Short-Term Assessment of Nitrous Oxide and Methane Emissions on a Crop Yield Basis in Response to Different Organic Amendment Types in Sichuan Basin
Abstract
:1. Introduction
2. Materials and Methods
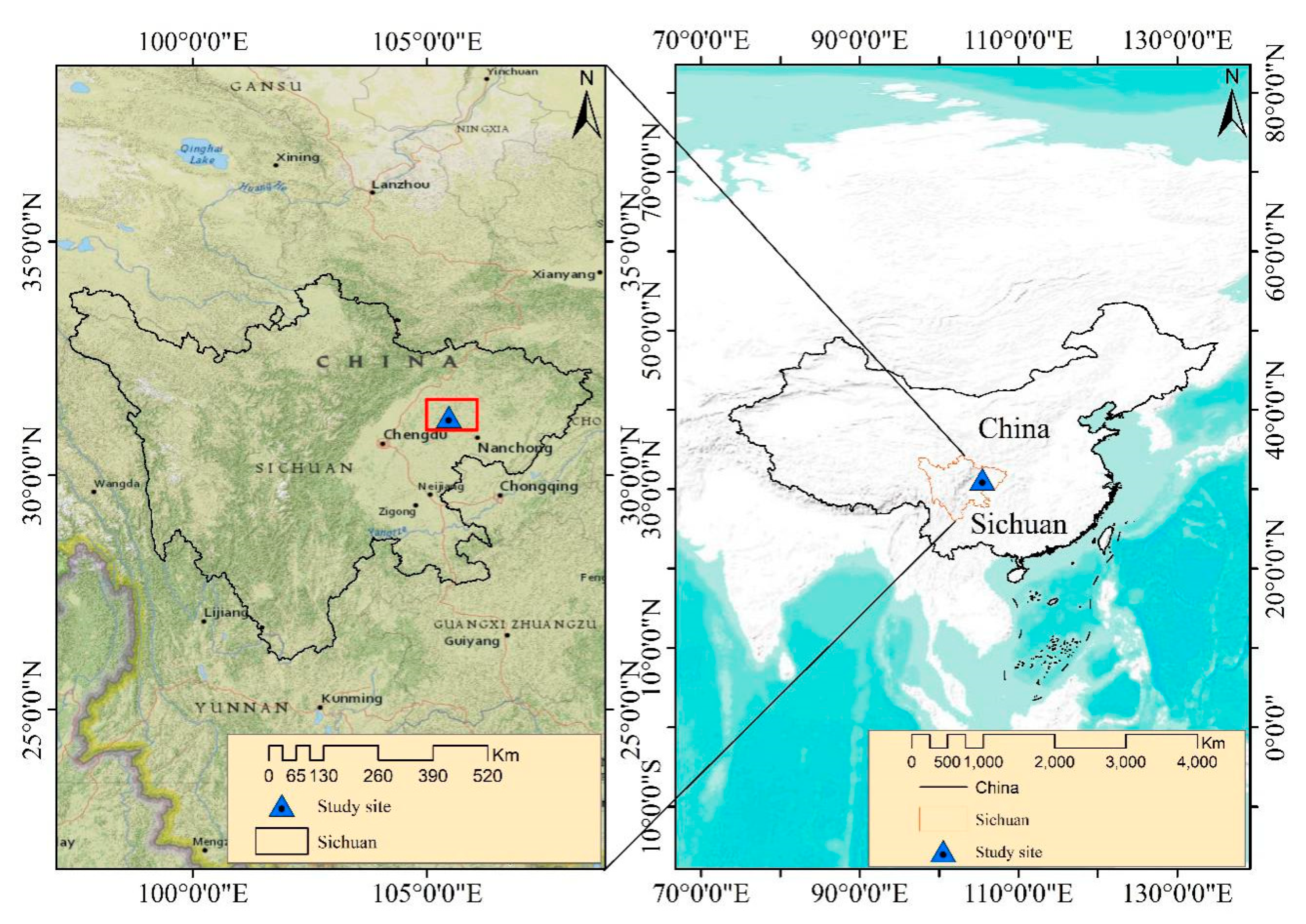
2.1. Experimental Site Description
2.2. Experimental Design
2.3. Gas Sampling and Flux Measurement
2.4. Soil Analysis and Environmental Variable Measurements
2.5. Crop Performance and Global Warming Potential
2.6. Statistical Analysis
3. Results
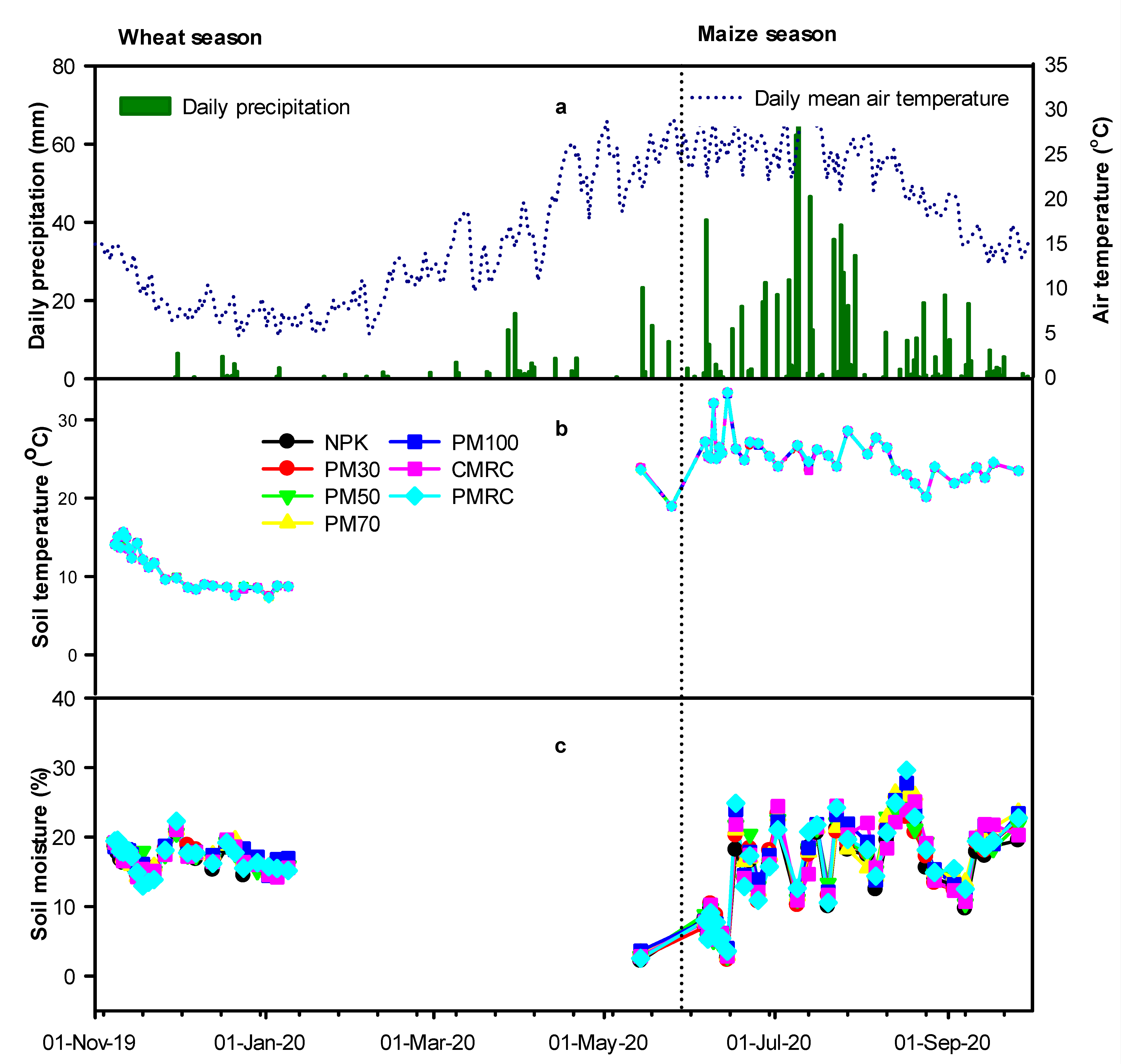
3.1. Environmental Variables
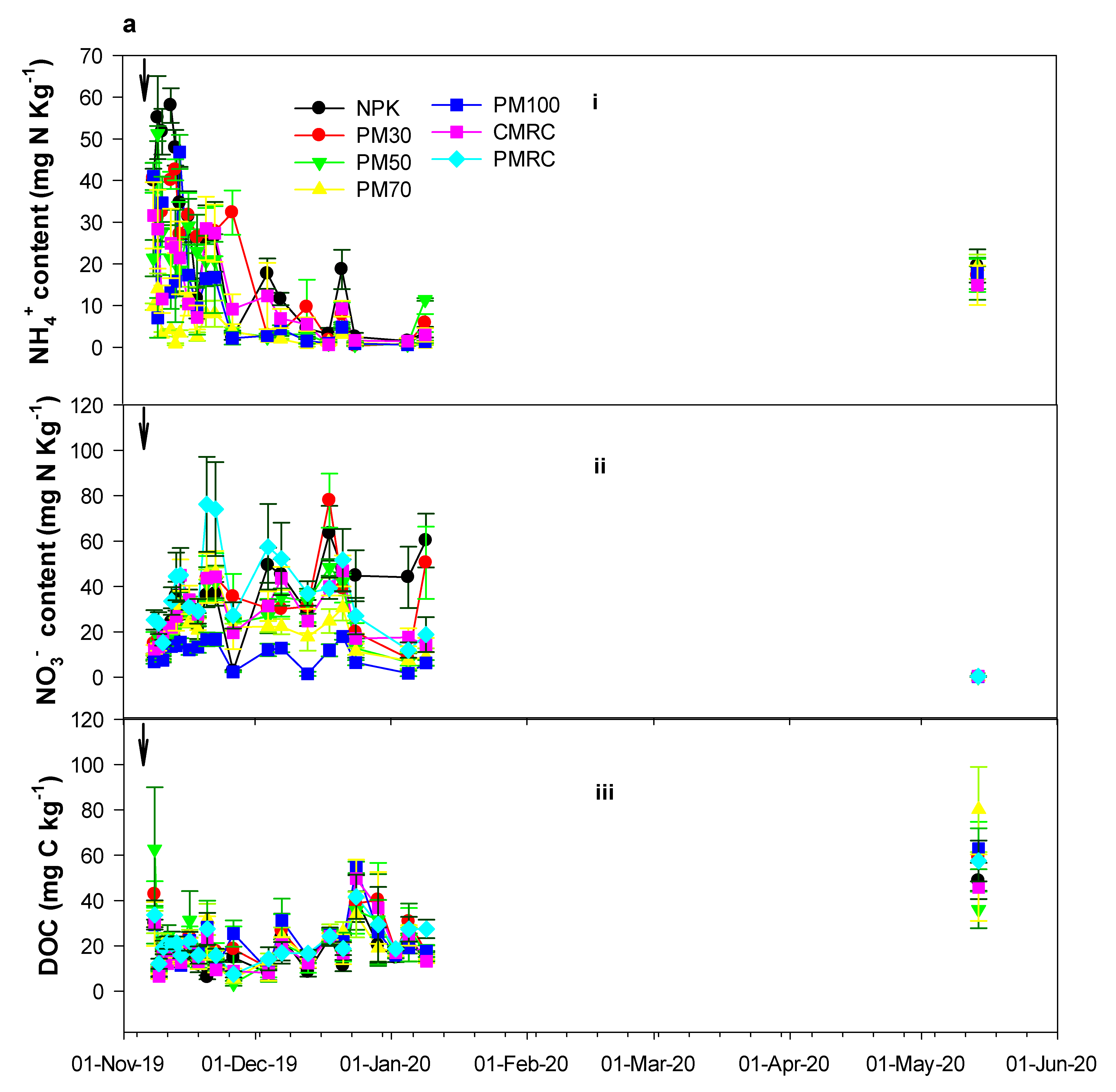
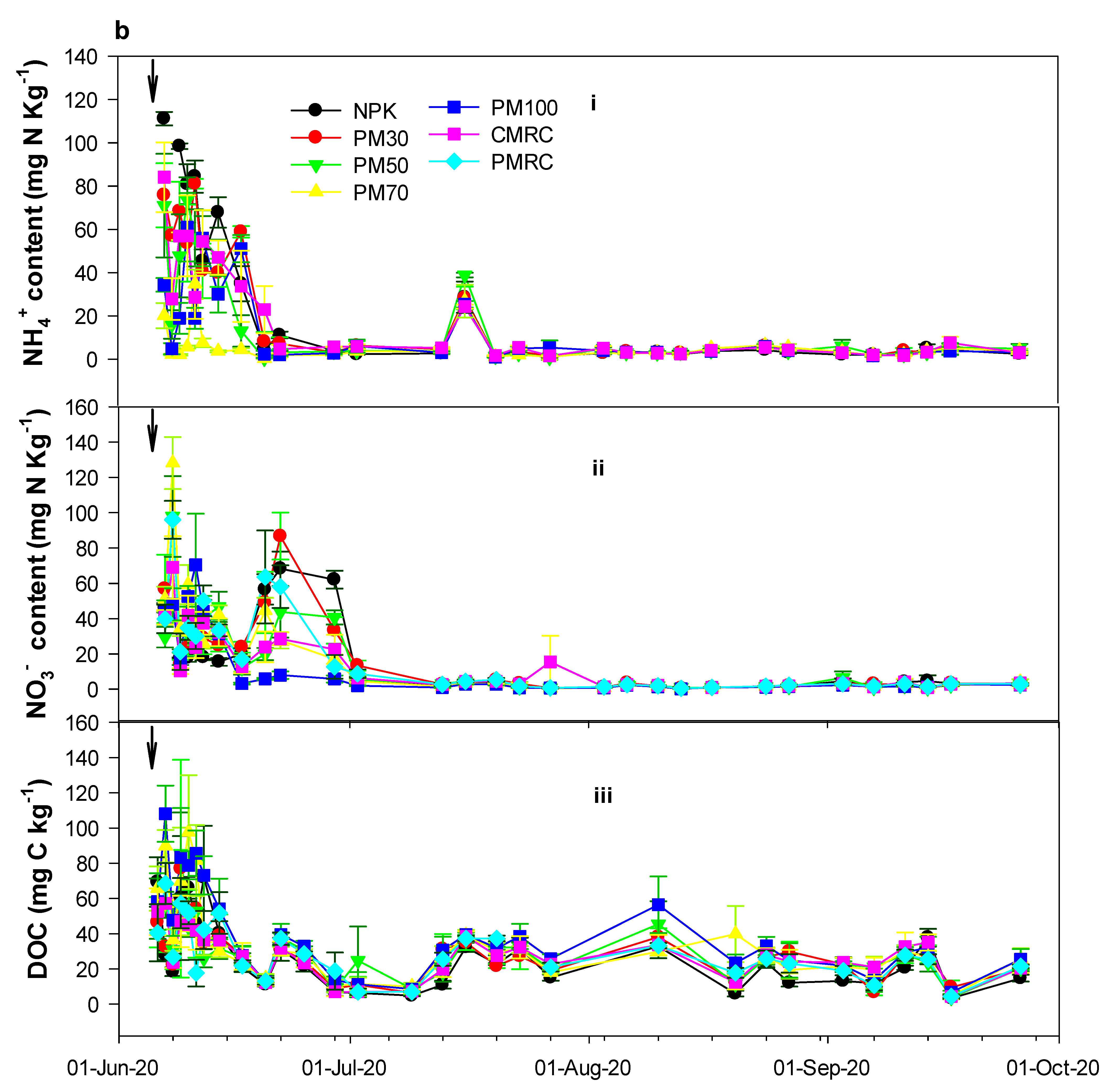
3.2. Effects of Different Fertilization Treatments on Soil Chemical Properties
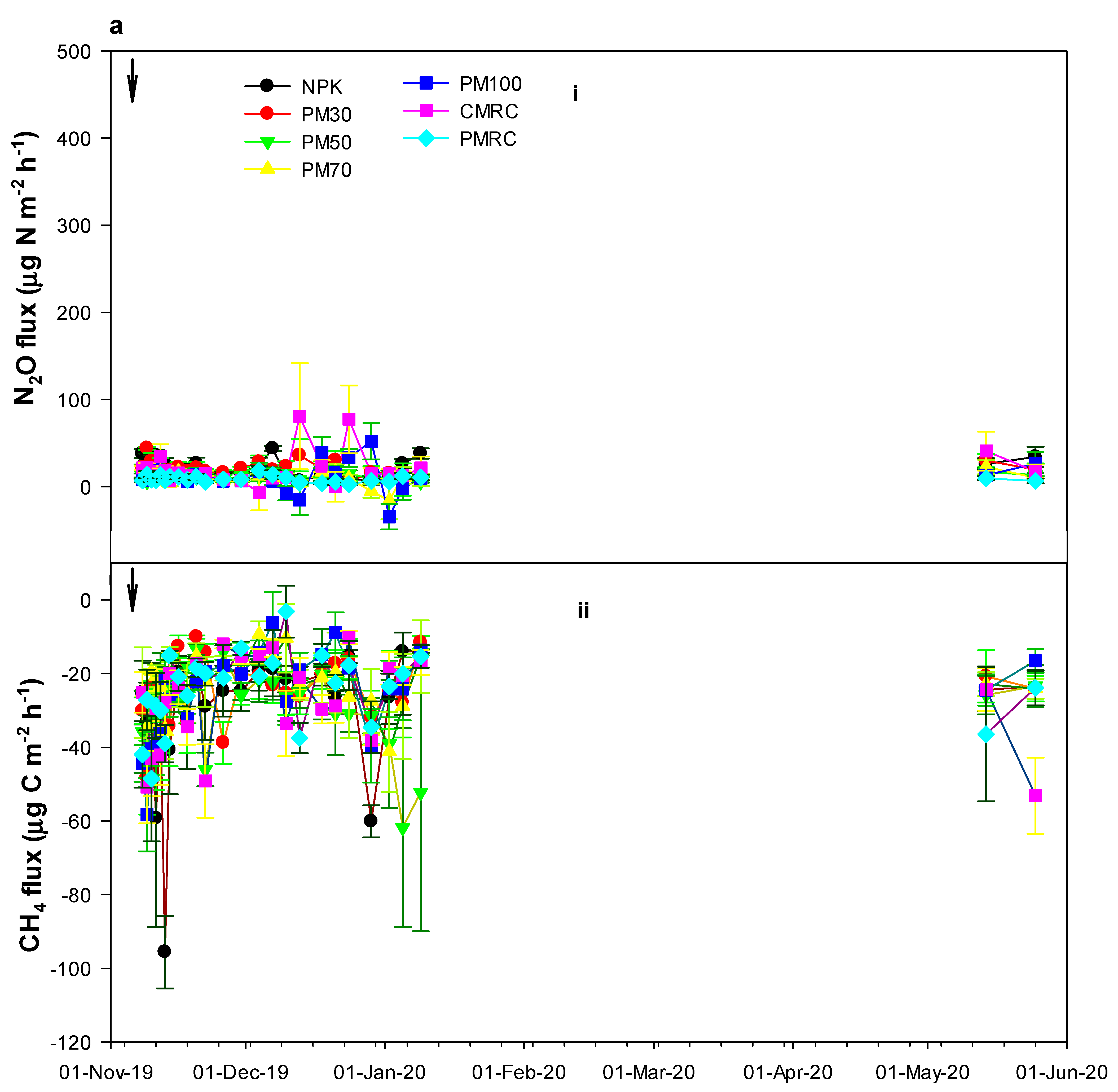
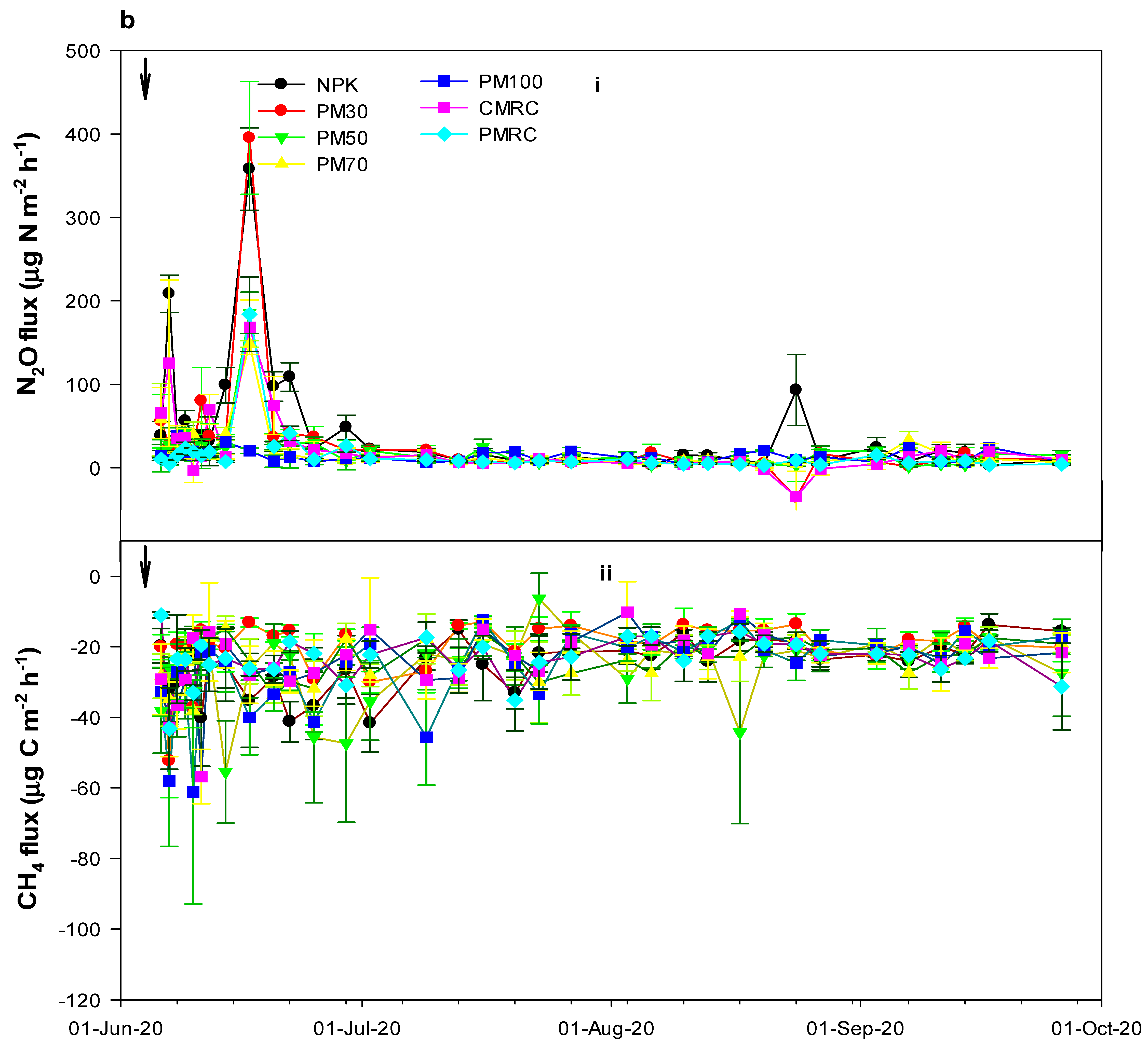
3.3. Direct N2O and CH4 Emissions under Different Fertilization Treatments
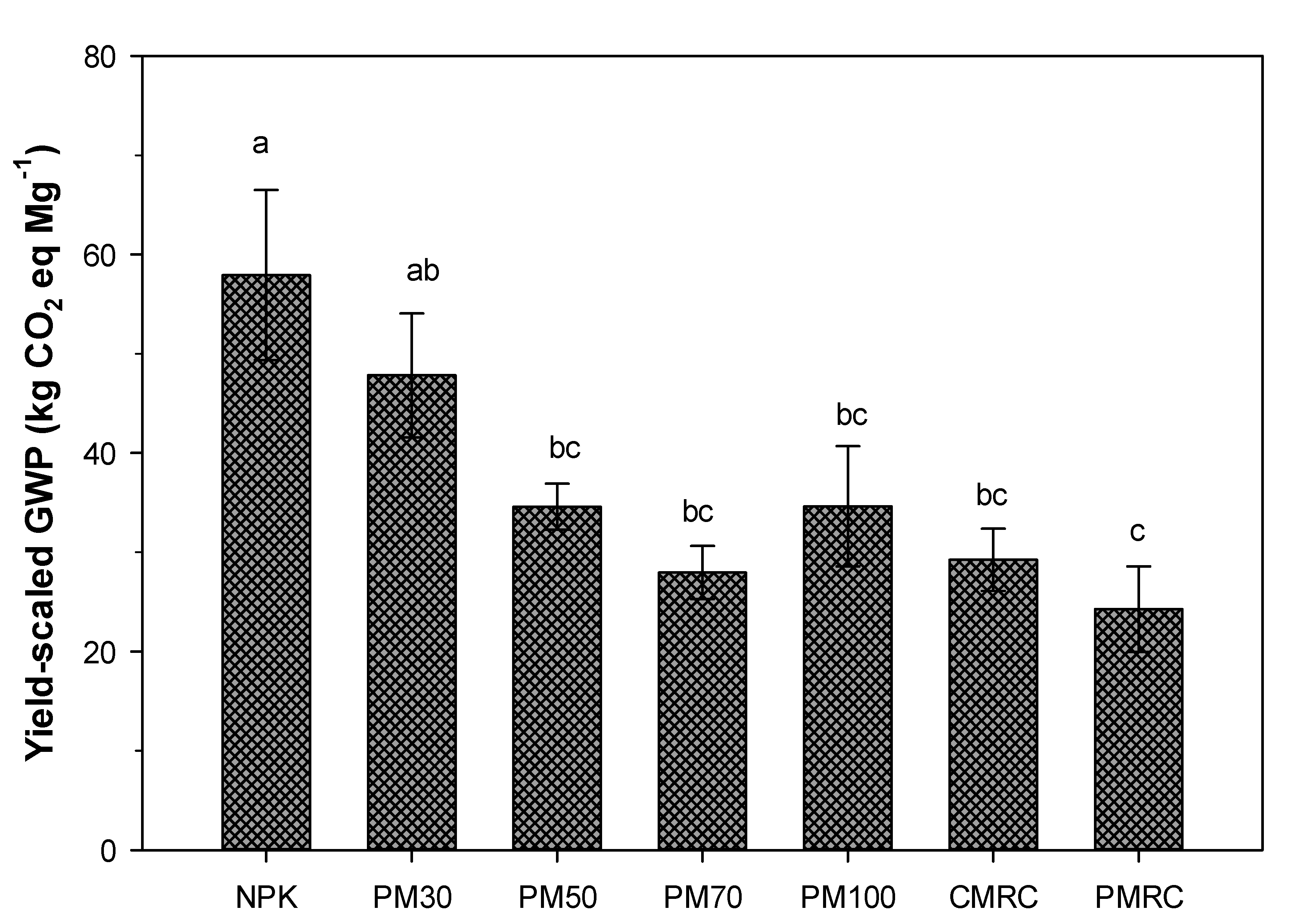
3.4. Crop Yield and Yield-Scaled Global Warming Potential (GWP) under Different Fertilization Treatments
3.5. Correlations between Greenhouse Gas and Soil Properties
4. Discussion
4.1. Factors Controlling N2O and CH4 Emissions
4.2. Effects of Different Fertilization Treatments on N2O and CH4 Fluxes
4.3. Impact of Different Fertilization Treatments on Yield-Scaled GWP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Organic Agriculture and Climate Change Mitigation—A Report of the Round Table on Organic Agriculture and Climate Change; FAO: Rome, Italy, 2011. [Google Scholar]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Agriculture. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Liu, H.; Li, J.; Li, X.; Zheng, Y.; Feng, S.; Jiang, G. Mitigating greenhouse gas emissions through replacement of chemical fertilizer with organic manure in a temperate farmland. Sci. Bull. 2015, 60, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Nayak, D.R.; Saetnan, E.; Cheng, K.; Wang, W.; Koslowski, F.; Cheng, Y.-F.; Zhu, W.Y.; Wang, J.-K.; Liu, J.-X.; Moran, D.; et al. Management opportunities to mitigate greenhouse gas emissions from Chinese agriculture. Agric. Ecosyst. Environ. 2015, 209, 108–124. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zhu, B.; Brüggemann, N.; Bergmann, J.; Wang, Y.; Butterbach-Bahl, K. N2O and CH4 Emissions, and NO3—Leaching on a Crop-Yield Basis from a Subtropical Rain-fed Wheat–Maize Rotation in Response to Different Types of Nitrogen Fertilizer. Ecosystems 2013, 17, 286–301. [Google Scholar] [CrossRef]
- Xiu, Y.; Cheng, Y. The Comprehensive Utilization of Crops Straw Stalk and the Rural Circulation Economy. J. Agric. Mech. Res. 2006, 10, 31–33. (In Chinese) [Google Scholar]
