atmospheric bioaerosols, there are still limitations associated with the fact that not all microorganisms during cultivation are able to initiate the growth of a colony, plaques, or infection center, since microorganisms of other species that have entered the sample or some components of bioaerosols are able to suppress the reproduction of the studied microorganism [195,196,197,198,199,200]. In addition, some growing colonies of microorganisms on a nutrient medium can mask others [72,195,198,199,200], making it difficult to determine the concentration of viable microorganisms by a culture method. All of the above also contribute to the high variability of cultural methods.
5. Conclusions
Thus, the conducted studies revealed long-term negative trends in the concentrations of total protein and culturable microorganisms in the atmospheric aerosol of the south of Western Siberia. For culturable microorganisms, the reported reductions in concentrations are about an order of magnitude over about 20 years of observations for all sites. For the total protein, a similar drop in concentration was found only for high-altitude samples, while for ground-based samples it was much smaller. Based on the results of airborne sounding, vertical profiles of the concentrations of total protein and culturable microorganisms in the atmospheric aerosol of the south of Western Siberia were constructed. These concentrations change slowly with sampling altitude. The variability of the obtained dependencies is not great and amounts to 20–30%. Significantly greater variability was found in the composition of culturable microorganisms in samples. To reduce it, it is necessary to continue long-term studies to accumulate data that will probably reduce the variability, averaged over a large number of samples, to acceptable values.
The conducted studies not only revealed the main patterns of changes in the concentrations of total protein and culturable microorganisms in the atmospheric aerosol of the south of Western Siberia and the observed biodiversity of microorganisms in these aerosols but also identified gaps in our knowledge of the ongoing processes. Firstly, it is not clear how the sources and power of bioaerosols change and how much the spectrum of biogenic molecules and microorganisms emitted by them changes. Secondly, it is not known how much the atmospheric flows, their powers, and their trajectories have changed. Thirdly, it is not evident how much temperature and humidity have changed in these flows. Finally, it remains to be learned how much the inactivation rates of culturable microorganisms carried in the flows will change. This list of gaps is supported in [81,196,197,201,202,203,204,205], which identified the similar gaps. The answers to these questions will allow researchers not only to understand the ongoing changes in the observed concentrations of bioaerosols in different regions but also to carry out mathematical simulations of the ongoing processes.
In the meantime, the long-term changes identified in this work in the biological components of atmospheric aerosol show a decrease in their contribution to the air quality index.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos13050651/s1, Figure S1: Photo of Antonov-30 aircraft. Insert is the samplers’ inlets, Figure S2: Photo of Tupolev-134 aircraft. Insert is the samplers’ Inlets, Figure S3: The map of sampling sites and aircraft’s typical flight trajectory, Figure S4: Vertical concentration profiles of total protein averaged over the year of measurements, Figure S5: Vertical concentration profiles of culturable microorganisms averaged over the year of measurements, Figure S6: Vertical concentration profiles of total protein averaged over selected months of the years of measurements, Figure S7: Vertical concentration profiles of culturable microorganisms averaged over selected months of the years of measurements, Figure S8: The composition of culturable microorganisms in samples that combine the results of one experiment (airborne sounding of the atmosphere at eight altitudes during one day), Figure S9: The composition of culturable microorganisms in samples that combine the results of one experiment (a 7-day sampling session in the village of Klyuchi), Figure S10: The composition of culturable microorganisms in samples that combine the results of one experiment (four samples of one day at Vector’s site).
Author Contributions
Conceptualization, A.S.S., B.D.B. and M.V.P.; methodology, A.S.S.; formal analysis, G.A.B.; investigation, A.S.S., B.D.B., I.S.A., S.E.O., I.K.R. and D.V.S.; data curation, G.A.B. and A.S.S.; writing—original draft preparation, A.S.S. and G.A.B.; writing—review and editing, A.S.S.; supervision, A.S.S.; project administration, A.S.S.; funding acquisition, A.S.S. and B.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Russian Federation Rospotrebnadzor, grant numbers 141-00069-18-01 and 141-00102-21-02 and RFFI grant № 19-05-50024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are presented in Excel files in local database and can be supplied by request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization, Regional Office for Europe. References Air Quality Guidelines for Europe, 2nd ed.; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2000; Available online: https://apps.who.int/iris/handle/10665/107335/ (accessed on 28 January 2022).
- Lv, M.; Li, Z.; Jiang, Q.; Chen, T.; Wang, Y.; Hu, A.; Cribb, M.; Cai, A. Contrasting trends of surface PM2.5, O3, and NO2 and their relationships with meteorological parameters in typical coastal and inland cities in the Yangtze River Delta. Int. J. Environ. Res. Public Health 2021, 18, 12471. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Han, L.; Zhang, X.; Zhou, W.; Li, W.; Qian, Y. A review of current air quality indexes and improvements under the multi-contaminant air pollution exposure. J. Environ. Manag. 2021, 279, 111681. [Google Scholar] [CrossRef] [PubMed]
- Safatov, A.S.; Agafonov, A.P.; Arshinov, M.Y.; Baklanov, A.M.; Belan, B.D.; Buryak, G.A.; Fofonov, A.V.; Generalov, V.M.; Kozlov, A.S.; Lapteva, N.A.; et al. Complex assessment of atmospheric air quality in the city of Gelendzhik. Atmos. Ocean. Opt. 2018, 31, 519–531. [Google Scholar] [CrossRef]
