Abstract
(1) Background: Continuous monitoring of the tree stem increment throughout the year is crucial for the understanding of trees’ reactions to changes in meteorology, solar radiation and surface ozone and evaluating the adaptive capacity of prevailing tree species to recent environmental global changes; (2) Methods: Data on tree intra-annual sequences based on electronic dendrometer data of Picea abies (L.) Karst, Pinus sylvestris L., Betula pendula, and Betula pubescens, growing under different nutritional and humidity conditions in the north-eastern part of Lithuania, together with their stem sap flow intensity, common meteorology and O3 fluxes, were used to meet the objectives of the study; (3) Results: Stem shrinking/contraction during the day, due to transpiration, and the swelling/expansion during the night was significantly related to meteorology, sun activity and O3 flux intensity. These variations were negatively related to current time and temperature, but positively to precipitation and relative humidity. O3 fluxed through the stomata stimulated the shrinking process more intensively than it inhibited the swelling process, but only for pine and birch trees. Spruce trees demonstrated the highest sensitivity to O3 impact due to its significant effect on the stem swelling process. Pine trees were less sensitive to O3 damages and birch trees were the least sensitive. An over-moisture regime at measoeutrophic organic soil forest site increased the significance of the effect of O3 on the tree increment of the considered tree species; (4) Conclusion: The most intensive tree ring formation of Scots pine trees in relation to recent environmental changes indicated their high resiliencies and adaptations to a local specific condition. Reduced tree growth intensity and weak relationships between the birch tree radios increment and main meteorological parameters indicated the lowest adaptive capacity of this tree species to recent environmental changes.
1. Introduction
The growth of forests under changing environmental conditions has been extensively studied during recent decades [1]. Special attention is being paid to the effects of climate changes, which have become the main drivers of environmental changes owing to a significant rise in global mean surface temperatures and extreme weather events [2]. Their potential impacts and the risks to forest ecosystems are best studied and understood as they relate to wood production [3]. Tree-ring width and its formation are considered to be appropriate indicators of changes in environmental conditions [4,5] and are a proxy for ecosystem health [6].
Climate change is expected to increasingly impact forest ecosystems in the present century [7,8,9]. It may increase growth rates in temperate and boreal forests [10,11], while temperature-induced drought stress endangering the survival of trees and forest communities could determine the opposite effect, reducing it [10,12], especially in some southernmost areas [13]. The state of knowledge revealed that this effect varies across geographic areas, species, stand composition, tree age, and soil fertility [14]. In a hemiboreal vegetation zone, which is a transition zone between boreal and temperate zones such as the Lithuanian forest, this effect on tree growth is suggested to depend on site conditions and can be either positive or negative [1]. Therefore, the accurate prediction of climate effects on forest ecosystems represents a critical research gap [15].
Field-measured forest productivity and its time-series are crucial for understanding the impact of climate change on the main tree species increment and stand productivity in general [16]. Climate warming is the main factor responsible for the increase in the tree-growth intensity of hemi-boreal European forest tree species, mainly Scots pine and Birch sp., with the exception of the Norway spruce, whose growth has significantly decreased in central Europe [17]. Recurrent drought events reinforced the effect of climate warming on spruce growth through changes in tree physiological processes, modifying the functioning and vitality of individual trees [18,19] and increasing the susceptibility to bark beetle attacks [20] and, finally, tree mortality [21]. Therefore, resilience to extreme meteorological factors is of the greatest concern due to high vulnerability under the pressure of recent global changes [22,23,24,25]. Despite this, our earlier obtained results confirmed that the hemi-boreal forest in the north-eastern part of Europe is favorable for the growth of Norway spruce trees [26]. On-going temperature and precipitation increases, which are typical for this region of Europe, had essential impacts on the recovery of spruce tree vitality and increased their incremental growth in Lithuanian forests up to a mature age.
Based on our results, Scots pine trees are likely to be the tree species that are the most sensitive and most resilient to environmental changes in hemi-boreal forests. These results also contradict the findings obtained for central or southern Europe, where the incremental growth of Scots pine trees was found to be least sensitive to environmental changes and differed least under different growth conditions [27]. Pine trees growing on dry mineral oligotrophic soils, with a natural moisture regime, had no higher requirements for precipitation, which indicated their very high resistance to the drought effect. At this forest site, which is typical for Scots pine growth, only positive effects on pine increment from temperature during the dormant and vegetative periods were detected [26]. Only drought may significantly reduce Scots pine growth, especially at the southernmost distribution limit forest sites in the future [13].
In central Europe, above-average summer temperatures have resulted in the positive tree-ring formation of birch trees, whereas below-average summer temperatures and a dry winter have resulted in negative growth intensity [28]. These findings also contradict our obtained results on birch tree-ring formation in Lithuania. The increment of mature and over-mature birch trees decreased significantly. Meteorological factors, which resulted in the increment increase of coniferous tree species, were responsible for reductions in birch trees [26].
In Europe, divergent forest productivity trends have recently been reported both at the local and regional levels, challenging the projections of boreal tree growth dynamics, which are mainly detected on the annual increment scale [29]. Continuous monitoring of the tree ring formation throughout the year is crucial for the understanding of tree reactions to changes in environmental conditions, such as temperature, sun radiation, soil water potential (SWP), vapor pressure deficit (VPD) and rainfall [30].
Zweifel et al. [31], analyzing published results, presumed that stem growth, including cell division and cell enlargement, is attributable to the activity in the cambium that is strongly related to water content in storage tissues [32]. The replenishment of water in storage tissues [31], that is, potential water friction losses, which leads to increasing the potential fluctuation of water, resulting in xylem diameter variation along the hydraulic pathway [33]. Therefore, both these physiological processes—tree stem growth and water uptake—finally resulted in stem circumference rhythm, which is difficult to detect with empirical treatment of stem radius variation records [34].
Automated dendrometers at intra-daily resolution offer great potential to link environmental conditions with tree physiology at the seasonal scale [35]. These dendrometers are believed to provide a better estimation of the average radial growth than point dendrometers because they summarize the growth of all radii [36]. The main disadvantage lies in the difficulty of interpreting the obtained results. Dendrometer measurements do not distinguish between xylem, phloem and periderm increments, and these are also confounded with the overall swelling and shrinkage of the stem [37,38].
High-resolution analysis of stem circumference variation on an hourly scale provides insights into the temporal patterns in reversible tree water-related stem swelling and shrinking processes due to changing water potentials, including irreversible stem increments due to cell division and cell enlargement in the cambium in relation to environmental variables [32,39,40,41,42]. These two processes need to be separated to obtain drivers of growth and tree water deficits [35]. Therefore, automatic measurements of stem radius variations provide an effective and sensitive proxy, not only for plant water status assessment, but also for tree stem increment and its modelling in relation to environmental changes [35]. This process, based on graphical analyses of the time series on the stem increment has recently been extensively studied [30,35,38,42] and remains an intriguing issue given the potential impact of climate change on plant adaptive capacity to recent environmental changes [43].
Recently, climate change in the north-eastern part of Europe, including Lithuania, is associated with an increase in sun irradiance, temperature and precipitation amount vs. its decrease in middle and southern Europe, and also a decrease in surface ozone concentration [26,44]. Such environmental changes contributed to the regeneration of forest health and an increase in productivity [26,45]. However, the independent effect of each individual predicted variable on tree growth is still under investigation. Cambial phenological phases, which are influenced by the prevailing climatic conditions in forest stands at different forest sites of different fertility and humidity levels, have also scarcely been explored [39].
Notwithstanding this, in this study I tried to better understand how the daily stem radial activity of the prevailing forest tree species in Lithuania—Scots pine, Norway spruce and silver and downy birch were affected by variations in relative humidity, air temperature, solar radiation, wind speed and SWP as well as VPD. Special attention was paid to surface ozone concentrations and their fluxes through the stomata, which were detected by analyzing tree sap flow data. These data related to the seasonal growth behavior of the major tree species for forest sites in response to O3 exposure are still lacking, especially when applying an hourly data approach [46,47].
