Low- and Medium-Cost Sensors for Tropospheric Ozone Monitoring—Results of an Evaluation Study in Wrocław, Poland
Abstract
:1. Introduction
2. Material and Methods
2.1. Measurement Site Description
2.2. Reference Ozone Data
2.3. O3 Sensors and Measurement Setup
3. Data Analysis
3.1. Data Preparation
3.2. Reproducibility between Units
3.3. Relationship between O3 Sensors and Reference Analyser
3.4. Attempts to Test the Equivalence of Sensor Measurements
4. Results and Discussion
4.1. Long-Term Sensor Performance
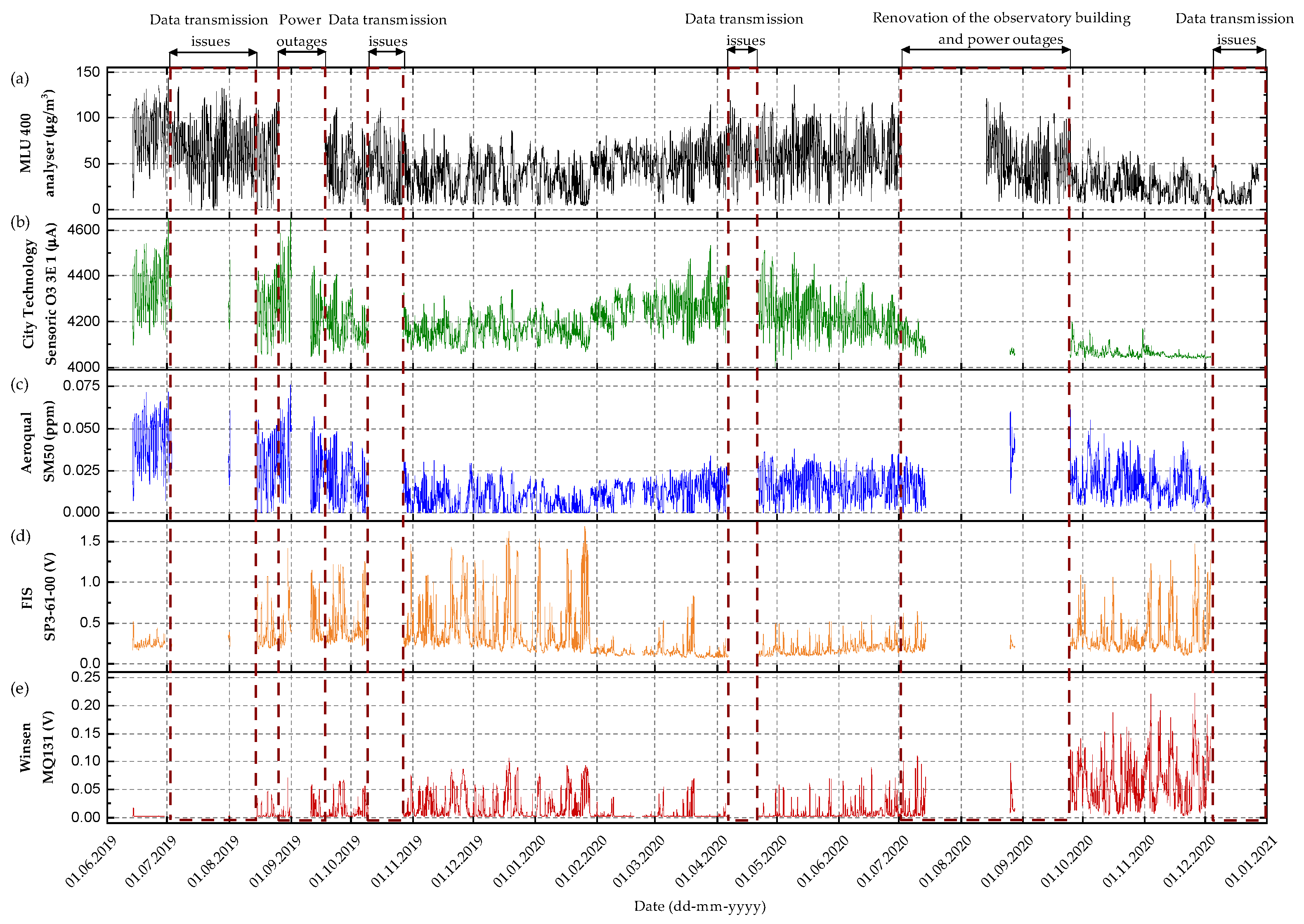
4.1.1. Evaluation of Operation Stability
4.1.2. Meteorological Conditions and Ozone Concentrations during the Study
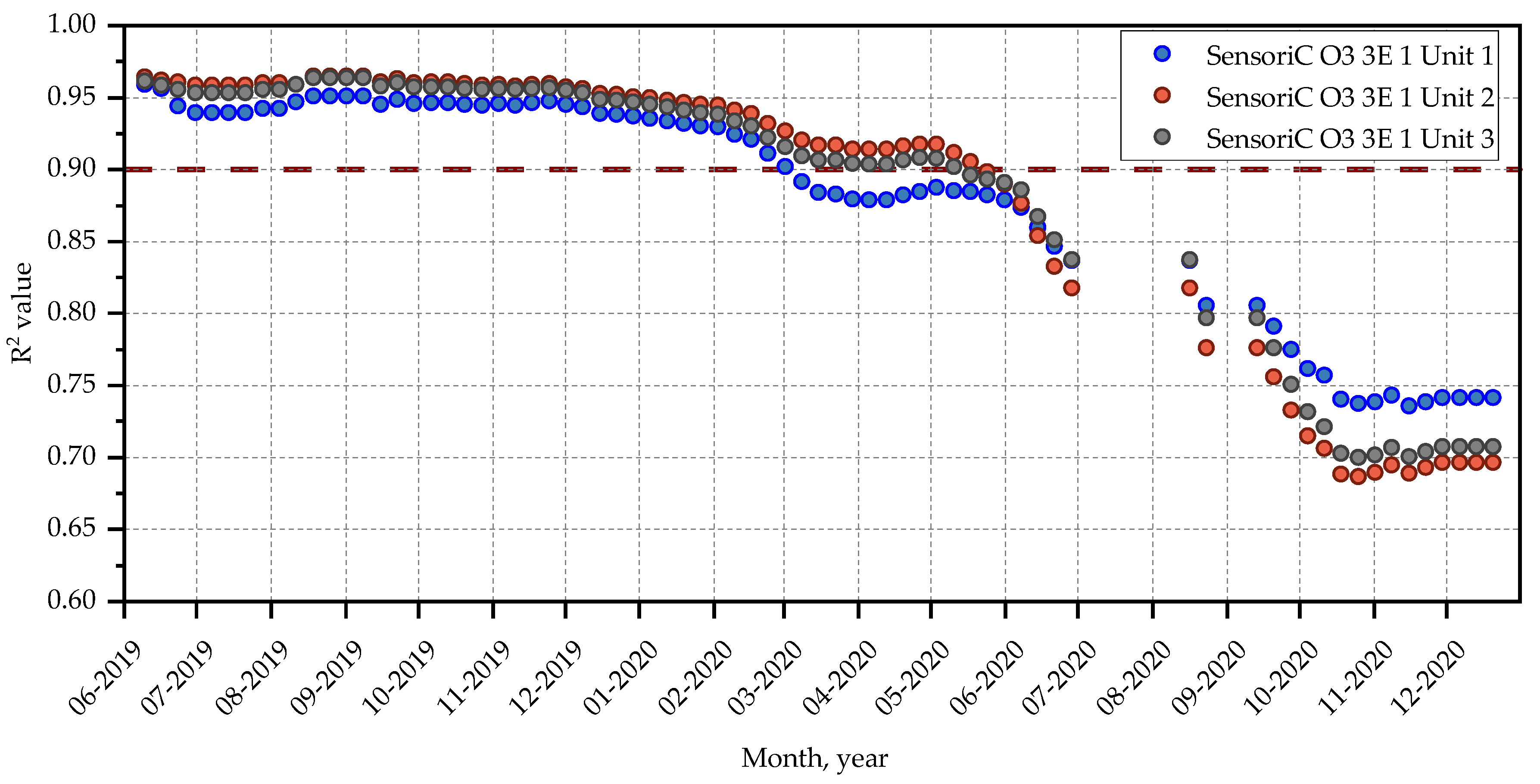
4.2. Reproducibility between Units
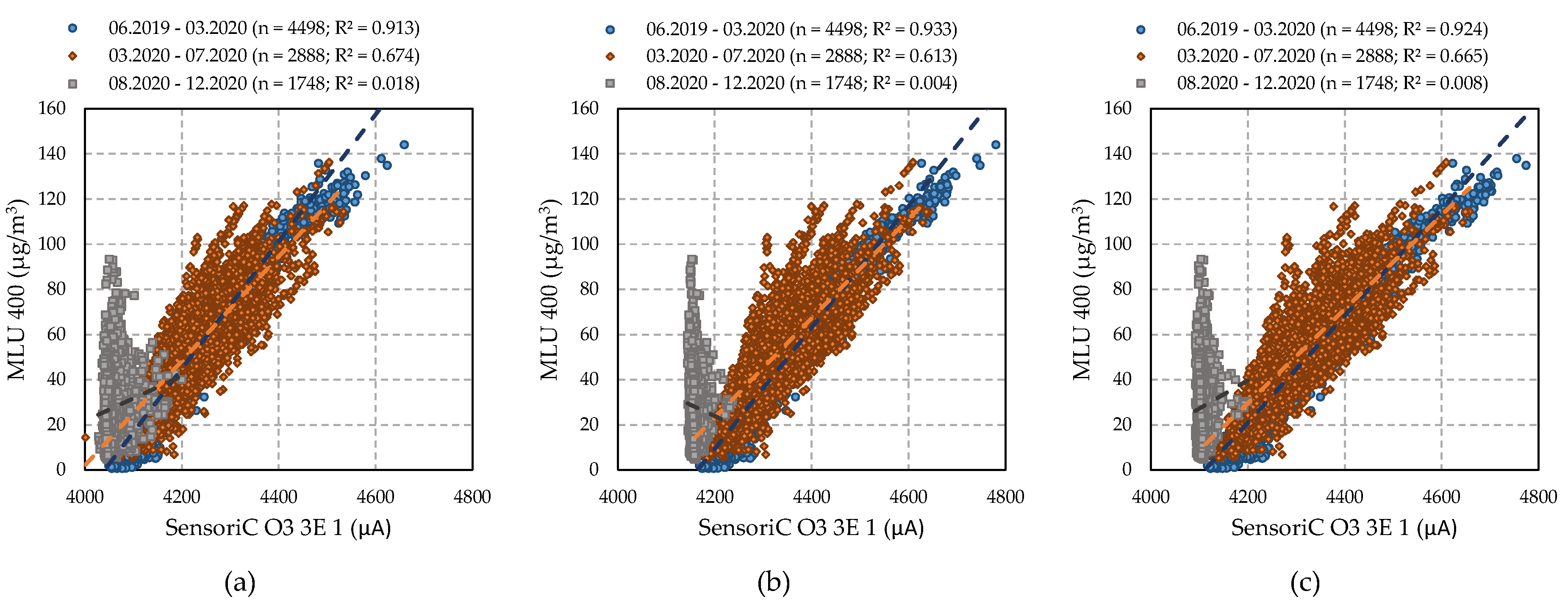
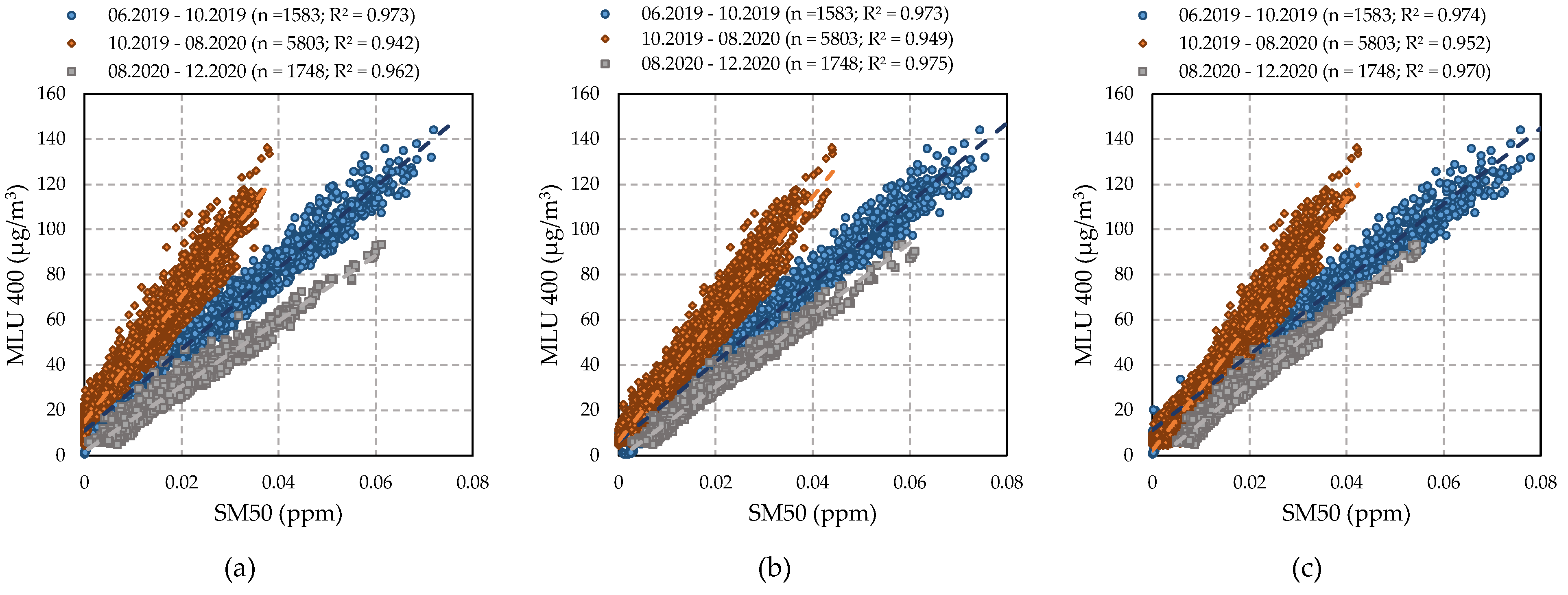
4.3. Relationship between O3 Sensors and Reference Analyser
4.3.1. Performance of SensoriC O3 3E 1 Sensors (City Technology)
4.3.2. Performance of SM50 OZU Sensors (Aeroqual)
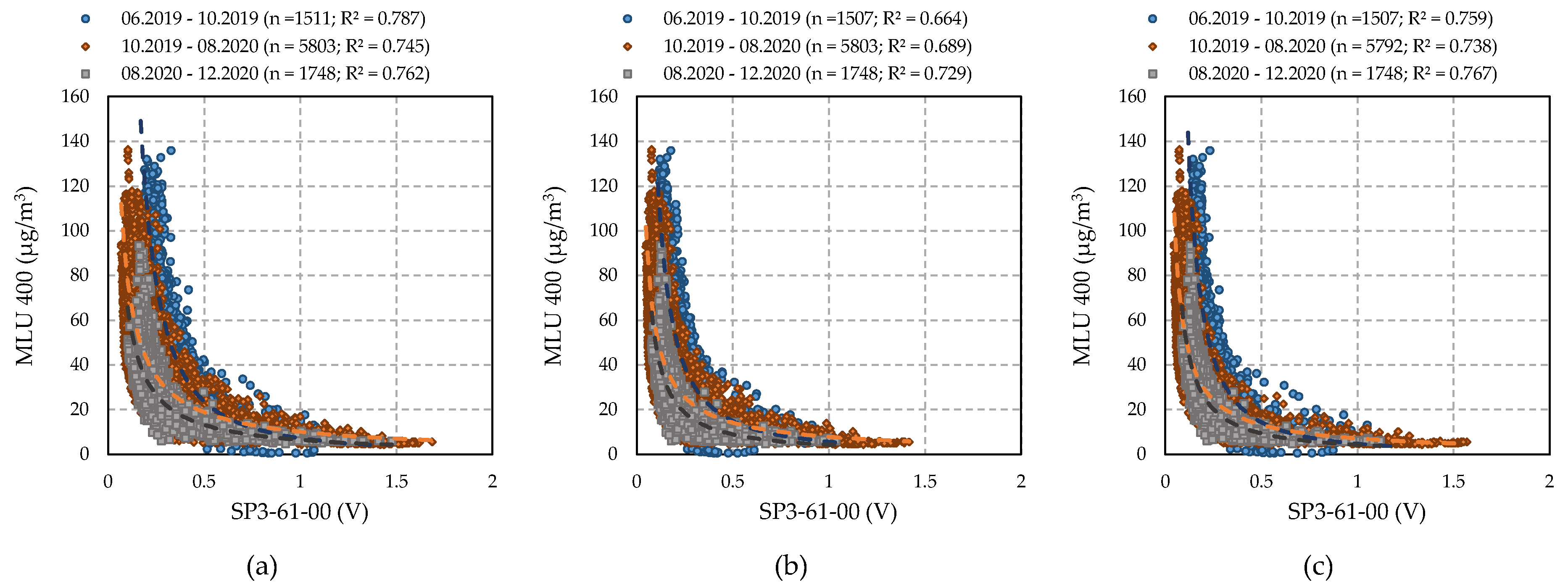
4.3.3. Performance of SP3-61-00 Sensors (FIS)
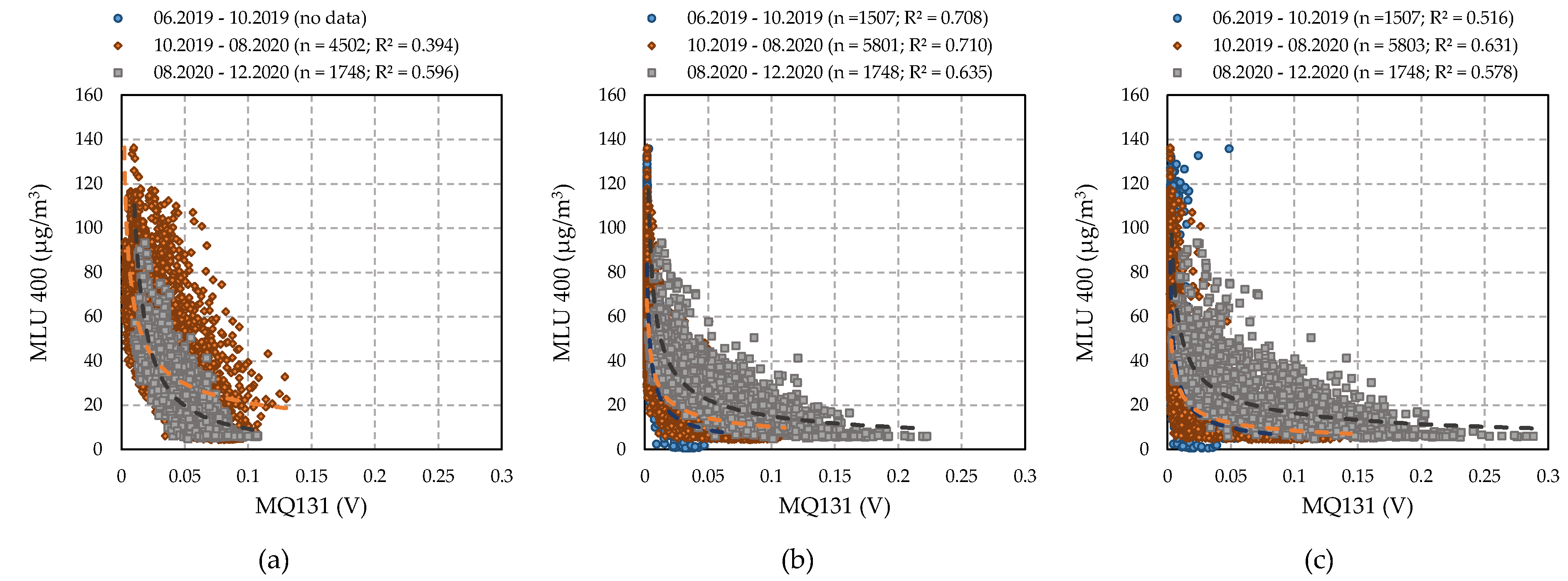
