Preparation Methods and Performance Analysis of Polyanthra-Quinone/Carbon Nanotube Composites for Capturing Carbon Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Preparation of Materials
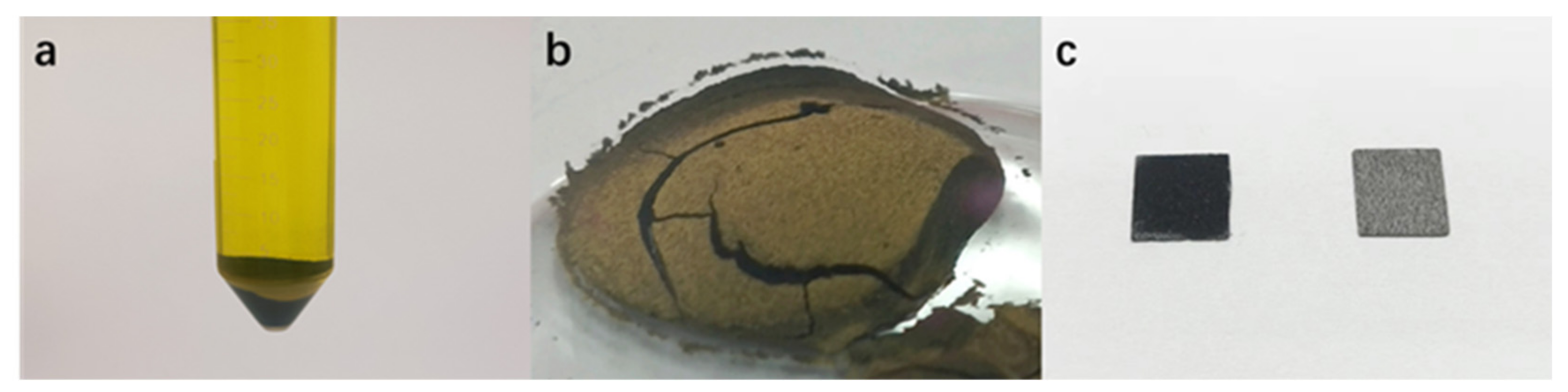
2.2.1. Preparation of PAQ/MWCNTs Composites
2.2.2. Preparation of PAQ/MWCNTs Modified Electrode
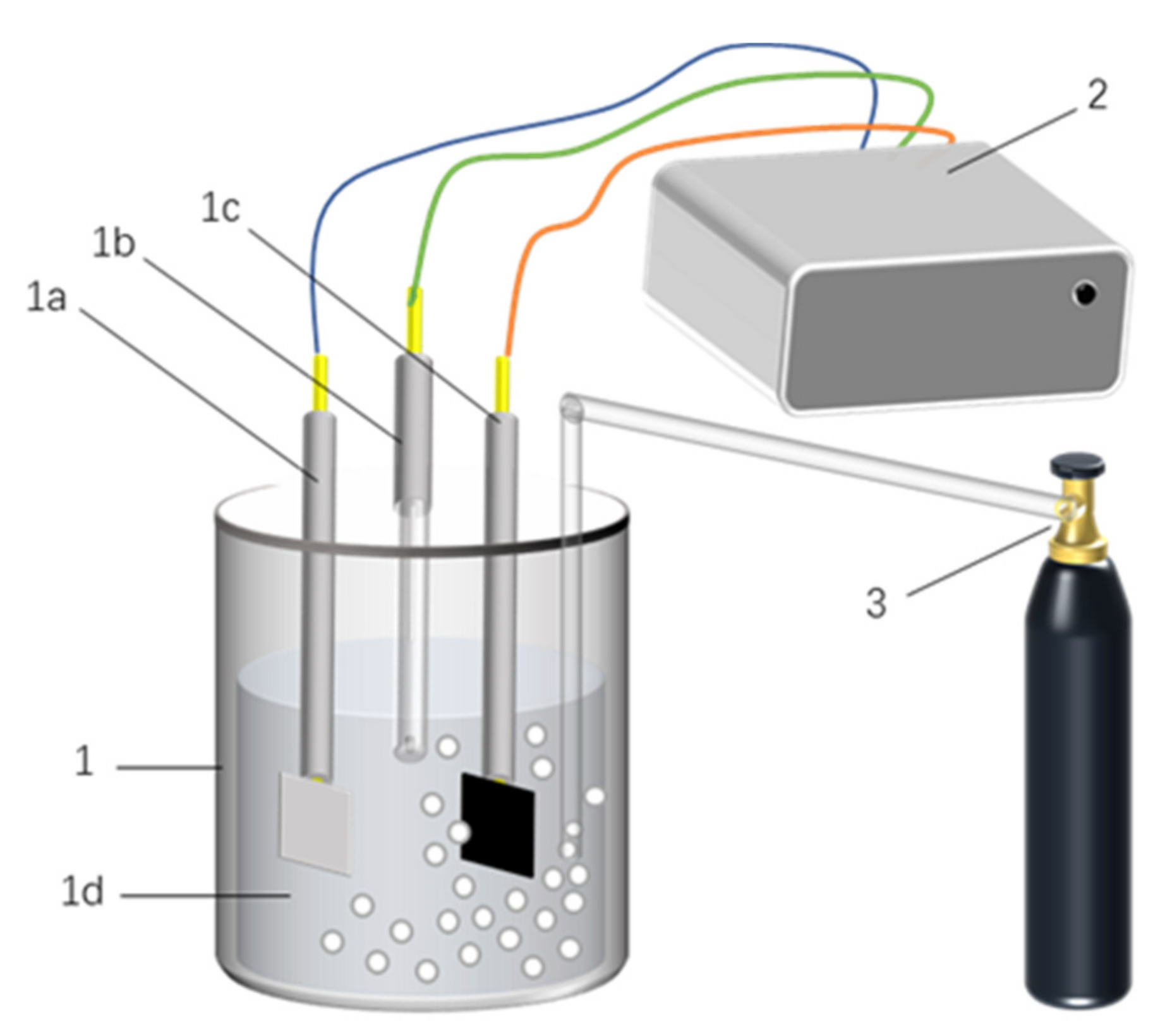
2.3. Electrochemical Experiments
2.3.1. Electrochemical Performance Test
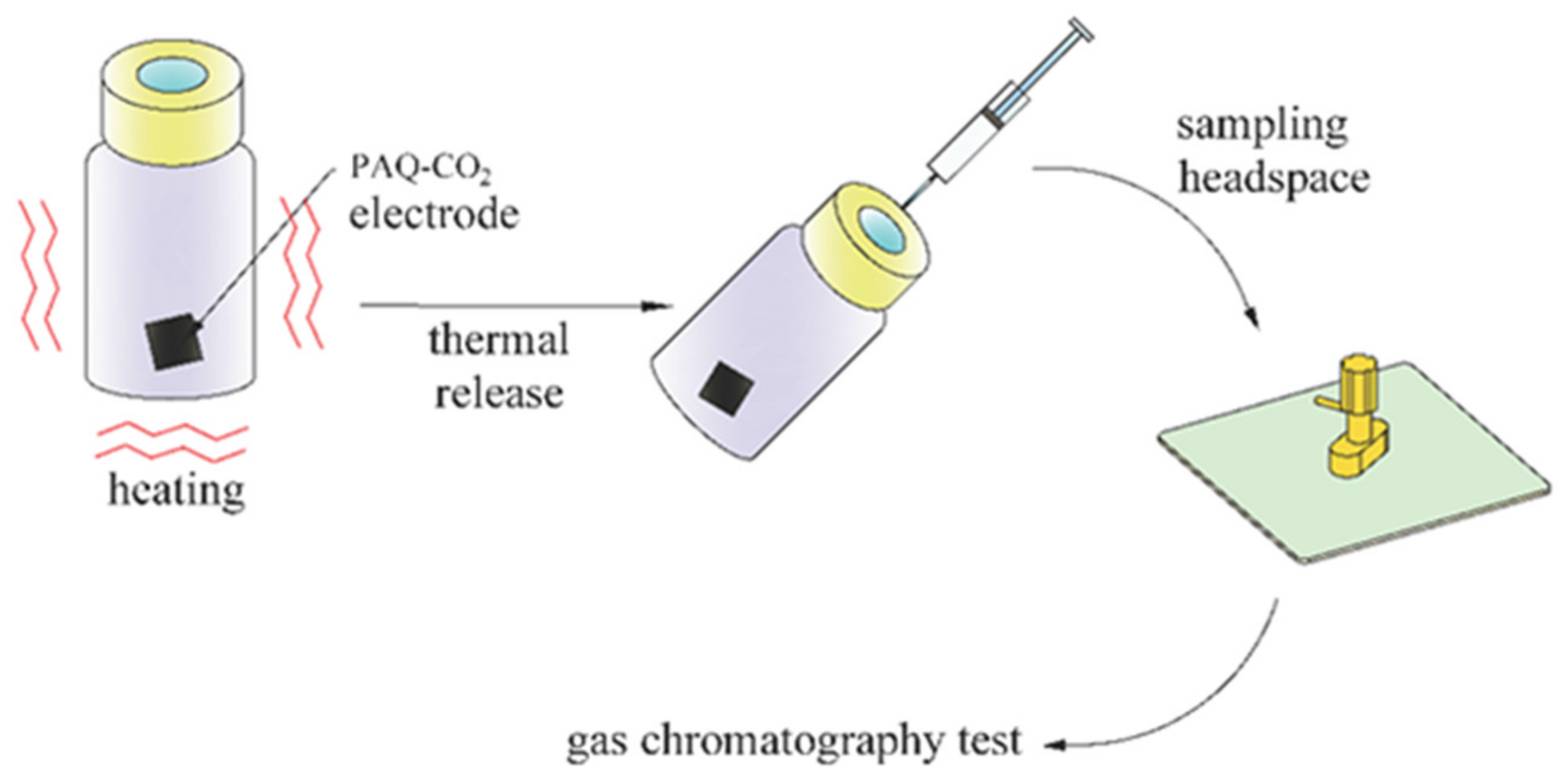
2.3.2. Carbon Dioxide Capture and Thermal Regeneration Experiments
2.4. Test Equipment and Conditions
3. Results and Discussion
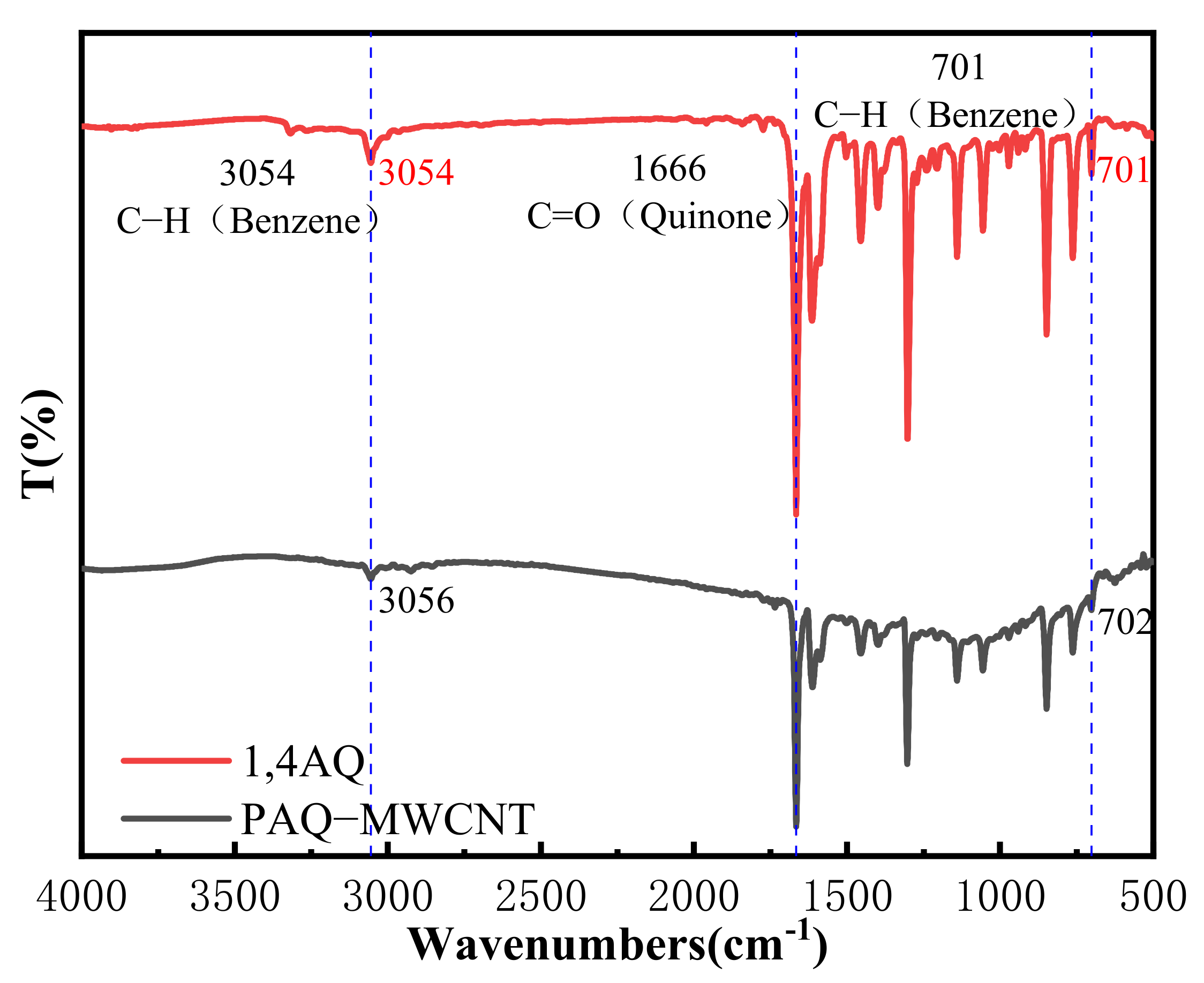
3.1. FTIR Spectra of 1,4-AQ and PAQ/MWCNTs Composites
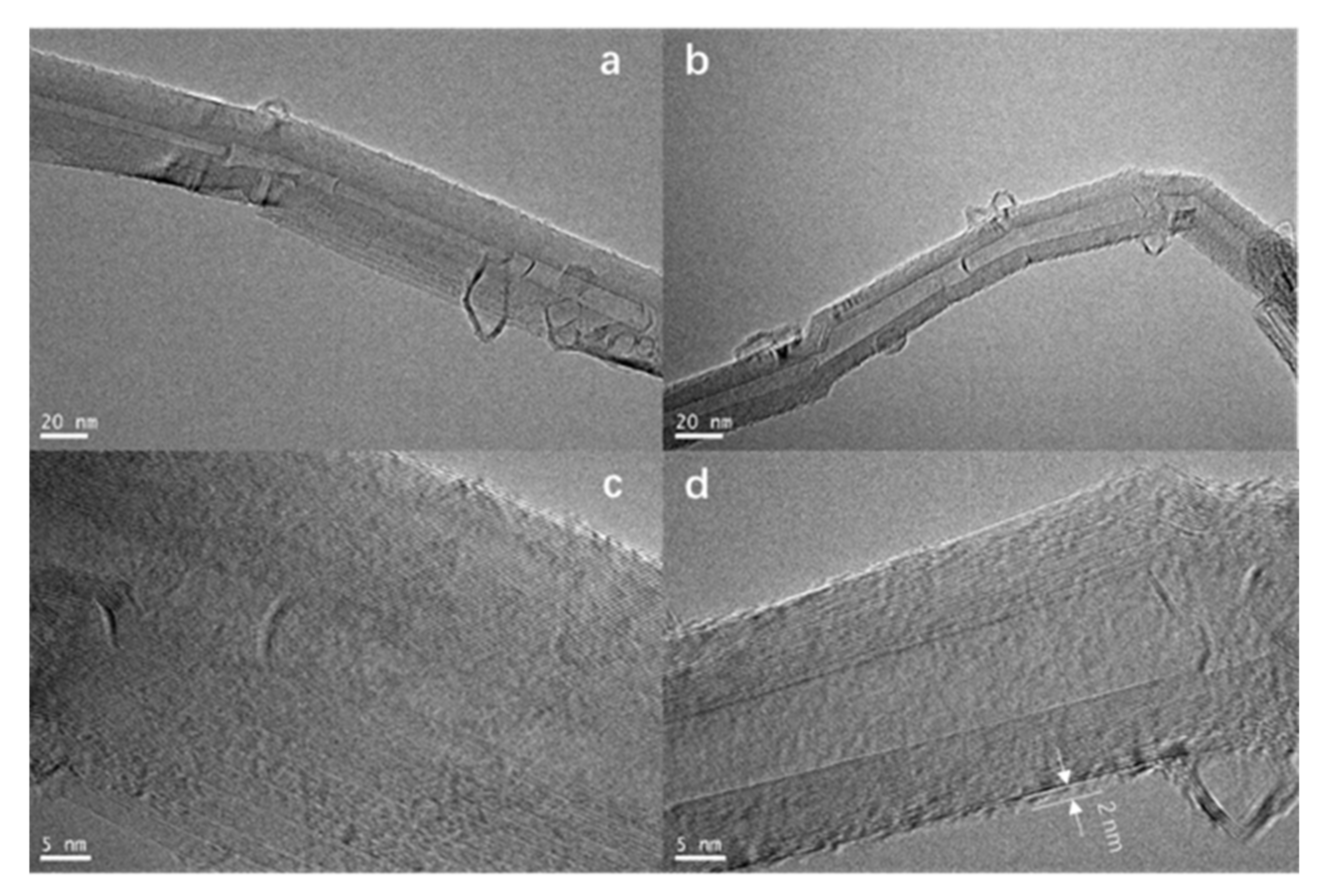
3.2. TEM Characterization of PAQ/MWCNTs Composites
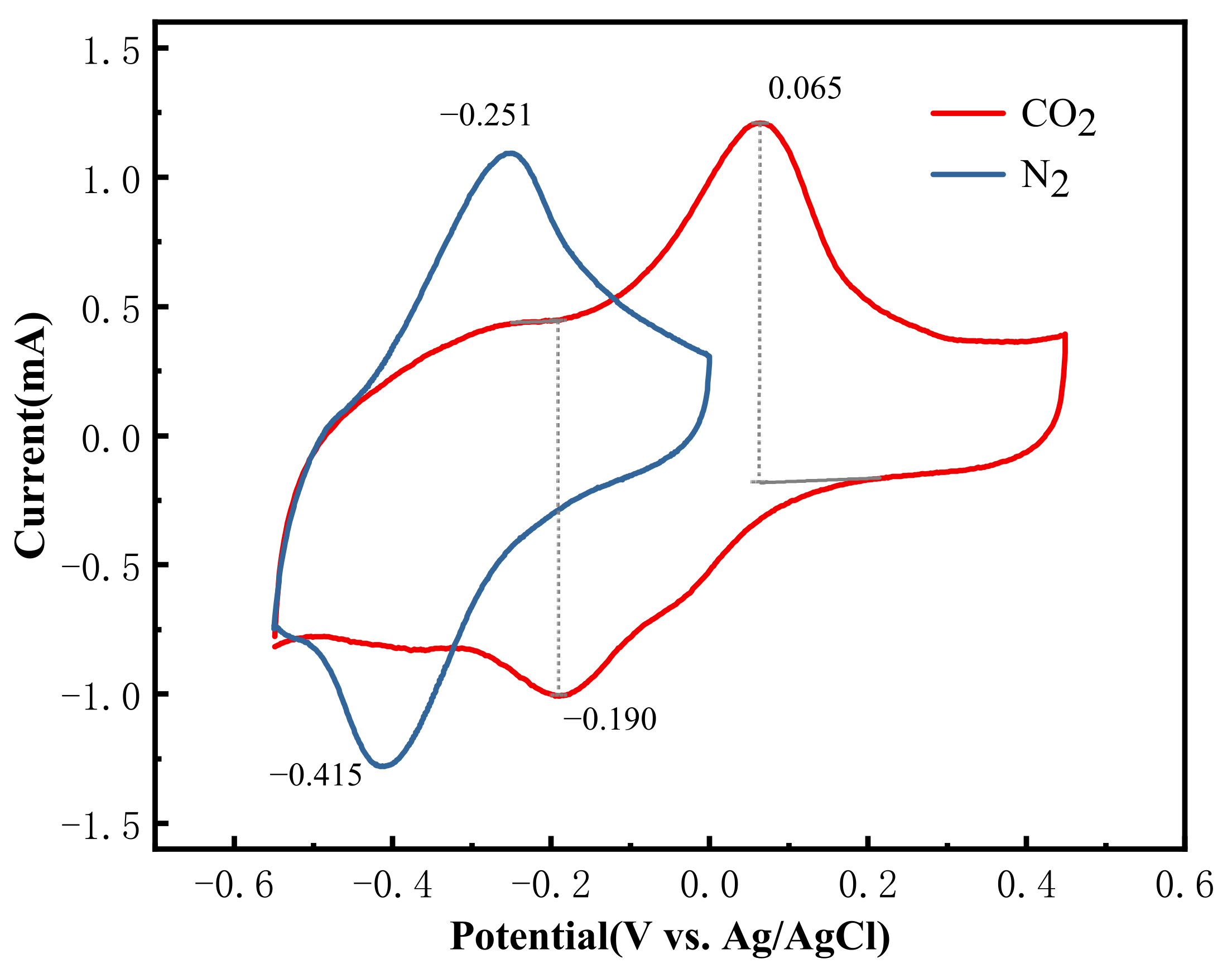
3.3. CV Testing of PAQ/MWCNTs Composites
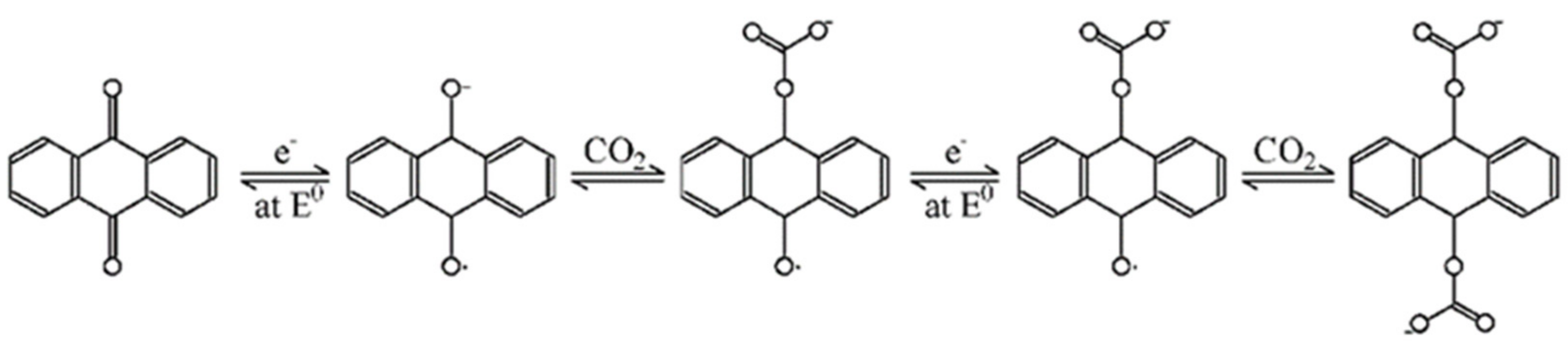
3.4. Electrochemical CO2 Capture Effect Test
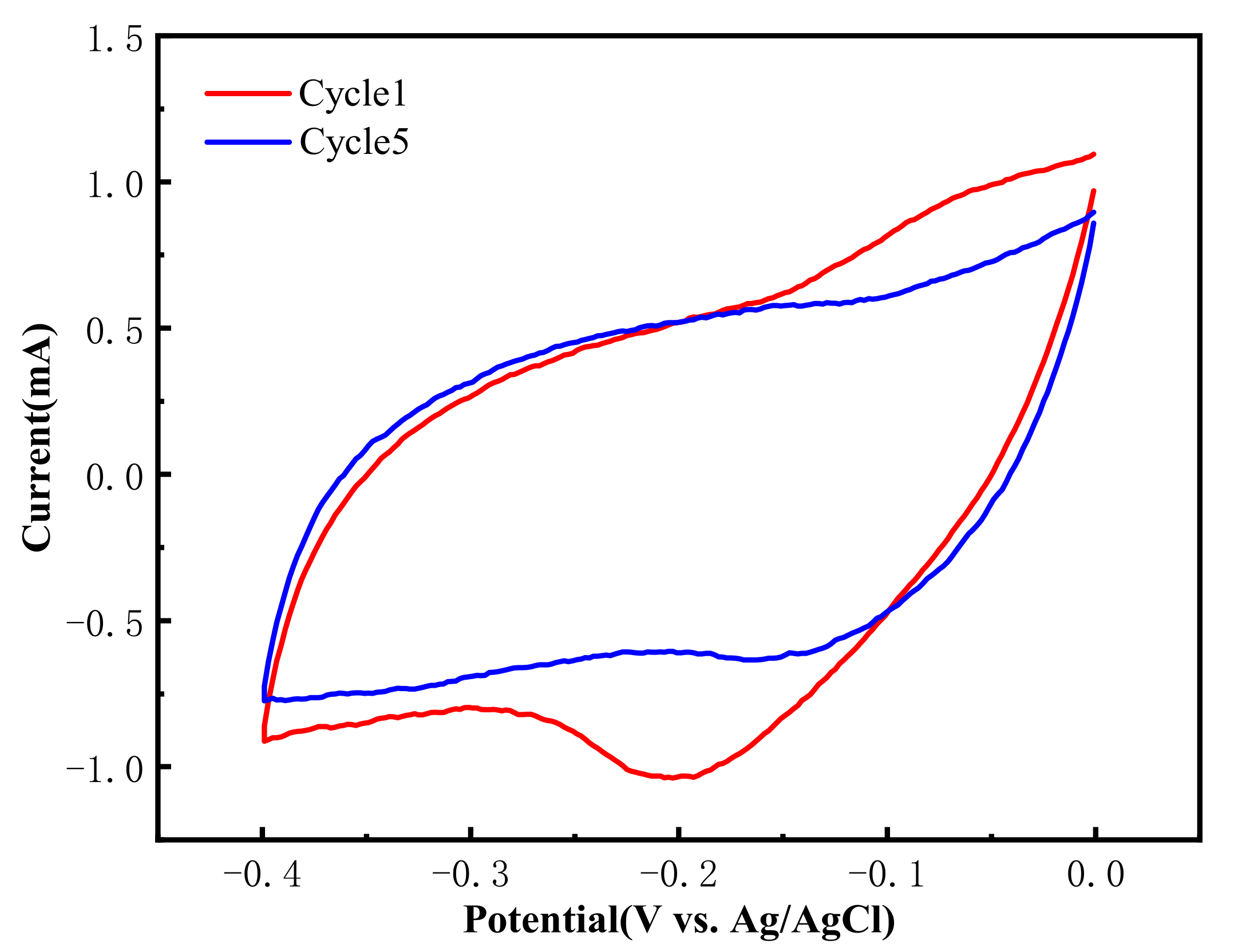
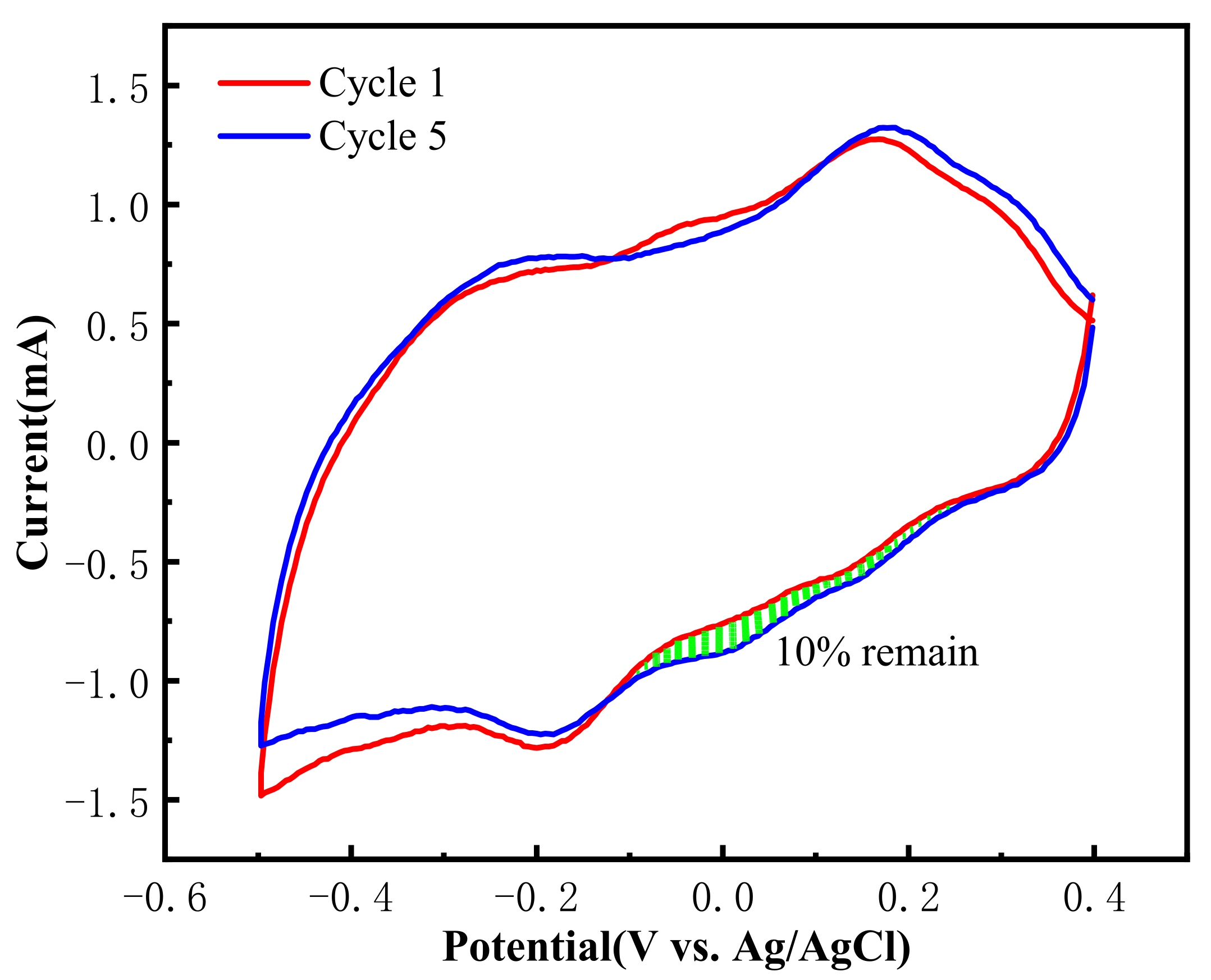