- Bai, Z.; Ma, L.; Jin, S.; Ma, W.; Velthof, G.L.; Oenema, O.; Liu, L.; Chadwick, D.; Zhang, F. Nitrogen, Phosphorus, and Potassium Flows through the Manure Management Chain in China. Environ. Sci. Technol. 2016, 50, 13409–13418. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, D.; Wei, J.; Yan’An, T.; Guanghui, Y.; Qirong, S.; Qing, C. Improving manure nutrient management towards sustainable agricultural intensification in China. Agric. Ecosyst. Environ. 2015, 209, 34–46. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Wang, X.; Wang, Y. Long-term field measurements of annual methane and nitrous oxide emissions from a Chinese subtropical wheat-rice rotation system. Soil Biol. Biochem. 2017, 115, 21–34. [Google Scholar] [CrossRef]
- Shan, J.; Yan, X. Effects of crop residue returning on nitrous oxide emissions in agricultural soils. Atmos. Environ. 2013, 71, 170–175. [Google Scholar] [CrossRef]
- Yao, Z.; Zheng, X.; Xie, B.; Mei, B.; Wang, R.; Butterbach-Bahl, K.; Zhu, J.; Yin, R. Tillage and crop residue management significantly affects N-trace gas emissions during the non-rice season of a subtropical rice-wheat rotation. Soil Biol. Biochem. 2009, 41, 2131–2140. [Google Scholar] [CrossRef]
- Intergovernmental Panel Climate Change. Fourth Assessment Report: Climate Change 2007: The AR4 Synthesis Report; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Firestone, M.K.; Davidson, E.A. Microbiological basis of NO and N2O production and consumption in soil. In Exchange of Trace Gases Between Terrestrial Ecosystems and the Atmosphere, 1st ed.; Andreae, M., Schimel, D., Eds.; John Wiley and Sons Ltd Chichester: New York, NY, USA, 1990; Volume 47, pp. 7–21. [Google Scholar]
- Malhi, S.S.; Lemke, R. Tillage, Crop Residue and N Fertilizer Effects on Crop Yield, Nutrient Uptake, Soil Quality and Nitrous Oxide Gas Emissions in a Second 4-Yr Rotation Cycle. Soil Tillage Res. 2007, 96, 269–283. [Google Scholar] [CrossRef]
- Rowlings, D.W.; Grace, P.R.; Kiese, R.; Weier, K.L. Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Glob. Chang. Biol. 2012, 18, 726–738. [Google Scholar] [CrossRef]
- Vallejo, A.; Skiba, U.M.; Garcia, A.V.; Arce, A.; Lopez-Fernandez, S.; Sanchez-Martin, L. Nitrogen oxides emission from soils bearing a potato crop as influenced by fertilization with treated pig slurries and composts. Soil Biol. Biochem. 2006, 38, 2782–2793. [Google Scholar] [CrossRef]
- Jones, S.; Rees, R.; Skiba, U.; Ball, B. Influence of organic and mineral N fertiliser on N2O fluxes from a temperate grassland. Agric. Ecosyst. Environ. 2007, 121, 74–83. [Google Scholar] [CrossRef]
- Miller, M.; Zebarth, B.; Dandie, C.; Burton, D.; Goyer, C.; Trevors, J. Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biol. Biochem. 2008, 40, 2553–2562. [Google Scholar] [CrossRef]
- Meijide, A.; Díez, J.A.; Sanchez-Martin, L.; López-Fernández, S.; Vallejo, A. Nitrogen oxide emissions from an irrigated maize crop amended with treated pig slurries and composts in a Mediterranean climate. Agric. Ecosyst. Environ. 2007, 121, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Meijide, A.; García-Torres, L.; Arce, A.; Vallejo, A. Nitrogen oxide emissions affected by organic fertilization in a non-irrigated Mediterranean barley field. Agric. Ecosyst. Environ. 2009, 132, 106–115. [Google Scholar] [CrossRef]
