Ammonia Volatilization, Forage Accumulation, and Nutritive Value of Marandu Palisade Grass Pastures in Different N Sources and Doses
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Treatments
2.3. Ammonia Volatilization Measurement
2.4. Forage Accumulation
2.5. Forage Chemical Composition
2.6. Statistical Analysis
3. Results
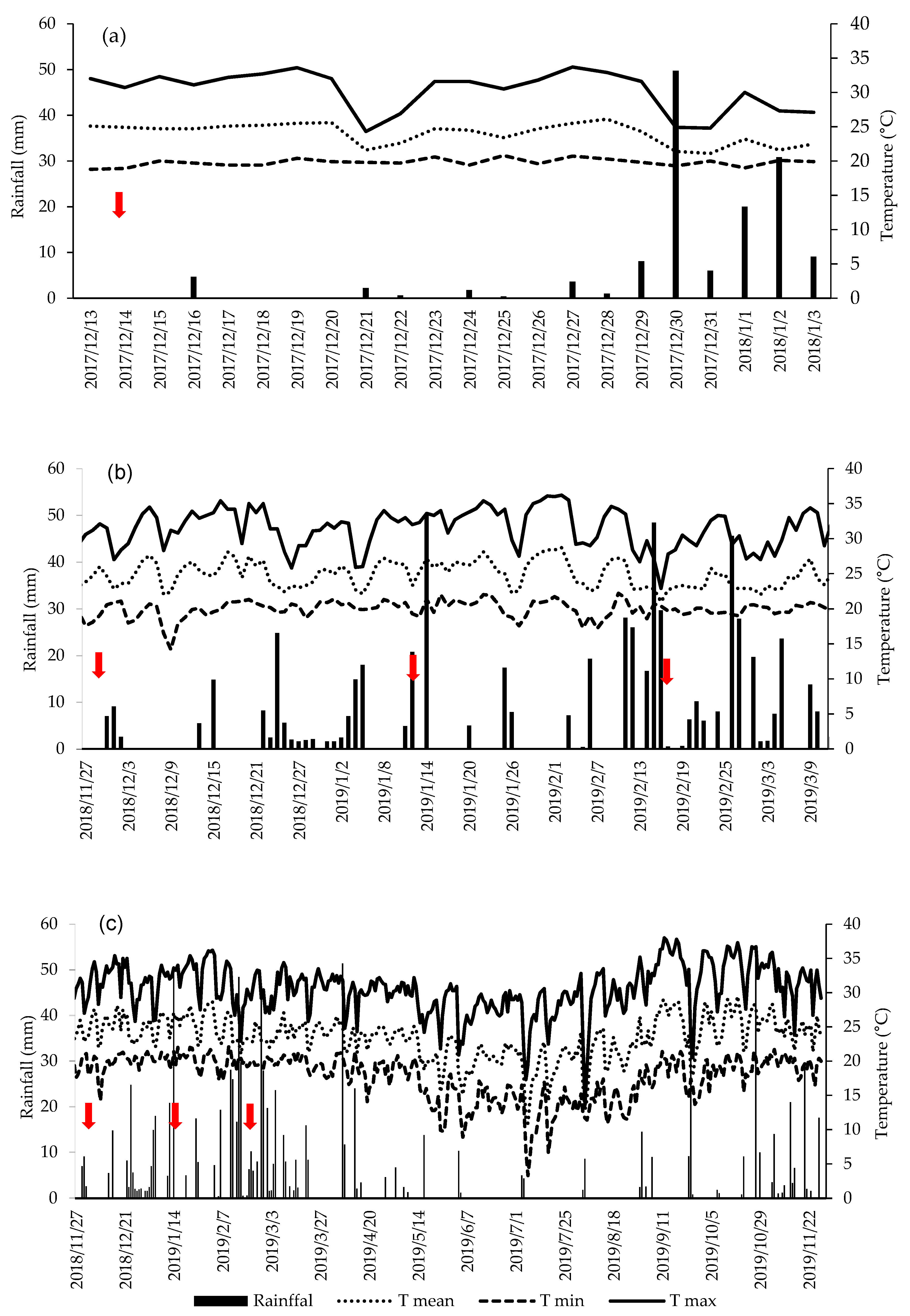
3.1. Environmental Factors
3.2. Ammonia Volatilization
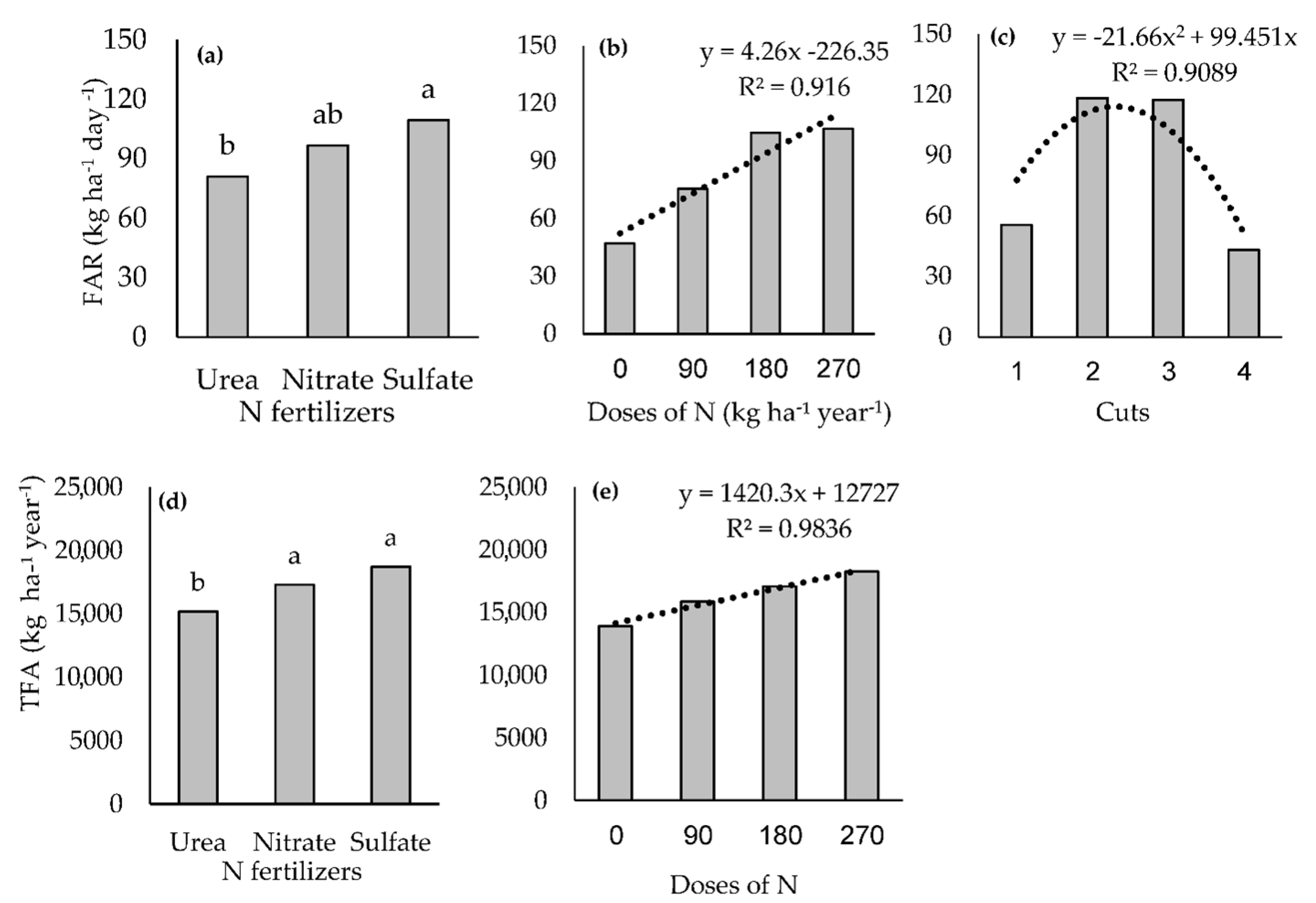
3.3. Forage Accumulation
3.4. Chemical Composition of Forage
4. Discussion
4.1. Ammonia Volatilization
4.2. Forage Accumulation
4.3. Chemical Composition of Forage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruulsema, T.; Lemunyon, J.; Herz, B. Know your fertilizer rights. Crop. Soils 2009, 42, 13–18. [Google Scholar]
- Cardoso, A.S.; Barbero, R.P.; Romanzini, E.P.; Teobaldo, R.W.; Ongaratto, F.; Fernandes, M.H.M.R.; Ruggieri, A.C.; Reis, R.A. Intensification: A Key Strategy to Achieve Great Animal and Environmental Beef Cattle Production Sustainability in Brachiaria Grasslands. Sustainability 2020, 12, 6656. [Google Scholar] [CrossRef]
- IFA e IPNI. Assessment of Fertilizer Use by Crop at the Global Level. International Fertilizer Association (IFA) and International Plant Nutrition Institute (IPNI). 2017. Available online: https://www.ifastat.org/plant-nutrition (accessed on 19 July 2021).
