Abstract
The emergence of new super-insulated buildings to reduce energy consumption can lead to a degradation of the indoor air quality. While some studies were carried out to assess the air quality in these super-insulated buildings, they were usually focused on the measurement of gas phase pollutants such as carbon dioxide and volatile organic compounds. This work reports the first measurements of Polycyclic Aromatic Hydrocarbons (PAHs) associated with particles as a function of time and particle size in a low-energy building. The airborne particles were collected indoors and outdoors over three to four days of sampling using two three-stage cascade impactors allowing to sample simultaneously particles with aerodynamic diameter Dae > 10 µm, 2.5 µm < Dae < 10 µm, 1 µm < Dae < 2.5 µm, and Dae < 1 µm. The 16 US-EPA priority PAHs were then extracted and quantified by high-performance liquid chromatography (HPLC) coupled to fluorescence detection. The resulting total particle concentrations were low, in the ranges 3.73 to 9.66 and 0.60 to 8.83 µg m-3 for indoors and outdoors, respectively. Thirteen PAHs were always detected in all the samples. The total PAH concentrations varied between 290 and 415 pg m−3 depending on the particle size, the environment (indoors or outdoors) and the sampling period considered. More interestingly, the temporal variations of individual PAHs highlighted that high molecular weight PAHs were mainly associated to the finest particles and some of them exhibited similar temporal behaviors, suggesting a common emission source. The indoor-to-outdoor concentration ratios of individual PAH were usually found close to or less than 1, except during the event combining rainy conditions and limited indoor ventilation rate.
1. Introduction
Air quality has become, in two decades, a subject of interest that concerns all audiences. Nevertheless, indoor air quality (IAQ) remains a complex subject due to the presence of many pollutants with various chemical properties and health impacts but also because of the physicochemical processes including the chemical reactions that govern the concentrations of pollutants in buildings [1,2].
In addition, for several decades, new energy saving policies have been established resulting in better insulation of buildings which limits air exchange between indoors and outdoors. Moreover, the thermal regulations of 2005 and 2012 [3] impose new constraints on the construction of buildings which must consume less and less energy involving the installation of efficient insulation and ventilation, heat recovery, etc. These new practices can have a significant impact on the concentrations of pollutants in indoor air and therefore on the health of occupants. Thus, it makes sense to carry out an IAQ assessment in the first low-energy buildings built. Knowing the impact of these new constructions on IAQ would allow them to validate their designs or correct bad practices in terms of low-energy building construction or to avoid the widespread dissemination of air management problems.
In the case of schools installed in an energy efficient building designed with reinforced sealing, it is therefore essential to use an efficient controlled ventilation system to ensure a healthy atmosphere inside. Indeed, several recent studies have shown that pollutants’ concentrations in schools were high due to the use of insufficient or even unsuitable ventilation systems, resulting in a reduction in the work performance of students [4,5,6,7]. In fact, Mumovic et al. were interested in the IAQ of nine newly built schools in England and in particular in the comparison of their ventilation systems. They showed that classrooms with a mechanical system were much better ventilated than those with a natural ventilation system, which showed large variations in CO2 concentration [7].
While many studies have been focused on the study of VOCs emitted by construction materials, furniture [8] or the influence of ventilation through the air exchange rate [9,10] in European schools, only a few studies have recently targeted PAHs associated with particulate matter in European preschool and school buildings [11,12,13,14,15,16] (see Table 1). Among these studies, Wei et al. reported a very extensive study in which they measured, in 308 nurseries and elementary schools, semi-volatile organic compounds such as PAHs, pesticides, phthalates or polybrominated diphenyl ethers (PBDEs) in both the air and dust [14]. Oliveira et al. reported levels of PM1 and PM2.5 bound PAHs in indoor and outdoor air of two preschool environments from April to June 2013 in the North of Portugal [15]. The same authors assessed also PM2.5–bound PAHs in 10 Portuguese primary schools [13]. In Sweden, Lim et al. reported PAH concentrations in indoor and outdoor air particulate matter (PM10) and indoor dust at five preschools in Stockholm [16]. Other recent studies performed around the world have reported measurements of PAHs in schools for example in Beijing [17] or in Urban vs. Industrial ‘oil and gas’ zones in Kuwait [18]. Recently, Oliveira et al. published a review on children’s environmental exposure to particulate matter and polycyclic aromatic hydrocarbons, where they described their major sources and health impacts [19].

Table 1.
List of previous studies dedicated to PAHs measurements in European schools.