The state of knowledge revealed that the current O3 levels in the Northern Hemisphere are already high enough to negatively affect trees, especially fast-growing deciduous trees [48]. It can reduce the photosynthetic capacity and growth of forest trees [49,50] due to the humid climate increasing stomatal conductance and long light days extending diurnal periods with open stomata, both facilitating ozone uptake [47]. The degree of damages depends on the actual amount of pollutants that reach the target sites, as well as the capacity of the cells to restore homeostatic equilibrium by adapting to metabolic changes. Stomata conductance plays a fundamental role in determining the flux of O3 into the apoplastic region of plants [47,50]. Plants that show more rapid stomatal closure are reported to be resistant to O3 [51].
Recently, ozone flux after the passage of O3 through the stomata into the leaf intercellular space is also of the greatest concern when evaluating the effect of O3 on forest trees [44,52,53]. Transpiration rate, which is established by applying tree sap flow data obtained from the tissue heat balance method, allows the detection of O3 flux through the stomata and the detection of its effect on the increment formation of the prevailing forest tree species in Lithuania under different site conditions. It is also very beneficial to detect tree water-use efficiency (WUE), which is a key plant response mechanism to moderate severe soil water deficits and is therefore quite often used to assess the adaptive capacity of trees to recent environmental changes [54].
The present study was conducted with the aim of confirming the hypothesis that the boreal forest coniferous species, Scot pine and Norway spruce, are adaptive to recent climate changes and their capacity to mitigate climate change is higher than that of deciduous tree species, mainly Birch spp., which was established based on annual increment sequences relating to the monthly mean time series of meteorology and environmental contaminants, including surface ozone [7]. Norway spruce, with the rapid increases recorded in growth intensity since 1980, was found to be well adapted to recent environmental changes, which makes it one of the most favorable tree species for silviculture in the northeastern part of Europe. Scot pine demonstrated the highest level of resilience and capacity to adapt to recent global changes because its reaction to both negative and favorable environmental factors was best expressed. The sap flux of these considered coniferous tree species was lowest, which increased their WUF event during the drought period [54]. Therefore, the requirement to create well-designed process-based models for climate responsibility to cambium phenology, adaptation, distribution or the replacement of these tree species in response to climate change is of great concern not only in Lithuania but also across all of Europe.
Only a significant decline in the growth intensity of silver and downy birch trees and the absence of significant reactions to environmental factors indicated that these tree species demonstrated a reduced resistance to recent changes in environmental conditions, especially in the mature and over-mature age groups at the driest site [26]. Their WUE was also the lowest there [54].
To validate these results at the hourly scale, it is suggested that a stem-cycle approach should be used, separating increment formation changes into distinct phases [30,35,41,55] and generalizing the obtained data by applying a diurnal approach that extracts the summary metrics per day [42]. Based on this, the presented study was conducted on stem shrinking and swelling processes separately, detecting key predicted variables resulting in the intensity of these opposite processes, when the reliability of the obtained results was checked through a comparative analysis with the common results obtained at the diurnal scale.
To meet the aims of this study the objectives were as follows:
- To detect differences in the tree stem increment of the prevailing tree species in Lithuania, growing under different site conditions;
- To detect key environmental factors that have the most significant effect on tree stem shrinking and swelling processes during vegetation;
- To detect the direct effect of surface ozone fluxes on the tree stem radius increment;
- To evaluate the adaptation capacity of the prevailing tree species to recent climate changes.
The obtained data should allow an explanation of the difference in tree stem increments and evaluation of the adaptive capacity of the prevailing tree species in Lithuania to recent environmental changes.
2. Materials and Methods
The investigation was conducted in the coniferous–deciduous forest in the north-eastern part of Lithuania at the Aukstaitija Integrated monitoring station (IMS), which was established in Aukstaitija National Park (NP) in 1993 (Figure 1). It was established in the strict reserve zone of NP in the Azvinciai mature and over matured natural forest. Bearing in mind differences in the physiological reactions of the tree species to environmental stressors at sites with different moisture and fertility availabilities, seasonal reactions of the prevailing tree species in Lithuania—Scots pine (Pinus sylvestris L.), Norway spruce (Picea abies Karst.) Silver and Downy birch (Betula pendula Roth. and B. pubescens Ehrh.)—were investigated at three different forest sites (FS). A more detailed description of FSs can be found in our earlier studies [26,56,57,58].
Figure 1.
Location of the studied object and Aukstaitija National Park in Lithuania.
2.1. Site Description
The main dendrometric characteristics of the monitored FSs are compiled in Table 1. The sample trees chosen for the intensive investigation of fluctuations in their stem circumference suitably reflected the mean dendrometric parameters of each considered tree species.

Table 1.
Main dendrometric characteristics of the considered mixed stands.
Site FS-1 was established on oligotrophic mineral soil. There, Pinus sylvestris with Betula pendula and Picea abies dominated in the first stand layer. Sorbus aucuparia, Frangula alnus and Juniperus communis were present in the shrub layer. Vaccinium myrtillus, V. vitis-idaea and Melampyrum pratense dominated in the herb layer. Pleurozium schreberi, Hylocomium splendens and Dicranum polysetum dominated in the moss layer. The soil type of FS-1 is Haplic Arenosol, the water table is deeper than 6 m [26,54]. The pure and mixed groups of the considered tree species, which were indicated as “pure” or “mix,” were selected for the hourly estimation of stem increment. Several suppressed spruce trees from the second stand layer comprised the group called “press”.
FS-2 was established on mesoeutrophic peatland organic soil. Pinus sylvestris with Betula pendula and Picea abies also dominated in the stand. Sorbus aucuparia, Frangula alnus and Corylus avellana were present in shrub layer. Vaccinium myrtillus, Maianthemum bifolium, Oxalis acetosella, Mercurialis perennis, Trientalis europea and Equisetum pratense were present in the herb layer. Pleurozium schreberi, Hylocomium splendens and Plagiomnium affine dominated in the moss layer. The soil type of FS-2 is Terric Histosol, the depth of the water table was approximately 0.5 m [26,54]. Only the pure groups of the considered tree species were selected for the analysis of the stem increment. These groups were called “peat”.
FS-3 was established in the spring of 2017 as a pure spruce stand, typical for this region, located 200 m from the FS-2 at a mesoeutrophic mineral forest soil site. Frangula alnus and Corylus avellana were present in the shrub layer. Oxalis acetosella, Vaccinium myrtillus, Maianthemum bifolium, Trientalis europea, Dryoptetis filix-mas and Equisetum were present in the herb layer. Pleurozium schreberi, Hylocomium splendens, Eurhynchium angustirete and Plagiomnium affine dominated in the moss layer. The soil type of FS-3 is Gleyic Arenosol; the water table was about 1.5 m. Only one pure spruce tree group was selected for the present study and it was called “pure”.
Data on soil water potential suitably reflected periods with sufficient humidity in the soil and periods with a lack of moisture, that is, drought periods (Figure 2). Soil water potential at a depth of 40 cm was higher than that at depths of 10 and 20 cm and, in general, agreed well with the precipitation amount at all FS.
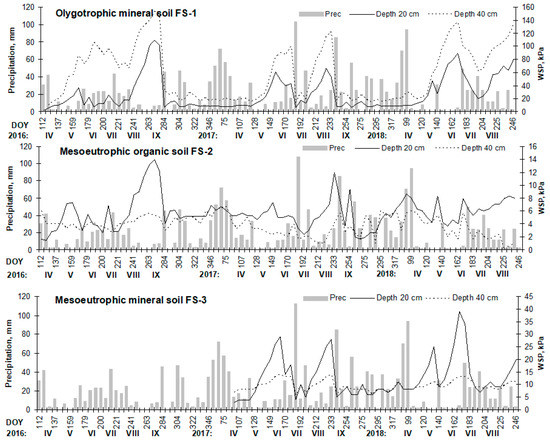
Figure 2.
Seasonal dynamics in water soil potential (WSP) and precipitation amount per week at the considered FS during 2016–2018 period. (Roman numbers represent month).