- Lara Cabezas, W.A.R.; Korndörfer, G.H.; Motta, S.A. Volatilização de N-NH3 na cultura de milho: I. Efeito da irrigação e substituição parcial da ureia por sulfato de amônio. Rev. Bras. Ciênc. Solo 1997, 21, 481–487. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, J.J.; Liu, S.; Zhang, Z.Q.; Syam, K.D.; Gerald, M. Application effects of coated urea and urease and nitrification inhibitors on ammonia and greenhouse gas emissions from a subtropical cotton field of the Mississippi delta region. Sci. Total Environ. 2015, 533, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Dupas, E.; Buzetti, S.; Rabêlo, F.H.S.; Sarto, A.L.; Cheng, N.C.; Galindo, F.S.; Dinalli, R.P.; de Niro Gazola, R. Nitrogen re-covery, use efficiency, dry matter yield, and chemical composition of palisade grass fertilized with nitrogen sources in the Cerrado biome. Aust. J. Crop. Sci. 2016, 10, 1330–1338. [Google Scholar] [CrossRef]
- McRoberts, K.C.; Parsons, D.; Ketterings, Q.M.; Hai, T.T.; Quan, N.H.; Ba, N.X.; Nicholson, C.F.; Cherney, D.J.R. Urea and composted cattle manure affect forage yield and nutritive value in sandy soils of south-central Vietnam. Grass Forage Sci. 2017, 73, 1–14. [Google Scholar] [CrossRef]
- Delevatti, L.M.; Cardoso, A.S.; Barbero, R.P.; Leite, R.G.; Romanzini, E.P.; Ruggieri, A.C.; Reis, R.A. Effect of nitrogen appli-cation rate on yield, forage quality, and animal performance in a tropical pasture. Sci. Rep. 2019, 9, 7596. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.J.; Tian, Z.; Wang, X.; Harrison, S. Ammonia and greenhouse gas emissions from a subtropical wheat field under different nitrogen fertilization strategies. J. Environ. Sci. 2017, 57, 196–210. [Google Scholar] [CrossRef]
- Pereira, E.I.; Nogueira, A.R.; Cruz, C.C.; Guimarães, G.G.; Foschini, M.M.; Bernardi, A.C.; Ribeiro, C. Controlled urea release employing nanocomposites increases the efficiency of nitrogen use by forage. ACS Sustain. Chem. Eng. 2017, 5, 9993–10001. [Google Scholar] [CrossRef]
- Longhini, V.Z.; Cardoso, A.S.; Berça, A.S.; Boddey, R.M.; Reis, R.A.; Dubeux, J.C.B., Jr.; Ruggieri, A.C. Nitrogen supply and rainfall affect ammonia emissions from dairy cattle excreta and urea applied on warm-climate pastures. J. Environ. Qual. 2020, 40, 1453–1456. [Google Scholar] [CrossRef]
- Haynes, R.J.; Sherlock, R.R. Gaseous losses of nitrogen. In Mineral Nitrogen in the Plant–Soil System; Haynes, R.J., Ed.; Academic Press: New York, NY, USA, 1986; pp. 242–302. [Google Scholar]
- Cardoso, A.S.; Oliveira, S.C.; Janusckiewicz, E.R.; Brito, L.F.; Morgado, E.S.; Reis, R.A.; Ruggieri, A.C. Seasonal effects on ammonia, nitrous oxide, and methane emissions for beef cattle excreta and urea fertilizer applied to a tropical pasture. Soil Tillage Res. 2019, 194, 104341. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Macdonald, J.D.; Gasser, M.O.; Bertrand, N. Reducing ammonia volatilization in a no-till soil by incorporating urea and pig slurry in shallow bands. Nutr. Cycl. Agroecosystems 2009, 84, 71–80. [Google Scholar] [CrossRef]
