Abstract
Ventilation air methane (VAM) is the main cause of greenhouse gas emissions in coal mining. Catalytic flow reverse reactor (CFRR) is widely used in VAM to mitigate methane emissions. In this study, palladium (Pd) and La1−xSrxMnO3 were used as catalysts in a CFRR. Different types of catalysts were prepared by loading La0.8Sr0.2MnO3, La0.9Sr0.1MnO3, and 0.1%Pd-La0.9Sr0.1MnO3 on a cordierite honeycomb reactor coated with γ-Al2O3 to compare their performances. In addition, this study compared the performance of the three catalysts in an 800 °C reactor based on different methane inlet concentrations, inlet speeds, and conversion times. The results showed: (1) 0.1% addition of Pd increased methane conversion. (2) La0.8Sr0.2MnO3 had higher efficiency at lower methane inlet concentrations, whereas La0.9Sr0.1MnO3 was more efficient at higher methane concentrations. This study demonstrates that a higher Sr loading is worth implementing only when the methane concentration of VAM is lower than 0.6%. (3) To achieve a higher methane conversion efficiency, the inlet velocity of methane should also be considered.
1. Introduction
Methane (CH4) emissions from the coal mining industry contribute to 11% of anthropogenic methane emissions [1,2]. Air ventilated from coal shafts contains methane in low concentrations (0.1–1 vol.%) called ventilation air methane (VAM). VAM is the main source of coal-related methane emissions, and the abatement of VAM is one of the most important measures to achieve the goal of global greenhouse gas emission mitigation by 2050 [2]. Since the warming potential of methane is 28 times higher than that of CO2, the main idea of VAM treatment is to oxidize methane to CO2 before releasing it into the atmosphere [3]. Compared to traditional flame combustion, catalytic flameless combustion has a clear advantage. For instance, it features a lower temperature, higher conversion, no energy loss by flame [4], and negligible formation of noxious by-products such as NOx [5].
One of the most promising utilization technologies of VAM is the use of a reverse flow reactor (RFR). RFR consists of a catalytic fixed-bed reactor in which the feed flow direction is periodically reversed [6]. A great amount of heat released in the reaction can be stored inside the reactor by regularly switching the feed flow direction, so that auto-thermal reaction can be achieved even under very lean feed conditions [7]. Differentiated by whether or not catalysts are used, RFR includes two main systems: the thermal flow reverse reactor (TFRR) and the catalytic flow reverse reactor (CFRR) [4]. Without using any catalyst, TFRR works at a much higher temperature (above 1200 °C) [8]. The use of the catalyst in CFRR significantly decreases the reactor temperature and allows better control of reaction over a wider fuel/air ratio without affecting the flammability [9,10].
The most commonly used catalysts include noble metal catalysts and non-noble metal oxides [11]. Palladium (Pd) is characterized as one of the most active catalysts for methane oxidation [12,13,14]. However, the broad application of noble metal catalysts was limited due to their high cost and some considerable problems such as volatility, sintering, and susceptibility to poisoning [15,16]. Non-noble metal oxides are considered to be one of the better choices due to their high activity for deep oxidation and low cost [17,18]. Perovskite-type mixed oxides are promising alternatives to noble metal oxides. Such materials offer better features such as elevated chemical and thermal ability [19,20]. However, bulk perovskite has low porosity and a strong tendency to sinter [20]. Alumina Al2O3 was the most widely used support for perovskite to increase their surface area and mechanical strength [21]. A range of lanthanum strontium manganates (La1-xSrxMnO3) supported on Al2O3 was proved to be efficient for methane combustion [22,23,24].
The monolithic catalytic reactor is widely used in the methane combustion industry. A common configuration of the monolith reactor is the ‘honeycomb’ reactor, which consists of large amounts of parallel passageways with 1–10 mm inside dimension [25]. Cordierite is a low-cost monolith with high porosity and heat stability that is a common substrate of honeycomb reactors [26]. In this study, we used cordierite honeycomb monolith as supporter, coated with γ-Al2O3, and we prepared three different perovskite catalysts (La0.8Sr0.2MnO3 γ-Al2O3/cord (cat1), La0.9Sr0.1MnO3 γ-Al2O3/cord (cat2), and 0.1%Pd-La0.9Sr0.1MnO3 γ-Al2O3/cord (cat3)). The efficiency of three catalysts was evaluated on a self-designed CFRR system with different methane inlet concentrations, inlet velocities, and reverse cycles. The main objective of this work is to comprehensively evaluate the efficacy of methane combustion with different ratios of Sr substitution on LaMnO3 and their combination with Pd.