2.2. Variation in Meteorology, Solar Activity and Surface Ozone during 2016–2018 Period
Meteorological parameters, temperature, precipitation amount, humidity, atmospheric pressure, wind speed, solar radiation, soil water potential (SWP) and vapor pressure deficit (VPD), as well as data on surface ozone, were obtained from Aukstaitija IMS (Figure 2). A positive diurnal stem circumference increment was applied to identify the start of the growing period, and a negative applied to identify the end of the growing period. This period is marked by a textbox in Figure 3.
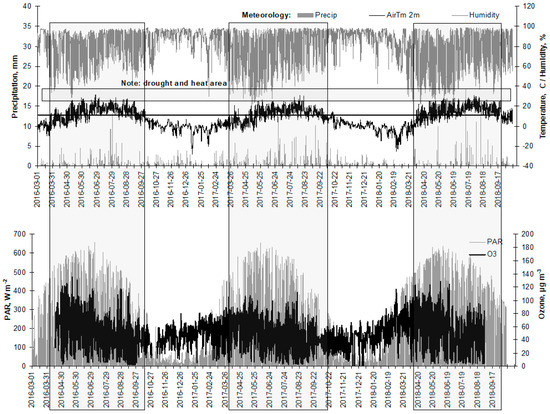
Figure 3.
Values of the main meteorological parameters (precipitation, mm per h; temperature, °C; humidity, %) and sun radiation (photo synthetically active radiation, W m−2 and surface ozone, µg m−3) on hourly scale in Aukstaitija IMS, 2016–2018. Periods when mean temperature exceeded 5 °C are in box.
The drought effect was most expressed in 2018. In that year, sun PAR radiation together with air temperature was the highest. The maximal value of surface ozone was registered in 2016. Drought was detected during the period when mean daily humidity decreased below 40% and air temperature exceeded 25 °C.
Soil water potential was analyzed by applying data from 4 × 3 watermark soil moisture sensors (10, 20, 40 cm depth), which were installed under the canopy at all forest sites.
The vapor pressure deficit in hPa was obtained from relative humidity and air temperature data.
Ozone uptake was evaluated by combining hourly canopy conductance data with ambient ozone concentrations. Canopy conductance for water vapor was derived from xylem sap flow data measured in tree trunks. Sap flow density was estimated with the heat dissipation method [59], using SFM1 Sap Flow Meters (SFM1 instrument, ICT International, Australia). Sap flow sensors were installed on all monitored trees at approximately 120 cm stem height in N-exposition and sheltered with aluminum foil caps. Sensor needles measuring at two different depths were inserted into the sapwood with a bark depth of 10 mm (bark removed or spacer set). Sapwood cores were taken with a conventional coring tool at breast height from 10 trees per species and plot to determine wood properties (fresh, dry weight; thermal diffusivity) and to measure sapwood thickness by dye indication or microscopic analysis (acc. to SFM1 manual, ICT international). Measuring intervals of 15 min were summarized to hourly values as the basis for all further calculations [54].
For conversion to sap velocity, sap flow and total plant water use, these data were computed using ICT International Sap Flow Tool Software (Sap Flow Tool software for HFD and HRMl, ICT international).
Using the tree stem sap wood area obtained at the measured height, sap flow density was upscaled to the whole tree. Using the total leaf area for pine and spruce trees and the projected leaf area for birch trees, the whole tree sap flow was scaled to the ground area related whole-tree transpiration (EC). EC data were time shifted according to the ambient solar radiation and VPD, in order to obtain current, more accurate ozone uptake results.
Whole-tree canopy conductance to water vapor scaled to ground area (GC) was determined from the time ratio of the time shifted ground area related to whole-tree transpiration (EC) versus ambient (VPD) according to [60]:
GC = EC/VPD.
The whole tree O3 uptake was then calculated according to the flux equation:
where FCO3 is the whole-tree canopy O3 flux or uptake rate scaled to ground area, O3 is the ozone concentration of the ambient air, GC is the whole-tree canopy conductance for water vapor scaled to ground area, and 0.613 is a conversion factor to account for the lower diffusivity of O3 relative to water vapor in air [61].
FCO3 = O3 × GC × 0.613,
2.3. Data Sampling Methods
Ten averaged data series of tree ring stem increments on an hourly scale based on electronic dendrometers data (DRL 26, EMS Brno Regio 621 00 Czech Republic) were prepared to detect the regional peculiarities of tree increment including reversible stem shrinking and swelling and irreversible increment in relation to meteorology and surface ozone. At least three hourly sequences of stem circumference variation of each considered tree species obtained from DRL 26 were used to calculate the stem basal area increment (BAI) and complete their averaged data series.
The obtained data allowed for examining the reversible and irreversible fluctuations in stem circumference, which were related to daily changes in stem water potential and fluctuations in bark, phloem and in xylem [62,63,64]. To meet the objectives of the presented study stem basal area increment (BAI) based on stem circumference data was detected.
2.4. Data Analyses
Pearson correlation analysis was used to examine the relationship between stem BAI and meteorological parameters on an hourly scale and to detect the key parameters most significantly related to changes in stem BA: its shrinking and swelling including increments during 2016–2018 vegetation periods. The period of stem growth was separated into two distinct phases based on changes in stem circumference: data with negative values were used to analyze the key environmental factors responsible for stem shrinking, and positive values were used to analyze the key environmental factors responsible for stem swelling. Generalizing the obtained results, a diurnal approach was applied to all data to meet the objectives of presented study.
To solve this problem, a linear multiple regression technique implemented in the statistical software STATISTICA version 8.0 www.statsoft.com (accessed on 21 June 2021) was applied. Single hourly values on the considered predict variables during the vegetation season were included in the multiple regression models. While developing the models, the selected parameters were excluded from the regression model by a stepwise procedure based on the lowest level of significance [65]. Finally, variables with a high level of significance (p < 0.05) were used to run the models. These parameters were evaluated as key factors limiting hourly fluctuations in the stem BAI of the prevailing tree species in Lithuania under different growth conditions. The goodness-of-fit of each model was assessed by determining the coefficient of determination (R2) and the level of statistical significance (p).
The effect of surface ozone was detected by using different methodological approaches: firstly, detecting relationships between ozone concentration and variation in stem BAI; secondly, analyzing the effect of ozone fluxes through stomata; and finally, the significance of the O3 effect was established by developing regression models and calculating the differences in R2 and p with the ozone effect and without it.
3. Results
3.1. Annual Increment, Sap Flow Intensity and WUE of the Prevailing Tree Species
The swelling and shrinking processes of stem circumference during the dormant period, that is, from the beginning of September up to the beginning of new vegetation in April or May, were not included in the analysis. The period of the investigation was characterized by an over moisture regime in 2017, when the precipitation during the vegetation period exceeded 680 mm, which was close to the long-term annual average of precipitation at this location and the longest duration of vegetation period, which exceeded 220 days (Table 2). That year, the vegetation period was the coolest and least polluted by surface ozone if compared with the other two considered years (Figure 3).

Table 2.
Duration and main meteorological characteristics (precipitation, Pr and temperature, Tm) of the considered vegetation periods.
Precise measurements of the stem circumference at an hourly scale indicated that, on the 10–12 May, the first signs of the tree ring formation of coniferous trees were detected, first of all at the mineral soil forest site (FS) (Figure 4). After a few days, their growth started at the organic peatland FS. Birch tree growth started a few days after the coniferous tree species at both considered FSs. Rather, regular growth with stem shrinking during the daytime and swelling at night, including growth, was observed until the middle of June, when the drought period started. Drought, which continued for about two weeks during each considered year, that is, from approximately the middle or end of June up to the beginning of July, had a significant impact on the tree stem increment.
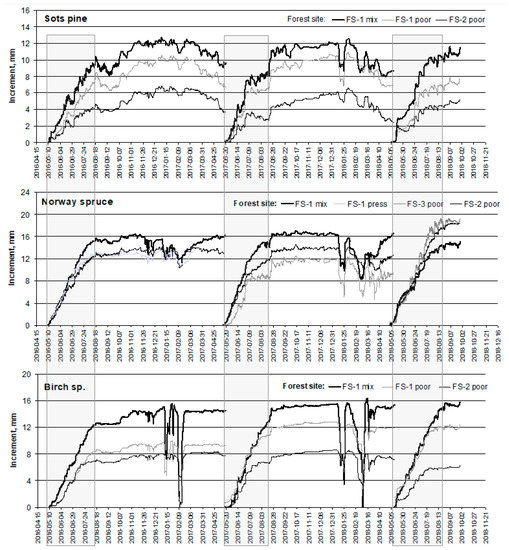
Figure 4.