- Souza, T.L.D.; Guelfi, D.R.; Silva, A.L.; Andrade, A.B.; Chagas, W.F.T.; Cancellier, E.L. Ammonia and carbon dioxide emissions by stabilized conventional nitrogen fertilizers and controlled release in corn crop. Ciênc. Agrotecnologia 2017, 41, 494–510, (In Portuguese, with English abstract). [Google Scholar] [CrossRef][Green Version]
- Martha, B.G., Jr.; Corsi, M.; Trivelin, P.C.O.; Vilela, L.; Pinto, T.L.F.; Barioni, L.G. Ammonia volatilization loss in Tanzânia grass pasture fertilized with urea in the summer. Rev. Bras. Zootec. 2004, 33, 2240–2247, (In Portuguese, with English abstract). [Google Scholar] [CrossRef]
- De Morais, R.F.; Boddey, R.M.; Urquiaga, S.; Jantalia, C.P.; Alves, B.J. Ammonia volatilization and nitrous oxide emissions during soil preparation and N fertilization of elephant grass (Pennisetum purpureum Schum.). Soil Biol. Biochem. 2013, 64, 80–88. [Google Scholar] [CrossRef]
- Jank, L.; Barrios, S.C.; do Valle, C.B.; Simeão, R.M.; Alves, G.F. The value of improved pastures to Brazilian beef production. Crop Pasture Sci. 2014, 65, 1132–1137. [Google Scholar] [CrossRef]
- Pagano, M.C.; Correa, E.J.A.; Duarte, N.F.; Yelikbayev, B.; O’Donovan, A.; Gupta, V.K. Advances in eco-efficient agriculture: The plant-soil mycobiome. Agriculture 2017, 7, 14. [Google Scholar] [CrossRef]
- Pontes, L.D.S.; Baldissera, T.C.; Giostri, A.F.; Stafin, G.; dos Santos, B.R.C.; Carvalho, P.D.F. Effects of nitrogen fertilization and cutting intensity on the agronomic performance of warm-season grasses. Grass Forage Sci. 2017, 72, 663–675. [Google Scholar] [CrossRef]
- Ruggieri, A.C.; Cardoso, A.S.; Ongaratto, F.; Casagrande, D.R.; Barbero, R.P.; Brito, L.F.; Azenha, M.V.; Oliveira, A.A.; Koscheck, J.F.W.; Reis, R.A. Grazing Intensity impacts on herbage mass, sward structure, greenhouse gas emissions, and animal performance: Analysis of brachiaria Pastureland. Agronomy 2020, 10, 1750. [Google Scholar] [CrossRef]
- Claessen, M.E.C. Manual de Métodos de Análise de Solo/Centro Nacional de Pesquisa de Solos (Manual of Soil Analysis Meth-ods/National Soil Research Center), 2nd ed.; EMBRAPA-CNPS: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- Araújo, E.S.; Marsola, T.; Miyazawa, M.; Soares, L.H.B.; Urquiaga, S.; Oddey, R.M.; Alves, B.J.R. Calibração de Câmara Semiaberta Estática para Quantificação de Amônia Volatilizada do Solo. Pesqui. Agropecu. Bras. 2009, 44, 769–776. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen: Inorganic forms. In Methods of Soil Analysis. Part. 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monographs: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- ANKOM. Acid Detergent Fiber in Feeds. Filter Bag Technique (for A200, A200I). In Ankom Technology Method 8; Ankom Technology Corp.: Macedon, NY, USA, 2006; pp. 1–15. [Google Scholar]
- Sansigolo, A.S. Variabilidade Interanual da estação chuvosa em São Paulo. Climanálise 1989, 4, 40–43. [Google Scholar]
- André, R.G.B.; Anunciação, Y.M.T. A precipitação pluvial provável em Jaboticabal, São Paulo. Agrometeoros 2017, 25, 347–359. [Google Scholar] [CrossRef]