2. Materials and Methods
2.1. Introduction of the Experimental Station
This study was conducted on a self-designed VAM thermal CFRR system. The working principle of CFRR was shown by Li et al. [10]. The self-built CFRR experimental system (Figure 1) mainly includes five parts: intake system, reaction system, heating system, piping system, and measurement and control system. The intake system includes the air compressor, pressure-reducing valve, methane cylinder, rotameter, and mass flow meter (1–5 in Figure 1). The air compressor was connected with the rotameter, and the methane cylinder was connected with the mass flow meter. Air and methane flow were controlled by the flow meter, and the air was mixed and flowed into the reaction system. The reaction system includes the heating exchange tube, differential pressure gauge, and regenerator (9–11 in Figure 1). The regenerator was connected with the heat exchange tube, and a U-formed differential pressure gauge was connected to measure the pressure of the inlet and outlet gas. The heating system includes the heating furnace. The highest available temperature was 1000 °C. The pipeline system is composed of two three-way valves, four solenoid valves, two on–off valves, and pipelines. The flow direction of the VAM air in the device is controlled by adjusting the solenoid valve. The measurement and control system consists of 5 temperature measurement points, thermocouples, infrared online gas analyzer, acquisition card, and computer (13–17 in Figure 1). The 5 thermocouples are distributed at 5 temperature measurement points. The thermocouples are connected to the data acquisition card. The temperature data of the temperature measurement point are transmitted to the computer, and a branch is connected to the gas analyzer on the inlet and outlet pipelines, so as to perform real-time monitoring of the inlet and outlet methane concentration. Methane conversion (%) was therefore calculated as:
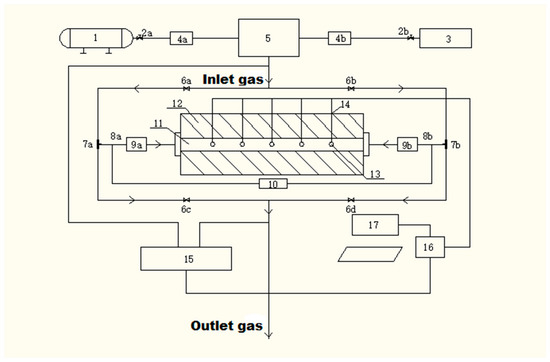
Figure 1.
Apparatus flow chart of methane oxidation experiment. (1) Air compressor; (2a, 2b) pressure-reducing valve; (3) methane cylinder; (4a) rotameter; (4b) mass flow meter; (5) mixing tank; (6a, 6b, 6c, 6d) solenoid valve; (7a, 7b) three-way valve; (8a, 8b) differential pressure interface; (9a, 9b) heat exchange tube; (10) differential pressure gauge; (11) regenerator; (12) heating furnace; (13) temperature measurement point; (14) thermocouple; (15) methane gas analyzer; (16) acquisition card; (17) computer.
2.2. Preparation of the Catalysts
The catalyst preparation includes three steps: acid treatment of the cordierite, coating of γ-Al2O3, and impregnation of the active ingredients.
2.2.1. Acid Treatment of the Cordierite
Catalysts were supported by cordierite honeycomb ceramic (COR), COR was washed and air-dried for 2 h at 110 °C, rinsed into 20% (w/w) oxalic acid solution for 4 h at 80 °C, and then cleaned by ultrasonic treatment for 20 min. Air-dried COR was again dried in the oven for 2 h at 110 °C, then roasted in a muffle furnace at 450 °C for 2 h, and later weighed after cooling.
2.2.2. Coating of γ-Al2O3
Ten grams of Sasol high-purity dispersible alumina hydrates (Brunsbüttel, Germany) was added into 100 mL of deionized water. The solution was blended for 15 min, the pH was adjusted to 3–4, and a small amount of polyethylene glycol was added as a dispersant. Subsequently, it was stirred at a higher speed of 1600 rpm for 2 h. The sol was stored until used. For γ-Al2O3 coating, cordierite was then rinsed into the sol for 20 min. Then, it was pulled out and let stand to dry. After that, the cordierite was oven-dried for 3 h at 120 °C. Lastly, it was roasted in a muffle furnace at 550 °C for 3 h. The processes above were repeated 4 times to obtain high-quality γ-Al2O3/cord supporters.
2.2.3. Impregnation of Catalytically Active Ingredient
The incipient impregnation was performed by immersing the γ-Al2O3-coated cordierite honeycomb in La0.8Sr0.2MnO3 and La0.9Sr0.1MnO3 solution with a certain concentration. The impregnated coating was dried at room temperature for 24 h, dried in the oven for 18 h at 80 °C, and then roasted in a muffle furnace for 6 h at 800 °C. The preparation of 0.1%Pd-La0.9Sr0.1MnO3 γ-Al2O3/cord was performed by rinsing the La0.9Sr0.1MnO3 γ-Al2O3/cord in PdCl2 solution, and the solution was heated until dried. Then, it was air-dried in the oven for 2 h at 120 °C. After this, it was roasted in a muffle furnace for 4 h at 500 °C.