4.3.4. Performance of MQ131 Sensors (Winsen)
4.3.5. Influence of Environmental Factors on Sensors Calibration
4.4. Influence of Interfering Compounds on Performance of Sensors
4.5. Attempts to Test the Equivalence of Sensor Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change and Land: An IPCC Special Report On Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; IPCC: Geneva, Switzerland, 2019.
- Monks, P.S.; Archibald, A.T.; Colette, A.; Cooper, O.; Coyle, M.; Derwent, R.; Williams, M.L. Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmos. Chem. Phys. 2015, 15, 8889–8973. [Google Scholar] [CrossRef] [Green Version]
- Nowack, P.J.; Abraham, N.L.; Maycock, A.C.; Braesicke, P.; Gregory, J.M.; Joshi, M.M.; Osprey, A.; Pyle, J.A. A large ozone-circulation feedback and its implications for global warming assessments. Nat. Clim. Chang. 2015, 5, 41–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ. Int. 2020, 142, 105876. [Google Scholar] [CrossRef] [PubMed]
- Nuvolone, D.; Petri, D.; Voller, F. The effects of ozone on human health. Environ. Sci. Pollut. Res. 2018, 25, 8074–8088. [Google Scholar] [CrossRef] [PubMed]
- Lefohn, A.S.; Malley, C.S.; Smith, L.; Wells, B.; Hazucha, M.; Simon, H.; Lewis, A. Tropospheric ozone assessment report: Global ozone metrics for climate change, human health, and crop/ecosystem research. Elem. Sci. Anth. 2018, 6, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, Z.L.; Doherty, R.M.; Von Schneidemesser, E.; Malley, C.S.; Cooper, O.R.; Pinto, J.P.; Lewis, A. Tropospheric Ozone Assessment Report: Present-day ozone distribution and trends relevant to human health. Elem. Sci. Anth. 2018, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, S.; Xue, B.; Lv, Z.; Meng, Z.; Yang, X.; He, K. Ground-level ozone pollution and its health impacts in China. Atmos. Environ. 2018, 173, 223–230. [Google Scholar] [CrossRef]
- Malley, C.S.; Henze, D.K.; Kuylenstierna, J.C.I.; Vallack, H.W.; Davila, Y.; Anenberg, S.C.; Turner, M.C.; Ashmore, M.R. Updated Global Estimates of Respiratory Mortality in Adults ≥30Years of Age Attributable to Long-Term Ozone Exposure. Environ. Health Perspect. 2017, 125, 087021. [Google Scholar] [CrossRef] [Green Version]