- Vlek, P.L.G.; Stumpe, J.M. Effects of solution chemistry and environmental conditions on ammonia volatilization losses from aqueous systems. Soil Sci. Soc. Am. J. 1978, 42, 416–421. [Google Scholar] [CrossRef]
- Fenn, L.B.; Hossner, L.R. Ammonia volatilization from ammonium or ammonium-forming nitrogen fertilizers. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1985; pp. 123–169. [Google Scholar]
- Harrison, R.; Webb, J. A review of the effect of N fertilizer type on gaseous emissions. Adv. Agron. 2001, 73, 65–108. [Google Scholar] [CrossRef]
- Otto, R.; Zavaschi, E.; Netto, S.; Machado, B.D.A.; Mira, A.B.D. Ammonia volatilization from nitrogen fertilizers applied to sugarcane straw. Rev. Ciênc. Agron. 2017, 48, 413–418. [Google Scholar] [CrossRef]
- Baumont, R.; Lewis, E.; Delaby, L.; Prache, S.; Horan, B. Sustainable intensification of grass-based ruminant production. Grassl. Sci. Eur. 2014, 19, 521–532. [Google Scholar]
- Viero, F.; Bayer, C.; Fontoura, S.M.V.; de Moraes, R.P. Ammonia volatilization from nitrogen fertilizers in no-till wheat and maize in southern Brazil. Rev. Bras. Ciênc. Solo 2014, 38, 1515–1525. [Google Scholar] [CrossRef]
- Sommer, S.G.; Schjorring, J.K.; Denmead, O.T. Ammonia emission from mineral fertilizers and fertilized crops. Adv. Agron. 2004, 82, 557–622. [Google Scholar]
- Sanz-Cobena, A.; Misselbrook, T.; Camp, V.; Vallejo, A. Effect of water addition and the urease inhibitor NBPT on the abatement of ammonia emission from surface applied urea. Atmos. Environ. 2011, 45, 1517–1524. [Google Scholar] [CrossRef]
- Kissel, D.E.; Cabrera, M.L.; Vaio, N.; Craig, J.R.; Rema, J.A.; Morris, L.A. Rainfall timing and ammonia loss from urea in a loblolly pine plantation. Soil Sci. Soc. Am. J. 2004, 68, 1744–1750. [Google Scholar] [CrossRef]
- IPCC. Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Guidelines for National Greenhouse Gas Inventories. International Panel on Climate Change (IPCC); IPCC: Geneva, Switzerland, 2019; pp. 546–554. [Google Scholar]
- Forrestal, P.J.; Harty, M.; Carolan, R.; Lanigan, G.J.; Watson, C.J.; Laughlin, R.J.; McNeill, G.; Chambers, B.J.; Richards, K.G. Ammonia emissions from urea, stabilized urea and calcium ammonium nitrate: Insights into loss abatement in temperate grassland. Soil Use Manag. 2016, 32, 92–100. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Zhang, H.; Wang, M.; Liang, Z. Assessment of ammonia volatilization losses and nitrogen utilization during the rice growing season in alkaline salt-affected soils. Sustainability 2017, 9, 132. [Google Scholar] [CrossRef]
- Artur, A.G.; Monteiro, F.A. Marandu palisade grass growth and nutrient accumulation as affect by nitrogen and sulfur ferti-lizations. Aust. J. Crop Sci. 2014, 8, 422–429. [Google Scholar]
- De Bona, F.D.; Monteiro, F.A. Marandu palisade grass growth under nitrogen and sulphur for replacing signal grass in de-graded tropical pasture. Sci. Agric. 2010, 67, 570–578, (In Portuguese, with English abstract). [Google Scholar] [CrossRef]