2.3. Coating Adhesion Test
The weight loss of the substrate was calculated based on its weight difference before and after the loading. The theoretical coating was 20%. However, after calculation, the coated catalysts of La0.8Sr0.2MnO3 γ-Al2O3/cord (cat1), La0.9Sr0.1MnO3 γ-Al2O3/cord (cat2), and 0.1% Pd-La0.9Sr0.1MnO3 γ-Al2O3/cord (cat3) were 10.8%, 15.7%, and 14.8%, respectively.
2.4. Brunauer–Emmett–Teller (BET) Surface Area Analysis
The surface area was analyzed with a JW-BK static nitrogen adsorption apparatus (Beijing, China). The samples were evaluated by N2 adsorption at 77 K according to the BET method after degassing under vacuum at 250 °C. The result of each catalyst is shown in Table 1. The results showed that the surface area of cat3 > cat2 > cat1.

Table 1.
Results of the BET specific surface curves of each catalyst.
2.5. Data Analysis
Set a complete cycle period by switching the flow direction twice, for example, when the switching frequency is 5 min, a cycle period is 10 min. We collected the mean methane conversion of the first 6 cycle periods to perform linear regression analysis. We first checked normal distribution with the Shapiro–Wilk test, and then linear correlations between methane conversion with different methane inlet concentrations, methane inlet velocities, and switch times were tested with Pearson correlation coefficients. Data analyses were performed using IBM SPSS 21.0 software package. Graphs were prepared in Sigmaplot 10.0.
3. Results and Discussion
3.1. Comparison of the Conversion of Methane with or without Catalyst
Figure 2 shows the conversion of methane without or with catalyst. The conversion of methane in treatment without catalyst was high at the beginning, but after 30 min, the conversion was reduced to only 20%. In contrast, treatment using La0.8Sr0.2MnO3 γ-Al2O3/cord showed a stable conversion of methane between 50–80%. The results demonstrate that the reaction cannot be sustained at 800 °C without catalysts, and the use of catalysts is essential for self-maintenance operation of the reactor.
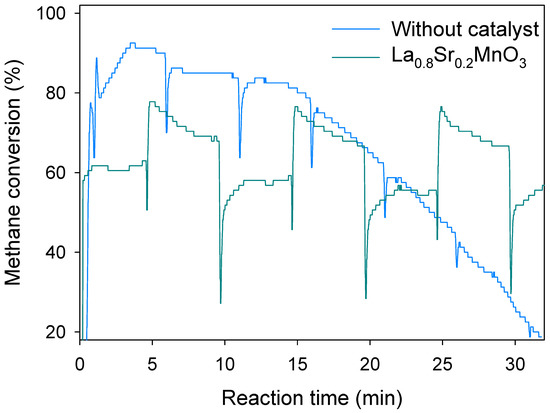
Figure 2.
The conversion of methane without catalyst or with La0.8Sr0.2MnO3 catalyst. Start-up conditions: methane inlet concentration: 0.8%, reaction temperature: 800 °C, inlet flow velocity: 1.2 m3/h, switch time: 5 min.
3.2. Effect of Methane Inlet Concentration on Methane Conversion
Figure 3 shows a clear positive correlation between methane inlet concentration and methane conversion. The reason might be: (1) the reaction is determined by the concentration of methane: r = [CH4]x[O2]y; (2) a higher methane concentration in the reactor allows the methane particles to more easily be exposed to the surface area of the catalysts; and (3) the reaction with higher methane concentration produced more heat to sustain further reaction. The addition of Pd had a higher conversion efficiency than without Pd at all methane concentrations. The increased surface area by the addition of Pd might be an important reason for higher catalysis efficiency (Table 1). The activity of Pd to increase catalysis efficiency has been proved in other studies [27,28,29]. When added to La1−xSrxMnO3, Pd particles become catalytic reaction centers, affecting the activity of the entire catalyst, which might play an important role in methane oxidation.
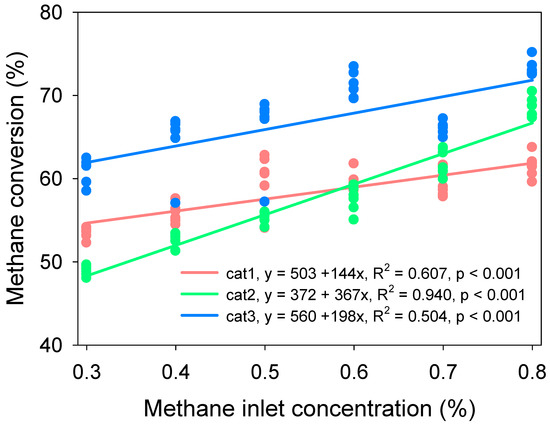
Figure 3.
Correlation of methane inlet concentration and methane conversion. Start-up conditions: switch frequency: 5 min, reaction temperature: 800 °C, gas flow: 1.2 m3/h.