3.4.1. CV Tests before and after Thermal Regeneration of the Material Electrode
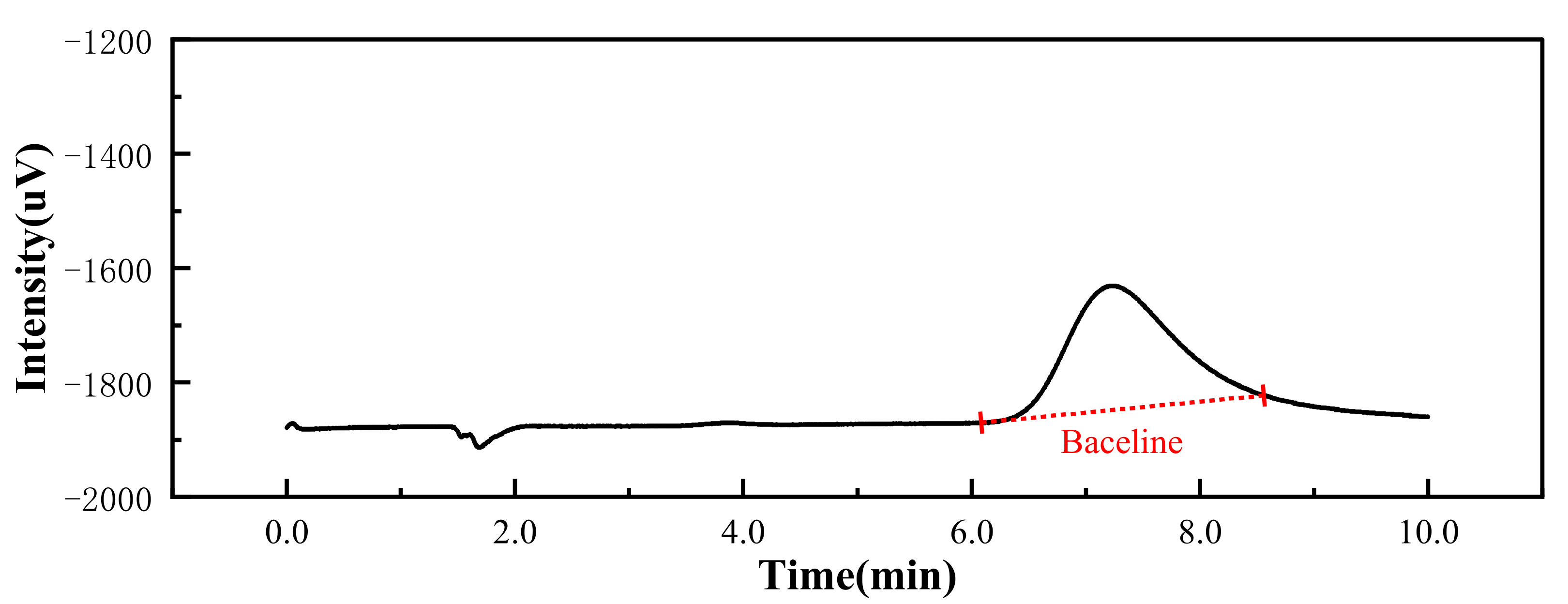
3.4.2. CO2 Capture Capacity
4. Conclusions
- The material is structurally composed of poly(anthraquinone) with a width of about 2 nm covered by multi-walled carbon nanotubes. Besides, electrochemically, the material had good redox reversibility, its carbon dioxide capture capacity was 7.80 mmol·g−1 with 73.4% material utilization;
- The magnetic stirring method proposed in this experiment was simpler in terms of steps as compared with the metal-organic dehalogenated polycondensation and the combined ultrasonication method used by previous researchers.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- People’s Network. Database of Xi Jinping’s Series of Important Speeches. Available online: http://jhsjk.people.cn/ (accessed on 5 September 2021).
- Wang, C.; Zhang, Y. The path to carbon neutral vision and policy system. China Environ. Manag. 2020, 12, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Xian, X.; Guo, S.; Kong, H.; Zhao, W.; Li, J.; Liu, J.; Zhong, P. Science and technology needs and technology pathways for carbon neutral vision. China Environ. Manag. 2021, 13, 65–70. [Google Scholar] [CrossRef]