- Moura, B.B.; Alves, E.S.; Marabesi, M.A.; de Souza, S.R.; Schaub, M.; Vollenweider, P. Ozone affects leaf physiology and causes injury to foliage of native tree species from the tropical Atlantic Forest of southern Brazil. Sci. Total Environ. 2018, 610–611, 912–925. [Google Scholar] [CrossRef]
- Mills, G.; Pleijel, H.; Malley, C.S.; Sinha, B.; Cooper, O.R.; Schultz, M.G.; Lewis, A. Tropospheric Ozone Assessment Report: Present-day tropospheric ozone distribution and trends relevant to vegetation. Elem. Sci. Anth. 2018, 6, 47. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Yendrek, C.R.; Sitch, S.; Collins, W.J.; Emberson, L.D. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu. Rev. Plant Biol. 2012, 63, 637–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rim, D.; Gall, E.T.; Maddalena, R.L.; Nazaroff, W.W. Ozone reaction with interior building materials: Influence of diurnal ozone variation, temperature and humidity. Atmos. Environ. 2016, 125, 15–23. [Google Scholar] [CrossRef] [Green Version]
- EEA. Air Pollution: How It Affects Our Health. 2021. Available online: https://www.eea.europa.eu/themes/air/health-impacts-of-air-pollution (accessed on 4 January 2022).