- Ball, B.; McTaggart, I.; Scott, A. Mitigation of greenhouse gas emissions from soil under silage production by use of organic manures or slow-release fertilizer. Soil Use Manag. 2006, 20, 287–295. [Google Scholar] [CrossRef]
- Snyder, C.; Bruulsema, T.; Jensen, T.; Fixen, P. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Aronson, E.L.; Helliker, B.R. Methane flux in non-wetland soils in response to nitrogen addition: A meta-analysis. Ecology 2010, 91, 3242–3251. [Google Scholar] [CrossRef] [PubMed]
- Hütsch, B.W. Methane oxidation in non-flooded soils as affected by crop production—Invited paper. Eur. J. Agron. 2001, 14, 237–260. [Google Scholar] [CrossRef]
- Bodelier, P.L.; Laanbroek, H. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- Raza, S.T.; Zhu, B.; Tang, J.L.; Ali, Z.; Anjum, R.; Bah, H.; Iqbal, H.; Ren, X.; Ahmad, R. Nutrients Recovery during Vermicomposting of Cow Dung, Pig Manure, and Biochar for Agricultural Sustainability with Gases Emissions. Appl. Sci. 2020, 10, 8956. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, G.; Chen, Y.; Yu, M.; Yu, Z.; Li, H.; Yu, Y.; Huang, H. Effects of physico-chemical parameters on the bacterial and fungal communities during agricultural waste composting. Bioresour. Technol. 2011, 102, 2950–2956. [Google Scholar] [CrossRef]
- Magrí, A. Research Trends on Nutrient Management From Digestates Assessed Using a Bibliometric Approach. Front. Sustain. Food Syst. 2018, 2. [Google Scholar] [CrossRef]
- Walsh, J.J.; Jones, D.L.; Edwards-Jones, G.; Williams, A.P. Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. J. Plant Nutr. Soil Sci. 2012, 175, 840–845. [Google Scholar] [CrossRef]
- Cui, Y.-F.; Meng, J.; Wang, Q.-X.; Zhang, W.-M.; Cheng, X.-Y.; Chen, W.-F. Effects of straw and biochar addition on soil nitrogen, carbon, and super rice yield in cold waterlogged paddy soils of North China. J. Integr. Agric. 2017, 16, 1064–1074. [Google Scholar] [CrossRef]
- Bah, H.; Zhou, M.; Ren, X.; Hu, L.; Dong, Z.; Zhu, B. Effects of organic amendment applications on nitrogen and phosphorus losses from sloping cropland in the upper Yangtze River. Agric. Ecosyst. Environ. 2020, 302, 107086. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, M.; Lin, H.; Ntacyabukura, T.; Wang, Y.; Zhu, B. Effects of different long-term crop straw management practices on ammonia volatilization from subtropical calcareous agricultural soil. Atmos. Ocean. Sci. Lett. 2020, 13, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Wang, T.; Kuang, F.; Luo, Z.; Tang, J.; Xu, T. Measurements of Nitrate Leaching from a Hillslope Cropland in the Central Sichuan Basin, China. Soil Sci. Soc. Am. J. 2009, 73, 1419–1426. [Google Scholar] [CrossRef]