The cumulative seasonal prolongation of stem circumference (mm) of the prevailing tree species in Lithuania at three forest sites in Aukstaitija IMS, 2016–2018. Trees growth periods are in box.
Precipitation, which interrupted the effect of drought on tree growth, affected the tree increment remarkably. After episodes with abundant precipitation, the highest growth rate, including stem swelling, was recorded for spruce trees and the lowest for birch trees at all investigated FSs. The end of tree ring width formation was detected at approximately the same time, that is, 15–18 August.
FS conditions also had no impact on this phenological stage of monitored trees. The obtained data revealed that, in the middle of August, tree ring width formation stopped and afterwards only stem circumference fluctuations with higher shrinking rates were recorded.
Therefore, the 95-day period from tree ring width formation, starting on the 12 May up to its end on 18 August, was chosen for the detailed investigation of the causative effects of meteorology and surface ozone at the level of stem circumference fluctuations at the hourly scale. The exceptional case was the end of the growth of spruce trees at the peatland FS in 2018 when the stem increment/circumference swelling continued until the beginning of October (Table 3).

Table 3.
Main characteristics of the monitored trees at different forest sites, their transpiration, water use efficiency and O3 flux intensity.
The obtained results revealed that spruce trees demonstrated the highest growth rate during this period at both considered forest sites. Only the growth rate of the pine and birch trees differed significantly. At the mineral oligotropic soil FS, the growth rate of the pine trees exceeded the growth rate of silver birch trees, while at the organic soil FS, the growth rate of downy birch trees exceeded the growth rate of Scots pine trees.
Winter shrinkage exceeded the growth rate of a whole season, which has been mentioned by other scientists [40], and was especially noticeable in the birch tree growth series in January and February.
The mean parameters of the monitored trees revealed that pine trees demonstrated the biggest stem increment in 2018 after a very humid vegetation period in 2017 (Table 3). This tendency was detected at both pine FSs. The comparison of tree increments among FSs showed that pine trees growing together with deciduous trees at oligotrophic mineral FS-1 were the most productive. Their increment was the biggest. The pine increment at FS-1, growing in group with other pine trees, was a little lower and the least increment was detected for pine trees growing at the mesoeutrophic organic (peatland) FS-2, which is not typical for pine growth in Lithuania.
Different results were obtained comparing sap flow intensity data, which were in a close relationship with water contents at the site. The highest sap flow intensity was observed for pine trees growing at the peatland FS-2. There, the water use efficiency (WUE) of pine trees was the lowest, that is, for 1 dm3 of timber production pine used up to 500 L of water. The highest WUE was detected for pine trees growing at the oligotrophic mineral FS-1 together with deciduous trees, that is, WUE reached 200–300 L of water for 1 dm3 of timber.
Spruce trees demonstrated different results. The biggest annual increment was observed for spruce trees growing at mesoeutrophic mineral FS-3, while their WUE was one of the lowest, that is, about 150–200 L per 1 dm3 of timber. The highest WUE was detected at oligotrophic mineral FS-1 with a lack of humidity, that is, only 80–150 L of water for 1 dm3 of timber. At mesoeutrophic organic FS-2 with an over-moisture regime, WUE was the lowest and made 180–240 L per dm3 of timber. Water availability was the key factor responsible for spruce tree productivity and WUE.
Birch trees growing in pure stands at oligotrophic mineral FS-1 demonstrated the highest productivity and their WUE made about 200 L per dm3 of timber and did not differ significantly from the WUE of pine trees. Significant differences among birch tree WUE on FS-2 were not detected.
3.2. Intra-Annual Fluctuation in Stem Basal Area of the Prevailing Tree Species
Maximal growth rate, which exceeded the regular growth rate, was recorded in periods with rather abundant precipitation and a lower temperature. Such conditions were first of all favorable for stem swelling including the increment. The time and duration of these processes differed among the considered tree species. An increase in the stem circumference of pine trees was recorded at 6 p.m. and reached its maximal value at 8 p.m.; meanwhile, for spruce trees, the maximal value in increment was reached at midnight. Such expansion of circumference including growth/new cells formation continued for pine trees up to 5–6 a.m. meanwhile for spruce trees up to 8–9 a.m., that is, a few hours longer than for pine trees. By contrast, birch tree stem swelling started at 3 p.m. and reached its maximal value at 7 p.m. and stopped at 7 a.m., that is, earliest if compared with coniferous species. At the organic soil forest site, no significant difference in stem increment formation was detected.
Exceptionally different growth rates were established for the considered tree species during the drought period. During this period, the mean daily temperature exceeded 25 °C, humidity decreased by up to 40% (Figure 3). These environmental conditions were unfavorable for tree growth and mainly resulted in stem shrinking and the inhibition of ring increment over a 10 h period, that is, from 8 a.m. up to 6 p.m., and the suppression of the stem swelling during the night.
The results obtained on the balance of stem BA shrinking and swelling at the diurnal scale were rather surprising. Spruce trees demonstrated the greatest decrease in stem BA during the daytime and the greatest increase during the nighttime at both FSs. An approximately twofold lower reduction and increase in stem BA were recorded for pine trees during these time periods and only birch stem BA shrinking and swelling processes were close to zero. At organic soil FS-2, the intensity of these processes was less expressed for all considered tree species than at mineral soils FS-1. There, spruce stem BA swelling was significantly higher than shrinking, which indicated that, even during drought episodes, more humid site conditions were more favorable for spruce tree growth than for pine and birch tree growth.
Generalizing the shrinking–swelling processes during the vegetation, it was established that stem the BA shrinking process continued for up to 35% of the hours while the swelling process was up to 65% of the hours (Table 4). This means that during the day shrinking occurred for 8 h and swelling was two-fold longer, that is, 16 h. No significant differences among tree species and forest site conditions were established.

Table 4.
Swelling and shrinking of tree stem basal area of the considered tree species under different growth conditions during 2016–2018.
Differences in the annual increment resulted in changes in the hourly rate of BA shrinking and swelling processes. For birch trees, the hourly rate of stem shrinking was significantly lower than the hourly rate of stem swelling. For pine trees, the hourly rate of stem shrinking was lower than or equal to the hourly rate of stem swelling. For spruce trees, the hourly rate of stem shrinking was lower than or equal to the hourly rate of stem swelling at FS-1 and FS-2 and higher at FS-3.
3.3. Intensity of Shrinking and Swelling of Stem Basal Area on Hourly Scale in Relation to Environmental Factors
It is well established that the timing and magnitude of diurnal variations in stem size are mainly determined by the course of transpiration and soil water content [37], both of which are mainly related to precipitation amount and air humidity, that is, to microclimatic conditions, and can rapidly change under different weather conditions [66].
To detect the key environmental parameters responsible for water contents in xylem and cambium, which resulted in fluctuations in stem circumference, a separate analysis of stem BA shrinking and swelling processes in response to environmental conditions was performed. The considered predicted variables had a more significant effect on variation in stem BA shrinking than its swelling. The key factor that stimulated the shrinking process for spruce trees was low humidity level and a higher temperature together with SWP and VPD. The other considered variables only stimulated stem shrinking, the most significant of which was the negative effect of PAR (Figure 5). The effect of O3 fluxes on stem shrinking was not significant, especially at FS-1 for spruce trees suppressed by neighboring trees (Figure 5, Norway spruce, Press).