- Seah, G.L.; Wang, L.; Tan, L.F.; Tipjanrawee, C.; Sasangka, W.A.; Usadi, A.K.; McConnachie, J.M.; Tan, K.W. Ordered Mesoporous Alumina with Tunable Morphologies and Pore Sizes for CO2 Capture and Dye Separation. ACS Appl. Mater. Interfaces 2021, 13, 36117–36129. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Zhou, P.; Xu, L. Impacts of CaO solid particles in carbon dioxide absorption process from ship emission with NaOH solution. J. Shanghai Jiaotong Univ. 2018, 23, 320–326. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; He, S.; Qin, X.; Li, C.; Li, T. Interfacial engineering in metal–organic framework-based mixed matrix membranes using covalently grafted polyimide brushes. J. Am. Chem. Soc. 2018, 140, 17203–17210. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Arenas, L.F.V.; Navarrete, B. Carbon capture and utilization technologies: A literature review and recent advances. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Xu, S.; Han, C.; Zhang, L.; Zhang, Y.; Du, D.; He, S.; Luo, Y. Advances in carbon dioxide separation and capture. Nat. Gas Chem. Ind. 2011, 36, 72–78. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, X.; Deng, J. Ionic liquids based membranes for CO2 separation: A review. CIESC J. 2016, 7, 248–257. [Google Scholar] [CrossRef]
- Mizen, M.B.; Wrighton, M.S. Reductive Addition of CO2 to 9, 10-Phenanthrenequinone. J. Electrochem. Soc. 1989, 136, 941. [Google Scholar] [CrossRef]
- Rheinhardt, J.H.; Singh, P.; Tarakeshwar, P.; Buttry, D.A. Electrochemical capture and release of carbon dioxide. ACS Energy Lett. 2017, 2, 454–461. [Google Scholar] [CrossRef]
- Scovazzo, P.; Poshusta, J.; DuBois, D.; Koval, C.; Noble, R. Electrochemical separation and concentration of<1% carbon dioxide from nitrogen, J. Electrochem. Soc. 2013, 150, D91. [Google Scholar] [CrossRef]