- Omirou, M.; Anastopoulos, I.; Fasoula, D.A.; Ioannides, I.M. The effect of chemical and organic N inputs on N2O emission from rain-fed crops in Eastern Mediterranean. J. Environ. Manag. 2020, 270, 110755. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, B.; Zhang, J.; Müller, C.; Cai, Z. Mechanisms of soil N dynamics following long-term application of organic fertilizers to subtropical rain-fed purple soil in China. Soil Biol. Biochem. 2015, 91, 222–231. [Google Scholar] [CrossRef]
- Yuesi, W.; Yinghong, W. Quick measurement of CH4, CO2 and N2O emissions from a short-plant ecosystem. Adv. Atmos. Sci. 2003, 20, 842–844. [Google Scholar] [CrossRef]
- Zheng, X.; Xie, B.; Liu, C.; Zhou, Z.; Yao, Z.; Wang, Y.; Wang, Y.; Yang, L.; Zhu, J.; Huang, Y.; et al. Quantifying net ecosystem carbon dioxide exchange of a short-plant cropland with intermittent chamber measurements. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Parkin, T.B.; Venterea, R.T. USDA-ARS GRACEnet Project Protocols, Chapter 3. Chamber-Based Trace Gas Flux Measure-ments. In Sampling Protocols; USDA-ARS: Beltsville, MD, USA, 2010; pp. 1–39. [Google Scholar]
- Pelster, D.; Rufino, M.; Rosenstock, T.; Mango, J.; Saiz, G.; Diaz-Pines, E.; Baldi, G.; Butterbach-Bahl, K. Smallholder farms in eastern African tropical highlands have low soil greenhouse gas fluxes. Biogeosciences 2017, 14, 187–202. [Google Scholar] [CrossRef] [Green Version]
- Pelster, D.; Chantigny, M.H.; Rochette, P.; Angers, D.A.; Rieux, C.; Vanasse, A. Nitrous Oxide Emissions Respond Differently to Mineral and Organic Nitrogen Sources in Contrasting Soil Types. J. Environ. Qual. 2012, 41, 427–435. [Google Scholar] [CrossRef]
- Hillel, D. Soil and Water. In Physical Principles and Processes; Academic Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Linquist, B.; van Groenigen, K.J.; Adviento-Borbe, M.A.; Pittelkow, C.; Van Kessel, C. An agronomic assessment of greenhouse gas emissions from major cereal crops. Glob. Chang. Biol. 2011, 18, 194–209. [Google Scholar] [CrossRef]
- Grassini, P.; Cassman, K.G. High-yield maize with large net energy yield and small global warming intensity. Proc. Natl. Acad. Sci. USA 2012, 109, 1074–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Sun, H.; Liao, X.; Luo, J.; Lindsey, S.; Yuan, J.; He, T.; Zaman, M.; Ding, W. N2O and NO Emissions as Affected by the Continuous Combined Application of Organic and Mineral N Fertilizer to a Soil on the North China Plain. Agronomy 2020, 10, 1965. [Google Scholar] [CrossRef]
- Liu, L.; Estiarte, M.; Peñuelas, J. Soil moisture as the key factor of atmospheric CH4 uptake in forest soils under environmental change. Geoderma 2019, 355, 113920. [Google Scholar] [CrossRef]