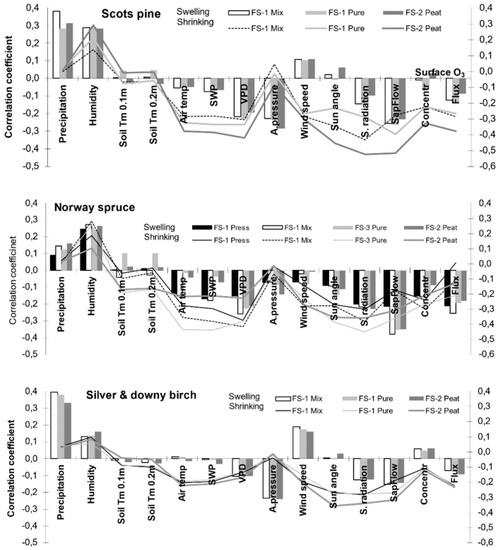
Figure 5.
Relationship between values of the stem basal area fluctuations (shrinking and swelling) on hourly scale of the considered tree species and meteorology at different forest sites. Silver birch on FS-1 and downy birch on FS-2.
Quite a similar relationship was detected between fluctuations in pine stem BA and the considered environmental factors. The significant adverse effect of atmospheric pressure is noticeable, but this effect can be explained mainly through the collinearity of this parameter with the rest of the considered parameters, first of all, temperature and humidity. O3 fluxes had a more significant effect on stem shrinking than on stem swelling.
Contrary to the results obtained for coniferous trees, the direct effects of temperature and humidity were lower for both stem swelling and shrinking processes of the birch tree (Figure 5). The direct effect of surface ozone fluxes had a more significant effect on stem shrinking than on stem swelling. The effect of precipitation on stem swelling process intensity was expressed most significantly. A much lower effect of PAR and ozone fluxes was detected on stem shrinking during daytime in comparison with their effect on the stem shrinking of pine trees. The obtained data revealed that, in general, the effect of the considered environmental factors on birch stem BAI was less expressed than that on coniferous trees.
Multi regression models of tree BAI revealed that wind speed, PAR, soil water potential, vapor pressure deficit and ozone fluxes are the key factors resulting in pine tree stem BA shrinking (Table 5). They explained up to 20% of the variation in shrinking intensity at oligotrophic mineral soil FS-1 and up to 30% of its variation at mesoeutrophic organic soil FS-2.

Table 5.
Key factors contributing to the shrinking of tree stem basal area of the considered tree species under different growth conditions and their significance expressed by means of multiple regression model statistics.
The predicted variables, with the exception of O3 fluxes, explained up to 24% of the variation in spruce stem BA shrinking at FS-1. O3 fluxes had no significant effect on spruce tree stem shrinking at this FS-1, while their effect at FS-2 and FS-3 was significant. In addition, temperature, PAR and VPD were also key variables at these FSs. These parameters explained about 12% and 22% of the variation in spruce tree stem shrinking, respectively.
Wind speed, temperature, PAR and O3 fluxes were the key parameters resulting in birch tree stem shrinking variation. However, their explanation rate was the lowest if compared with the rate obtained for coniferous tree species, that is, only up to 10%.
None of the considered parameters with the effect of mitigating the stem shrinking process remained in the models.
The multi-regression models created revealed that only the direct effect of precipitation amounts, relative humidity and wind speed resulted in statistically significant intensity of stem BA swelling (Table 6). SWP was responsible for the mitigation of the swelling process only for spruce trees at FS-1. Surface ozone fluxes demonstrated highly significant inhibition of stem BA swelling of all tree species at all considered FSs.

Table 6.
Key factors contributing to swelling of tree stem basal area of the considered tree species under different growth conditions and their significance expressed by means of multiple regression model statistics.
3.4. Integrated Effect of Environmental Factors on Hourly Fluctuation in Stem Basal Area
During the entire period of tree ring formation, only precipitation and relative humidity stimulated tree stem increment (Figure 6). Based on the obtained results, birch trees were least tolerant to the lack of precipitation at both considered FSs, while spruce trees demonstrated the highest resiliencies to this factor. The key factor resulting in spruce stem increment was relative humidity.
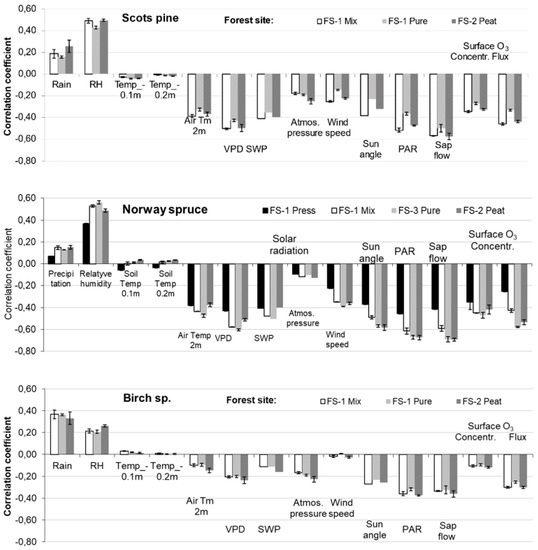
Figure 6.
Relationship between data on meteorology on hourly scale and growth rates of the prevailing tree species under different growth conditions in 2016–2018.
Lower values of VPD and SWP also stimulated spruce tree stem increment most significantly if compared with the other considered tree species. On the other hand, higher temperature together with solar PAR also most significantly resulted in a decrease in spruce stem increment through its shrinking.
Different results were obtained when investigating the effect of air humidity on the stem increment of the considered tree species. Both coniferous tree species demonstrated higher requirements for humidity for a greater rate of tree stem increment than birch trees, and surprisingly at the organic soil forest site. Temperature and sun characteristics, that is, photoperiods (sun angle) and PAR, which stimulated stem BA shrinking processes, inhibited the tree stem increment process. The effect of these factors was reinforced by higher wind speed and atmosphere pressure. Consequently, surface ozone, especially its fluxes, also reduced the stem increment.
The created generalized multiple regression models of tree increment based on an hourly scale allowed for the evaluation of the main detected environmental factors responsible for fluctuations in the stem BA of the considered tree species under different growth conditions (Table 7). Multivariate regression models showed that variations in the stem increment of all considered tree species were positively related to the amount of precipitation and relative humidity, especially for pine trees at all FSs and spruce trees at FS-1. A negative direct effect of temperature was not responsible for the tree stem increment.

Table 7.
Key factors of tree ring formation of the considered tree species on hourly scale under different growth conditions and their significance expressed by means of multiple regression models statistics.
Precise measurements of ring width formation indicated close temporal linkages between PAR and patterns of tree stem increment during diurnal cycles, confirming the state of knowledge in this area [67]. O3 fluxes also remained among the key factors resulting in the BA increment of the considered tree species, inhibiting it. Finally, I can conclude that surface ozone flaxes stimulated shrinking and inhibited the swelling of the tree stem, which resulted in the reduction of tree ring width in general.
4. Discussion and Conclusions
Seasonal variability of environmental contaminants and the main meteorological parameters, such as air temperature and soil water regime, are suggested as key research areas for investigating climate change effects on forest ecosystems [53] including the intra-annual variability of environmental effects on tree health and productivity. This means that the continuous monitoring of stem circumference variation throughout the year is crucial for the understanding of tree reactions to short-term changes under environmental conditions such as temperature, air humidity, rainfall and parameters of solar activity as well as air contaminants, including surface ozone.
During the vegetation period, the reversible shrinking and swelling of stems contribute to a significant part of the variability in stem size changes, reflecting the use of water stored in tree tissues [68,69]. This higher variation in stem circumference allows us to gain a deeper insight into stem growth variations in relation to changing environmental conditions including meteorology, natural solar radiation, and surface ozone (O3) [41,70,71]. Specific parameters, such as changes in hourly rate in the stem basal area, can only be obtained with the stem cycle approach [41,55,70]. To my mind, even the shrinking process cannot be ignored when investigating these rather complicated processes of tree ring width formation. It is most likely that, during deep drought processes, some part of the stem shrinking could not be reversible due to the limited plasticity of the cell membranes. Therefore, this stem-cycle methodological approach allows for separating the high-resolution dendrometer records into distinct phases of contraction, expansion and stem-radius increment. It is supported by many scientists investigating increment formation on the basis of short time sequences [41,66,70,72,73,74] and therefore was used to meet the main tasks in the presented study.