In France, the major emissions of total PAHs, i.e., 63.3 % in 2012, are related to the tertiary and residential sector [20]. These emissions, which are mainly due to atmospheric emissions due to domestic heating by combustion of biomass (wood, coal), tend to decrease. Road transport contributes to 25.8% of PAH emissions, which is mainly caused by diesel emissions releasing mainly phenanthrene (PHE), pyrene (PYR) and fluoranthene (FLN) [21]. Besides, it was demonstrated that FLN is the major emitted PAH with 57.8% of total PAH emissions from the tertiary and transport sectors [20]. Emissions from the industrial sector only represent 5.5% of total PAH emissions, which is partly explained by the numerous regulations limiting emissions for aluminum production, the petrochemical industry, the incineration of household and industrial waste, cement factories, bitumen, and tar industries, etc. [22].
In indoor air, similarly to VOCs, a large part of PAHs can originate outdoors [13,23]. Specific indoor sources of PAHs have also been reported such as different means of domestic heating, in particular open fireplaces, cooking [24], and other practices such as smoking [23,25,26,27], burning of sticks’ incense or candles [28,29]. For example, Pey et al. [25] showed that the ban on smoking in public places resulted in a decrease in the concentration of benzo[a]pyrene (B[a]P) by 90%, the concentration having dropped from 1 to 0.1 ng m−3 after its implementation in a cafeteria, while the chrysene (CHR) concentration decreased by 83%. Similarly, in 2011, Orecchio [28] demonstrated that the combustion of decorative candles caused emissions of PAHs responsible for b[a]P concentrations varying between 0.1 and 7.5 ng m−3. In their study, the concentrations of total PAHs varied from 7 ng m−3 for the insecticidal candle (citronella) to 267 ng m−3 for a scented candle.
The main objective of this work was to monitor the temporal variations of indoor levels of the 16 US-EPA particle-bound PAHs in a low-energy building for the first time over two weeks and compare them with those measured simultaneously outdoors. In this context, the particles were collected for three to four days using two three-stage cascade impactors enabled to sample PM1, PM2.5, and PM10. PAHs were then quantified according an off-line analytical method developed in a previous study [30] and already used for field measurements [31].
Contrary to the studies reported in the literature, which report single PAH measurement performed in several buildings and only on a single particle fraction, i.e., PM2.5 or PM10, this study focused on a single building to monitor its concentrations according to the time and simultaneously on several fractions of particles by means of cascade impactors. The present study reports the indoor and outdoor particle concentrations, the indoor and outdoor average PAH concentrations on particles over the field campaign duration, the indoor and outdoor temporal variation of total and individual PAH concentrations according to the particle size fractions, i.e., PM1, PM2.5, and PM10. This novel experimental approach is discussed in terms of advantages and drawbacks. Our results are also discussed to highlight the effect of ventilation on indoor air PAH levels in a low energy building and are compared with those found in the literature.
In addition, the risk assessment due to PAHs’ exposure was investigated by using the B[a]P equivalent. It should be noted that this same school has been widely studied within the framework of the MERMAID (Mesures Expérimentales Représentatives et Modélisation Air Intérieur Détaillée) project aiming at performing some representative experimental measurements coupled to detailed indoor air modelling, notably with continuous measurements of formaldehyde [10] and Benzene, Toluene, Ethyl benzene and Xylenes (BTEX) [9].
2. Materials and Methods
In this section, we described the indoor and outdoor particles sampling methods, the chemical analysis performed to determine the particle-bound PAH concentrations. Some information related to the investigated school, especially to its ventilation system and air sampling conditions, is also given.
2.1. Building Location and Description
After a preliminary field campaign performed in 10 low consumption energy school buildings [32], the present building was selected for the intensive field campaign of the MERMAID project because it was considered representative, i.e., among those where the indoor air pollution was average. In addition, its access was relatively easy for the installation of the equipment with an adjoining terrace to the classroom and an adjoining classroom for the installation of the numerous real-time analysers.
The intensive field campaign was performed from 14 April to 6 May 2014, during the spring school holidays to avoid disturbing the teachings, in a junior high school of Maubeuge, a city of about 30,000 inhabitants located in North of France, as shown in Figure 1. The investigated school was in the peri-urban area of the city. The building was recently constructed according to the French energy regulation of 2005 (RT 2005) using low emission building materials and was equipped with a dual-flow controlled mechanical ventilation system with heat recovery, to limit both energy consumption and indoor air pollution.
Figure 1.
Picture and location of the junior high school investigated in this work. The city of Maubeuge (North of France, 50°16′39″ North, 3°58′24″ East) has about 31,000 inhabitants.