- Shimizu, M.; Hatano, R.; Arita, T.; Kouda, Y.; Mori, A.; Matsuura, S.; Niimi, M.; Jin, T.; Desyatkin, A.R.; Kawamura, O.; et al. The effect of fertilizer and manure application on CH4 and N2O emissions from managed grasslands in Japan. Soil Sci. Plant Nutr. 2013, 59, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.-K.; Su, F.; Ju, X.-T.; Gao, B.; Oenema, O.; Christie, P.; Huang, B.-X.; Jiang, R.-F.; Zhang, F.-S. Greenhouse gas emissions from a wheat–maize double cropping system with different nitrogen fertilization regimes. Environ. Pollut. 2013, 176, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Kusa, K.; Sawamoto, T.; Hatano, R. Nitrous oxide emissions for 6 years from a gray lowland soil cultivated with onions in Hokkaido, Japan. Nutr. Cycl. Agroecosyst. 2002, 63, 239–247. [Google Scholar] [CrossRef]
- Saggar, S.; Jha, N.; Deslippe, J.; Bolan, N.; Luo, J.; Giltrap, D.; Kim, D.-G.; Zaman, M.; Tillman, R. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total. Environ. 2012, 465, 173–195. [Google Scholar] [CrossRef]
- Li, M.; Shimizu, M.; Hatano, R. Evaluation of N2O and CO2 hot moments in managed grassland and cornfield, southern Hokkaido, Japan. Catena 2015, 133, 1–13. [Google Scholar] [CrossRef]
- Millar, N.; Robertson, G.; Grace, P.R.; Gehl, R.J.; Hoben, J.P. Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (Maize) production: An emissions reduction protocol for US Midwest agriculture. Mitig. Adapt. Strat. Glob. Chang. 2010, 15, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Parkin, T.B.; Helmers, M.J.; Zhou, X.; Castellano, M. Denitrification and Nitrous Oxide Emissions in Annual Croplands, Perennial Grass Buffers, and Restored Perennial Grasslands. Soil Sci. Soc. Am. J. 2014, 79, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Burchill, W.; Li, D.; Lanigan, G.J.; Williams, M.; Humphreys, J. Interannual variation in nitrous oxide emissions from perennial ryegrass/white clover grassland used for dairy production. Glob. Chang. Biol. 2014, 20, 3137–3146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterbach-Bahl, K.; Baggs, L.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Dasselaar, A.V.D.P.-V.; Van Beusichem, M.L.; Oenema, O. Effects of soil moisture content and temperature on methane uptake by grasslands on sandy soils. Plant Soil 1998, 204, 213–222. [Google Scholar] [CrossRef]
- Wu, X.; Wang, F.; Li, T.; Fu, B.; Lv, Y.; Liu, G. Nitrogen additions increase N2O emissions but reduce soil respiration and CH4 uptake during freeze–thaw cycles in an alpine meadow. Geoderma 2020, 363, 114157. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, S.; Fang, H.; Yu, G.; Xu, M.; Dang, X.; Li, L.; Wang, L. Simulated Nitrogen Deposition Reduces CH4 Uptake and Increases N2O Emission from a Subtropical Plantation Forest Soil in Southern China. PLoS ONE 2014, 9, e93571. [Google Scholar] [CrossRef]