The obtained data confirmed the background knowledge in the area of short time tree stem increment formation. Stem increment is positively related to precipitation and relative humidity and negatively to temperature, sunshine hours and drought [41,75,76]. Nighttime temperature was more effective for stem increment than daytime temperature [38]. This means that stems contract during the day, due to transpiration and photosynthesis, and expand during the night and on rainy days when water reserves are gradually replenished [66] and assimilated carbohydrates are allocated along the stem. These ecophysiological processes differed significantly under different growth conditions due to their different strategies when surviving different (favorable or unfavorable) environmental conditions [77,78]; first of all, heat and drought episodes. Therefore, in general, the obtained data confirmed the statement that weather fluctuations very well reflect seasonal stem increment variation and that different environmental conditions result in statistically significant differences in tree stem increment during the growing season.
Long term investigation of tree growth intensity revealed that, recently, Norway spruce trees have demonstrated greater stem radial increment in Lithuania than pine and birch trees [26]. An increase in precipitation amount followed by a decrease in soil water potential and vapor pressure deficit resulted in significantly higher spruce stem increment. Their stem shrinking was higher than that of pine and birch trees; however, stem swelling at nighttime exceeded the intensity of this process in pine and birch trees. These growth reactions indicated their better adaptation to recent climatic conditions in hemi-boreal forest, which contradicts the knowledge of the previous century, that is, that climate change reduces radial growth in spruce stems and especially in the subsequent vegetation period [4,78,79].
Pine trees also demonstrated a very sensitive reaction to environmental changes. Their stem shrinking and swelling processes in general were most expressed during the entire vegetation period. The highest reversible fluctuations in water storage in xylem, phloem and bark did not mask the signal caused by wood formation, which has been referred to by many authors [62,63,80,81], and this is why this species became most sensitive to changes in meteorology and air pollutants including surface ozone in the northeastern part of Europe. The considered meteorological parameters explained the variation in both stem shrinking and swelling most significantly. These results contradict the findings obtained in Central or Southern Europe, where the increment of Scots pine was least sensitive to environmental changes and differed least between different growth conditions [27]. Our data obtained earlier indicated that meteorology and acidifying compounds explained the variation in annual pine tree ring width most significantly [56,57,58]. On an hourly scale, temperature, precipitation, humidity, sun activity and surface ozone play a major role in pine intra-annual tree ring width formation, confirming our data obtained on annual pine increment.
The integrated effect of meteorology and ozone fluxes on the intra-annual tree ring width formation of birch trees expressed by means of multi regression models was found to be remarkably lower than the effect on spruce or pine annual tree ring width formation. Information on the direct positive hourly effect of temperature and solar radiation on birch tree growth rate is more limited [67,82,83]. Stem shrinking, as well as the whole tree stem increment during the vegetation, was the least explained by meteorological factors. These data very well reflect data obtained at the annual scale. The air temperature of months also had no significant effect on birch growth and only the precipitation amounts during June at the mineral soil forest site stimulated birch tree ring width formation [26]. A very weak relationship between meteorological parameters and birch stem swelling and shrinking could be presented as a key factor which resulted in the gradual decrease in tree ring width in Lithuania.
Finally, the adaptive capacity of the considered tree species to the recent global changes was evaluated based on the reaction of these tree species to variations in hourly concentrations of surface ozone. Among air pollutants, ground-level ozone (O3) is one of the main drivers of changes in forest conditions [53]. During the considered time, mean ozone concentration reached about 60 µg m−3, meanwhile its maximal values rarely exceeded 125 µg m−3 with the exception of 2016. This concentration could be evaluated as low with a negligible effect on tree growth; however, earlier results indicated that maximal values in surface ozone had a negative and significant effect on pine stem increment [52]. Despite this, our data confirmed the statement obtained by McLaughlin et al. [67] and the negative effect of ozone fluxes on short time tree increment formation was found to be weak but statistically significant (p < 0.05), especially under more humid conditions. Based on the obtained results, the coniferous tree species seems to be more sensitive to ozone damage than birch trees, and these data also agreed well with data obtained at the hourly scale [26].
On the basis of these results, I could conclude that birch trees, which demonstrated the lowest (positive or negative) reaction to the environmental changes, should be evaluated as not having capacity to adapt to existing environmental conditions and their prospects of composing sustainable succession is very problematic, especially when their annual growth gradually decreases. The hypothesis that the coniferous species are more adaptive to recent climate changes in Lithuania and their capacity to mitigate the threats of global changes is higher than that of deciduous tree species was confirmed on an hourly scale of variation in stem circumference.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data on environmental condition were obtained performing ICP IM programme since 1993 and are stored at Finnish Environmental Institute and DEIMS data base: https://deims.org/fad7f221-25f3-4286-a1b3-43a5f010a3e3 (accessed on 4 August 2021). Hourly data on tree stem increment and sap flow intensity are stored at local data base at VDU and could be presented only under personal request.
Conflicts of Interest
I declare no conflict of interest.
References
- Matisons, R.; Elferts, D.; Krišāns, O.; Schneck, V.; Gärtner, H.; Bast, A.; Wojda, T.; Kowalczyk, J.; Jansons, Ā. Non-linear regional weather-growth relationships indicate limited adaptability of the eastern Baltic Scots pine. For. Ecol. Manag. 2021, 479, 118600. [Google Scholar] [CrossRef]
- Bolte, A.; Hilbrig, L.; Grundmann, B.; Kampf, F.; Brunet, J.; Roloff, A. Climate change impacts on stand structure and competitive interactions in a southern Swedish spruce–beech forest. Eur. J. For. Res. 2009, 129, 261–276. [Google Scholar] [CrossRef] [Green Version]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Eckstein, D.; Krause, C.; Bauch, J. Dendroecological Investigation of SpruceTrees (Picea abies (L.) Karst.) of Different Damage and Canopy Classes. Holzforschung 1989, 43, 411–417. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Shortle, W.C.; Smith, K.T. Dendroecological applications in air pollution and environmental chemistry: Research needs. Dendrochronologia 2002, 20, 133–157. [Google Scholar] [CrossRef] [Green Version]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Matala, J.; Ojansuu, R.; Peltola, H.; Raitio, H.; Kellomaki, S. Modelling the response of tree growth to temperature and CO2 elevation as related to the fertility and current temperature sum of a site. Ecol. Model. 2006, 199, 39–52. [Google Scholar] [CrossRef]
- Scholze, M.; Knorr, W.; Arnell, N.W.; Prentice, I.C. A climate-change risk analysis for world ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 13116–13120. [Google Scholar] [CrossRef] [Green Version]