- De Bona, F.D.; Schmidt, F.; Monteiro, F.A. Importance of the nitrogen source in the grass species Brachiaria brizantha responses to sulfur limitation. Plant Soil 2013, 373, 201–216. [Google Scholar] [CrossRef]
- Lima, J.E.; Nascente, A.S.; Leandro, W.M.; Silveira, P.M.D. Urochloa ruziziensis responses to sources and doses of urea. Rev. Bras. Eng. Agrícola Ambient. 2016, 20, 401–407. [Google Scholar] [CrossRef][Green Version]
- Pinho Costa, K.A.; Severiano, E.C.; Simon, G.A.; Epifanio, P.S.; Silva, A.G.; Costa, R.R.G.; Santos, C.B.; Rodrigues, C.R. Nu-tritional Characteristics of Brachiaria brizantha Cultivars Subjected to Different Intensities Cutting. Am. J. Plant Sci. 2014, 5, 1961–1972. [Google Scholar] [CrossRef]
- Avelino, A.C.D.; de Faria, D.A.; Penso, S.; Lima, D.D.O.S.; Rodrigues, R.C.; de Abreu, J.G.; Cabral, L.S.; Peixoto, W.M. Ag-ronomic and bromatological traits of Brachiaria brizantha cv. Piatã as affected by nitrogen rates and cutting heights. J. Exp. Agric. Int. 2019, 36, 1–11. [Google Scholar] [CrossRef]
- Benett, C.G.S.; Buzetti, S.; Silva, K.S.; Bergamaschine, A.F.; Fabricio, J.A. Yield and bromatologic composition of marandu grass as function of sources and doses of nitrogen. Ciênc. Agrotecnologia 2008, 32, 1629–1636, (In Portuguese, with English abstract). [Google Scholar] [CrossRef]
- Campos, F.P.; Nicácio, D.R.O.; Sarmento, P.; Cruz, M.C.P.; Santos, T.M.; Faria, A.F.G.; Ferreira, M.E.; Conceição, M.R.G.; Lima, C.G. Chemical composition and in vitro ruminal digestibility of hand-plucked samples of Xaraes palisade grass fertilized with incremental levels of nitrogen. Anim. Feed Sci. Technol. 2016, 215, 1–12. [Google Scholar] [CrossRef]
- McWilliam, J.R. Response of pasture plants to temperature. In Plant Relation in Pasture; Wilson, J.R., Ed.; CSIRO: Melbourne, Australia, 1978; pp. 17–34. [Google Scholar]
- Marques, D.L.; de Souza Franca, A.F.; Oliveira, L.G.; Arnhold, E.; Ferreira, R.N.; Correa, D.S.; Bastos, D.C.; Brunes, L.C. Production and chemical composition of hybrid Brachiaria cv. Mulato II under a system of cuts and nitrogen fertilization. Bio-Sci. J. 2017, 33, 685–696. [Google Scholar] [CrossRef]
- Carvalho, Z.G.; Sales, E.C.J.D.; Monção, F.P.; Vianna, M.C.M.; Silva, E.A.; Queiroz, D.S. Morphogenic, structural, productive and bromatological characteristics of Brachiaria in silvopastoral system under nitrogen doses. Acta Scientiarum. Anim. Sci. 2019, 41, e39190. [Google Scholar] [CrossRef]

| Experiment 1 | Experiment 2 | ||
|---|---|---|---|
| N Fertilizers (F) | Doses (kg ha−1 year−1) (D) | % NH3 | |
| Urea | 90 | 13.92 | 6.30 |
| 180 | 24.37 | 10.87 | |
| 270 | 44.58 | 24.10 | |
| Mean | 27.62 | 13.76 | |
| Ammonium nitrate | 90 | 2.02 | 1.05 |
| 180 | 3.17 | 2.58 | |
| 270 | 7.71 | 5.67 | |
| Mean | 4.30 | 3.10 | |
| Ammonium sulfate | 90 | 2.22 | 0.78 |
| 180 | 3.10 | 1.88 | |
| 270 | 5.27 | 3.65 | |
| Mean | 3.53 | 2.10 | |
| SEM | 0.04 | 0.03 | |
| p-value | |||
| F | <0.0001 | <0.0001 | |
| D | <0.0001 | <0.0001 | |
| F × D | 0.0003 | <0.0001 | |
| Crude Protein (g kg−1 DM) | ||||
| Factors | ||||