- Yin, W.; Grimaud, A.; Azcarate, I.; Yang, C.; Tarascon, J.M. Electrochemical reduction of CO2 mediated by quinone derivatives: Implication for Li–CO2 battery. J. Phys. Chem. C 2018, 122, 6546–6554. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, H.Z.; Diederichsen, K.M.; van Voorhis, T.; Hatton, T.A. Electrochemically mediated carbon dioxide separation with quinone chemistry in salt-concentrated aqueous media. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, G.; Wei, X. Electrocatalytic activity of multi-walled carbon nanotubes modified electrode for p-Benzenediol. Chin. J. Anal. Chem. 2004, 08, 1057–1060. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Li, J.; Guan, Y.; Fu, K.; Su, Y.; Bao, L.; Wu, F. Applications of Carbon Nanotubes and Graphene in the Energy Storage Batteries. Prog. Chem. 2014, 26, 1233–1243. [Google Scholar] [CrossRef]
- Voskian, S.; Hatton, T.A. Faradaic electro-swing reactive adsorption for CO2 capture. Energy Environ. Sci. 2019, 12, 3530–3547. [Google Scholar] [CrossRef]
- Yamamoto, T.; Etori, H. Poly (anthraquinone) s having a. pi.-conjugation system along the main chain. Synthesis by organometallic polycondensation, redox behavior, and optical properties. Macromolecules 1995, 28, 3371–3379. [Google Scholar] [CrossRef]