- Bouwman, M.; Forrester, D.; Ouden, J.D.; Nabuurs, G.-J.; Mohren, G. Species interactions under climate change in mixed stands of Scots pine and pedunculate oak. For. Ecol. Manag. 2021, 481, 118615. [Google Scholar] [CrossRef]
- Pretzsch, H. The course of tree growth. Theory and reality. For. Ecol. Manag. 2020, 478, 118508. [Google Scholar] [CrossRef]
- Mensah, A.A.; Holmström, E.; Petersson, H.; Nyström, K.; Mason, E.G.; Nilsson, U. The millennium shift: Investigating the relationship between environment and growth trends of Norway spruce and Scots pine in northern Europe. For. Ecol. Manag. 2021, 481, 118727. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, Á.; Camarero, J.J.; Gómez, C.; Cañellas, I.; Aulló-Maestro, I.; Gil, L.; Montes, F. Scots pine plantations growth adaptation to climate warming in locations at the southernmost distribution limit of the species. Dendrochronologia 2020, 63, 125745. [Google Scholar] [CrossRef]
- Toochi, E.C. Forest and environment: Developments in global change ecology. For. Res. Eng. Int. J. 2017, 1, 100–105. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Climate warming will reduce growth and survival of Scots pine except in the far north. Ecol. Lett. 2008, 11, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, X.; Liang, P.; Wu, Y.; An, H.; Sun, H.; Wu, P.; Wu, X.; Li, Q.; Guo, X.; et al. A new tree-ring sampling method to estimate forest productivity and its temporal variation accurately in natural forests. For. Ecol. Manag. 2019, 433, 217–227. [Google Scholar] [CrossRef]
- Laubhann, D.; Sterba, H.; Reinds, G.J.; De Vries, W. The impact of atmospheric deposition and climate on forest growth in European monitoring plots: An individual tree growth model. For. Ecol. Manag. 2009, 258, 1751–1761. [Google Scholar] [CrossRef]
- Mina, M.; Martin-Benito, D.; Bugmann, H.; Cailleret, M. Forward modeling of tree-ring width improves simulation of forestgrowth responses to drough. Agric. For. Meteorol. 2016, 221, 13–33. [Google Scholar] [CrossRef] [Green Version]
- Lévesque, M.; Walthert, L.; Weber, P. Soil nutrients influence growth response of temperate tree species to drought. J. Ecol. 2016, 104, 377–387. [Google Scholar] [CrossRef]
- Vakula, J.; Zúbrik, M.; Galko, J.; Gubka, A.; Kunca, A.; Nikolov Ch Bošela, M. Influence of selected factors on bark beetle outbreak dynamics in the WesternCarpathians. Lesn. Cas. For. J. 2015, 61, 149–156. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought andheat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Lévesque, M.; Saurer, M.; Siegwolf, R.T.W.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns ofdrought tolerance in major European temperate forest trees: Climatic driversand levels of variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Suvanto, S.; Nöjd, P.; Henttonen, H.M.; Beuker, E.; Mäkinen, H. Geographical patterns in the radial growth response of Norwayspruce provenances to climatic variation. Agric. For. Meteorol. 2016, 222, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Kolár, T.; Cermák, P.; Trnka, M.; Zid, T.; Rybníček, M. Temporal changes in the climate sensitivity of Norway spruce and European beech along an elevation gradient in central Europe. Agric. For. Meteorol. 2017, 239, 24–33. [Google Scholar] [CrossRef]
- Augustaitis, A.; Augustaitienė, I.; Baumgarten, M.; Bičenkienė, S.; Girgždienė, R.; Kulbokas, G.; Linkevičius, E.; Marozas, V.; Mikalajūnas, M.; Mordas, G.; et al. Tree-ring formation as an indicator of forest capacity to adapt to the main threats of environmental changes in Lithuania. Sci. Total Environ. 2018, 615, 1247–1261. [Google Scholar] [CrossRef]
- Seidling, W.; Ziche, D.; Beck, W. Climate responses and interrelations of stem increment and crown transparency in Norway spruce, Scots pine, and common beech. For. Ecol. Manag. 2012, 284, 196–204. [Google Scholar] [CrossRef]
- Levanič, T.; Eggertsson, O. Climatic effects on birch (Betula pubescens Ehrh.) growth in Fnjoskadalur valley, northern Iceland. Dendrochronologia 2008, 25, 135–143. [Google Scholar] [CrossRef]
- Ols, C.; Kålås, I.H.; Drobyshev, I.; Söderström, L.; Hofgaard, A. Spatiotemporal variation in the relationship between boreal forest productivity proxies and climate data. Dendrochronologia 2019, 58, 125648. [Google Scholar] [CrossRef]
- Deslauriers, A.; Rossi, S.; Anfodillo, T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia 2007, 25, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Fonti, P.; Rossi, S.; Rathgeber, C.B.K.; Griča, L. Ecophysiology and Plasticity of Wood and Phloem Formation. Dendroecology 2017, 231, 13–33. [Google Scholar] [CrossRef]
- Zweifel, R.; Haeni, M.; Buchmann, N.; Eugster, W. Are trees able to grow in periods of stem shrinkage? New Phytol. 2016, 211, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Offenthaler, I.; Hietz, P.; Richter, H. Wood diameter indicates diurnal and long-term patterns of xylem water potential in Norway spruce. Trees 2001, 15, 215–221. [Google Scholar] [CrossRef]
- Cocozzaa, C.; Giovannellib, A.; Lasserrea, B.; Cantinib, C.; Lombardia, F.; Tognettia, R. A novel mathematical procedure to interpret the stem radius variation in olive trees. Agric. For. Meteorol. 2012, 161, 80–93. [Google Scholar] [CrossRef]
- Knüsel, S.; Peters, R.L.; Haeni, M.; Wilhelm, M.; Zweifel, R. Processing and Extraction of Seasonal Tree Physiological Parameters from Stem Radius Time Series. Forests 2021, 12, 765. [Google Scholar] [CrossRef]
- Tardif, J.; Flannigan, M.; Bergeron, Y. An analysis of the daily activity of 7 boreal tree species, Nothwestern Quebec. Environ. Monit. Assess. 2001, 7, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.T.; Winget, C.H. Diurnal and seasonal variation in radii of tree stems. Ecology 1964, 45, 149–155. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, R.; Item, H.; Häsler, R. Link between diurnal stem radius changes and tree water relations. Tree Physiol. 2001, 21, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweifel, R.; Häsler, R. Dynamics of water storage in mature, subalpine Picea abies: Temporal and spatial patterns of change in stem radius. Tree Physiol. 2001, 21, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 2005, 19, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Maaten, E.; Bouriaud, O.; van der Maaten-Theunissen, M.; Mayer, H.; Spiecker, H. Meteorological forcing of day-to-day stem radius variationsof beech is highly synchronic on opposing aspects of a valley. Agric. For. Meteorol. 2013, 181, 85–93. [Google Scholar] [CrossRef]
- Huang, J.-G.; Ma, Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.; Mäkinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Augustaitis, A.; Belyazid, S.; Calfapietra, C.; De Marco, A.; Fenn, M.E.; Bytnerowicz, A.; Grulke, N.E.; He, S.; Matyssek, R.; et al. Global topics and novel approaches in the study of air pollution, climate change and forest ecosystems. Environ. Pollut. 2016, 213, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Augustaitis, A.; Augustaitienė, I.; Mozgeris, G.; Juknys, R.; Vitas, A.; Jasinevičienė, D. Growth patterns of Scots pine (Pinus sylvestris L.) under the current regional pollution load in Lithuania. iForest 2015, 8, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Timonen, U.; Huttunen, S.; Manninen, S. Ozone sensitivity of wild field layer plant species of northern Europe. A review. Plant Ecol. 2004, 172, 27–39. [Google Scholar] [CrossRef]
- Matyssek, R.; Bytnerowicz, A.; Karlsson, P.-E.; Paoletti, E.; Sanz, M.; Schaub, M.; Wieser, G. Promoting the O3 flux concept for European forest trees. Environ. Pollut. 2007, 146, 587–607. [Google Scholar] [CrossRef]
- Matyssek, R.; Wieser, G.; Calfapietra, C.; de Vries, W.; Dizengremel, P.; Ernst, D.; Jolivet, Y.; Mikkelsen, T.N.; Mohren, G.M.J.; Le Thiec, D.; et al. Forests under climate change and air pollution: Gaps in understanding and future directions for research. Environ. Pollut. 2012, 160, 57–65. [Google Scholar] [CrossRef]