The investigated classroom was located on the southern first floor and had three doors and three double windows. The investigated classroom is shown in Figure 2 together with the analytical instruments. The walls were painted, and vinyl covered the floor. Its volume was approximately equal to 140 m3 (6.6 m width × 7.9 m length × 2.7 m height).

Figure 2.
Pictures of the cascade impactors installed either inside the investigated classrooms (red) or outdoors (blue) during the field campaign.
The ventilation system was programmed to be only activated at the normal time of occupants’ presence to minimize the energy consumption. According to the European standard NF EN 779: 2012 relating to “general ventilation air filters for the removal of particles and the determination of filtration performance”, outdoor air is coarsely filtered with a G4 filter and mixed with a part of recycled indoor air. Then, the mixture is further filtered using a F7 fine filter to remove most of the submicron particles, heated, and then propelled into the classrooms. The resulting indoor air renewal rate was found to be 0.2 and 2.1 h−1 when ventilation was off and on, respectively [33].
2.2. Sampling Methodology
Indoor and outdoor pollutants’ levels, temperature, and relative humidity were monitored by numerous instruments with typical high time resolution of 1 min during this field campaign [9,10,34]. Most of the analytical instruments were placed in the adjacent room of the studied classroom.
PAH samplings were performed with two commercial manual 3-stages size fractionating cascade impactors provided by DEKATI@ (Impactor PM-10/PM-2.5/PM-1, Kangasala, Finland) placed either inside the investigated classroom or outdoors. Collected fractions corresponded to particles with aerodynamic diameter (Dae) in the ranges Dae > 10 µm, 10 µm > Dae > 2.5 µm, 2.5 µm > Dae > 1 µm and a backup filter which allowed the collection of particles with Dae < 1 µm. DEKATI PM-10 impactor operation is based on inertial classification and gravimetric analysis of the aerosol particles. This device classifies particles according to their aerodynamic diameter for 50% of efficiency (D50%) for each stage, explaining why bigger particles can be present in theoretical smaller fractions. D50% is given by the manufacturer with 2.8% of accuracy for each impaction stage.
Samples were collected onto glass microfiber filter (GFF) (Whatmann, Ø 47 mm) and sizes were adapted to the calibrated collection plates, i.e., Ø 25 mm for the three first plates and 47 mm for the backup filter. The chemical analyses were performed on particles impacted on filters for three to four days. The sampling flow rates being 0.5 and 1.8 m3 h−1 (i.e., 8.3 and 30 L min−1) in indoor and outdoor air, respectively, the corresponding total sampling air volumes varied in the ranges 31.7 to 48.8 m3 and 119.0 to 181.3 m3 for indoors and outdoors, respectively. Sampling details are presented in Table S1. After sampling, filters were stored in Petri dishes covered with aluminum foil and kept in a refrigerator (4 °C) until they were further analyzed (maximum seven days after sampling).
Before PAH analysis, Particulate matter (PM) masses were measured gravimetrically by subtracting the initial mean mass of the blank filter from the final mean mass of the same filter after sampling. The difference was then divided by the total air volume that passed through the filter to obtain mass concentration. Each filter was weighed 3 times and the mean result was then used for further calculations. The microbalance (XSR105, Mettler Toledo, France) integrated automatic internal adjustment and the resulting accuracy was 10 µg.
2.3. Chemical Analysis
The 16 US-EPA priority polycyclic aromatic hydrocarbons (PAHs) and their abbreviation are listed in Supplementary Materials (see Table S2). The extraction, analysis and limits of detection (LOD) and quantification (LOQ) of individual PAHs are also given in Table S2, these values being derived from our previous work [30]. Briefly, the particulate matter impacted on GFF was extracted with approximately 70 mL of acetonitrile (ACN) by means of an accelerated solvent extractor. The extracts were then carefully filtered through a PVDF filter (0.45 µm) and reduced to 1 mL using a rotary evaporator (Büchi@, Villebon-sur-Yvette, France) at 45 °C and 220 mbar. A gentle stream of nitrogen was finally used to concentrate the extracts in the range 100–150 µL where the exact volume was determined by weighing. Extracts were analyzed using a high-performance liquid chromatography equipped with a C18 Pinnacle II PAH (Restek@, Lisses, France) 150 mm × 3.0 mm ID × 4 µm (particle size), a diode array detector and a fluorescence detector (Thermo Fisher Scientific@, Illkirch-Graffenstaden, France). Each compound (or group of PAHs compounds) was detected at its optimum emission/excitation wavelength: 270/330 nm (NAP, ACE, FLU), 250/370 nm (PHE), 250/400 nm (ANT), 270/440 nm (FLN), 270/400 nm (PYR), 270/390 nm (B[a]A, CHR), 290/430 nm (B[b]F, B[k]F, B[a]P, DB[a,h]A, B[g,h,i]P) and 305/500 nm (IND). ACY, which is not fluorescent, was quantified with the DAD at its optimum absorbance wavelength λ = 229 nm. LOQ were in the range 0.05–0.47 µg L−1 corresponding to 0.37–3.02 pg m−3 for 40 m3 of pumped air, this value being representative of the indoor air sample volume (see Table S2).