- Després, V.R.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.O.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Bhowmik, H.S.; Naresh, S.; Bhattu, D.; Rastogi, N.; Prévôt, A.S.H.; Tripathi, S.N. Temporal and spatial variability of carbonaceous species (EC.; OC.; WSOC and SOA) in PM2.5 aerosol over five sites of Indo-Gangetic Plain. Atmos. Pollut. Res. 2021, 12, 375–390. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, C.-Q.; Li, L.; Ren, L.; Ren, H.; Zhang, Z.; Li, Q.; Wang, S.; Hu, W.; Deng, J.; et al. Large contributions of biogenic and anthropogenic sources to fine organic aerosols in Tianjin, North China. Atmos. Chem. Phys. 2020, 20, 117–137. [Google Scholar] [CrossRef]
- Huang, W.; Saathoff, H.; Shen, X.; Ramisetty, R.; Leisner, T.; Mohr, C. Seasonal characteristics of organic aerosol chemical composition and volatility in Stuttgart, Germany. Atmos. Chem. Phys. 2019, 19, 11687–11700. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.; Kang, M.; Kim, D.; Batmunkh, T.; Bae, M.-S.; Park, K. Chemical characteristics of aerosols in coastal and urban ambient atmospheres. Aerosol Air Qual. Res. 2017, 17, 908–919. [Google Scholar] [CrossRef]
- Li, L.; Ren, L.; Ren, H.; Yue, S.; Xie, Q.; Zhao, W.; Kang, M.; Li, J.; Wang, Z.; Sun, Y.; et al. Molecular characterization and seasonal variation in primary and secondary organic aerosols in Beijing, China. J. Geophys. Res. Atmos. 2018, 123, 12394–12412. [Google Scholar] [CrossRef]
- Nozière, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgić, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef]
- Ramli, N.A.; Yusof, N.F.F.M.; Shith, S.; Suroto, A. Chemical and biological compositions associated with ambient respirable particulate matter: A review. Water Air Soil Pollut. 2020, 231, 120. [Google Scholar] [CrossRef]
- Samaké, A.; Bonin, A.; Jaffrezo, J.-L.; Taberlet, P.; Weber, S.; Uzu, G.; Jacob, V.; Conil, S.; Martins, J.M.F. High levels of primary biogenic organic aerosols in the atmosphere in summer are driven by only a few microbial taxa from the leaves of surrounding plants. Atmos. Chem. Phys. 2020, 20, 5609–5628. [Google Scholar] [CrossRef]
- Feltracco, M.; Barbaro, E.; Tedeschi, S.; Spolaor, A.; Turetta, C.; Vecchiato, M.; Morabito, E.; Zangrando, R.; Barbante, C.; Gambaro, A. Interannual variability of sugars in Arctic aerosol: Biomass burning and biogenic inputs. Sci. Total Environ. 2020, 706, 136089. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Vogel, A.L.; Esmaeilirad, S.; Cao, L.; Zheng, J.; Jaffrezo, J.-L.; Fermo, P.; Kasper-Giebl, A.; Daellenbach, K.R.; Chen, M.; et al. A 1-year characterization of organic aerosol composition and sources using an extractive electrospray ionization time-of-flight mass spectrometer (EESI-TOF). Atmos. Chem. Phys. 2020, 20, 7875–7893. [Google Scholar] [CrossRef]
- Haque, M.; Kawamura, K.; Deshmukh, D.K.; Fang, C.; Song, W.; Mengying, B.; Zhang, Y.-L. Characterization of organic aerosols from a Chinese megacity during winter: Predominance of fossil fuel combustion. Atmos. Chem. Phys. 2019, 19, 5147–5164. [Google Scholar] [CrossRef]
- Hunter, J.F.; Day, D.A.; Palm, B.B.; Yatavelli, R.L.N.; Chan, A.W.H.; Kaser, L.; Cappellin, L.; Hayes, P.L.; Cross, E.S.; Carrasquillo, A.J.; et al. Comprehensive characterization of atmospheric organic carbon at a forested site. Nat. Geosci. 2017, 10, 748–753. [Google Scholar] [CrossRef]
- Samaké, A.; Jaffrezo, J.-L.; Favez, O.; Weber, S.; Jacob, V.; Albinet, A.; Riffault, V.; Perdrix, E.; Waked, A.; Golly, B.; et al. Polyols and glucose particulate species as tracers of primary biogenic organic aerosols at 28 French sites. Atmos. Chem. Phys. 2019, 19, 3357–3374. [Google Scholar] [CrossRef]
- Samaké, A.; Jaffrezo, J.-L.; Favez, O.; Weber, S.; Jacob, V.; Canete, T.; Albinet, A.; Charron, A.; Riffault, V.; Perdrix, E.; et al. Arabitol, mannitol, and glucose as tracers of primary biogenic organic aerosol: The influence of environmental factors on ambient air concentrations and spatial distribution over France. Atmos. Chem. Phys. 2019, 19, 11013–11030. [Google Scholar] [CrossRef]
- Golly, B.; Waked, A.; Weber, S.; Samake, A.; Jacob, V.; Conil, S.; Rangognio, J.; Chrétien, E.; Vagnot, M.-P.; Robic, P.-Y.; et al. Organic markers and OC source apportionment for seasonal variations of PM2.5 at 5 rural sites in France. Atmos. Environ. 2019, 198, 142–157. [Google Scholar] [CrossRef]
- Safatov, A.S.; Buryak, G.A.; Andreeva, I.S.; Olkin, S.E.; Reznikova, I.K.; Sergeev, A.N.; Belan, B.D.; Panchenko, M.V. Atmospheric bioaerosols. In Aerosols—Science and Technology; Agranovski, I., Ed.; Wiley–VCH Verlag GmbH & Co. KGaA: Wienheim, Germany, 2010; pp. 407–454. [Google Scholar]
- Yao, M.; Wu, Y.; Zhen, S.; Mainelis, G. Comparison of electrostatic collection and liquid impinging methods when collecting airborne house dust allergens, endotoxin and (1,3)-β-d-glucans. J. Aerosol Sci. 2009, 40, 492–502. [Google Scholar] [CrossRef]
- Pashynska, V.; Vermeylen, R.; Vas, G.; Maenhaut, W.; Claeys, M. Development of a gas chromatographic/ion trap mass spectrometric method for the determination of levoglucosan and saccharidic compounds in atmospheric aerosols. Application to urban aerosols. J. Mass Spectrom. 2002, 37, 1249–1257. [Google Scholar] [CrossRef]
- Axelsson, B.-O.; Saraf, A.; Larsson, L. Determination of ergosterol in organic dust by gas chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1995, 666, 77–84. [Google Scholar] [CrossRef]
- Larsson, L. Determination of microbial chemical markers by gas chromatography-mass spectrometry—Potential for diagnosis and studies on metabolism in situ. Apmis 1994, 102, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kunit, M.; Puxbaum, H. Enzymatic determination of the cellulose content of atmospheric aerosols. Atmos. Environ. 1996, 30, 1233–1236. [Google Scholar] [CrossRef]
- Xu, S.; Ren, L.; Lang, Y.; Hou, S.; Ren, H.; Wei, L.; Wu, L.; Deng, J.; Hu, W.; Pan, X.; et al. Molecular markers of biomass burning and primary biological aerosols in urban Beijing: Size distribution and seasonal variation. Atmos. Chem. Phys. 2020, 20, 3623–3644. [Google Scholar] [CrossRef]
- Viidanoja, J.; Kerminen, V.-M.; Hillamo, R. Measuring the size distribution of atmospheric organic and black carbon using impactor sampling coupled with thermal carbon analysis: Method development and uncertainties. Aerosol Sci. Technol. 2002, 36, 607–616. [Google Scholar] [CrossRef][Green Version]
- Bencardino, M.; Andreoli, V.; D’Amore, F.; De Simone, F.; Mannarino, V.; Castagna, J.; Moretti, S.; Naccarato, A.; Sprovieri, F.; Pirrone, N. Carbonaceous aerosols collected at the observatory of Monte Curcio in the Southern Mediterranean Basin. Atmosphere 2019, 10, 592. [Google Scholar] [CrossRef]