Interestingly, La0.8Sr0.2MnO3 had a higher efficiency than La0.9Sr0.1MnO3 at lower methane influx concentrations, but the efficiency of cat2 became lower than cat1 as the methane concentration increased (Figure 3). The result reveals that higher Sr substitution receives more benefit in VAM treatment only with lower methane concentrations (<vol. 0.6%). Gao et al. also demonstrated that x = 0.1 was the optimal degree of substitution [30], that lower substitution leads to insufficient oxygen vacancy, and that higher substitution leads to an excessive number of Mn4+ ions, which was unfavorable to activation of gaseous oxygen. The dynamic of three catalysts at 0.8% methane inlet concentration, 5 min switch frequency, 800 °C reaction temperature, and 1.2 m3/h inlet flow velocity is shown in Figure 4, and the dynamic of different methane inlet concentrations on methane conversion is shown in Figure 5.
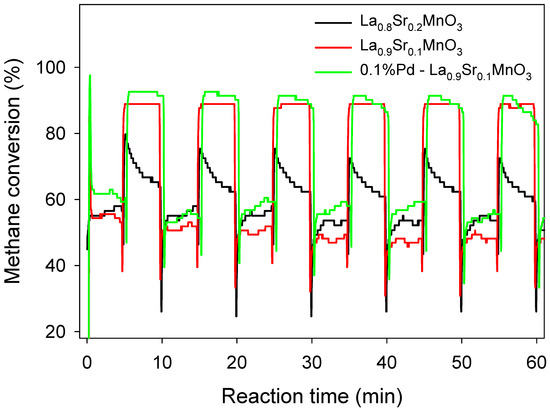
Figure 4.
Methane conversion of La0.8Sr0.2MnO3, La0.9Sr0.1MnO3, and 0.1%Pd-La0.9Sr0.1MnO3 catalyst. Start-up conditions: methane inlet concentration: 0.8%, reaction temperature: 800 °C, inlet flow velocity: 1.2 m3/h, switch time: 5 min.
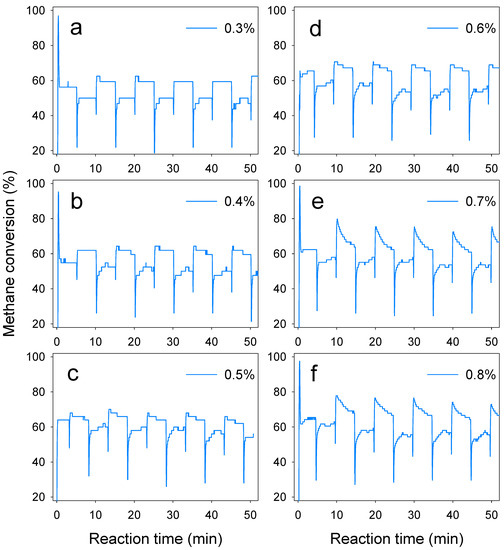
Figure 5.
Methane conversion of La0.8Sr0.2MnO3 catalyst with different methane inlet concentrations. Start-up conditions: reaction temperature: 800 °C, inlet flow velocity: 1.2 m3/h, switch time: 5 min. The methane inlet concentration were (a) 0.3%, (b) 0.4%, (c) 0.5%, (d) 0.6%, (e) 0.7%, (f) 0.8%.
3.3. Effect of Methane Inlet Velocity on Methane Conversion
The addition of Pd (cat3) shows the highest methane conversion efficiency, when the inlet flow velocity was lower than 1.8 m3/h (Figure 6). Increased inlet velocity clearly decreased methane conversion in cat1 and cat3, whereas methane conversion had no clear response to inlet velocity by cat2. The results indicate that enough time for the contact of CH4 and surface area is required to ensure the efficiency of methane conversion in VAM treatment. The dynamic of methane conversation of La0.8Sr0.2MnO3 catalyst with different methane inlet velocities is shown in Figure 7.
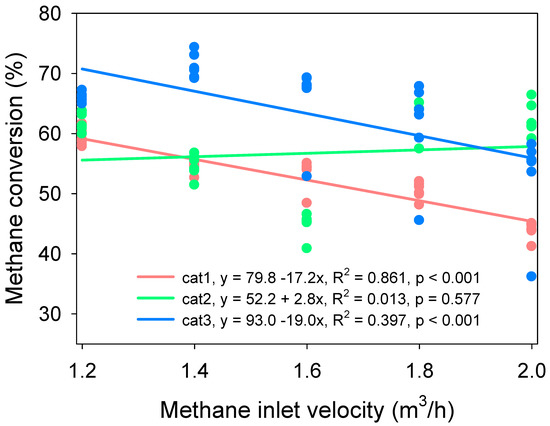
Figure 6.
Correlation of methane inlet velocity and methane conversion. Start-up conditions: switch frequency: 5 min, reaction temperature: 800 °C, methane inlet concentration: 0.7%.