- Castro, M.S.; Steudler, P.A.; Melillo, J.M.; Aber, J.D.; Bowden, R.D. Factors controlling atmospheric methane consumption by temperate forest soils. Glob. Biogeochem. Cycles 1995, 9, 1–10. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Brüggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Glob. Chang. Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, X.; Liu, J.; Sun, N.; Wu, L.; Li, Z.; Xu, M. A synthetic analysis of greenhouse gas emissions from manure amended agricultural soils in China. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Musafiri, C.M.; Macharia, J.M.; Kiboi, M.N.; Ng’Etich, O.K.; Shisanya, C.A.; Okeyo, J.M.; Mugendi, D.N.; Okwuosa, E.A.; Ngetich, F.K. Soil greenhouse gas fluxes from maize cropping system under different soil fertility management technologies in Kenya. Agric. Ecosyst. Environ. 2020, 301, 107064. [Google Scholar] [CrossRef]
- Nyamadzawo, G.; Shi, Y.; Chirinda, N.; Olesen, J.E.; Mapanda, F.; Wuta, M.; Wu, W.; Meng, F.; Oelofse, M.; de Neergaard, A.; et al. Combining organic and inorganic nitrogen fertilisation reduces N2O emissions from cereal crops: A comparative analysis of China and Zimbabwe. Mitig. Adapt. Strat. Glob. Chang. 2014, 22, 233–245. [Google Scholar] [CrossRef]
- Mukumbuta, I.; Shimizu, M.; Hatano, R. Mitigating Global Warming Potential and Greenhouse Gas Intensities by Applying Composted Manure in Cornfield: A 3-Year Field Study in an Andosol Soil. Agriculture 2017, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Shimizu, M.; Marutani, S.; Desyatkin, A.R.; Iizuka, N.; Hata, H.; Hatano, R. Effect of chemical fertilizer and manure application on N2O emission from reed canary grassland in Hokkaido, Japan. Soil Sci. Plant Nutr. 2010, 56, 53–65. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Yuan, J.; Luo, J.; Wang, W.; Fan, J.; Liu, D.; Ding, W. Organic fertilizers have divergent effects on soil N2O emissions. Biol. Fertil. Soils 2019, 55, 685–699. [Google Scholar] [CrossRef]
- Masunga, R.H.; Uzokwe, V.N.; Mlay, P.D.; Odeh, I.; Singh, A.; Buchan, D.; De Neve, S. Nitrogen mineralization dynamics of different valuable organic amendments commonly used in agriculture. Appl. Soil Ecol. 2016, 101, 185–193. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, S.; Shen, G.; Shaaban, M.; Ju, W.; Cui, Y.; Duan, C.; Fang, L. Effects of inorganic and organic fertilizers on CO2 and CH4 fluxes from tea plantation soil. Elem. Sci. Anth. 2021, 9. [Google Scholar] [CrossRef]
- Bah, H.; Ren, X.; Wang, Y.; Tang, J.; Zhu, B. Characterizing Greenhouse Gas Emissions and Global Warming Potential of Wheat-Maize Cropping Systems in Response to Organic Amendments in Eutric Regosols, China. Atmosphere 2020, 11, 614. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, B.; Butterbach-Bahl, K.; Zheng, X.; Wang, T.; Wang, Y. Nitrous oxide emissions and nitrate leaching from a rain-fed wheat-maize rotation in the Sichuan Basin, China. Plant Soil 2012, 362, 149–159. [Google Scholar] [CrossRef]