- Choi, J.-K.; Ban, S.-J.; Kim, Y.-P.; Kim, Y.-H.; Yi, S.-M.; Zoh, K.-D. Molecular marker characterization and source appointment of particulate matter and its organic aerosols. Chemosphere 2015, 134, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.U.; Kistler, M.; Shahid, I.; Alam, K.; Chishtie, F.; Mahmud, T.; Kasper-Giebl, A. Composition and source apportionment of saccharides in aerosol particles from an agro-industrial zone in the Indo-Gangetic Plain. Environ. Sci. Pollut. Res. 2020, 27, 14124–14137. [Google Scholar] [CrossRef]
- Majewski, G.; Rogula-Kozłowska, W.; Rozbicka, K.; Rogula-Kopiec, P.; Mathews, B.; Brandyk, A. Concentration, chemical composition and origin of PM1: Results from the first long-term measurement campaign in Warsaw (Poland). Aerosol Air Qual. Res. 2018, 18, 636–654. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Chen, X.; Kumari, D.; Achal, V. A Review on airborne microbes: The characteristics of sources, pathogenicity and geography. Atmosphere 2020, 11, 919. [Google Scholar] [CrossRef]
- Górny, R.L. Microbial aerosols: Sources, properties, health effects, exposure assessment—A review. KONA Powder Part. J. 2020, 37, 64–84. [Google Scholar] [CrossRef]
- Jing, W.; Liu, Q.; Wang, M.; Zhang, X.; Chen, J.; Sui, G.; Wang, L. A method for particulate matter 2.5 (PM2.5) biotoxicity assay using luminescent bacterium. Ecotoxicol. Environ. Saf. 2019, 170, 796–803. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar] [CrossRef]
- Mack, S.M.; Madl, A.K.; Pinkerton, K.E. Respiratory health effects of exposure to ambient particulate matter and bioaerosols. Compr. Physiol. 2020, 10, 1–20. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Lewandowska, A.U.; Śliwińska-Wilczewska, S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment—Review study. Environ. Int. 2019, 131, 104964. [Google Scholar] [CrossRef]
- Walser, S.M.; Gerstner, D.G.; Brenner, B.; Bünger, J.; Eikmann, T.; Janssen, B.; Kolb, S.; Kolk, A.; Nowak, D.; Raulf, M.; et al. Evaluation of exposure–response relationships for health effects of microbial bioaerosols—A systematic review. Int. J. Hyg. Environ. Health 2015, 218, 577–589. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chakraborty, A.; Ghosh, D.; Mandal, J.; Biswas, S.; Mukhopadhyay, U.K.; Bhattacharya, S.G. Effect of airborne Alternaria conidia, ozone exposure, PM10 and weather on emergency visits for asthma in school-age children in Kolkata city, India. Aerobiologia 2014, 30, 137–148. [Google Scholar] [CrossRef]
- O’Gorman, C.M.; Fuller, H.T. Prevalence of culturable airborne spores of selected allergenic and pathogenic fungi in outdoor air. Atmos. Environ. 2008, 42, 4355–4368. [Google Scholar] [CrossRef]
- Tham, R.; Dharmage, S.C.; Taylor, P.E.; Katelaris, C.H.; Vicendese, D.; Abramson, M.J.; Erbas, B. Outdoor fungi and child asthma health service attendances. Pediatr. Allergy Immunol. 2014, 25, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Reponen, T.; Hershey, G.K.K. Fungal exposure and asthma: IgE and non-IgE-mediated mechanisms. Curr. Allergy Asthma Rep. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.M.; Hovmøller, M.S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef]
- Chen, P.-S.; Chen, Y.-S.; Lin, H.-H.; Liu, P.-J.; Ni, W.-F.; Hsueh, P.-T.; Liang, S.-H.; Chen, C.; Chen, Y.-L. Airborne transmission of melioidosis to humans from environmental aerosols contaminated with B. pseudomallei. PLoS Negl. Trop. Dis. 2015, 9, e0003834. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, A.; Goldblatt, M. Aerobiology and its role in the transmission of infectious diseases. J. Pathog. 2013, 2013, 493960. [Google Scholar] [CrossRef]
- Paton, D.J.; Gubbins, S.; King, D.P. Understanding the transmission of foot-and-mouth disease virus at different scales. Curr. Opin. Virol. 2018, 28, 85–91. [Google Scholar] [CrossRef]
- Sharoni, S.; Trainic, M.; Schatz, D.; Lehahn, Y.; Flores, M.J.; Bidle, K.D.; Ben-Dor, S.; Rudich, Y.; Koren, I.; Vardi, A. Infection of phytoplankton by aerosolized marine viruses. Proc. Natl. Acad. Sci. USA 2015, 112, 6643–6647. [Google Scholar] [CrossRef]
- Ypma, R.J.F.; Jonges, M.; Bataille, A.; Stegeman, A.; Koch, G.; Van Boven, M.; Koopmans, M.; Van Ballegooijen, W.M.; Wallinga, J. Genetic data provide evidence for wind-mediated transmission of highly pathogenic avian influenza. J. Infect. Dis. 2013, 207, 730–735. [Google Scholar] [CrossRef]
- Alexandersen, S.; Zhang, Z.; Donaldson, A.I.; Garland, A.J.M. The pathogenesis and diagnosis of foot-and-mouth disease. J. Comp. Pathol. 2003, 129, 1–36. [Google Scholar] [CrossRef]
- Pasteur, L. Memoire sur les Corpuscles Organises qui Existent dans l’Atmosphere, Examen de la Doctrine des Generations Spontaneesm; Masson: Paris, France, 1861. [Google Scholar]
- Miquel, P. Les Organisms Vivants de l’Atmosphère; Gauthier-Villars: Paris, France, 1883. [Google Scholar]
- Safatov, A.S.; Andreeva, I.S.; Belan, B.D.; Buryak, G.A.; Emel’Yanova, E.K.; Jaenicke, R.; Panchenko, M.V.; Pechurkina, N.I.; Puchkova, L.I.; Repin, V.E.; et al. To what extent can viable bacteria in atmospheric aerosols be dangerous for humans? CLEAN Soil Air Water 2008, 36, 564–571. [Google Scholar] [CrossRef]
- Be, N.A.; Thissen, J.B.; Fofanov, V.Y.; Allen, J.E.; Rojas, M.; Golovko, G.; Fofanov, Y.; Koshinsky, H.; Jaing, C.J. Metagenomic analysis of the airborne environment in urban spaces. Microb. Ecol. 2015, 69, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Safatov, A.; Andreeva, I.; Buryak, G.; Ohlopkova, O.; Olkin, S.; Puchkova, L.; Reznikova, I.; Solovyanova, N.; Belan, B.; Panchenko, M.; et al. How has the hazard to humans of microorganisms found in atmospheric aerosol in the south of western Siberia changed over 10 years? Int. J. Environ. Res. Public Health 2020, 17, 51651. [Google Scholar] [CrossRef] [PubMed]
- Gusareva, E.S.; Acerbi, E.; Lau, K.J.X.; Luhung, I.; Premkrishnan, B.N.V.; Kolundžija, S.; Purbojati, R.W.; Wong, A.; Houghton, J.N.I.; Miller, D.; et al. Microbial communities in the tropical air ecosystem follow a precise diel cycle. Proc. Natl. Acad. Sci. USA 2019, 116, 23299–23308. [Google Scholar] [CrossRef] [PubMed]
- Hai, V.D.; Hoang, S.M.T.; Hung, N.T.Q.; Ky, N.M.; Gwi-Nam, B.; Ki-Hong, P.; Chang, S.W.; Bach, Q.-V.; Nhu-Trang, T.-T.; Nguyen, D.D. Characteristics of airborne bacteria and fungi in the atmosphere in Ho Chi Minh city, Vietnam—A case study over three years. Int. Biodeterior. Biodegrad. 2019, 145, 104819. [Google Scholar] [CrossRef]
- Karlsson, E.; Johansson, A.-M.; Ahlinder, J.; Lundkvist, M.J.; Singh, N.J.; Brodin, T.; Forsman, M.; Stenberg, P. Airborne microbial biodiversity and seasonality in northern and southern Sweden. PeerJ 2020, 8, e8424. [Google Scholar] [CrossRef]