| Cuts | 1 | 2 | 3 | 4 |
| N fertilizers | ||||
| Urea | 132.0 abcd | 147.1 ab | 121.9 cde | 105.9 e |
| Ammonium Nitrate | 142.1 abc | 142.5 abc | 130.5 bcd | 111.1d e |
| Ammonium Sulfate | 145.3 ab | 153.2 a | 140.4 abc | 108.4 e |
| Doses of N (kg ha−1 year−1) | ||||
| 0 | 126.0 | 100.3 | 99.4 | 119.4 |
| 90 | 133.3 | 133.2 | 111.1 | 103.2 |
| 180 | 141.0 | 146.7 | 137.0 | 112.8 |
| 270 | 145.1 | 163.0 | 144.8 | 109.3 |
| Mean | 136.3 | 135.8 | 123.1 | 111.2 |
| Effect | Linear | Linear | Linear | Cubic |
| Neutral Detergent Fiber (g kg−1 DM) | ||||
| N fertilizers | ||||
| Urea | 634.4 ab | 634.4 ab | 638.6 ab | 657.8 a |
| Ammonium Nitrate | 630.6 ab | 625.5 ab | 629.1 ab | 644.9 ab |
| Ammonium Sulfate | 634.3 ab | 624.5 b | 624.2 b | 650.4 ab |
| Doses of N (kg ha−1 year−1) | ||||
| 0 | 643.3 | 658.0 | 657.3 | 601.1 |
| 90 | 638.4 | 641.8 | 656.2 | 643.8 |
| 180 | 633.5 | 621.0 | 619.0 | 653.3 |
| 270 | 627.4 | 621.6 | 616.7 | 655.9 |
| Mean | 635.6 | 635.6 | 637.3 | 638.5 |
| Effect | Linear | Linear | Linear | Linear |
| Acid Detergent Fiber (g kg−1 DM) | ||||
| N fertilizers | ||||
| Urea | 305.4 a | 288.0 abc | 299.4 abc | 295.4 abc |
| Ammonium Nitrate | 302.7 ab | 280.2 c | 296.9 abc | 285.6 abc |
| Ammonium Sulfate | 297.0 abc | 283.0 bc | 290.6 abc | 301.2 abc |
| Doses of N (kg ha−1 year−1) | ||||
| 0 | 301.7 | 298.3 | 299.5 | 263.5 |
| 90 | 302.8 | 286.2 | 299.4 | 280.8 |
| 180 | 300.5 | 278.4 | 292.2 | 304.9 |
| 270 | 301.7 | 286.5 | 295.3 | 296.6 |
| Mean | 301.7 | 287.4 | 296.6 | 286.4 |
| Effect | NS | Quadratic | NS | Quadratic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa, D.C.d.C.; Cardoso, A.d.S.; Ferreira, M.R.; Siniscalchi, D.; Gonçalves, P.H.d.A.; Lumasini, R.N.; Reis, R.A.; Ruggieri, A.C. Ammonia Volatilization, Forage Accumulation, and Nutritive Value of Marandu Palisade Grass Pastures in Different N Sources and Doses. Atmosphere 2021, 12, 1179. https://doi.org/10.3390/atmos12091179
Corrêa DCdC, Cardoso AdS, Ferreira MR, Siniscalchi D, Gonçalves PHdA, Lumasini RN, Reis RA, Ruggieri AC. Ammonia Volatilization, Forage Accumulation, and Nutritive Value of Marandu Palisade Grass Pastures in Different N Sources and Doses. Atmosphere. 2021; 12(9):1179. https://doi.org/10.3390/atmos12091179
Chicago/Turabian StyleCorrêa, Darlena Caroline da Cruz, Abmael da Silva Cardoso, Mariane Rodrigues Ferreira, Débora Siniscalchi, Pedro Henrique de Almeida Gonçalves, Rodolfo Nussio Lumasini, Ricardo Andrade Reis, and Ana Cláudia Ruggieri. 2021. "Ammonia Volatilization, Forage Accumulation, and Nutritive Value of Marandu Palisade Grass Pastures in Different N Sources and Doses" Atmosphere 12, no. 9: 1179. https://doi.org/10.3390/atmos12091179
APA StyleCorrêa, D. C. d. C., Cardoso, A. d. S., Ferreira, M. R., Siniscalchi, D., Gonçalves, P. H. d. A., Lumasini, R. N., Reis, R. A., & Ruggieri, A. C. (2021). Ammonia Volatilization, Forage Accumulation, and Nutritive Value of Marandu Palisade Grass Pastures in Different N Sources and Doses. Atmosphere, 12(9), 1179. https://doi.org/10.3390/atmos12091179