- Witting, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Chang. Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Cho, K.; Tiwari, S.; Agrawal, S.B.; Torres, N.L.; Agrawal, M.; Sarkar, A.; Shibato, J.; Agrawal, G.K.; Kubo, A.; Rakwal, R. Tropospheric ozone and plants: Absorption, responses, and consequences. Rev. Environ. Contam. Toxicol. 2011, 212, 61–111. [Google Scholar]
- Sarkar, A.; Agrawal, S. Elevated ozone and two modern wheat cultivars: An assessment of dose dependent sensitivity with respect to growth, reproductive and yield parameters. Environ. Exp. Bot. 2010, 69, 328–337. [Google Scholar] [CrossRef]
- Paoletti, E.; Schaub, M.; Matyssek, R.; Wieser, G.; Augustaitis, A.; Bastrup-Birk, A.M.; Bytnerowicz, A.; Günthardt-Goergb, M.S.; Müller-Starck, G.; Serengil, Y. Advances of air pollution science: From forest decline to multiple-stress effects on forest ecosystem services. Environ. Pollut. 2010, 158, 1986–1989. [Google Scholar] [CrossRef]
- Serengil, Y.; Augustaitis, A.; Bytnerowicz, A.; Grulke, N.; Kozovitz, A.R.; Matyssek, R.; Müller-Starck, G.; Schaub, M.; Wieser, G.; Coskun, A.A.; et al. Adaptation of Forest Ecosystems to Air Pollution and Climate Change. iForest 2011, 4, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Baumgarten, M.; Hesse, B.D.; Augustaitienė, I.; Marozas, V.; Mozgeris, G.; Byčenkienė, S.; Mordas, G.; Pivoras, A.; Pivoras, G.; Juonytė, D.; et al. Responses of species-specific sap flux, transpiration and water use efficiency of pine, spruce and birch trees to temporarily moderate dry periods in mixed forests at a dry and wet forest site in the hemi-boreal zone. J. Agric. Meteorol. 2019, 75, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Rossi, S.; Turcotte, A.; Morin, H.; Krause, C. A three-step procedure in SAS to analyze the time series from automatic dendrometers. Dendrochronologia 2011, 29, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Augustaitis, A.; Augustaitienė, I.; Kliučius, A.; Pivoras, G.; Šopauskienė, D.; Girgždienė, R. The seasonal variability of air pollution effects on pine conditions under changing climates. Eur. J. For. Res. 2010, 129, 431–441. [Google Scholar] [CrossRef]
- Augustaitis, A.; Augustaitiene, I.; Kliucius, A.; Bartkevicius, E.; Mozgeris, G.; Sopauskiene, D.; Eitminaviciute, I.; Arbaciauskas, K.; Mazeikyte, R.; Bauziene, I. Impact of acidity components in the air and their deposition on biota in forest ecosystems. Balt. For. 2005, 2, 84–93. [Google Scholar]
- Augustaitis, A.; Šopauskienė, D.; Baužienė, I. Direct and Indirect Effects of Regional Air Pollution on Tree Crown Defoliation. Balt. For. 2010, 16, 23–34. [Google Scholar]
- Burges, S.S.O.; Adams, M.A.; Turner, N.C.; Beverly, C.R.; Ong, C.K.; Khan, A.A.H.; Bleby, T.M. An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol. 2001, 21, 589–598. [Google Scholar] [CrossRef]
- Köstner, B.M.M.; Schulze, E.D.; Kelliher, F.M.; Hollinger, D.Y.; Byers, J.N.; Hunt, J.E.; McSeveny, T.M.; Meserth, R.; Weir, P.L. Transpiration and canopy conductance in a pristine broad- leaved forest of Nothofagus: An analysis of xylem sap flow and eddy correlation measurements. Oecologia 1992, 91, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G.; Häsler, R.; Götz, B.; Koch, W.; Havranek, W.M. Role of climate, crown position, tree age and altitude in calculated ozone flux into needles of Picea abies and Pinus cembra: A synthesis. Environ. Pollut. 2000, 109, 415–422. [Google Scholar] [CrossRef]
- Perämäki, M.; Nikinmaa, E.; Sevanto, S.; Ilvesniemi, H.; Siivola, E.; Hari, P.; Vesala, T. Tree stem diameter variations and transpiration in Scots pine: An analysis using a dynamic sap flow model. Tree Physiol. 2001, 21, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Sevanto, S.; Vesala, T.; Peramaki, M.; Nikinmaa, E. Time lags for xylem and stem diameter variations in a Scots pine tree. Plant Cell Environ. 2002, 25, 1071–1077. [Google Scholar] [CrossRef]
- Nöjd, P.; Henttonen, H.M.; Mäkinen, H. Increment cores from the Finnish National Forest Inventory as a source of information for studying intra-annual wood formation. Dendrochronologia 2008, 26, 133–140. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis, 3rd ed.; Wiley: New York, NY, USA, 1998; p. 736. [Google Scholar]
- Vieira, J.; Rossi, S.; Campelo Freitas, H.; Nabais, C. Seasonal and daily cycles of stem radial variation of Pinus pinaster in a drought-prone environment. Agric. For. Meteorol. 2013, 180, 173–181. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, S.B.; Wullschleger, S.D.; Nosal, M. Diurnal and seasonal changes in stem increment and water use by yellow poplar trees in rponse to environmental stress. Tree Physiol. 2003, 23, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Cermák, J.; Kučera, J.; Bauerle, W.L.; Phillips, N.; Hinckley, T.M. Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol. 2007, 27, 181–198. [Google Scholar] [CrossRef]
- Ježík, M.; Blaženec, M.; Kučera, J.; Střelcová, K.; Ditmarová, L. The response of intra-annual stem circumference increase of young European beech provenances to 2012-2014 weather variability. iForest 2015, 9, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Drew, D.M.; O’Grady, A.P.; Downes, G.; Read, J.; Worledge, D. Daily patterns of stem size variation in irrigated and unirrigated Eucalyptus globulus. Tree Physiol. 2008, 28, 1573–1581. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.H. Possible impacts of changes in UV-B radiation on North American trees and forests. Environ. Pollut. 2005, 137, 380–389. [Google Scholar] [CrossRef]
- Herzog, K.M.; Häsler, R.; Thum, R. Diurnal changes in the radius of a subalpine Norway spruce stem: Their relation to the sap flow and their use to estimate transpiration. Trees 1995, 10, 94–101. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D.; D’Orangeville, L. Influence of climate on seasonal patterns of stem increment of balsam fir in a boreal forest of Québec, Canada. Agric. For. Meteorol. 2012, 162–163, 108–114. [Google Scholar] [CrossRef]
- Maaten, E.; Maaten-Theunissen, M.; Smiljanic, M.; Rossi, S.; Simard, S.; Wilmking, M.; Deslauriers, A.; Fonti, P.; Arx, G.; Bouriaud, O. Dendrometer: Analyzing the pulse of trees in R. Dendrochronologia 2016, 40, 12–16. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Plastic responses of Abies pinsapo xylogenesis to drought and competition. Tree Physiol. 2009, 29, 1525–1536. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.; Gou, X.; Zhang, T.; Zou, C.; Ji, C.; Fan, Z.; Qin, L.; Shang, H.; Li, X. Intra-annual radial growth of Schrenk spruce (Picea schrenkiana Fisch. et Mey) and its response to climate on the northern slopes of the Tianshan Mountains. Dendrochronologia 2016, 40, 36–42. [Google Scholar] [CrossRef]
- Lévesque, M.; Rigling, A.; Bugmann, H.; Weber, P.; Brang, P. Growth responseof five co-occurring conifers to drought across a wide climatic gradient inCentral Europe. Agric. For. Meteorol. 2014, 197, 1–12. [Google Scholar] [CrossRef]
- Zweifel, R.; Rigling, A.; Dobbertin, M. Species-specific stomatal response oftrees to drought—A link to vegetation dynamics? J. Veg. Sci. 2009, 20, 442–454. [Google Scholar] [CrossRef]
- Spiecker, H. Growth variation and environmental stresses: Long-term observations on permanent research plots in southwestern Germany. Water Air Soil Poll. 1991, 54, 247–256. [Google Scholar] [CrossRef]
- Brough, D.W.; Jones, H.G.; Grace, J. Diurnal changes in water content of the stems of apple trees, as influenced by irrigation. Plant Cell Environ. 1986, 9, 1–7. [Google Scholar]
- Neher, H.V. Effects of pressures inside Monterey pine trees. Trees 1993, 8, 9–17. [Google Scholar] [CrossRef]
- Saxe, H.; Cannell, M.G.R.; Johnsen, Ø.; Ryan, M.G.; Vourlitis, G. Tree and forest functioning in response to global warming. New Phytol. 2001, 149, 369–399. [Google Scholar] [CrossRef]
- Seo, J.W.; Eckstein, D.; Jalkanen, R.; Schmitt, U. Climatic control of intra- and inter-annual wood-formation dynamics of Scots pine in northern Finland. Environ. Exp. Bot. 2011, 72, 422–431. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).