- Polymenakou, P.N.; Mandalakis, M.; Macheras, M.; Oulas, A.; Kristoffersen, J.B.; Christakis, C.A.; Terzoglou, V.; Stavroulaki, M. High genetic diversity and variability of microbial communities in near-surface atmosphere of Crete island, Greece. Aerobiologia 2020, 36, 341–353. [Google Scholar] [CrossRef]
- Naznin, R.; Sultana, N.; Hossain, N.; Islam, M.N.; Tabassum, A.; Gani, A.; Jannat, M. Conventional and molecular identification of culturable airborne bacteria. Plant Tissue Cult. Biotechnol. 2020, 30, 15–25. [Google Scholar] [CrossRef]
- Banchi, E.; Pallavicini, A.; Muggia, L. Relevance of plant and fungal DNA metabarcoding in aerobiology. Aerobiologia 2020, 36, 9–23. [Google Scholar] [CrossRef]
- Zuev, V.E.; Belan, B.D.; Kabanov, D.M.; Kovalevskii, V.K.; Luk’yanov, O.Y.; Meleshkin, V.E.; Mikushev, M.K.; Panchenko, M.V.; Penner, I.E.; Pokrovskii, E.D.; et al. The “OPTIK–E” AN—30 aircraft—Laboratory for ecological investigations. Atmos. Ocean. Opt. 1992, 5, 658–663. [Google Scholar]
- Belan, B.D.; Zuev, V.E.; Panchenko, M.V. Main results of airborne sounding of aerosol conducted at the Institute of Atmospheric Optics from 1981 till 1991. Atmos. Ocean. Opt. 1995, 8, 131–156. [Google Scholar]
- Belan, B.D.; Ligotskii, A.V.; Luk’yanov, O.Y.; Mikushev, M.K.; Plokhikh, I.N.; Podanev, A.V.; Tolmachev, G.N. Database on the results of ecological survey of air basins. Atmos. Ocean. Opt. 1994, 7, 585–590. [Google Scholar]
- Belan, B.D. Airborne ecological sounding of the atmosphere. Atmos. Ocean. Opt. 1993, 6, 205–222. [Google Scholar]
- Zuev, V.E. Equipment for Remote Probing of Atmospheric Parameters; TP SB USSR AS, USSR: Tomsk, Russia, 1987. (In Russian) [Google Scholar]
- Nazarov, L.E. Isokinetic atmospheric aerosol sampling from an airplane. Tr. Inst. Exp. Meteor. 1985, 9, 76–81. (In Russian) [Google Scholar]
- Griffiths, W.D.; DeCosemo, G.A.L. The assessment of bioaerosols: A critical review. J. Aerosol Sci. 1994, 25, 1425–1458. [Google Scholar] [CrossRef]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1972. [Google Scholar]
- Saggie, J. The Methods of Soil Microbiology; Kolos Publishers: Moscow, Russia, 1983. (In Russian) [Google Scholar]
- Gerhardt, F.; Murray, R.G.E.; Wood, W.A.; Krieg, N.R. Methods of General Bacteriology, 2nd ed.; Publisher American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Starr, M.P.; Stolp, H.; Truper, H.G.; Balows, A.; Schlegel, H.G. The Prokaryotes. A Handbook on Habitats, Isolation, and Identification of Bacteria; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Lebedeva, M.N. A Guide for Practical Studies in Medical Microbiology; Medicine: Moscow, Russia, 1973. (In Russian) [Google Scholar]
- Ashmarin, I.P.; Vorobyov, A.A. Statistical Methods in Microbiological Studies; Medgiz: Leningrad, Russia, 1962. (In Russian) [Google Scholar]
- You, W.W.; Haugland, R.P.; Ryan, D.K.; Haugland, R.P. 3-(4-carboxybenzoyl)quinoline-2-carboxaldehyde, a reagent with broad dynamic range for the assay of proteins and lipoproteins in solution. Anal. Biochem. 1997, 244, 277–282. [Google Scholar] [CrossRef]
- State Committee of USSR for Hydrometeorology. The Detection of Polycyclic Aromatic Hydrocarbons (The Method of High-Performance Liquid Chromatography); Guidance for Atmospheric Pollution Control, RD 52.04.186-89; Ministry of Health of USSR: Moscow, Russia, 1991; pp. 647–657. (In Russian) [Google Scholar]
- Sachs, L. Statistische Methoden; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1972. [Google Scholar]
- Tignat-Perrier, R.; Dommergue, A.; Thollot, A.; Keuschnig, C.; Magand, O.; Vogel, T.M.; LaRose, C. Global airborne microbial communities controlled by surrounding landscapes and wind conditions. Sci. Rep. 2019, 9, 14441. [Google Scholar] [CrossRef]
- Xu, C.; Wei, M.; Chen, J.; Zhu, C.; Li, J.; Xu, X.; Wang, W.; Zhang, Q.; Ding, A.; Kan, H.; et al. Profile of inhalable bacteria in PM2.5 at Mt. Tai, China: Abundance, community, and influence of air mass trajectories. Ecotoxicol. Environ. Saf. 2019, 168, 110–119. [Google Scholar] [CrossRef]
- Cáliz, J.; Triadó-Margarit, X.; Camarero, L.; Casamayor, E.O. A long-term survey unveils strong seasonal patterns in the airborne microbiome coupled to general and regional atmospheric circulations. Proc. Natl. Acad. Sci. USA 2018, 115, 12229–12234. [Google Scholar] [CrossRef]
- Damialis, A.; Vokou, D.; Gioulekas, D.; Halley, J.M. Long-term trends in airborne fungal-spore concentrations: A comparison with pollen. Fungal Ecol. 2015, 13, 150–156. [Google Scholar] [CrossRef]
- Corden, J.M.; Millington, W.M. The long-term trends and seasonal variation of the aeroallergen Alternaria in Derby, UK. Aerobiologia 2001, 17, 127–136. [Google Scholar] [CrossRef]
- Millington, W.M.; Corden, J.M. Long term trends in indoor Aspergillus/Penicillum spore in Derby, UK from 1970 to 2003 and comparative study in 1994 and 1996 with indoor air of two local houses. Aerobiologia 2005, 21, 105–113. [Google Scholar] [CrossRef]
- Olsen, Y.; Skjøth, C.A.; Hertel, O.; Rasmussen, K.; Sigsgaard, T.; Gosewinkel, U. Airborne Cladosporium and Alternaria spore concentrations through 26 years in Copenhagen, Denmark. Aerobiologia 2020, 36, 141–157. [Google Scholar] [CrossRef]
- Schumacher, C.J.; Pöhlker, C.; Aalto, P.; Hiltunen, V.; Petäjä, T.; Kulmala, M.; Pöschl, U.; Huffman, J.A. Seasonal cycles of fluorescent biological aerosol particles in boreal and semi-arid forests of Finland and Colorado. Atmos. Chem. Phys. 2013, 13, 11987–12001. [Google Scholar] [CrossRef]
- Lighthart, B. Mini-review of the concentration variations found in the alfresco atmospheric bacterial populations. Aerobiologia 2000, 16, 7–16. [Google Scholar] [CrossRef]
- Burrows, S.M.; Elbert, W.; Lawrence, M.G.; Pöschl, U. Bacteria in the global atmosphere—Part 1: Review and synthesis of literature data for different ecosystems. Atmos. Chem. Phys. 2009, 9, 9263–9280. [Google Scholar] [CrossRef]
- Bovallius, Å.; Bucht, B.; Roffey, R.; Ånäs, P. Three-year investigation of the natural airborne bacterial flora at four localities in Sweden. Appl. Environ. Microbiol. 1978, 35, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Lighthart, B. The annual bacterial particle concentration and size distribution in the ambient atmosphere in a rural area of the Willamette Valley, Oregon. Aerosol Sci. Technol. 2000, 32, 393–403. [Google Scholar] [CrossRef]
- Bowers, R.M.; Clements, N.; Emerson, J.B.; Wiedinmyer, C.; Hannigan, M.P.; Fierer, N. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ. Sci. Technol. 2013, 47, 12097–12106. [Google Scholar] [CrossRef]