- Liu, R.; Luo, Z.; Wei, Q. Preparation and Electrochemical Performance of PDAAQ/MWCNTs Composite. J. Instrum. Anal. 2016, 35, 765–768. [Google Scholar] [CrossRef]
- Chen, Y.; Du, L.; Yang, P.; Sun, P.; Yu, X.; Mai, W. Significantly enhanced robustness and electrochemical performance of flexible carbon nanotube-based supercapacitors by electrodepositing polypyrrole. J. Power Sources 2015, 287, 68–74. [Google Scholar] [CrossRef]
- Nagaoka, T.; Nishii, N.; Fujii, K.; Ogura, K. Mechanisms of reductive addition of CO2 to quinones in acetonitrile. J. Electroanal. Chem. 1992, 322, 383–389. [Google Scholar] [CrossRef]
- Guin, P.S.; Das, S.; Mandal, P.C. Electrochemical reduction of quinones in different media: A review. Int. J. Electrochem. 2011, 2011, 1–22. [Google Scholar] [CrossRef]
- Apaydin, D.H.; Głowacki, E.D.; Portenkirchner, E.; Sariciftci, N.S. Direct electrochemical capture and release of carbon dioxide using an industrial organic pigment: Quinacridone. Angew. Chem. Int. Ed. 2014, 53, 6819–6822. [Google Scholar] [CrossRef] [PubMed]