- López-Fernández, S.; Díez, J.A.; Hernáiz, P.; Arce, A.; Garcia, A.V.; Vallejo, A. Effects of fertiliser type and the presence or absence of plants on nitrous oxide emissions from irrigated soils. Nutr. Cycl. Agroecosyst. 2007, 78, 279–289. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Velthof, G.; Oenema, O.; van Groenigen, K.J.; Van Kessel, C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
- Rahman, M. Carbon Dioxide Emission from Soil. Agric. Res. 2013, 2, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.M.; Chang, S.X.; Bork, E.W.; Carlyle, C.N. Enrichment Planting and Soil Amendments Enhance Carbon Sequestration and Reduce Greenhouse Gas Emissions in Agroforestry Systems: A Review. Forests 2018, 9, 369. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, R.; Sugiyama, C.; Yasuda, K.; Nagatake, A.; Yuan, Y.; Du, J.; Yamaki, N.; Taira, K.; Kawai, M.; Hatano, R. Effects of Three Types of Organic Fertilizers on Greenhouse Gas Emissions in a Grassland on Andosol in Southern Hokkaido, Japan. Front. Sustain. Food Syst. 2021, 5, 100. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Luo, J.; Wang, H.; Zhai, L.; Geng, Y.; Li, J.; Lei, Q.; Bashir, M.A.; Wu, S.; et al. Long-term manure application increased greenhouse gas emissions but had no effect on ammonia volatilization in a Northern China upland field. Sci. Total. Environ. 2018, 633, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Adair, E.C.; Barbieri, L.; Schiavone, K.; Darby, H.M. Manure Application Decisions Impact Nitrous Oxide and Carbon Dioxide Emissions during Non-Growing Season Thaws. Soil Sci. Soc. Am. J. 2019, 83, 163–172. [Google Scholar] [CrossRef]
| Winter Wheat Season | Summer Maize Season | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Urea (kg N ha−1) | Pig Manure (kg N ha−1) | Compost (kg N ha−1) | Total N Applied (kg N ha−1) | Urea (kg N ha−1) | Pig Manure (kg N ha−1) | Compost (kg N ha−1) | Total N Applied (kg N ha−1) |
| NPK | 130 | 0 | 0 | 130 | 150 | 0 | 0 | 150 |
| PM30 | 91 | 39 | 0 | 130 | 105 | 45 | 0 | 150 |
| PM50 | 65 | 65 | 0 | 130 | 75 | 75 | 0 | 150 |
| PM70 | 39 | 91 | 0 | 130 | 45 | 105 | 0 | 150 |
| PM100 | 0 | 130 | 0 | 130 | 0 | 150 | 0 | 150 |
| CMRC | 65 | 0 | 65 1 | 130 | 75 | 0 | 75 1 | 150 |
| PMRC | 65 | 0 | 65 2 | 130 | 75 | 0 | 75 2 | 150 |
| Organic Amendment Added | Total N Content 1 (%) | Total C Content 1 (%) | C:N Ratio | Total P Content 1 (g kg−1) | Total K Content 1 (g kg−1) |
|---|---|---|---|---|---|
| Pig Manure | 2.2 ± 0.1 | 31.4 ± 0.6 | 14.1:1 | 5.7 ± 0.4 | 16.8 ± 0.6 |
| Pig manure-crop residue compost | 2.0 ± 0.1 | 25.1 ± 0.7 | 12.5:1 | 7.7 ± 0.3 | 12.1 ± 2.2 |
| Cow manure-crop residue compost | 1.9 ± 0.0 | 24.9 ± 0.3 | 13.1:1 | 3.4 ± 0.3 | 13.8 ± 2.0 |
| Treatment | Cumulative N2O Emissions (kg N ha−1) * | Cumulative CH4 Fluxes (kg C ha−1) * | Wheat Grain Yield (kg ha−1) | Maize Grain Yield (kg ha−1) | Combined GWP (kg CO2 eq ha−1) * |
|---|---|---|---|---|---|
| NPK | 0.93 ± 0.1 a | −0.65 ± 0.1 a | 3106.6 ± 23.1 a | 7309.8 ± 46.4 a | 412.0 ± 38.8 a |
| PM30 | 0.63 ± 0.1 b | −0.52 ± 0.0 a | 2880.7 ± 20.6 a | 6170.8 ± 38.9 ab | 289.9 ± 26.7 b |