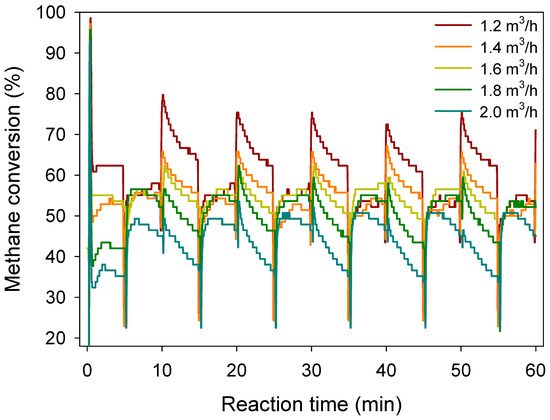
Figure 7.
Methane conversion of La0.8Sr0.2MnO3 catalyst with different methane inlet velocities. Start-up conditions: methane inlet concentration: 0.7%, reaction temperature: 800 °C, inlet flow velocity: 1.2 m3/h, switch time: 5 min.
3.4. Effect of Switch Frequency on Methane Conversion
The switch frequency of reverse flow reactors also determines the reaction center of feed gas [31]. In this study, only cat1 showed a clear negative correlation with increasing switch time (Figure 8). No clear difference in methane conversion for cat2 and cat3 was found as switch time increased from 1 min to 7 min. However, the dynamic of methane emission with different switch times (Figure 9) shows that treatment with the switch time of 1 min experienced transient peaks and also low conversion, which means the stability was not as good as with the switch time of 3 min or 5 min. A long switch time also reduced the methane conversion efficiency, while the efficiency of heat transfer was limited [32].
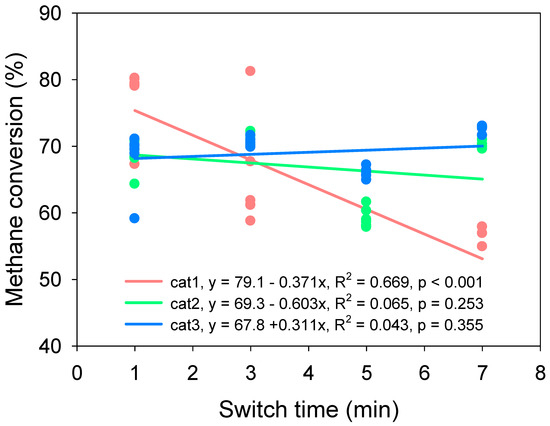
Figure 8.
Correlation of switch time and methane conversion. Start-up conditions: reaction temperature: 800 °C, methane inlet concentration: 0.7%, methane inlet velocity: 1.2 m3/h.
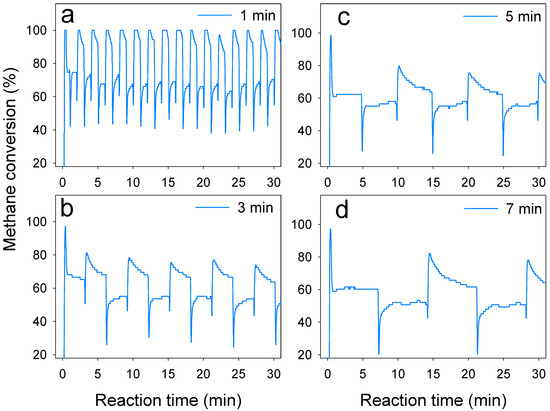
Figure 9.
Dynamic of methane conversion of La0.8Sr0.2MnO3 catalyst with different switch times. Start-up conditions: reaction temperature: 800 °C, methane inlet concentration: 0.7%, methane inlet velocity: 1.2 m3/h. The switch time were (a) 1 min, (b) 3 min, (c) 5 min, (d) 7 min.
3.5. Catalysis Characteristics and Catalytic Mechanism
Overall, the addition of Pd had the highest efficiency than other catalysts. One reason was its higher surface area on the supporters. Our previous study showed that Pd and PdO particles attached to the surface of perovskite, and the protruded structure can significantly increase the surface area of the catalysts (Figure 10) [29]. The other reason was that the combination of Pd and La1−xSrxMnO3 provided dual-site active centers for methane oxidation [28,33,34], which the Pd-perovskite efficiently combine as low- and high-temperature oxidation activity within a single system. At lower temperatures, the higher contribution of lattice oxygen and possible local change in the oxidation state of palladium possibly enhanced catalysis activity; at higher temperatures, the presence of perovskite provides higher activity and durability by promoting a self-regenerative activation mechanism for PdO-Pd equilibrium [28,33,35].
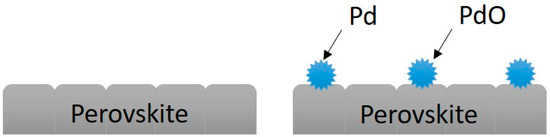
Figure 10.
Pd addition increased the surface area of the catalyst: spherical Pd particles protruded on the perovskite layer and thus increased surface area of the catalyst.
