Long-Term Dynamic of Cold Stress during Heading and Flowering Stage and Its Effects on Rice Growth in China
Abstract
:1. Introduction
2. Materials and Methods
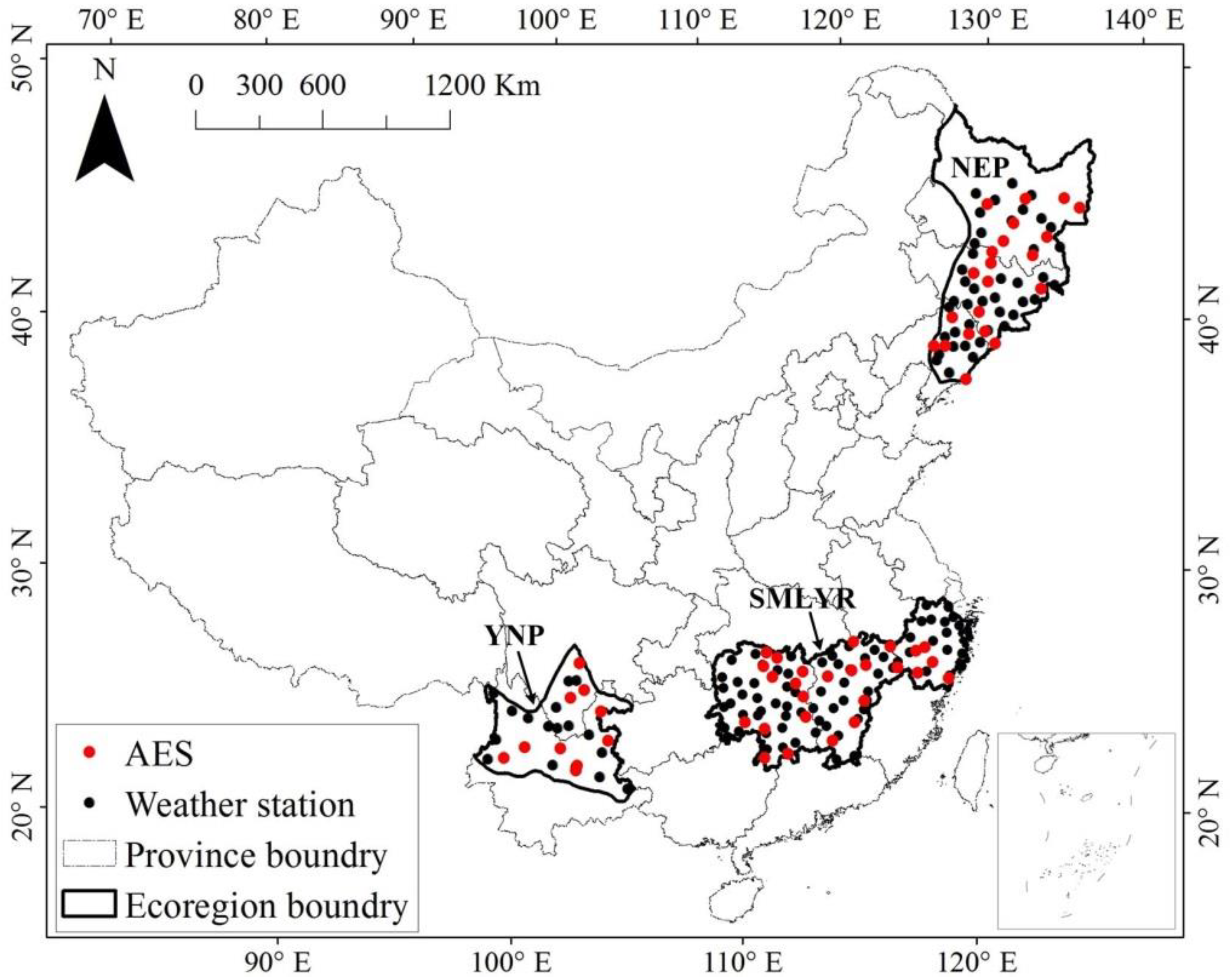
2.1. Study Region and Data Sources
2.2. Cold-Stress Indices
2.3. Characterizing the Spatio-Temporal Patterns of Cold Stress Indices during Heading and Flowering
2.4. Impact of Cold Stress on Rice Yield and Related Variables
3. Results
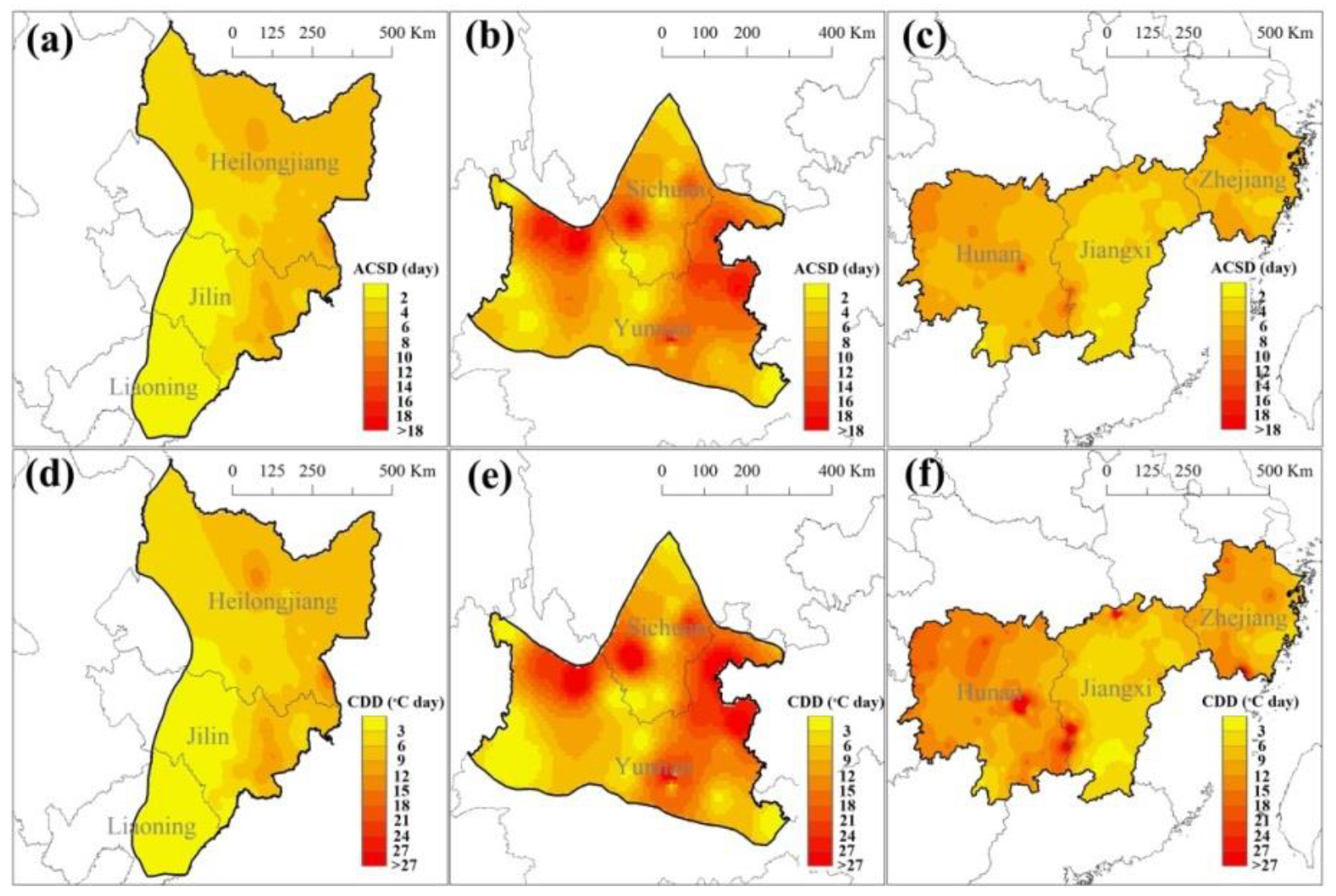
3.1. Spatial Variation of Cold Stress during Rice Heading and Flowering
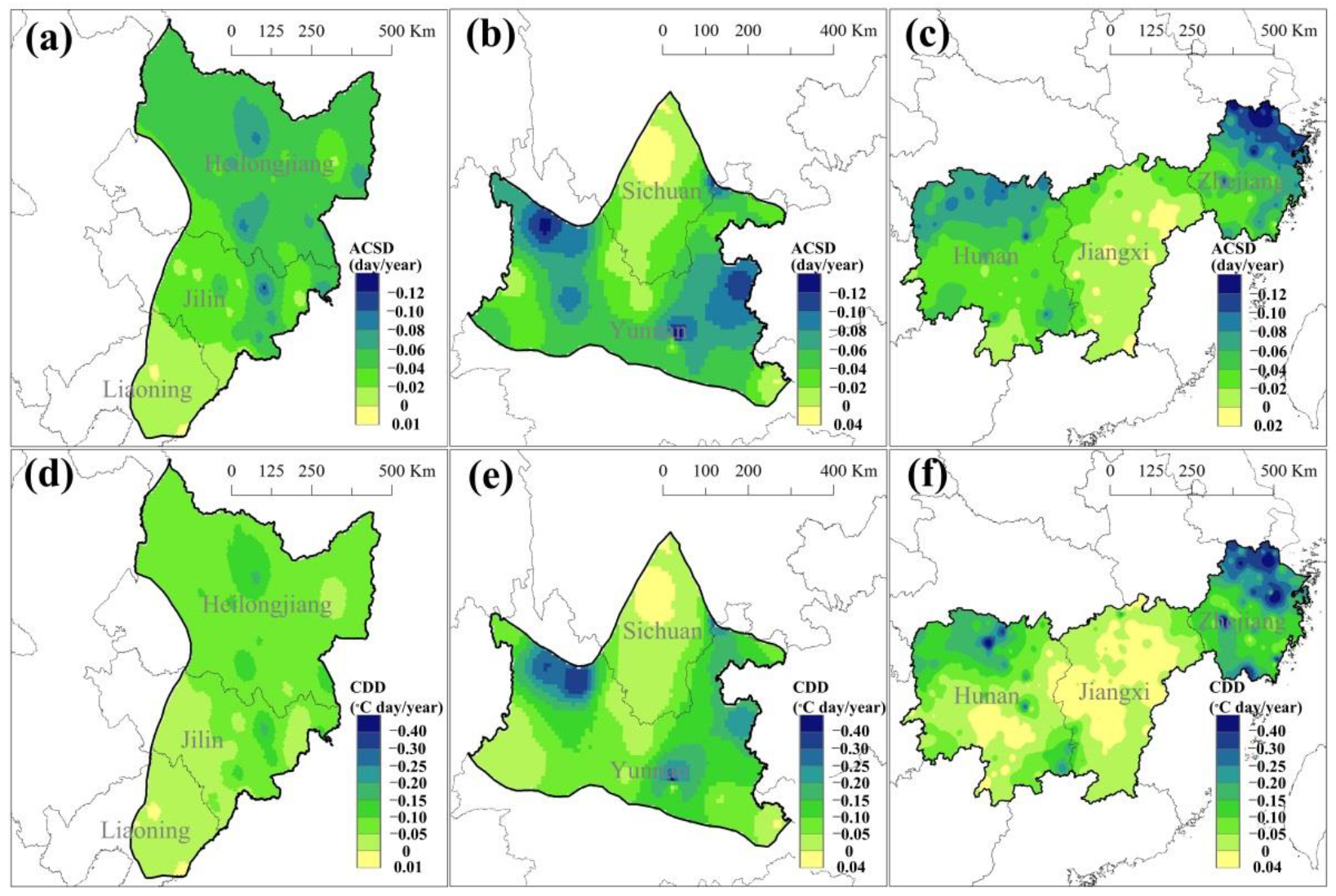
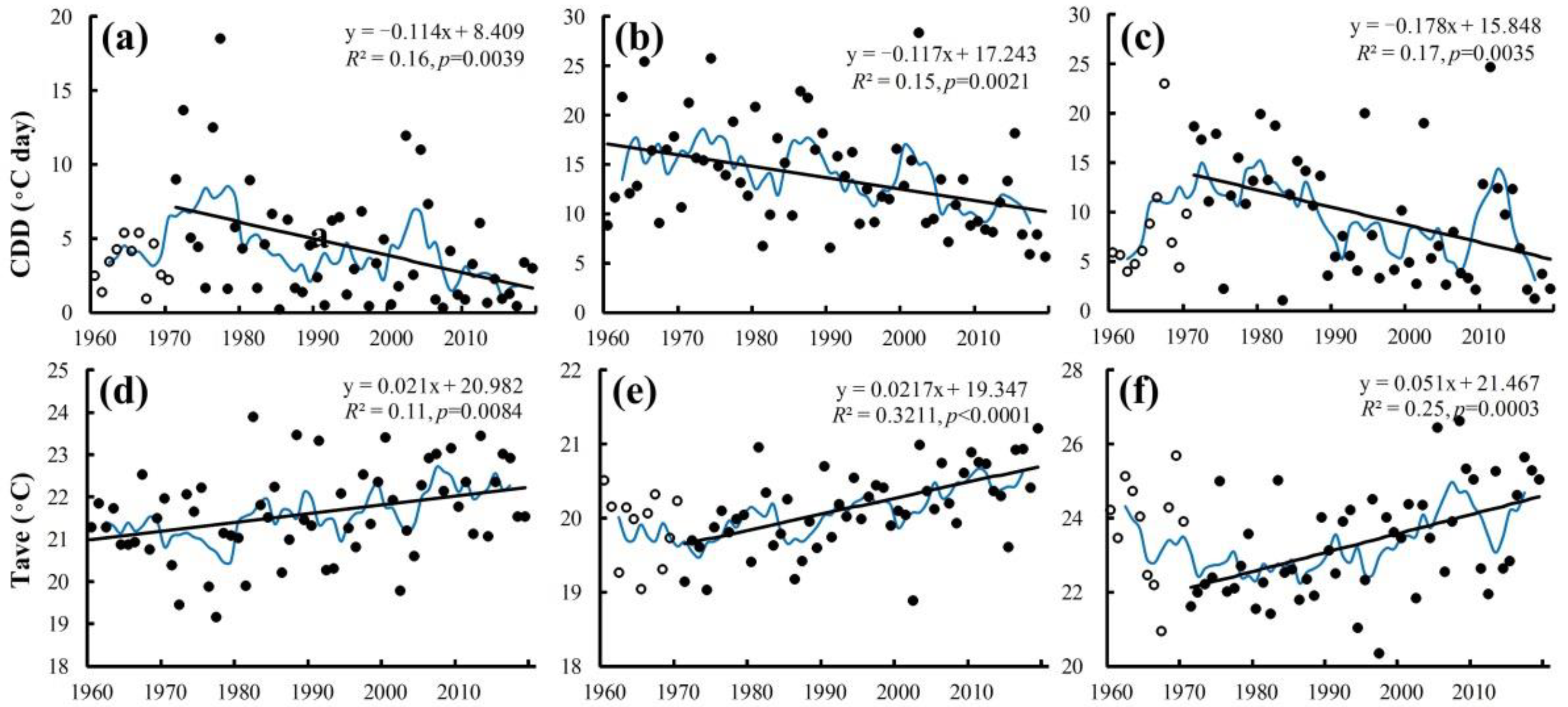
3.2. Temporal Trends of Cold Indices during Heading and Flowering in the Past 60 Years
3.3. Pearson’s Correlation
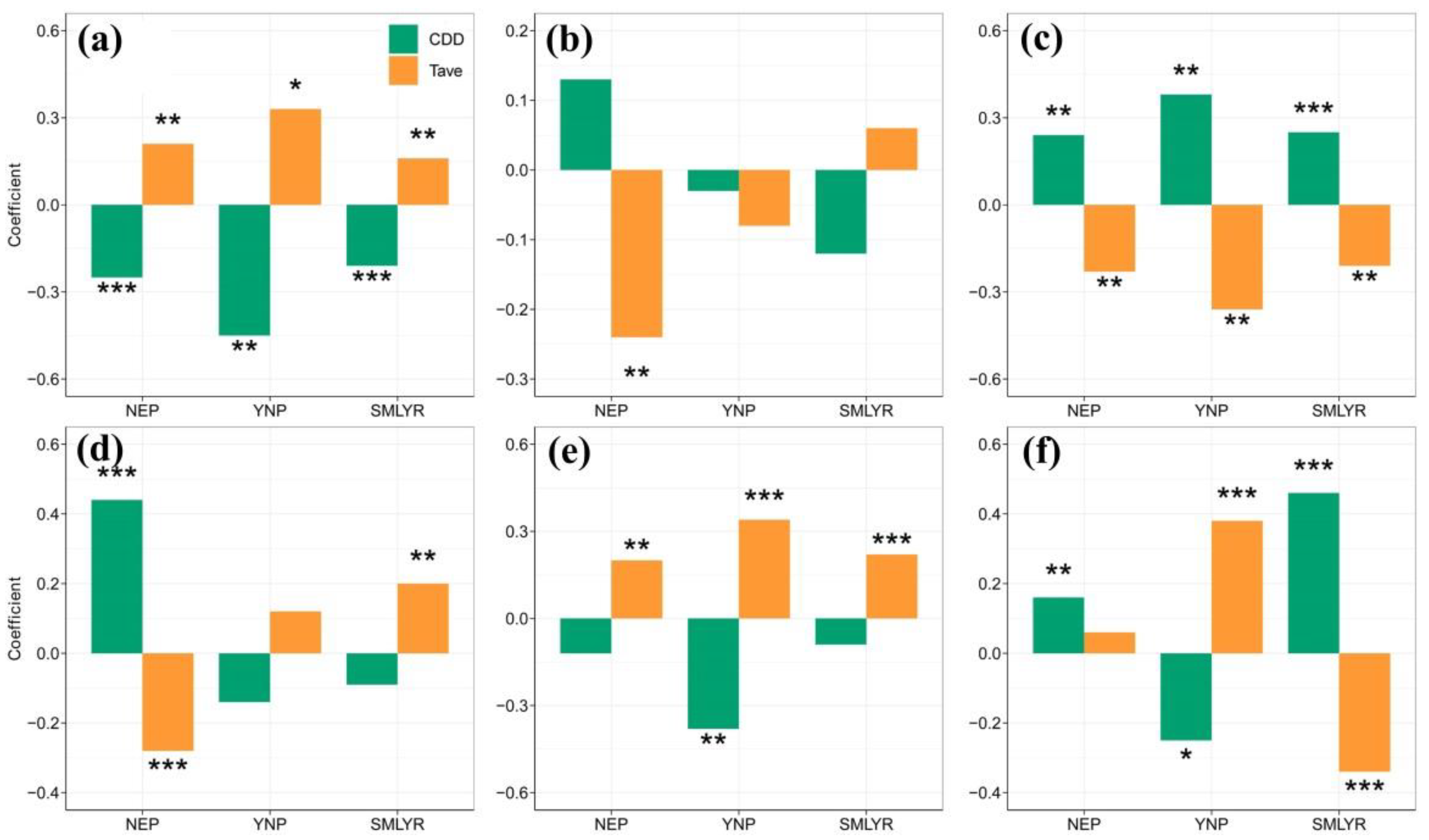
3.4. Impacts of Cold Stress on Rice Yield and Related Variables
4. Discussion
4.1. Spatial and Temporal Variations of Cold Stress
4.2. Impact of Cold Stress on Rice Yield and Related Variables
4.3. Potential Adaptation Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vogel, E.; Donat, M.G.; Alexander, L.V.; Meinshausen, M.; Ray, D.K.; Karoly, D.; Meinshausen, N.; Frieler, K. The effects of climate extremes on global agricultural yields. Environ. Res. Lett. 2019, 14, 054010. [Google Scholar] [CrossRef]
- Ben-Ari, T.; Adrian, J.; Klein, T.; Calanca, P.; Van der Velde, M.; Makowski, D. Identifying indicators for extreme wheat and maize yield losses. Agric. For. Meteorol. 2016, 220, 130–140. [Google Scholar] [CrossRef]
- Mäkinen, H.; Kaseva, J.; Trnka, M.; Balek, J.; Kersebaum, K.C.; Nendel, C.; Gobin, A.; Olesen, J.E.; Bindi, M.; Ferrise, R.; et al. Sensitivity of European wheat to extreme weather. Field Crops Res. 2018, 222, 209–217. [Google Scholar] [CrossRef]
- Ye, J.; Gao, Z.; Wu, X.; Lu, Z.; Li, C.; Wang, X.; Chen, L.; Cui, G.; Yu, M.; Yan, G.; et al. Impact of increased temperature on spring wheat yield in northern China. Food Energy Secur. 2021, 10, e283. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, C.; Wang, P.; Tao, F. Future extreme temperature and its impact on rice yield in China. Int. J. Climatol. 2017, 37, 4814–4827. [Google Scholar] [CrossRef]