| PM50 | 0.50 ± 0.1 bc | −0.68 ± 0.1 a | 2894.4 ± 27.1 a | 5979.0 ± 53.1 ab | 209.6 ± 29.9 bc |
| PM70 | 0.40 ± 0.0 c | −0.62 ± 0.0 a | 2478.2 ± 13.0 b | 5866.0 ± 40.3 b | 162.7 ± 17.0 c |
| PM100 | 0.43 ± 0.0 c | −0.65 ± 0.0 a | 1997.4 ± 11.6 b | 5384.5 ± 51.3 b | 177.8 ± 16.9 c |
| CMRC | 0.46 ± 0.1 bc | −0.59 ± 0.0 a | 2706.4 ± 11.5 a | 6700.0 ± 34.8 ab | 197.2 ± 27.0 bc |
| PMRC | 0.39 ± 0.1c | −0.60 ± 0.0 a | 3030.5 ± 12.2 a | 6657.3 ± 54.0 ab | 161.2 ± 28.1 c |
| Treatments | Soil Temperature | NH4+ | NO3− | Available N (NH4+ + NO3−) | DOC | Soil Moisture | |
|---|---|---|---|---|---|---|---|
| N2O | NPK | 0.248 | 0.576 ** | 0.677 ** | 0.634 ** | 0.430 * | −0.419 * |
| PM30 | 0.441 * | 0.716 ** | 0.733 ** | 0.422 * | 0.383 * | −0.393 * | |
| PM50 | 0.494 ** | 0.502 ** | 0.605 ** | 0.491 ** | 0.270 | −0.266 | |
| PM70 | 0.414 * | 0.525 ** | 0.586 ** | 0.516 ** | 0.221 | −0.401 * | |
| PM100 | 0.333 | 0.205 | 0.515 ** | 0.501 ** | 0.307 | −0.352 * | |
| CMRC | 0.367 * | 0.277 | 0.459 * | 0.420 * | 0.060 | −0.241 | |
| PMRC | 0.219 | 0.389 * | 0.482 ** | 0.406 * | 0.066 | −0.209 | |
| CH4 | NPK | −0.147 | −0.222 | −0.446 * | −0.361 | −0.073 | 0.214 |
| PM30 | −0.083 | −0.349 | −0.383 * | −0.405 * | −0.023 | 0.427 * | |
| PM50 | −0.364 * | −0.241 | −0.413 * | −0.366 * | −0.066 | 0.241 | |
| PM70 | −0.035 | −0.042 | −0.288 | −0.197 | −0.291 | 0.120 | |
| PM100 | −0.195 | −0.091 | −0.421 * | −0.444 * | −0.271 | 0.439 ** | |
| CMRC | −0.185 | −0.192 | −0.478 ** | −0.386 * | −0.238 | 0.488 ** | |
| PMRC | 0.320 | −0.361 | −0.497 ** | −0.224 | 0.004 | 0.454 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladipo, D.G.; Wei, K.; Hu, L.; Medaiyese, A.; Bah, H.; Gbadegesin, L.A.; Zhu, B. Short-Term Assessment of Nitrous Oxide and Methane Emissions on a Crop Yield Basis in Response to Different Organic Amendment Types in Sichuan Basin. Atmosphere 2021, 12, 1104. https://doi.org/10.3390/atmos12091104
Oladipo DG, Wei K, Hu L, Medaiyese A, Bah H, Gbadegesin LA, Zhu B. Short-Term Assessment of Nitrous Oxide and Methane Emissions on a Crop Yield Basis in Response to Different Organic Amendment Types in Sichuan Basin. Atmosphere. 2021; 12(9):1104. https://doi.org/10.3390/atmos12091104
Chicago/Turabian StyleOladipo, Dayo George, Kai Wei, Lei Hu, Ayodeji Medaiyese, Hamidou Bah, Lanre Anthony Gbadegesin, and Bo Zhu. 2021. "Short-Term Assessment of Nitrous Oxide and Methane Emissions on a Crop Yield Basis in Response to Different Organic Amendment Types in Sichuan Basin" Atmosphere 12, no. 9: 1104. https://doi.org/10.3390/atmos12091104
APA StyleOladipo, D. G., Wei, K., Hu, L., Medaiyese, A., Bah, H., Gbadegesin, L. A., & Zhu, B. (2021). Short-Term Assessment of Nitrous Oxide and Methane Emissions on a Crop Yield Basis in Response to Different Organic Amendment Types in Sichuan Basin. Atmosphere, 12(9), 1104. https://doi.org/10.3390/atmos12091104