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide: Executive Summary; World Health Organization: Geneva, Switzerland, 2021.
- Jenkin, M.E.; Clemitshaw, K.C. Ozone and other secondary photochemical pollutants: Chemical processes governing their formation in the planetary boundary layer. Atmos. Environ. 2000, 34, 2499–2527. [Google Scholar] [CrossRef]
- Berezina, E.; Moiseenko, K.; Skorokhod, A.; Pankratova, N.V.; Belikov, I.; Belousov, V.; Elansky, N.F. Impact of VOCs and NOx on Ozone Formation in Moscow. Atmosphere 2020, 11, 1262. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, J.; McElroy, M.B.; Munger, J.W.; Ma, H.; Chen, D.; Nielsen, C.P. Ozone Air Quality during the 2008 Beijing Olympics—Effectiveness of Emission Restrictions. Atmos. Chem. Phys. 2009, 9, 5237–5251. [Google Scholar] [CrossRef] [Green Version]
- Stowell, J.D.; Kim, Y.-M.; Gao, Y.; Fu, J.S.; Chang, H.H.; Liu, Y. The Impact of Climate Change and Emissions Control on Future Ozone Levels: Implications for Human Health. Environ. Int. 2017, 108, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Blande, J.D.; Holopainen, J.K.; Niinemets, Ü. Plant Volatiles in Polluted Atmospheres: Stress Responses and Signal Degradation. Plant Cell Environ. 2014, 37, 1892–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzuca, G.; Ren, X.; Loughner, C.; Estes, M.; Crawford, J.; Pickering, K.; Weinheimer, A.; Dickerson, R. Ozone production and its sensitivity to NOx and VOCs: Results from the DISCOVER-AQ field experiment, Houston 2013. Atmos. Chem. Phys. 2016, 16, 14463–14474. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sharma, S.K.; Mandal, T.K. Ozone sensitivity factor: NOX or NMHCs?: A case study over an urban site in Delhi, India. Urban Clim. 2021, 39, 100980. [Google Scholar]
- Zhan, J.; Liu, Y.; Ma, W.; Zhang, X.; Wang, X.; Bi, F.; Zhang, Y.; Wu, Z.; Li, H. Ozone formation sensitivity study using machine learning coupled with the reactivity of volatile organic compound species. Atmos. Meas. Tech. 2022, 15, 1511–1520. [Google Scholar] [CrossRef]
- Huo, H.; Zhang, Q.; He, K.; Wang, Q.; Yao, Z.; Streets, D.G. High-Resolution Vehicular Emission Inventory Using a Link-Based Method: A Case Study of Light-Duty Vehicles in Beijing. Environ. Sci. Technol. 2009, 43, 2394–2399. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Fontes, T.; Bandeira, J.M.; Pereira, S.R.; Tchepel, O.; Dias, D.; Sá, E.; Amorim, J.H.; Borrego, C. Assessment of Potential Improvements on Regional Air Quality Modelling Related with Implementation of a Detailed Methodology for Traffic Emission Estimation. Sci. Total Environ. 2014, 470–471, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.Y.O.; Leung, D.Y.C.; Liu, C.H. A Review on Ozone Evolution and Its Relationship with Boundary Layer Characteristics in Urban Environments. Water Air Soil Pollut. 2011, 214, 13–36. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Y.; Huang, X.; Wang, Y.; Hu, B.; Zhang, Y.; Song, T.; Li, X.; Wu, S.; Li, Q.; et al. Aggravated ozone pollution in the strong free convection boundary layer. Sci. Total Environ. 2021, 788, 147740. [Google Scholar] [CrossRef]
- Orru, H.; Åström, C.; Andersson, C.; Tamm, T.; Ebi, K.L.; Forsberg, B. Ozone and heat-related mortality in Europe in 2050 significantly affected by changes in climate, population and greenhouse gas emission. Environ. Res. Lett. 2019, 14, 074013. [Google Scholar] [CrossRef]
- Bell, M.L.; Goldberg, R.; Hogrefe, C.; Kinney, P.L.; Knowlton, K.; Lynn, B.; Rosenthal, J.; Rosenzweig, C.; Patz, J.A. Climate change, ambient ozone, and health in 50 US cities. Clim Chang. 2007, 82, 61–76. [Google Scholar] [CrossRef]
- Meehl, G.A.; Tebaldi, C.; Tilmes, S.; Lamarque, J.-F.; Bates, S.; Pendergrass, A.; Lombardozzi, D. Future heat waves and surface ozone. Environ. Res. Lett. 2018, 13, 064004. [Google Scholar] [CrossRef]
- Hertig, E.; Russo, A.; Trigo, R.M. Heat and Ozone Pollution Waves in Central and South Europe—Characteristics, Weather Types, and Association with Mortality. Atmosphere 2020, 11, 1271. [Google Scholar] [CrossRef]
- Kalisa, E.; Fadlallah, S.; Amani, M.; Nahayo, L.; Habiyaremye, G. Temperature and air pollution relationship during heatwaves in Birmingham, UK. Sustain. Cities Soc. 2018, 43, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Masiol, M.; Squizzato, S.; Chalupa, D.; Rich, D.Q.; Hopke, P.K. Spatial-temporal variations of summertime ozone concentrations across a metropolitan area using a network of low-cost monitors to develop 24 hourly land-use regression models. Sci Total Environ. 2019, 654, 1167–1178. [Google Scholar] [CrossRef]
- Kheirbek, I.; Wheeler, K.; Wlaters, S.; Kass, D.; Matte, T. PM2.5 and ozone health impacts and disparities in New York City: Sensitivity to spatial and temporal resolution. Air Qual. Atmos. Health 2013, 6, 473–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency (USEPA). Integrated Science Assessment for Ozone and Related Photochemical Oxidants. EPA 600/R-10/076F; 2013. Available online: https://www.epa.gov/isa/integrated-science-assessment-isa-ozone-and-related-photochemical-oxidants (accessed on 12 March 2022).