- Teixeira, E.I.; Fischer, G.; van Velthuizen, H.; Walter, C.; Ewert, F. Global hot-spots of heat stress on agricultural crops due to climate change. Agric. For. Meteorol. 2013, 170, 206–215. [Google Scholar] [CrossRef]
- Meyer, L.; Brinkman, S.; van Kesteren, L.; Leprince-Ringuet, N.; van Boxmeer, F. Climate Change 2014: Synthesis Report. Contribution of Working Groups I. II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Liu, M.; Guo, Y.; Wang, Y.; Hao, J. Changes of Extreme Agro-Climatic Droughts and Their Impacts on Grain Yields in Rain-Fed Agricultural Regions in China over the Past 50 Years. Atmosphere 2022, 13, 4. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Sridevi, V.D.; Chellamuthu, V. Impact of weather on rice—A review. Int. J. Appl. Res. 2015, 1, 825–831. [Google Scholar]
- Kuroki, M.; Saito, K.; Matsuba, S.; Yokogami, N.; Shimizu, H.; Ando, I.; Sato, Y. A quantitative trait locus for cold tolerance at the booting stage on rice chromosome 8. Theor. Appl. Genet. 2007, 115, 593–600. [Google Scholar] [CrossRef]
- Xu, P.; Cai, W. RAN1 is involved in plant cold resistance and development in rice (Oryza sativa). J. Exp. Bot. 2014, 65, 3277–3287. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.-M.; Zhou, L.; Zeng, Y.-W.; Wang, F.-M.; Zhang, H.-L.; Shen, S.-Q.; Li, Z.-C. Identification and mapping of quantitative trait loci for cold tolerance at the booting stage in a japonica rice near-isogenic line. Plant Sci. 2008, 174, 340–347. [Google Scholar] [CrossRef]
- Biswas, P.S.; Rashid, M.M.; Khatun, H.; Yasmeen, R.; Biswas, J.K. Chapter 11—Scope and Progress of Rice Research Harnessing Cold Tolerance. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 225–264. [Google Scholar]
- Sharifi, P. Evaluation on Sixty-eight Rice Germplasms in Cold Tolerance at Germination Stage. Rice Sci. 2010, 17, 77–81. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef]
- Mamun, E.A.; Alfred, S.; Cantrill, L.C.; Overall, R.L.; Sutton, B.G. Effects of chilling on male gametophyte development in rice. Cell Biol. Int. 2006, 30, 583–591. [Google Scholar] [CrossRef]