| Reagent Items | Characteristics and Manufacturers, etc. |
|---|---|
| 1,4-anthraquinone | purity > 99.5%, Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| Graphitized multi-walled carbon nanotubes | inner diameter: 5–10 nm, outer diameter: 20–30 nm, length: 10–30 μm, purity > 99.9%, Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| Lithium bis(trifluoromethanesulfonyl)imide | purity > 99.9%, Shanghai Eon Chemical Technology Co., Ltd. (Shanghai, China) |
| N-methyl-pyrrolidone | purity > 99.5%, Shanghai Maclean Biochemical Technology Co., Ltd. (Shanghai, China) |
| Carbon dioxide | purity ≥ 99.999%, Shanghai Mizheng Gas Co., Ltd. (Shanghai, China) |
| Nitrogen | purity ≥ 99.99%, Shanghai Mizheng Gas Co., Ltd. (Shanghai, China) |
| Carbon paper TGP-H-060 | thickness: 0.19 mm, density: 0.44 g/cm, resistivity: 5 mΩ-cm, Toray Corporation (Tokyo, Japan) |
| Ag/AgCl reference electrode | 0.1989 V vs. NHE |
| Individual Parts | Retention Time (min) | Peak Height (uV) | Peak Area (uV·s) | Actual Concentration (%) |