- Bertolini, V.; Gandolfi, I.; Ambrosini, R.; Bestetti, G.; Innocente, E.; Rampazzo, G.; Franzetti, A. Temporal variability and effect of environmental variables on airborne bacterial communities in an urban area of Northern Italy. Appl. Microbiol. Biotechnol. 2013, 97, 6561–6570. [Google Scholar] [CrossRef]
- Sarda-Estève, R.; Baisnée, D.; Guinot, B.; Sodeau, J.; O’Connor, D.; Belmonte, J.; Besancenot, J.-P.; Petit, J.-E.; Thibaudon, M.; Oliver, G.; et al. Variability and geographical origin of five years airborne fungal spore concentrations measured at Saclay, France from 2014 to 2018. Remote Sens. 2019, 11, 1671. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Li, C.-S. Collection efficiencies of aerosol samplers for virus-containing aerosols. J. Aerosol Sci. 2005, 36, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Negrin, M.M.; Del Panno, M.T.; Ronco, A.E. Study of bioaerosols and site influence in the La Plata area (Argentina) using conventional and DNA (fingerprint) based methods. Aerobiologia 2007, 23, 249–258. [Google Scholar] [CrossRef]
- Delort, A.-M.; Vaïtilingom, M.; Amato, P.; Sancelme, M.; Parazols, M.; Mailhot, G.; Laj, P.; Deguillaume, L. A short overview of the microbial population in clouds: Potential roles in atmospheric chemistry and nucleation processes. Atmos. Res. 2010, 98, 249–260. [Google Scholar] [CrossRef]
- Li, M.; Qi, J.; Zhang, H.; Huang, S.; Li, L.; Gao, D. Concentration and size distribution of bioaerosols in an outdoor environment in the Qingdao coastal region. Sci. Total Environ. 2011, 409, 3812–3819. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Mainka, A.; Pastuszka, J.S. Concentration and size distribution of culturable bacteria in ambient air during spring and winter in Gliwice: A typical urban area. Atmosphere 2017, 8, 239. [Google Scholar] [CrossRef]
- Sivri, N.; Bağcıgil, A.F.; Metiner, K.; Şeker, D.Z.; Orak, S.; Durak, S.G.; Sönmez, V.Z. Culturable airborne bacteria and isolation of methicillin-resistant coagulase-negative staphylococci from outdoor environments on European side of Istanbul, Turkey. Arch. Environ. Prot. 2016, 42, 77–86. [Google Scholar] [CrossRef]
- Striluk, M.L.; Aho, K.; Weber, C.F. The effect of season and terrestrial biome on the abundance of bacteria with plant growth-promoting traits in the lower atmosphere. Aerobiologia 2017, 33, 137–149. [Google Scholar] [CrossRef]
- Tanaka, D.; Terada, Y.; Nakashima, T.; Sakatoku, A.; Nakamura, S. Seasonal variations in airborne bacterial community structures at a suburban site of central Japan over a 1-year time period using PCR-DGGE method. Aerobiologia 2015, 31, 143–157. [Google Scholar] [CrossRef]
- Sarda-Estève, R.; Baisnée, D.; Guinot, B.; Mainelis, G.; Sodeau, J.; O’Connor, D.; Besancenot, J.P.; Thibaudon, M.; Monteiro, S.; Petit, J.-E.; et al. Atmospheric biodetection part I: Study of airborne bacterial concentrations from January 2018 to May 2020 at Saclay, France. Int. J. Environ. Res. Public Health 2020, 17, 76292. [Google Scholar] [CrossRef]
- Bowers, R.M.; McCubbin, I.B.; Hallar, A.G.; Fierer, N. Seasonal variability in airborne bacterial communities at a high-elevation site. Atmos. Environ. 2012, 50, 41–49. [Google Scholar] [CrossRef]
- Agarwal, S.; Mandal, P.; Majumdar, D.; Aggarwal, S.G.; Srivastava, A. Characterization of bioaerosols and their relation with OC, EC and carbonyl VOCs at a busy roadside restaurants-cluster in New Delhi. Aerosol Air Qual. Res. 2016, 16, 3198–3211. [Google Scholar] [CrossRef]
- Mentese, S.; Arısoy, M.; Rad, A.Y.; Güllü, G.; Arisoy, M. Bacteria and fungi levels in various indoor and outdoor environments in Ankara, Turkey. CLEAN Soil Air Water 2009, 37, 487–493. [Google Scholar] [CrossRef]
- Crawford, C.; Reponen, T.; Lee, T.; Iossifova, Y.; Levin, L.; Adhikari, A.; Grinshpun, S.A. Temporal and spatial variation of indoor and outdoor airborne fungal spores, pollen, and (1→3)-β-d-glucan. Aerobiologia 2009, 25, 147–158. [Google Scholar] [CrossRef]
- Ravva, S.V.; Hernlem, B.J.; Sarreal, C.Z.; Mandrell, R.E. Bacterial communities in urban aerosols collected with wetted-wall cyclonic samplers and seasonal fluctuations of live and culturable airborne bacteria. J. Environ. Monit. 2012, 14, 473–481. [Google Scholar] [CrossRef]
- Park, J.; Ichijo, T.; Nasu, M.; Yamaguchi, N. Investigation of bacterial effects of Asian dust events through comparison with seasonal variability in outdoor airborne bacterial community. Sci. Rep. 2016, 6, 35706. [Google Scholar] [CrossRef]
- Kallawicha, K.; Lung, S.-C.C.; Chuang, Y.-C.; Wu, C.-D.; Chen, T.-H.; Tsai, Y.-J.; Chao, H.J. Spatiotemporal distributions and land-use regression models of ambient bacteria and endotoxins in the Greater Taipei area. Aerosol Air Qual. Res. 2015, 15, 1448–1459. [Google Scholar] [CrossRef]
- Guan, T.; Yao, M.; Wang, J.; Fang, Y.; Hu, S.; Wang, Y.; Dutta, A.; Yang, J.; Wu, Y.; Hu, M.; et al. Airborne endotoxin in fine particulate matter in Beijing. Atmos. Environ. 2014, 97, 35–42. [Google Scholar] [CrossRef]
- Jones, A.M.; Harrison, R.M. The effects of meteorological factors on atmospheric bioaerosol concentrations—A review. Sci. Total Environ. 2004, 326, 151–180. [Google Scholar] [CrossRef]
- Di Giorgio, C.; Krempff, A.; Guiraud, H.; Binder, P.; Tiret, C.; Dumenil, G. Atmospheric pollution by airborne microorganisms in the city of Marseilles. Atmos. Environ. 1996, 30, 155–160. [Google Scholar] [CrossRef]
- Adhikari, A.; Sen, M.M.; Guptabhattacharya, S.; Chanda, S. Airborne viable, non-viable, and allergenic fungi in a rural agricultural area of India: A 2-year study at five outdoor sampling stations. Sci. Total Environ. 2004, 326, 123–141. [Google Scholar] [CrossRef]
- Abu-Dieyeh, M.H.; Barham, R.; Abu-Elteen, K.; Al-Rashidi, R.; Shaheen, I. Seasonal variation of fungal spore populations in the atmosphere of Zarqa area, Jordan. Aerobiologia 2010, 26, 263–276. [Google Scholar] [CrossRef]
- Almaguer, M.; Rojas-Flores, T.I.; Rodríguez-Rajo, F.J.; Aira, M.-J. Airborne basidiospores of Coprinus and Ganoderma in a Caribbean region. Aerobiologia 2014, 30, 197–204. [Google Scholar] [CrossRef]
- Bardei, F.; Bouziane, H.; del Mar Trigo, M.; Ajouray, N.; El Haskouri, F.; Kadiri, M. Atmospheric concentrations and intradiurnal pattern of Alternaria and Cladosporium conidia in Tétouan (NW of Morocco). Aerobiologia 2017, 33, 221–228. [Google Scholar] [CrossRef]
- De Antoni Zoppas, B.C.; Valencia-Barrera, R.M.; Vergamini Duso, S.M.; Fernández-González, D. Fungal spores prevalent in the aerosol of the city of Caxias do Sul, Rio Grande do Sul, Brazil, over a 2-year period (2001–2002). Aerobiologia 2006, 22, 117–124. [Google Scholar] [CrossRef]