- Susanti, Z.; Snell, P.; Fukai, S.; Mitchell, J.H. Importance of anther dehiscence for low-temperature tolerance in rice at the young microspore and flowering stages. Crop Pasture Sci. 2019, 70, 113–120. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Current Status, Challenges, and Opportunities in Rice Production. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–32. [Google Scholar]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, J.; Liu, B.; Peng, S.; Leung, H.; Zhao, J.; Wang, X.; Yang, T.; Huang, Z. Identification of QTLs for cold tolerance at seedling stage in rice (Oryza sativa L.) using two distinct methods of cold treatment. Euphytica 2014, 195, 95–104. [Google Scholar] [CrossRef]
- Nie, L.; Peng, S. Rice Production in China. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 33–52. [Google Scholar]
- Sun, W.; Huang, Y. Global warming over the period 1961–2008 did not increase high-temperature stress but did reduce low-temperature stress in irrigated rice across China. Agric. For. Meteorol. 2011, 151, 1193–1201. [Google Scholar] [CrossRef]
- Wang, P.; Hu, T.; Kong, F.; Xu, J.; Zhang, D. Rice exposure to cold stress in China: How has its spatial pattern changed under climate change? Eur. J. Agron. 2019, 103, 73–79. [Google Scholar] [CrossRef]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Dosio, A.; Naumann, G.; van den Berg, M.; Toreti, A. When Will Current Climate Extremes Affecting Maize Production Become the Norm? Earth’s Future 2019, 7, 113–122. [Google Scholar] [CrossRef]
- Hu, Y.; Li, L.; Tian, J.; Zhang, C.; Wang, J.; Yu, E.; Xing, Z.; Guo, B.; Wei, H.; Huo, Z.; et al. Effects of dynamic low temperature during the grain filling stage on starch morphological structure, physicochemical properties, and eating quality of soft japonica rice. Cereal Chem. 2020, 97, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Zhu, Y.; Tang, L.; Chen, J.; Sun, T.; Cao, W.; Tian, Y. Differential effects of temperature and duration of heat stress during anthesis and grain filling stages in rice. Environ. Exp. Bot. 2016, 132, 28–41. [Google Scholar] [CrossRef]