- Kumar, P.; Morawska, L.; Martani, C.; Biskos, G.; Neophytou, M.; Di Sabatino, S.; Bell, M.; Norford, L.; Britter, R. The rise of low-cost sensing for managing air pollution in cities. Environ. Int. 2015, 75, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badura, M.; Sówka, I.; Szymański, P.; Batog, P. Assessing the usefulness of dense sensor network for PM2.5 monitoring on an academic campus area. Sci Total Environ. 2020, 722, 137867. [Google Scholar]
- Pang, X.; Shaw, M.D.; Lewis, A.C.; Carpenter, L.J.; Batchellier, T. Electrochemical ozone sensors: A miniaturised alternative for ozone measurements in laboratory experiments and air-quality monitoring. Sens. Actuators B Chem. 2017, 240, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Karagulian, F.; Barbiere, M.; Kotsev, A.; Spinelle, L.; Gerboles, M.; Lagler, F.; Redon, N.; Crunaire, S.; Borowiak, A. Review of the Performance of Low-Cost Sensors for Air Quality Monitoring. Atmosphere 2019, 10, 506. [Google Scholar] [CrossRef] [Green Version]
- Peterson, P.J.D.; Aujla, A.; Grant, K.H.; Brundle, A.G.; Thompson, M.R.; Hey, J.V.; Leigh, R.J. Practical Use of Metal Oxide Semiconductor Gas Sensors for Measuring Nitrogen Dioxide and Ozone in Urban Environments. Sensors 2017, 17, 1653. [Google Scholar] [CrossRef]
- Arroyo, P.; Gómez-Suárez, J.; Suárez, J.I.; Lozano, J. Low-Cost Air Quality Measurement System Based on Electrochemical and PM Sensors with Cloud Connection. Sensors 2021, 21, 6228. [Google Scholar] [CrossRef]
- Zuidema, C.; Schumacher, C.S.; Austin, E.; Carvlin, G.; Larson, T.V.; Spalt, E.W.; Zusman, M.; Gassett, A.J.; Seto, E.; Kaufman, J.D.; et al. Deployment, Calibration, and Cross-Validation of Low-Cost Electrochemical Sensors for Carbon Monoxide, Nitrogen Oxides, and Ozone for an Epidemiological Study. Sensors 2021, 21, 4214. [Google Scholar] [CrossRef]
- Bart, M.; Williams, D.E.; Ainslie, B.; McKendry, I.; Salmond, J.; Grange, S.K.; Alavi-Shoshtari, M.; Steyn, D.; Henshaw, G.S. High density ozone monitoring using gas sensitive semi-conductor sensors in the Lower Fraser Valley, British Columbia. Environ. Sci. Technol. 2014, 48, 3970–3977. [Google Scholar] [CrossRef]
- Moltchanov, S.; Levy, I.; Etzion, Y.; Lerner, U.; Broday, D.M.; Fishbain, B. On the feasibility of measuring urban air pollution by wireless distributed sensor networks. Sci. Total. Environ. 2015, 502, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Weissert, L.F.; Salmond, J.A.; Miskell, G.; Alavi-Shoshtari, M.; Grange, S.K.; Henshaw, G.S.; Williams, D.E. Use of a dense monitoring network of low-cost instruments to observe local changes in the diurnal ozone cycles as marine air passes over a geographically isolated urban centre. Sci. Total. Environ. 2017, 575, 67–78. [Google Scholar] [CrossRef]
- Morawska, L.; Thai, P.K.; Liu, X.; Asumadu-Sakyi, A.; Ayoko, G.; Bartonova, A.; Bedini, A.; Chai, F.; Christensen, B.; Dunbabin, M.; et al. Applications of low-cost sensing technologies for air quality monitoring and exposure assessment: How far have they gone? Environ. Int. 2018, 116, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Adamiec, E.; Dajda, J.; Gruszecka-Kosowska, A.; Helios-Rybicka, E.; Kisiel-Dorohinicki, M.; Klimek, R.; Pałka, D.; Wąs, J. Using Medium-Cost Sensors to Estimate Air Quality in Remote Locations. Case Study of Niedzica, Southern Poland. Atmosphere 2019, 10, 393. [Google Scholar] [CrossRef] [Green Version]
- World Meteorological Organization. Low-Cost Sensors for the Measurement of Atmospheric Composition: Overview of Topic and Future Applications; WMO-No. 1215; Lewis, A.C., Schneidemesser, E., Peltier, R.E., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Alfonso, L.; Gharesifard, M.; When, U. Analysing the value of environmental citizen-generated data: Complementarity and cost per observation. J. Environ. Manag. 2022, 303, 114157. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, A.; Viana, M.; Padrosa, M.; Querol, X.; Minutolo, A.; Hou, K.M.; Barcelo-Ordinas, J.M.; Garcia-Vidal, J. Testing the performance of sensors for ozone pollution monitoring in a citizen science approach. Sci. Total. Environ. 2019, 651, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Spinelle, L.; Gerboles, M.; Aleixandre, M.; Bonavitacola, F. Evaluation of Metal Oxides Sensors for the Monitoring of O3 in Ambient Air at ppb Level. Chem. Eng. Trans. 2016, 54, 319–324. [Google Scholar]
- Suriano, D.; Cassano, G.; Penza, M. Design and Development of a Flexible, Plug-and-Play, Cost-Effective Tool for on-Field Evaluation of Gas Sensors. J. Sens. 2020, 2020, 8812025. [Google Scholar] [CrossRef]
- Sales-Lérida, D.; Bello, A.J.; Sánchez-Alzola, A.; Martínez-Jiménez, P.M. An Approximation for Metal-Oxide Sensor Calibration for Air Quality Monitoring Using Multivariable Statistical Analysis. Sensors 2021, 21, 4781. [Google Scholar] [CrossRef]