Whether Sr substitution can increase methane oxidation is still debated. Ciambelli et al. demonstrated that Sr substitution does not necessarily promote methane oxidation, while Sr might lead to the formation of Mn4+, which is easily reducible to Mn2+ even at low temperatures, thus hindering the redox mechanism [36]. However, McCarty suggests that Sr substitution results in more Mn4+ in the perovskite structure [37,38], and thus increases methane oxidation [39,40]. The different performances of La0.8Sr0.2MnO3 and La0.9Sr0.1MnO3 at different methane inlet concentrations and inlet velocities in our study provide a hint of the mechanisms. Our results showed that higher Sr substitution had a higher efficiency only at lower methane concentrations and lower inlet velocities (Figure 3 and Figure 6). Sr substitution alters the bond length of Mn–O and the Mn3+–O2−–Mn4+ bond angle, where the bond angle increases from 90° to nearly 180°, with the increase in Sr loading (Figure 11) [41]. At lower methane concentrations, Sr substitution benefits from expanded compositional space of a double exchange mechanism [42]. However, as the methane concentration increases, the reaction produces more heat, leading to a higher environment temperature, which may decrease the stability of Sr-substituted perovskite, whereas the Mn3+–O2−–Mn4+ bond angle was distorted by Sr substitution [41]. Thus, the efficiency of La0.8Sr0.2MnO3 decreased more prominently with the increase in methane concentration and inlet velocity. However, the effect of Sr substitution on perovskite on methane oxidation under different reaction conditions needs more elaborate research.
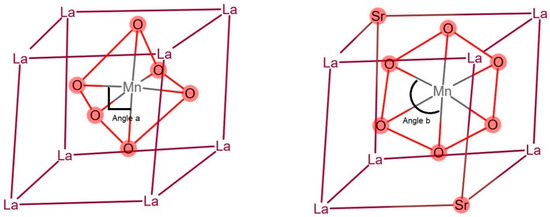
Figure 11.
Sr substitution increased the bond angle of Mn3+–O2−–Mn4+: the bonding angle of Mn3+–O2−–Mn4+ in LaMnO3 was 90° (left). However, Sr substitution distorted the structure; therefore, the angle was >90° (right).
4. Conclusions
We investigated the methane conversion of three catalysts: La0.8Sr0.2MnO3 γ-Al2O3/cord, La0.9Sr0.1MnO3 γ-Al2O3/cord, and 0.1%Pd-La0.9Sr0.1MnO3 γ-Al2O3/cord under different methane inlet concentrations, inlet velocities, and switch times. The addition of 0.1% Pd showed the highest efficiency in most cases. The highest methane conversion efficiencies could be obtained at both a lower methane inlet concentration (<0.6%) with La0.8Sr0.2MnO3 and a higher methane inlet concentration (>0.6%) with La0.9Sr0.1MnO3. Moreover, methane conversion was reduced, as the velocity of inlet methane increased. The experimental results of this study provide useful information for selecting suitable catalysts and reaction conditions in VAM treatment.
Author Contributions
Conceptualization: T.Z.; Methodology: T.Z.; Formal analysis and investigation: Y.W.; Writing—original draft preparation: Y.W.; Writing—review and editing: Y.W. and T.Z.; Funding acquisition: T.Z.; Resources: T.Z.; Supervision: T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Open Project Program of the State Key Laboratory of Petroleum Pollution Control (Grant No. PPC2019013), the CNPC Research Institute of Safety and Environmental Technology, the Open Foundation of Shaanxi Key Laboratory of Lacustrine Shale Gas Accumulation and Exploitation (under planning), and the Fundamental Research Funds for the Central Universities (No. 2009QH03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kholod, N.; Evans, M.; Pilcher, R.C.; Roshchanka, V.; Ruiz, F.; Coté, M.; Collings, R. Global Methane Emissions from Coal Mining to Continue Growing Even with Declining Coal Production. J. Clean. Prod. 2020, 256, 120489. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Global Non-CO2 Greenhouse Gas Emission Projections & Mitigation Potential: 2015–2050: Download the Report. Available online: https://www.epa.gov/global-mitigation-non-co2-greenhouse-gases/global-non-co2-greenhouse-gas-emission-projections (accessed on 30 October 2021).