- Wang, P.; Hu, T.; Kong, F.; Zhang, D. Changes in the spatial pattern of rice exposure to heat stress in China over recent decades. Clim. Chang. 2019, 154, 229–240. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, J.; Wassmann, R. Responses of rice yields to recent climate change in China: An empirical assessment based on long-term observations at different spatial scales (1981–2005). Agric. For. Meteorol. 2010, 150, 1128–1137. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, L.; Asseng, S.; Xia, Y.; Tang, L.; Liu, B.; Cao, W.; Zhu, Y. Estimating spring frost and its impact on yield across winter wheat in China. Agric. For. Meteorol. 2018, 260–261, 154–164. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, Z.; Shi, W.; Liu, Y.; Xiao, D.; Zhang, S.; Zhu, Z.; Wang, M.; Liu, F. Single rice growth period was prolonged by cultivars shifts, but yield was damaged by climate change during 1981–2009 in China, and late rice was just opposite. Glob. Chang. Biol. 2013, 19, 3200–3209. [Google Scholar] [CrossRef]
- Zhang, S.; Tao, F.; Zhang, Z. Changes in extreme temperatures and their impacts on rice yields in southern China from 1981 to 2009. Field Crops Res. 2016, 189, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Hirotsu, N.; Makino, A.; Ushio, A.; Mae, T. Changes in the Thermal Dissipation and the Electron Flow in the Water–Water Cycle in Rice Grown Under Conditions of Physiologically Low Temperature. Plant Cell Physiol. 2004, 45, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zhang, H.; Zhang, D.; Li, J.; Xiong, H.; Yu, J.; Li, J.; Rashid, M.A.R.; Li, G.; Ma, X.; et al. Genetic analysis of cold tolerance at the germination and booting stages in rice by association mapping. PLoS ONE 2015, 10, e0120590. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Cahill, K.N.; Field, C.B. Historical effects of temperature and precipitation on California crop yields. Clim. Chang. 2007, 81, 187–203. [Google Scholar] [CrossRef]