|---|---|---|---|---|
| Carbon dioxide | 7.228 | 217 | 13,754 | 0.137 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Feng, J.; Liu, X.; Guo, H. Preparation Methods and Performance Analysis of Polyanthra-Quinone/Carbon Nanotube Composites for Capturing Carbon Dioxide. Atmosphere 2022, 13, 543. https://doi.org/10.3390/atmos13040543
Wang Z, Feng J, Liu X, Guo H. Preparation Methods and Performance Analysis of Polyanthra-Quinone/Carbon Nanotube Composites for Capturing Carbon Dioxide. Atmosphere. 2022; 13(4):543. https://doi.org/10.3390/atmos13040543
Chicago/Turabian StyleWang, Zhongcheng, Jingsong Feng, Xiaoyu Liu, and Hao Guo. 2022. "Preparation Methods and Performance Analysis of Polyanthra-Quinone/Carbon Nanotube Composites for Capturing Carbon Dioxide" Atmosphere 13, no. 4: 543. https://doi.org/10.3390/atmos13040543
APA StyleWang, Z., Feng, J., Liu, X., & Guo, H. (2022). Preparation Methods and Performance Analysis of Polyanthra-Quinone/Carbon Nanotube Composites for Capturing Carbon Dioxide. Atmosphere, 13(4), 543. https://doi.org/10.3390/atmos13040543