- Masiol, M.; Squizzato, S.; Chalupa, D.; Rich, D.Q.; Hopke, P.K. Evaluation and Field Calibration of a Low-Cost Ozone Monitor at a Regulatory Urban Monitoring Station. Aerosol. Air Qual. Res. 2018, 18, 2029–2037. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Gillespie, J.; Schuder, M.D.; Duberstein, W.; Beverland, I.J.; Heal, M.R. Evaluation and calibration of Aeroqual series 500 portable gas sensors for accurate measurement of ambient ozone and nitrogen dioxide. Atmos. Environ. 2015, 100, 111–116. [Google Scholar] [CrossRef]
- Masey, N.; Gillespie, J.; Ezani, E.; Lin, C.; Wu, H.; Ferguson, N.S.; Hamilton, S.; Heal, M.R.; Beverland, I.J. Temporal changes in field calibration relationships for Aeroqual S500 O3 and NO2 sensor-based monitors. Sens. Actuators B Chem. 2018, 273, 1800–1806. [Google Scholar] [CrossRef]
- Sayahi, T.; Garff, A.; Quah, T.; Lê, K.; Becnel, T.; Powell, K.M.; Gaillardon, P.-E.; Butterfield, A.E.; Kelly, K.E. Long-term calibration models to estimate ozone concentrations with a metal oxide sensor. Environ. Pollut. 2020, 267, 115363. [Google Scholar] [CrossRef] [PubMed]
- Miskell, G.; Alberti, K.; Feenstra, B.; Henshaw, G.S.; Papapostolou, V.; Patel, H.; Polidori, A.; Salmond, J.; Weissert, L.; Williams, D. Reliable data from low cost ozone sensors in a hierarchical network. Atmos. Environ. 2019, 214, 116870. [Google Scholar] [CrossRef] [Green Version]
- Spinelle, L.; Gerboles, M.; Aleixandre, M. Performance evaluation of amperometric sensors for the monitoring of O3 and NO2 in ambient air at ppb level. Procedia Eng. 2015, 120, 480–483. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Villani, M.G.; Aleixandre, M.; Bonavitacola, F. Field calibration of a cluster of low-cost available sensors for air quality monitoring. Part A: Ozone and nitrogen dioxide. Sens. Actuators B Chem. 2015, 215, 249–257. [Google Scholar] [CrossRef]
- Badura, M.; Batog, P.; Drzeniecka-Osiadacz, A.; Modzel, P. Evaluation of Low-Cost Sensors for Ambient PM2.5 Monitoring. J. Sensors 2018, 2018, 5096540. [Google Scholar]
- Samad, A.; Nuñez, D.R.O.; Castillo, G.C.S.; Laquai, B.; Vogt, U. Effect of Relative Humidity and Air Temperature on the Results Obtained from Low-Cost Gas Sensors for Ambient Air Quality Measurements. Sensors 2020, 20, 5175. [Google Scholar] [CrossRef]
- Margaritis, D.; Keramydas, C.; Papachristos, I.; Lambropoulou, D. Calibration of Low-cost Gas Sensors for Air Quality Monitoring. Aerosol. Air Qual. Res. 2021, 21, 210073. [Google Scholar] [CrossRef]
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32008L0050&from=en (accessed on 28 February 2022).
- Guide to the Demonstration of Equivalence of Ambient Air Monitoring Methods—Report by an EC Working Group on Guidance for the Demonstration of Equivalence, January 2010. Available online: http://ec.europa.eu/environment/air/quality/legislation/pdf/equivalence.pdf (accessed on 15 March 2022).
- Jiao, W.; Hagler, G.; Williams, R.; Sharpe, R.; Brown, R.; Garver, D.; Judge, R.; Caudill, M.; Rickard, J.; Davis, M.; et al. Community Air Sensor Network (CAIRSENSE) project: Evaluation of low-cost sensor performance in a suburban environment in the southeastern United States. Atmos. Meas. Tech. 2021, 9, 5281–5292. [Google Scholar] [CrossRef] [Green Version]
- Cross, E.S.; Williams, L.R.; Lewis, D.K.; Magoon, G.R.; Onasch, T.B.; Kaminsky, M.L.; Worsnop, D.R.; Jayne, J.T. Use of electrochemical sensors for measurement of air pollution: Correcting interference response and validating measurements. Atmos. Meas. Tech. 2017, 10, 3575–3588. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.E.; Henshaw, G.S.; Bart, M.; Laing, G.; Wagner, J.; Naisbitt, S.; Salmond, J.A. Validation of low-cost ozone measurement instruments suitable for use in an air-quality monitoring network. Meas. Sci. Technol. 2013, 24, 065803. [Google Scholar] [CrossRef]
- DeWitt, H.L.; Crow, W.L.; Flowers, B. Performance evaluation of ozone and particulate matter sensors. J. Air Waste Manag. Assoc. 2020, 70, 292–306. [Google Scholar] [CrossRef]
- Kizel, F.; Etzion, Y.; Shafran-Nathan, R.; Levy, I.; Fishbain, B.; Bartonova, A.; Broday, D.M. Node-to-node field calibration of wireless distributed air pollution sensor network. Environ. Pollut. 2018, 233, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Masson, N.; Piedrahita, R.; Hannigan, M. Approach for quantification of metal oxide type semiconductor gas sensors used for ambient air quality monitoring. Sens. Actuators B Chem. 2015, 208, 339–345. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Shaw, M.D.; Gillot, S.; Lewis, A.C. The impacts of water vapour and co-pollutants on the performance of electrochemical gas sensors used for air quality monitoring. Sens. Actuators B Chem. 2018, 266, 674–684. [Google Scholar] [CrossRef]