- Bhatt, D.; Maskey, S.; Babel, M.S.; Uhlenbrook, S.; Prasad, K.C. Climate trends and impacts on crop production in the Koshi River basin of Nepal. Reg. Environ. Chang. 2014, 14, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Lin, H.; Wang, T.; Li, Y.; Liu, Y.; Chen, X.; Hu, X. Impact of climate change on cotton growth and yields in Xinjiang, China. Field Crops Res. 2020, 247, 107590. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Tian, L.; Cao, W.; Zhu, Y.; Asseng, S. Post-heading heat stress and yield impact in winter wheat of China. Glob. Chang. Biol. 2014, 20, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Hou, P.; Lobell, D.B.; Wang, H.; Cui, Z.; Zhang, F.; Chen, X. The benefits of recent warming for maize production in high latitude China. Clim. Chang. 2014, 122, 341–349. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, Y.; Yang, X. Climate warming over the past three decades has shortened rice growth duration in China and cultivar shifts have further accelerated the process for late rice. Glob. Chang. Biol. 2013, 19, 563–570. [Google Scholar] [CrossRef]
- Shimono, H. Earlier rice phenology as a result of climate change can increase the risk of cold damage during reproductive growth in northern Japan. Agric. Ecosyst. Environ. 2011, 144, 201–207. [Google Scholar] [CrossRef]
- Ishii, A.; Kuroda, E.; Shimono, H. Effect of high water temperature during vegetative growth on rice growth and yield under a cool climate. Field Crops Res. 2011, 121, 88–95. [Google Scholar] [CrossRef]
- Kumagai, E.; Yamada, T.; Hasegawa, T. Is the yield change due to warming affected by photoperiod sensitivity? Effects of the soybean E4 locus. Food Energy Secur. 2020, 9, e186. [Google Scholar] [CrossRef]
- Shi, P.; Tang, L.; Wang, L.; Sun, T.; Liu, L.; Cao, W.; Zhu, Y. Post-Heading Heat Stress in Rice of South China during 1981–2010. PLoS ONE 2015, 10, e0130642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, T.; Zong, S.; Kleidon, A.; Yuan, W.; Wang, Y.; Shi, P. Impacts of climate warming, cultivar shifts, and phenological dates on rice growth period length in China after correction for seasonal shift effects. Clim. Chang. 2019, 155, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Jagadish, S.; Craufurd, P.; Wheeler, T. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Malone, R.W.; Meek, D.W.; Hatfield, J.L.; Mann, M.E.; Jaquis, R.J.; Ma, L. Quasi-biennial corn yield cycles in Iowa. Agric. For. Meteorol. 2009, 149, 1087–1094. [Google Scholar] [CrossRef]