- Su, S.; Beath, A.; Guo, H.; Mallett, C. An Assessment of Mine Methane Mitigation and Utilisation Technologies. Prog. Energy Combust. Sci. 2005, 31, 123–170. [Google Scholar] [CrossRef]
- Su, S.; Agnew, J. Catalytic Combustion of Coal Mine Ventilation Air Methane. Fuel 2006, 85, 1201–1210. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, P.; Zhang, L.; Guo, M.; Ran, J. Experiment and Modeling of Low-Concentration Methane Catalytic Combustion in a Fluidized Bed Reactor. Appl. Therm. Eng. 2016, 93, 660–667. [Google Scholar] [CrossRef]
- Marín, P.; Ordóñez, S.; Díez, F.V. Procedures for Heat Recovery in the Catalytic Combustion of Lean Methane–Air Mixtures in a Reverse Flow Reactor. Chem. Eng. J. 2009, 147, 356–365. [Google Scholar] [CrossRef]
- Li, Z.; Qin, Z.; Zhang, Y.; Wu, Z.; Wang, H.; Li, S.; Shi, R.; Dong, M.; Fan, W.; Wang, J. A Control Strategy of Flow Reversal with Hot Gas Withdrawal for Heat Recovery and Its Application in Mitigation and Utilization of Ventilation Air Methane in a Reverse Flow Reactor. Chem. Eng. J. 2013, 228, 243–255. [Google Scholar] [CrossRef]
- Gosiewski, K.; Matros, Y.S.; Warmuzinski, K.; Jaschik, M.; Tanczyk, M. Homogeneous vs. Catalytic Combustion of Lean Methane—Air Mixtures in Reverse-Flow Reactors. Chem. Eng. Sci. 2008, 63, 5010–5019. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A. Catalytic or Thermal Reversed Flow Combustion of Coal Mine Ventilation Air Methane: What Is Better Choice and When? Chem. Eng. J. 2014, 238, 78–85. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Qin, Z.; Zhu, H.; Wu, J.; Wang, R.; Lei, L.; Chen, J.; Dong, M.; Fan, W.; et al. Demonstration of Mitigation and Utilization of Ventilation Air Methane in a Pilot Scale Catalytic Reverse Flow Reactor. Fuel Process. Technol. 2017, 160, 102–108. [Google Scholar] [CrossRef]
- Ruiz, J.A.C.; Oliveira, E.C.; Fraga, M.A.; Pastore, H.O. Performance of Pd Supported on Mesoporous Molecular Sieves on Methane Combustion. Catal. Commun. 2012, 4, 1–6. [Google Scholar] [CrossRef]
- Fan, X.; Wang, F.; Zhu, T.; He, H. Effects of Ce on Catalytic Combustion of Methane over Pd-Pt/Al2O3 Catalyst. J. Environ. Sci. China 2012, 24, 507–511. [Google Scholar] [CrossRef]
- Lee, J.H.; Trimm, D.L. Catalytic Combustion of Methane. Fuel Process. Technol. 1995, 42, 339–359. [Google Scholar] [CrossRef]
- Xiong, H.; Lester, K.; Ressler, T.; Schlögl, R.; Allard, L.F.; Datye, A.K. Metastable Pd ↔ PdO Structures During High Temperature Methane Oxidation. Catal. Lett. 2017, 147, 1095–1103. [Google Scholar] [CrossRef]
- Ojala, S.; Pitkäaho, S.; Laitinen, T.; Niskala Koivikko, N.; Brahmi, R.; Gaálová, J.; Matejova, L.; Kucherov, A.; Päivärinta, S.; Hirschmann, C.; et al. Catalysis in VOC Abatement. Top. Catal. 2011, 54, 1224. [Google Scholar] [CrossRef]
- Tang, W.; Liu, G.; Li, D.; Liu, H.; Wu, X.; Han, N.; Chen, Y. Design and Synthesis of Porous Non-Noble Metal Oxides for Catalytic Removal of VOCs. Sci. China Chem. 2015, 58, 1359–1366. [Google Scholar] [CrossRef]
- Lahousse, C.; Bernier, A.; Grange, P.; Delmon, B.; Papaefthimiou, P.; Ioannides, T.; Verykios, X. Evaluation of γ-MnO2 as a VOC Removal Catalyst: Comparison with a Noble Metal Catalyst. J. Catal. 1998, 178, 214–225. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic Combustion of VOCs on Non-Noble Metal Catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Milt, V.G.; Spretz, R.; Ulla, M.A.; Lombardo, E.A.; Fierro, J.L.G. The Nature of Active Sites for the Oxidation of Methane on La-Based Perovskites. Catal. Lett. 1996, 42, 57–63. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G.; Tascón, J.M.D. Structure and Reactivity of Perovskite-Type Oxides. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1989; Volume 36, pp. 237–328. [Google Scholar] [CrossRef]
- Cimino, S.; Pirone, R.; Russo, G. Thermal Stability of Perovskite-Based Monolithic Reactors in the Catalytic Combustion of Methane. Ind. Eng. Chem. Res. 2001, 40, 80–85. [Google Scholar] [CrossRef]
- Arai, H.; Yamada, T.; Eguchi, K.; Seiyama, T. Catalytic Combustion of Methane over Various Perovskite-Type Oxides. Appl. Catal. 1986, 26, 265–276. [Google Scholar] [CrossRef]