- Vîrghileanu, M.; Săvulescu, I.; Mihai, B.-A.; Nistor, C.; Dobre, R. Nitrogen Dioxide (NO2) Pollution Monitoring with Sentinel-5P Satellite Imagery over Europe during the Coronavirus Pandemic Outbreak. Remote Sens. 2020, 12, 3575. [Google Scholar] [CrossRef]

| Sensor Model | SensoriC O3 3E 1 | SM50 (OZU) | SP3-61-00 | MQ131 |
|---|---|---|---|---|
| Manufacturer (country of origin) | City Technology | Aeroqual | Nissha FIS | Winsen |
| (Great Britain/Germany) | (New Zealand) | (Japan) | (China) | |
| Cost level Approximate price ($) | Medium cost | Low-cost | ||
| 400 | 400 | 20 | 20 | |
| Sensor type | Electrochemical | Metal oxide semiconductor | ||
| Amperometric 3-electrode sensor cell | WO3 | ITO: In2O3/SnO2 | WO3 | |
| Concentration range (ppm) | 0–1 | 0–0.150 | ~0.025–0.6 | 0.01–1 |
| Output signal | Current: 4–20 mA | Digital: ppm Voltage: 0–5 V | Voltage: 0–5 V | Voltage: 0–5 V |
| Factory-fabricated accessories | Transmitter board | Board with microcontroller and micro fan | − | − |
| Dimensions (mm) | Sensor: h ≈ 14.5, φ = 15.6 Board: 25 x 44.5 | Sensor: h ≈ 15, φ ≈ 16 Board: 60 x 75 | Sensor: h = 13 (plus pins), φ = 14 | Sensor: h = 12 (plus pins), φ = 16 |
| Approximate weight (g) | 13 a | 65 a | 1.2 | 2 |
| Operating conditions | −20 °C to +40 °C 15% to 90% RH | −20°C to +50 °C 5% to 90% RH | −10 °C to +50 °C <95% | −10 °C to +50 °C |
| SensoriC O3 3E 1 | SM50 | SP3-61-00 | MQ131 | T | RH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit 1 | Unit 2 | Unit 3 | Unit 1 | Unit 2 | Unit 3 | Unit 1 | Unit 2 | Unit 3 | Unit 1 | Unit 2 | Unit 3 | |||
| O3 | 0.86 | 0.83 | 0.84 | 0.82 | 0.86 | 0.89 | −0.60 | −0.68 | −0.57 | −0.64 | −0.56 | −0.54 | 0.69 | −0.80 |
| Sensor | SensoriC O3 3E 1 a | SM50 OZU b | SP3-61-00 c | MQ131 d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Slope | 0.956 | 0.970 | 0.964 | 0.982 | 0.985 | 0.988 | 0.908 | 0.911 | 0.906 | 0.943 | 0.965 | 0.939 |
| Evaluation of uncorrected data | ||||||||||||
| Uncertainty of slope | 0.004 | 0.004 | 0.004 | 0.002 | 0.002 | 0.002 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 |
| Intercept | 1.183 | 0.902 | 1.184 | 0.846 | 0.738 | 0.563 | 3.823 | 3.756 | 3.884 | 2.515 | 1.890 | 2.854 |
| Uncertainty of intercept | 0.224 | 0.199 | 0.214 | 0.113 | 0.111 | 0.106 | 0.222 | 0.224 | 0.212 | 0.203 | 0.177 | 0.196 |
| Number of data pairs | 4248 | 4248 | 4248 | 8606 | 8606 | 8606 | 8281 | 8273 | 8255 | 5843 d | 8264 | 8273 |
| Bias at limit value, µg/m3 | −4.1 | −2.6 | −3.1 | −1.3 | −1.1 | −0.8 | −7.2 | −6.89 | −7.45 | −4.4 | −2.3 | −4.4 |
| Combined uncertainty, µg/m3 | 7.84 | 6.42 | 7.08 | 4.99 | 4.84 | 4.58 | 11.7 | 11.6 | 11.5 | 7.91 | 7.55 | 9.14 |
| Expanded relative uncertainty, % | 13.1 | 10.7 | 11.8 | 8.3 | 8.1 | 7.6 | 19.4 | 19.3 | 19.1 | 13.2 | 12.6 | 15.2 |
| Evaluation of uncorrected data | ||||||||||||
| Slope | 1.002 | 1.001 | 1.001 | 1.000 | 1.000 | 1.000 | 1.010 | 1.010 | 1.010 | 1.004 | 1.002 | 1.004 |
| Uncertainty of slope | 0.004 | 0.004 | 0.004 | 0.002 | 0.002 | 0.002 | 0.005 | 0.005 | 0.005 | 0.004 | 0.004 | 0.004 |
| Intercept | −0.094 | −0.047 | −0.068 | −0.002 | −0.019 | −0.014 | −0.484 | −0.474 | −0.452 | −0.161 | −0.100 | −0.181 |
| Uncertainty of intercept | 0.234 | 0.205 | 0.222 | 0.115 | 0.112 | 0.107 | 0.244 | 0.246 | 0.234 | 0.215 | 0.184 | 0.208 |
| Bias at limit value, µg/m3 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.8 | 0.8 | 0.7 | 0.3 | 0.2 | 0.3 |
| Combined uncertainty, µg/m3 | 7.06 | 6.07 | 6.66 | 4.91 | 4.79 | 4.57 | 10.3 | 10.3 | 9.79 | 7.08 | 7.48 | 8.59 |
| Expanded relative uncertainty, % | 11.8 | 10.1 | 11.1 | 8.2 | 8.0 | 7.6 | 17.1 | 17.2 | 16.3 | 11.8 | 12.5 | 14.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badura, M.; Batog, P.; Drzeniecka-Osiadacz, A.; Modzel, P. Low- and Medium-Cost Sensors for Tropospheric Ozone Monitoring—Results of an Evaluation Study in Wrocław, Poland. Atmosphere 2022, 13, 542. https://doi.org/10.3390/atmos13040542
Badura M, Batog P, Drzeniecka-Osiadacz A, Modzel P. Low- and Medium-Cost Sensors for Tropospheric Ozone Monitoring—Results of an Evaluation Study in Wrocław, Poland. Atmosphere. 2022; 13(4):542. https://doi.org/10.3390/atmos13040542
Chicago/Turabian StyleBadura, Marek, Piotr Batog, Anetta Drzeniecka-Osiadacz, and Piotr Modzel. 2022. "Low- and Medium-Cost Sensors for Tropospheric Ozone Monitoring—Results of an Evaluation Study in Wrocław, Poland" Atmosphere 13, no. 4: 542. https://doi.org/10.3390/atmos13040542
APA StyleBadura, M., Batog, P., Drzeniecka-Osiadacz, A., & Modzel, P. (2022). Low- and Medium-Cost Sensors for Tropospheric Ozone Monitoring—Results of an Evaluation Study in Wrocław, Poland. Atmosphere, 13(4), 542. https://doi.org/10.3390/atmos13040542