- Lobell, D.B.; Bänziger, M.; Magorokosho, C.; Vivek, B. Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat. Clim. Chang. 2011, 1, 42–45. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Zhang, J.; Tao, F.; Chen, Y.; Ding, H. The effect of terrain factors on rice production: A case study in Hunan Province. J. Geogr. Sci. 2019, 29, 287–305. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Jin, J.; Wang, L. Modeling the effects of elevation and precipitation on Rice (Oryza sativa L.) production considering multiple planting methods and cultivars in Central China. Sci. Total Environ. 2021, 813, 152679. [Google Scholar] [CrossRef]
- Krishnan, P.; Ramakrishnan, B.; Reddy, K.R.; Reddy, V.R. Chapter three—High-Temperature Effects on Rice Growth, Yield, and Grain Quality. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 111, pp. 87–206. [Google Scholar]
- Lobell, D.B. Changes in diurnal temperature range and national cereal yields. Agric. For. Meteorol. 2007, 145, 229–238. [Google Scholar] [CrossRef]
- Ugarte, C.; Calderini, D.F.; Slafer, G.A. Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crops Res. 2007, 100, 240–248. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021, 12, 1234. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.P.d.; Sperotto, R.A.; Cargnelutti, D.; Adamski, J.M.; de FreitasTerra, T.; Fett, J.P. Avoiding damage and achieving cold tolerance in rice plants. Food Energy Secur. 2013, 2, 96–119. [Google Scholar] [CrossRef]
- Moshelion, M.; Altman, A. Current challenges and future perspectives of plant and agricultural biotechnology. Trends Biotechnol. 2015, 33, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Waraich, E.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.A.; Aziz, T.; Rahmatullah. Silicon-Induced Growth and Yield Enhancement in Two Wheat Genotypes Differing in Salinity Tolerance. Commun. Soil Sci. Plant Anal. 2011, 42, 395–407. [Google Scholar] [CrossRef]
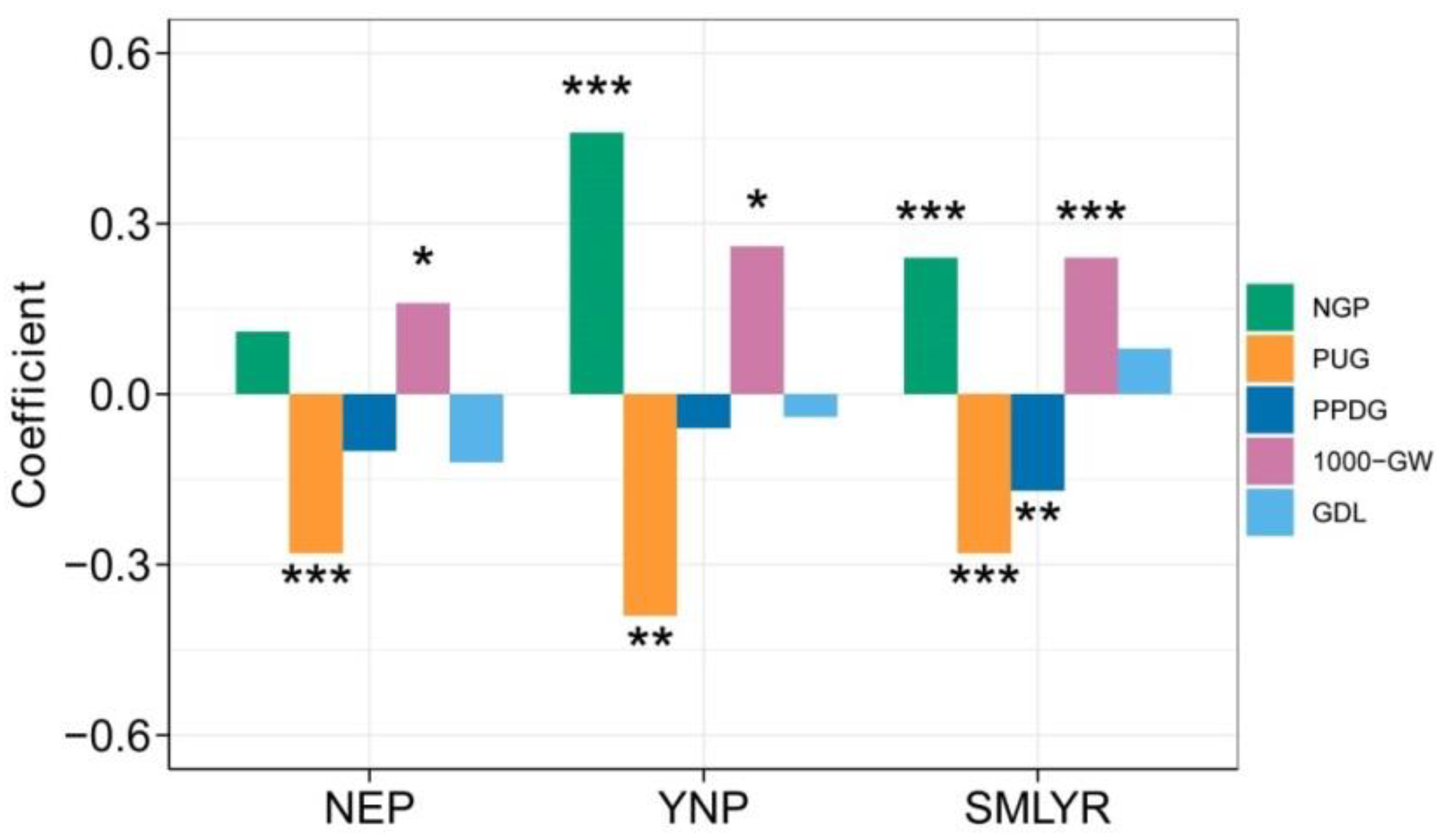
| Ecoregion | No. of AES | Average Temperature (°C) | Yield (ton/ha) | NGP | PUG (%) | PPDG (%) | 1000-GW (g) |