- Kucharczyk, B.; Tylus, W. Metallic Monolith Supported LaMnO3 Perovskite-Based Catalysts in Methane Combustion. Catal. Lett. 2007, 115, 122–132. [Google Scholar] [CrossRef]
- Zhang, H.M.; Teraoka, Y.; Yamazoe, N. Preparation of Supported La1−xSrxMnO3 Catalysts by the Citrate Process. Appl. Catal. 1988, 41, 137–146. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowskib, S.T.; Li, P.K.C.; Awdry, S. Evaluating the Effective Diffusivity of Methane in the Washcoat of a Honeycomb Monolith. Appl. Catal. B Environ. 2000, 25, 93–104. [Google Scholar] [CrossRef]
- Al-Harbi, O.A.; Özgür, C.; Khan, M.M. Fabrication and Characterization of Single Phase Cordierite Honeycomb Monolith with Porous Wall from Natural Raw Materials as Catalyst Support. Ceram. Int. 2015, 41 Pt A, 3526–3532. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, F.; Niu, X.; Zhu, Y. Preparation of Pd Supported on La(Sr)-Mn-O Perovskite by Microwave Irradiation Method and Its Catalytic Performances for the Methane Combustion. Sci. Rep. 2016, 6, 19511. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Casaletto, M.P.; Lisi, L.; Russo, G. Pd–LaMnO3 as Dual Site Catalysts for Methane Combustion. Appl. Catal. Gen. 2007, 327, 238–246. [Google Scholar] [CrossRef]
- Eyssler, A.; Mandaliev, P.; Winkler, A.; Hug, P.; Safonova, O.; Figi, R.; Weidenkaff, A.; Ferri, D. The Effect of the State of Pd on Methane Combustion in Pd-Doped LaFeO3. J. Phys. Chem. C 2010, 114, 4584–4594. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, R. Catalytic Activity for Methane Combustion of the Perovskite-Type La1−xSrxCoO3−δ Oxide Prepared by the Urea Decomposition Method. Appl. Catal. B Environ. 2010, 98, 147–153. [Google Scholar] [CrossRef]
- Budhi, Y.W.; Hoebink, J.H.B.J.; Schouten, J.C. Reverse Flow Operation with Reactor Side Feeding: Analysis, Modeling, and Simulation. Ind. Eng. Chem. Res. 2004, 43, 6955–6963. [Google Scholar] [CrossRef]
- Zhikai, L.; Zhangfeng, Q.; Yagang, Z.; Zhiwei, W.; Hui, W.; Shuna, L.; Mei, D.; Weibin, F.; Jianguo, W. A Logic-Based Controller for the Mitigation of Ventilation Air Methane in a Catalytic Flow Reversal Reactor. Front. Chem. Sci. Eng. 2013, 7, 347–356. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Pirone, R.; Russo, G. Dual-Site Pd/Perovskite Monolithic Catalysts for Methane Catalytic Combustion. Ind. Eng. Chem. Res. 2004, 43, 6670–6679. [Google Scholar] [CrossRef]
- Civera, A.; Negro, G.; Speccia, S.; Saracco, G.; Soecchia, V. Optimal compositional and structural design of a LaMnO3/ZrO2/Pd-based catalyst for methane combustion. Catal. Today 2005, 100, 275–281. [Google Scholar] [CrossRef]
- Petrović, S.; Karanović, L.; Stefanov, P.K.; Zdujić, M.; Terlecki-Baričević, A. Catalytic Combustion of Methane over Pd Containing Perovskite Type Oxides. Appl. Catal. B Environ. 2005, 58, 133–141. [Google Scholar] [CrossRef]
- Ciambelli, P.; Cimino, S.; De Rossi, S.; Faticanti, M.; Lisi, L.; Minelli, G.; Pettiti, I.; Porta, P.; Russo, G.; Turco, M. AMnO3 (A = La, Nd, Sm) and Sm1−xSrxMnO3 Perovskites as Combustion Catalysts: Structural, Redox and Catalytic Properties. Appl. Catal. B Environ. 2000, 24, 243–253. [Google Scholar] [CrossRef]
- McCarty, J.G.; Wise, H. Perovskite Catalysts for Methane Combustion. Catal. Today 1990, 8, 231–248. [Google Scholar] [CrossRef]
- Miniajluk, N.; Trawczyński, J.; Zawadzki, M.; Tylus, W. LaMnO3 (La0.8Sr0.2MnO3) Perovskites for Lean Methane Combustion: Effect of Synthesis Method. Adv. Mater. Phys. Chem. 2018, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Zwinkels, M.F.M.; Järås, S.G.; Menon, P.G.; Griffin, T.A. Catalytic Materials for High-Temperature Combustion. Catal. Rev. 1993, 35, 319–358. [Google Scholar] [CrossRef]
- Quinlan, M.A.; Wise, H.; McCarty, J.G. Basic Research on Natural Gas Combustion Phenomena-Catalytic Combustion. Final Report, 15 January 1986–14 January 1989; PB-90-106493/XAB; SRI International: Menlo Park, CA, USA, 1989. [Google Scholar]
- McBride, K.; Cook, J.; Gray, S.; Felton, S.; Stella, L.; Poulidi, D. Evaluation of La1−xSrxMnO3 (0 ≤ x < 0.4) Synthesised via a Modified Sol–Gel Method as Mediators for Magnetic Fluid Hyperthermia. Cryst. Eng. Comm. 2016, 18, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(Electro)Catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).