- Fang, Z.; Ouyang, Z.; Hu, L.; Wang, X.; Zheng, H.; Lin, X. Culturable airborne fungi in outdoor environments in Beijing, China. Sci. Total Environ. 2005, 350, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.M.; Fatima, K.; Al-Frayh, A.; Al-Sedairy, S.T. Prevalence of airborne basidiospores in three coastal cities of Saudi Arabia. Aerobiologia 2005, 21, 139–145. [Google Scholar] [CrossRef]
- Kallawicha, K.; Tsai, Y.-J.; Chuang, Y.-C.; Lung, S.-C.C.; Wu, C.-D.; Chen, T.-H.; Chen, P.-C.; Chompuchan, C.; Chao, H.J. The spatiotemporal distributions and determinants of ambient fungal spores in the Greater Taipei area. Environ. Pollut. 2015, 204, 173–180. [Google Scholar] [CrossRef]
- Lang-Yona, N.; Dannemiller, K.; Yamamoto, N.; Burshtein, N.; Peccia, J.; Yarden, O.; Rudich, Y. Annual distribution of allergenic fungal spores in atmospheric particulate matter in the Eastern Mediterranean; a comparative study between ergosterol and quantitative PCR analysis. Atmos. Chem. Phys. 2012, 12, 2681–2690. [Google Scholar] [CrossRef]
- Lugauskas, A.; Šveistyte, L.; Ulevičius, V. Concentration and species diversity of airborne fungi near busy streets in Lithuanian urban areas. Ann. Agric. Environ. Med. 2003, 10, 233–239. [Google Scholar]
- Medrela-Kuder, E. Seasonal variations in the occurrence of culturable airborne fungi in outdoor and indoor air in Craców. Int. Biodeterior. Biodegrad. 2003, 52, 203–205. [Google Scholar] [CrossRef]
- Mullins, J.; Hutcheson, P.S.; Slavin, R.G. Aspergillus fumigatus spore concentration in outside air: Cardiff and St. Louis compared. Clin. Exp. Allergy 1984, 14, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Nayar, T.S.; Jothish, P.S. An assessment of the air quality in indoor and outdoor air with reference to fungal spores and pollen grains in four working environments in Kerala, India. Aerobiologia 2013, 29, 131–152. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, H.; Abreu, I. Annual variation of fungal spores in atmosphere of Porto: 2003. Ann. Agric. Environ. Med. 2005, 12, 309–315. [Google Scholar] [PubMed]
- Oliveira, M.; Ribeiro, H.; Delgado, J.L.; Abreu, I. Seasonal and intradiurnal variation of allergenic fungal spores in urban and rural areas of the North of Portugal. Aerobiologia 2009, 25, 85–98. [Google Scholar] [CrossRef]
- Palmas, F.; Cosentino, S. Comparison between fungal airspore concentration at two different sites in the South of Sardinia. Grana 1990, 29, 87–95. [Google Scholar] [CrossRef]
- Pashley, C.H.; Fairs, A.; Free, R.C.; Wardlaw, A.J. DNA analysis of outdoor air reveals a high degree of fungal diversity, temporal variability, and genera not seen by spore morphology. Fungal Biol. 2012, 116, 214–224. [Google Scholar] [CrossRef]
- Priyamvada, H.; Singh, R.K.; Akila, M.; Ravikrishna, R.; Verma, R.S.; Gunthe, S.S. Seasonal variation of the dominant allergenic fungal aerosols—One year study from southern Indian region. Sci. Rep. 2017, 7, 11171. [Google Scholar] [CrossRef]
- Shelton, B.G.; Kirkland, K.H.; Flanders, W.D.; Morris, G.K. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 2002, 68, 1743–1753. [Google Scholar] [CrossRef]
- Takahashi, T. Airborne fungal colony-forming units in outdoor and indoor environments in Yokohama, Japan. Mycopathologia 1997, 139, 23–33. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gilbert, G.S. Meteorological factors associated with abundance of airborne fungal spores over natural vegetation. Atmos. Environ. 2017, 162, 87–99. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, H.; Delgado, J.L.; Abreu, I. The effects of meteorological factors on airborne fungal spore concentration in two areas differing in urbanisation level. Int. J. Biometeorol. 2009, 53, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.M.; Lauber, C.L.; Wiedinmyer, C.; Hamady, M.; Hallar, A.G.; Fall, R.; Knight, R.; Fierer, N. Characterization of airborne microbial communities at a high-elevation site and their potential to act as atmospheric ice nuclei. Appl. Environ. Microbiol. 2009, 75, 5121–5130. [Google Scholar] [CrossRef] [PubMed]
- Imshenetsky, A.A.; Lysenko, S.V.; Kazakov, G.A. Upper boundary of the biosphere. Appl. Environ. Microbiol. 1978, 35, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Hara, K.; Kobayashi, F.; Kurosaki, Y.; Kakikawa, M.; Matsuki, A.; Chen, B.; Shi, G.; Hasegawa, H.; Iwasaka, Y. Vertical distribution of airborne bacterial communities in an Asian-dust downwind area, Noto Peninsula. Atmos. Environ. 2015, 119, 282–293. [Google Scholar] [CrossRef]
- Maki, T.; Hara, K.; Iwata, A.; Lee, K.C.; Kawai, K.; Kai, K.; Kobayashi, F.; Pointing, S.B.; Archer, S.; Hasegawa, H.; et al. Variations in airborne bacterial communities at high altitudes over the Noto Peninsula (Japan) in response to Asian dust events. Atmos. Chem. Phys. 2017, 17, 11877–11897. [Google Scholar] [CrossRef]
- Núñez, A.; Moreno, D.A. The differential vertical distribution of the airborne biological particles reveals an atmospheric reservoir of microbial pathogens and aeroallergens. Microb. Ecol. 2020, 80, 322–333. [Google Scholar] [CrossRef]
- Maki, T.; Bin, C.; Kai, K.; Kawai, K.; Fujita, K.; Ohara, K.; Kobayashi, F.; Davaanyam, E.; Noda, J.; Minamoto, Y.; et al. Vertical distributions of airborne microorganisms over Asian dust source region of Taklimakan and Gobi Desert. Atmos. Environ. 2019, 214, 116848. [Google Scholar] [CrossRef]
- Rosen, J.M. The vertical distribution of dust to 30 kilometers. J. Geophys. Res. 1964, 69, 4673–4676. Available online: https://www.patarnott.com/atms749/pdf/dustSoundingByBalloon.pdf (accessed on 28 January 2022). [CrossRef]
- Mamta; Shrivastava, J.N.; Satsangi, G.P.; Kumar, R. Assessment of bioaerosol pollution over Indo-Gangetic plain. Environ. Sci. Pollut. Res. 2015, 22, 6004–6009. [Google Scholar] [CrossRef]
- Abdel Hameed, A.A.; Khoder, M.I.; Yuosra, S.; Osman, A.M.; Ghanem, S. Diurnal distribution of airborne bacteria and fungi in the atmosphere of Helwan area, Egypt. Sci. Total Environ. 2009, 407, 6217–6222. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Shamy, M.; Redal, M.A.; Khoder, M.; Awad, A.H.; Elserougy, S. Microorganisms associated particulate matter: A preliminary study. Sci. Total Environ. 2014, 479–480, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ran, P.; Ho, K.F.; Lu, W.; Li, B.; Gu, Z.; Song, C.; Wang, J. Concentrations and size distributions of airborne microorganisms in Guangzhou during summer. Aerosol Air Qual. Res. 2012, 12, 1336–1344. [Google Scholar] [CrossRef]