|---|---|---|---|---|---|---|---|
| NEP | 21 | 21.31 ± 10.61 | 8.11 ± 1.47 | 99.37 ± 23.62 | 6.27 ± 3.36 | 5.33 ± 4.15 | 24.80 ± 2.57 |
| SMLYR | 26 | 24.22 ± 2.02 | 6.19 ± 1.25 | 130.36 ± 36.96 | 16.41 ± 7.37 | 8.03 ± 4.98 | 25.95 ± 2.92 |
| YNP | 7 | 20.28 ± 6.17 | 9.11 ± 2.74 | 115.46 ± 29.17 | 16.39 ± 8.94 | 9.58 ± 6.87 | 25.16 ± 2.22 |
| Parameters | Region | Linear | Nonlinear | ||
|---|---|---|---|---|---|
| Formula | R2adj | Formula | R2adj | ||
| Yield (ton/ha) | NEP | ΔY = −0.042 × ΔCDD + 0.109 | 0.057 *** | ΔY = −0.046 × ΔCDD − 0.001 × ΔCDD2 + 0.109 | 0.056 ** |
| YNP | ΔY = −0.053 × ΔCDD − 0.051 | 0.184 ** | ΔY = −0.053 × ΔCDD + 0.003 × ΔCDD × ΔTave + 0.003 | 0.168 ** | |
| SMLYR | ΔY = −0.016 × ΔCDD + 0.065 | 0.040 *** | ΔY = −0.016 × ΔCDD + 0.001 × ΔTave × ΔCDD + 0.099 | 0.041 ** | |
| NGP | NEP | ΔY = −2.097 × ΔTave + 1.141 | 0.052 ** | ΔY = −2.032 × ΔTave − 0.239 × ΔTave2 + 1.582 | 0.049 ** |
| YNP | ΔY = −1.277 × ΔTave − 1.056 | −0.016 | ΔY = −1.839 × ΔTave − 0.125 × ΔCDD × ΔTave − 3.026 | −0.012 | |
| SMLYR | ΔY = −0.233 × ΔCDD + 2.393 | 0.011 | ΔY = −0.228 × ΔCDD − 0.003 × ΔCDD × ΔCDD + 3.075 | 0.01 | |
| PUG (%) | NEP | ΔY = 0.089 × ΔCDD − 0.307 × ΔTave − 0.016 | 0.056 ** | ΔY = 0.079 × ΔCDD − 0.323 × ΔTave − 0.003 × ΔCDD2 + 0.095 × ΔTave2 − 0.091 | 0.054 * |
| YNP | ΔY = 0.262 × ΔCDD + 0.593 | 0.129 ** | ΔY = 0.271 × ΔCDD + 0.005 × ΔCDD2 − 1.170 | 0.179 ** | |
| SMLYR | ΔY = 0.137 × ΔCDD − 0.174 | 0.059 *** | ΔY = 0.109 × ΔCDD − 0.255 × ΔTave − 0.015 × ΔCDD × ΔTave − 0.557 | 0.063 *** | |
| PPDG (%) | NEP | ΔY = 0.243 × ΔCDD + 0.090 | 0.193 *** | ΔY = 0.230 × ΔCDD − 0.006 × ΔCDD2 + 0.090 | 0.225 *** |
| YNP | ΔY = −0.058 × ΔCDD − 0.365 | −0.002 | ΔY = −0.056 × ΔCDD + 0.001 × ΔCDD2 − 0.579 | −0.019 | |
| SMLYR | ΔY = 0.389 × ΔTave + 0.347 | 0.037 ** | ΔY = 0.381 × ΔTave − 0.004 × ΔCDD × ΔTave + 0.243 | 0.034 ** | |
| 1000-GW (g) | NEP | ΔY = 0.243ΔTave − 0.079 | 0.033 * | ΔY = 0.238 × ΔTave + 0.002 × ΔCDD2 − 0.069 × ΔTave2 − 0.003 | 0.038 * |
| YNP | ΔY = −0.054 × ΔCDD + 0.237 | 0.128 ** | ΔY = −0.056 × ΔCDD − 0.001 × ΔCDD2 + 0.516 | 0.161 ** | |