- Fierer, N.; Liu, Z.; Rodríguez-Hernández, M.; Knight, R.; Henn, M.; Hernandez, M.T. Short-term temporal variability in airborne bacterial and fungal populations. Appl. Environ. Microbiol. 2008, 74, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.J.; Kim, H.B.; Lee, B.U. Concentration of environmental fungal and bacterial bioaerosols during the monsoon season. J. Aerosol Sci. 2014, 77, 31–37. [Google Scholar] [CrossRef]
- Vilavert, L.; Nadal, M.; Figueras, M.J.; Kumar, V.; Domingo, J.L. Levels of chemical and microbiological pollutants in the vicinity of a waste incineration plant and human health risks: Temporal trends. Chemosphere 2011, 84, 1476–1483. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Z.; Qian, L.; Zhao, Z.; Zhang, C.; Fu, Y.; Li, J.; Zhang, C.; Lu, B.; Qian, J. Biological and chemical compositions of atmospheric particulate matter during hazardous haze days in Beijing. Environ. Sci. Pollut. Res. 2018, 25, 34540–34549. [Google Scholar] [CrossRef]
- Faridi, S.; Hassanvand, M.S.; Naddafi, K.; Yunesian, M.; Nabizadeh, R.; Sowlat, M.H.; Kashani, H.; Gholampour, A.; Niazi, S.; Zare, A.; et al. Indoor/outdoor relationships of bioaerosol concentrations in a retirement home and a school dormitory. Environ. Sci. Pollut. Res. 2015, 22, 8190–8200. [Google Scholar] [CrossRef]
- Abdel Hameed, A.A.; Habeeballah, T. Air microbial contamination at the Holy Mosque, Makkah, Saudi Arabia. Curr. World Environ. 2013, 8, 179–187. [Google Scholar] [CrossRef]
- Ghimire, P.S.; Kang, S.; Sajjad, W.; Ali, B.; Tripathee, L.; Chen, P. Microbial community composition analysis in spring aerosols at urban and remote sites over the Tibetan Plateau. Atmosphere 2020, 11, 527. [Google Scholar] [CrossRef]
- Madhwal, S.; Prabhu, V.; Sundriyal, S.; Shridhar, V. Ambient bioaerosol distribution and associated health risks at a high traffic density junction at Dehradun city, India. Environ. Monit. Assess. 2020, 192, 196. [Google Scholar] [CrossRef]
- Makut, M.D.; Nyam, M.A.; Shehu, L.; Anzaku, S.J. A survey of the microflora of the outdoor air environment of Keffi Metropolis, Nasarawa State, Nigeria. Afr. J. Microbiol. Res. 2014, 8, 2650–2655. [Google Scholar] [CrossRef]
- Morakinyo, O.M.; Mokgobu, M.I.; Mukhola, M.S.; Godobedzha, T. Biological composition of respirable particulate matter in an industrial vicinity in south Africa. Int. J. Environ. Res. Public Health 2019, 16, 40629. [Google Scholar] [CrossRef] [PubMed]
- Tignat-Perrier, R.; Dommergue, A.; Thollot, A.; Magand, O.; Vogel, T.M.; Larose, C. Microbial functional signature in the atmospheric boundary layer. Biogeosciences 2020, 17, 6081–6095. [Google Scholar] [CrossRef]
- Fulton, J.D.; Mitchell, R.B. Microorganisms of the upper atmosphere. II. Microorganisms in two types of air masses at 690 meters over a city. Appl. Microbiol. 1966, 14, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J.D. Microorganisms of the upper atmosphere. III. Relationship between altitude and micropopulation. Appl. Microbiol. 1966, 14, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.D.; Pady, S.M. Microbiological studies of air masses over Montreal during 1950 and 1951. Can. J. Bot. 1954, 32, 591–600. [Google Scholar] [CrossRef]
- Li, K.; Dong, S.; Wu, Y.; Yao, M. Comparison of the biological content of air samples collected at ground level and at higher elevation. Aerobiologia 2010, 26, 233–244. [Google Scholar] [CrossRef]
- Kaarakainen, P.; Meklin, T.; Rintala, H.; Hyvärinen, A.; Kärkkäinen, P.; Vepsäläinen, A.; Hirvonen, M.-R.; Nevalainen, A. Seasonal variation in airborne microbial concentrations and diversity at landfill, urban and rural sites. CLEAN Soil Air Water 2008, 36, 556–563. [Google Scholar] [CrossRef]
- Lee, B.U.; Lee, G.; Heo, K.J. Concentration of culturable bioaerosols during winter. J. Aerosol Sci. 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jo, W.-K. Characteristics of indoor and outdoor bioaerosols at Korean high-rise apartment buildings. Environ. Res. 2006, 101, 11–17. [Google Scholar] [CrossRef]
- Rajput, P.; Anjum, M.H.; Gupta, T. One year record of bioaerosols and particles concentration in Indo-Gangetic Plain: Implications of biomass burning emissions to high-level of endotoxin exposure. Environ. Pollut. 2017, 224, 98–106. [Google Scholar] [CrossRef]
- Vaïtilingom, M.; Attard, E.; Gaiani, N.; Sancelme, M.; Deguillaume, L.; Flossmann, A.I.; Amato, P.; Delort, A.-M. Long-term features of cloud microbiology at the puy de Dôme (France). Atmos. Environ. 2012, 56, 88–100. [Google Scholar] [CrossRef]
- Agarwal, S. Seasonal variability in size-segregated airborne bacterial particles and their characterization at different source-sites. Environ. Sci. Pollut. Res. 2017, 24, 13519–13527. [Google Scholar] [CrossRef]
- Abdel Hameed, A.A.; Khodr, M.I. Suspended particulates and bioaerosols emitted from an agricultural non-point source. J. Environ. Monit. 2001, 3, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Balyan, P.; Ghosh, C.; Das, S.; Banerjee, B.D. Spatio-temporal characterisation of bioaerosols at diverse outdoor land-use sites in an urban environment. Aerobiologia 2020, 36, 77–81. [Google Scholar] [CrossRef]
- DasSarma, P.; DasSarma, S. Survival of microbes in Earth’s stratosphere. Curr. Opin. Microbiol. 2018, 43, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ščevková, J.; Kováč, J. First fungal spore calendar for the atmosphere of Bratislava, Slovakia. Aerobiologia 2019, 35, 343–356. [Google Scholar] [CrossRef]
- Haddrell, A.E.; Thomas, R.J. Aerobiology: Experimental considerations, observations, and future tools. Appl. Environ. Microbiol. 2017, 83, e00809-17. [Google Scholar] [CrossRef]
- Clauß, M.; Springorum, A.C.; Schulz, J.; Hartung, J. Zeitlich hochauflösende Messungen von Pilzsporen und Bakterien in der Außenluft: Einfluss von kurzzeitigen Konzentrationsveränderungen auf die Ergebnisse verschiedener Probenahmeverfahren zur Messung der Hintergrundkonzentration. Gefahrst.-Reinhalt. Luft 2012, 72, 155–161. [Google Scholar]
- Hughes, J.B.; Hellmann, J.J.; Ricketts, T.H.; Bohannan, B.J.M. Counting the uncountable: Statistical approaches to estimating microbial diversity. Appl. Environ. Microbiol. 2001, 67, 4399–4406. [Google Scholar] [CrossRef]
- Marcy, Y.; Ouverney, C.; Bik, E.M.; Lösekann, T.; Ivanova, N.; Martin, H.G.; Szeto, E.; Platt, D.; Hugenholtz, P.; Relman, D.A.; et al. Dissecting biological “dark matter” with single-cell genetic analysis of rare and uncultivated TM7 microbes from the human mouth. Proc. Natl. Acad. Sci. USA 2007, 104, 11889–11894. [Google Scholar] [CrossRef] [PubMed]
- Zengler, K.; Toledo, G.; Rappé, M.; Elkins, J.; Mathur, E.J.; Short, J.M.; Keller, M. Cultivating the uncultured. Proc. Natl. Acad. Sci. USA 2002, 99, 15681–15686. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Balasubramanian, R. Discrimination of viable from non-viable Gram-negative bacterial pathogens in airborne particles using propidium monoazide-assisted qPCR. Sci. Total Environ. 2013, 449, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liang, W.; Kan, B. Enumeration of viable non-culturable Vibrio cholerae using propidium monoazide combined with quantitative PCR. J. Microbiol. Methods 2015, 115, 147–152. [Google Scholar] [CrossRef]
- Hara, K.; Zhang, D. Bacterial abundance and viability in long-range transported dust. Atmos. Environ. 2012, 47, 20–25. [Google Scholar] [CrossRef]
- Tong, Y.; Lighthart, B. Diurnal distribution of total and culturable atmospheric bacteria at a rural site. Aerosol Sci. Technol. 1999, 30, 246–254. [Google Scholar] [CrossRef]
- Amato, P.; Parazols, M.; Sancelme, M.; Mailhot, G.; Laj, P.; Delort, A.-M. An important oceanic source of micro-organisms for cloud water at the Puy de Dôme (France). Atmos. Environ. 2007, 41, 8253–8263. [Google Scholar] [CrossRef]
- Bauer, H.; Schueller, E.; Weinke, G.; Berger, A.; Hitzenberger, R.; Marr, I.L.; Puxbaum, H. Significant contributions of fungal spores to the organic carbon and to the aerosol mass balance of the urban atmospheric aerosol. Atmos. Environ. 2008, 42, 5542–5549. [Google Scholar] [CrossRef]
- Blais-Lecours, P.; Perrott, P.; Duchaine, C. Non-culturable bioaerosols in indoor settings: Impact on health and molecular approaches for detection. Atmos. Environ. 2015, 110, 45–53. [Google Scholar] [CrossRef]
- Colwell, R.R. Viable but nonculturable bacteria: A survival strategy. J. Infect. Chemother. 2000, 6, 121–125. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, S.; Tormo-Molina, R.; Maya-Manzano, J.M.; Silva-Palacios, I.; Gonzalo-Garijo, Á. Outdoor airborne fungi captured by viable and non-viable methods. Fungal Ecol. 2014, 7, 16–26. [Google Scholar] [CrossRef]
- Hasegawa, N.; Yamasaki, S.; Horiguchi, Y. A study of bacterial culturability during bioaerosol challenge test using a test chamber. J. Aerosol Sci. 2011, 42, 397–407. [Google Scholar] [CrossRef]
- Hubad, B.; Lapanje, A. The efficient method for simultaneous monitoring of the culturable as well as nonculturable airborne microorganisms. PLoS ONE 2013, 8, e82186. [Google Scholar] [CrossRef] [PubMed]
- Hýsek, J.; Fisar, Z.; Binek, B. Long-run monitoring of bacteria, yeasts and other micromycetes in the air of an industrial conurbation. Grana 1991, 29, 450–453. [Google Scholar] [CrossRef]
- Nasrabadi, A.M.; An, S.; Kwon, S.-B.; Hwang, J. Investigation of live and dead status of airborne bacteria using UVAPS with LIVE/DEAD® BacLight Kit. J. Aerosol Sci. 2018, 115, 181–189. [Google Scholar] [CrossRef]
- Roszak, D.B.; Colwell, R.R. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 1987, 51, 365–379. [Google Scholar] [CrossRef]
- Staley, J.T.; Konopka, A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu. Rev. Microbiol. 1985, 39, 321–346. [Google Scholar] [CrossRef]
- Wainwright, M.; Wickramasinghe, N.C.; Narlikar, J.V.; Rajaratnam, P.; Perkins, J. Confirmation of the presence of viable but non-cultureable bacteria in the stratosphere. Int. J. Astrobiol. 2004, 3, 13–15. [Google Scholar] [CrossRef][Green Version]
- Wilson, M.; Lindow, S.E. Relationship of total viable and culturable cells in epiphytic populations of Pseudomonas syringae. Appl. Environ. Microbiol. 1992, 59, 3908–3913. [Google Scholar] [CrossRef]
- Hu, W.; Murata, K.; Fan, C.; Huang, S.; Matsusaki, H.; Fu, P.; Zhang, D. Abundance and viability of particle-attached and free-floating bacteria in dusty and nondusty air. Biogeosciences 2020, 17, 4477–4487. [Google Scholar] [CrossRef]
- Hu, W.; Murata, K.; Fukuyama, S.; Kawai, Y.; Oka, E.; Uematsu, M.; Zhang, D. Concentration and viability of airborne bacteria over the Kuroshio extension region in the northwestern Pacific Ocean: Data from three cruises. J. Geophys. Res. Atmos. 2017, 122, 12892–12905. [Google Scholar] [CrossRef]
- Chen, C.-C.; Yu, T.-S.; Chang, J.-Y.; Chang, C.-W.; Shih, T.-S.; Hwang, J.-S. A computer simulation study on bioaerosol colony counting error due to masking effect. Ann. Occup. Hyg. 1998, 42, 501–510. [Google Scholar] [CrossRef]
- Archer, S.D.J.; Lee, K.C.; Caruso, T.; Maki, T.; Lee, C.K.; Cary, S.C.; Cowan, D.A.; Maestre, F.T.; Pointing, S.B. Airborne microbial transport limitation to isolated Antarctic soil habitats. Nat. Microbiol. 2019, 4, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Droprinchinski Martins, L.; Hallak, R.; Cruz Alves, R.; de Almeida, D.S.; Squizzato, R.; Moreira, C.A.B.; Beal, A.; Da Silva, I.; Rudke, A.; Martins, J.A. Long-range transport of aerosols from biomass burning over southeastern south America and their implications on air quality. Aerosol Air Qual. Res. 2018, 18, 1734–1745. [Google Scholar] [CrossRef]
- Cox, C.S.; Wathes, C.M. Bioaerosols Handbook; CRC Press: Boca Raton, FL, USA; Lewis Publ.: London, UK, 1995. [Google Scholar]
- Pyrri, I.; Kapsanaki-Gotsi, E. A comparative study on the airborne fungi in Athens, Greece, by viable and non-viable sampling methods. Aerobiologia 2007, 23, 3–15. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Emelko, M.B.; Reilly, P.M. Quantification of analytical recovery in particle and microorganism enumeration methods. Environ. Sci. Technol. 2010, 44, 1705–1712. [Google Scholar] [CrossRef]
- Li, X.; Cheng, X.; Wu, W.; Wang, Q.; Tong, Z.; Zhang, X.; Deng, D.; Li, Y. Forecasting of bioaerosol concentration by a back propagation neural network model. Sci. Total Environ. 2020, 698, 134315. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Smith, D.J.; Griffin, D.W.; Jaffe, D.A.; Wawrik, B.; Burrows, S.M.; Christner, B.C.; Gonzalez-Martin, C.; Lipp, E.K.; Schmale, D.G.S., III; et al. Science questions and knowledge gaps to study microbial transport and survival in Asian and African dust plumes reaching North America. Aerobiologia 2018, 34, 425–435. [Google Scholar] [CrossRef]
- Smith, D.J.; Jaffe, D.A.; Birmele, M.N.; Griffin, D.W.; Schuerger, A.C.; Hee, J.; Roberts, M.S. Free tropospheric transport of microorganisms from Asia to North America. Microb. Ecol. 2012, 64, 973–985. [Google Scholar] [CrossRef]
- Van Leuken, J.P.G.; Swart, A.N.; Droogers, P.; Van Pul, A.; Heederik, D.; Havelaar, A.H. Climate change effects on airborne pathogenic bioaerosol concentrations: A scenario analysis. Aerobiologia 2016, 32, 607–617. [Google Scholar] [CrossRef]
- Hanson, M.C.; Petch, G.M.; Ottosen, T.-B.; Skjøth, C.A. Climate change impact on fungi in the atmospheric microbiome. Sci. Total Environ. 2022, 830, 154491. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).