| SMLYR | ΔY = 0.186 × ΔTave − 0.092 | 0.044 *** | ΔY = 0.181 × ΔTave − 0.004 × ΔCDD × ΔTave − 0.192 | 0.048 *** | |
| GDL (day) | NEP | ΔY = 0.149 × ΔCDD + 0.550 × ΔTave + 0.004 | 0.057 *** | ΔY = 0.140 × ΔCDD + 0.529 × ΔTave − 0.033 × ΔCDD × ΔTave − 0.236 | 0.076 *** |
| YNP | ΔY = 0.132 × ΔTave + 0.506 | 0.138 *** | ΔY = 0.131 × ΔTave − 0.008 × ΔCDD × ΔTave + 0.406 | 0.130 *** | |
| SMLYR | ΔY = 0.117 × ΔCDD − 0.132 × ΔTave + 0.284 | 0.213 *** | ΔY = 0.114 × ΔCDD − 0.144 × ΔTave + 0.001 × ΔCDD × ΔCDD + 0.150 | 0.214 *** | |
| NEP | YNP | SMLYR | |
|---|---|---|---|
| Start of heading (SOH) data (day) | 216.97 ± 3.41 | 213.37 ± 9.99 | 255.85 ± 3.99 |
| SOH data trend (day·yr−1) | −0.05 ± 0.32 | −0.002 ± 0.32 | −0.03 ± 0.34 |
| End of flowering (EOF) data (day) | 235.26 ± 3.98 | 237.09 ± 12.98 | 271.04 ± 4.17 |
| EOF data trend (day·yr−1) | 0.06 ± 0.30 | 0.37 ± 0.42 | 0.17 ± 0.36 |
| Duration (day) | 18.29 ± 3.15 | 23.72 ± 4.43 | 15.15 ± 2.55 |
| Duration trend (day·yr−1) | 0.11 ± 0.31 | 0.38 ± 0.20 | 0.20 ± 0.21 |
| Trendobs (°C·day·year−1) | 0.045 ± 0.201 | −0.131 ± 0.551 | −0.152 ± 0.424 |
| Trendtem (°C·day·year−1) | −0.002 ± 0.173 | −0.172 ± 0.421 | −0.381 ± 0.251 |
| Trendphe (°C·day·year−1) | 0.046 ± 0.205 | 0.068 ± 0.212 | 0.229 ± 0.528 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Qiu, Z.; Ge, H.; Du, C. Long-Term Dynamic of Cold Stress during Heading and Flowering Stage and Its Effects on Rice Growth in China. Atmosphere 2022, 13, 103. https://doi.org/10.3390/atmos13010103
Li Z, Qiu Z, Ge H, Du C. Long-Term Dynamic of Cold Stress during Heading and Flowering Stage and Its Effects on Rice Growth in China. Atmosphere. 2022; 13(1):103. https://doi.org/10.3390/atmos13010103
Chicago/Turabian StyleLi, Zhenwang, Zhengchao Qiu, Haixiao Ge, and Changwen Du. 2022. "Long-Term Dynamic of Cold Stress during Heading and Flowering Stage and Its Effects on Rice Growth in China" Atmosphere 13, no. 1: 103. https://doi.org/10.3390/atmos13010103
APA StyleLi, Z., Qiu, Z., Ge, H., & Du, C. (2022). Long-Term Dynamic of Cold Stress during Heading and Flowering Stage and Its Effects on Rice Growth in China. Atmosphere, 13(1), 103. https://doi.org/10.3390/atmos13010103