For these measurements, three field blanks were considered for each filter size, i.e., 25 mm for the intermediate plates and 47 mm for the last plate of the impactor. These filters were weighed and stored in the same way as the filters used for the samples. They were transported to the location of the field campaign where they stayed for two weeks. They were then weighed, extracted, and analyzed under the same conditions used for other samples. When PAHs were detected on the blanks, the average quantities (n = 3) were then subtracted to the PAHs masses obtained from field measurements.
3. Results and Discussion
During the investigated period, the average temperatures were 24.0 ± 0.6 °C and 13.6 ± 1.4 °C for indoors and outdoors, respectively. The relative humidity in indoor and outdoor air were 35.6 ± 5.0% and 63.5 ± 9.6% respectively, the quoted errors corresponding to the standard deviation.
The PAH concentrations were simultaneously measured in indoor and outdoor air using two cascade impactors, from April 14th and May 6th, 2014. The filters were changed every three to four days depending on the accessibility to the sampling systems leading to six sampling periods on the total duration of the field campaign.
Using four glass microfiber filters (GFF) per impactor and per sampling period in addition to the six blank measurements, 54 filters were thus extracted and analyzed according to the method described above [30] for both indoor and outdoor air.
The results are presented and discussed in the different sub-sections below.
3.1. Indoor and Outdoor Particle Concentrations
3.1.1. Results
The particulate matter mass concentrations (µg m−3) collected with the three-stages cascade impactors as a function of time are displayed in Figure 3 where both indoor and outdoor values are shown related to the collection plate and therefore the particulate size (Dae). Indoor and outdoor PM concentrations are very low with values varying in the ranges 3.73 to 9.66 and 0.60 to 8.83 µg m−3, respectively. As shown in Figure 3, the finest particles, i.e., Dae < 1 mm (PM1), represent the most important part of the sampled particles in indoor and outdoor air. If the masses collected on the ultimate filter were always quantifiable with regard to the uncertainty of the microbalance used, i.e., 10 µg, it was often impossible to determine with accuracy the quantities of particles impacted on the upper plates (Dae > 10 µm, 10 µm > Dae > 2.5 µm and 2.5 µm > Dae > 1 µm).
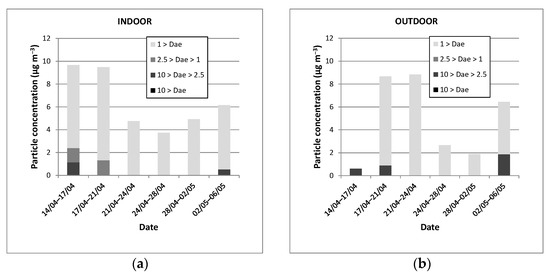
Figure 3.
Particulate matter mass concentrations (µg m−3) measured in 2014 vs. time obtained either indoors (a) or outdoors (b) related to the collection plate and therefore the particulate size (Dae).
3.1.2. Comparison with Literature
If chemical speciation of PAHs is not often studied in schools, measurements of particle concentrations, often PM10 or PM2.5 are very widespread [11,12,35,36,37,38,39,40,41]. Particulate matter is one of the major air pollutants due to their notorious health effects. Thus, these studies tend to highlight potential sources and show the important contribution of outdoor air to indoor air pollution, especially when the schools are in urban areas. In terms of particle concentration levels, they are very variable depending on the location of the schools with, for example, these PM2.5 values (in µg m−3): 11–27 in winter and 12–27 in spring in six Italian schools located near high traffic areas [12], 13–84 in Barcelona in Spain [38], 4.3–73.1 (median: 37.0) in winter and 9.8–55.1 (median: 22.1) in summer in 64 schools located in North of Munich in Germany [42]. In 10 Portuguese schools’ environments, Oliveira et al. reported mean concentrations of PM2.5 in the ranges 9.2–66 μg m−3 and 25–67 μg m−3 for indoors and outdoors, respectively [13]. Inside two preschools located also in Portugal, the same authors found mean PM2.5 values of 14.2 and 18.4 μg m−3. Outdoors, the corresponding mean PM2.5 concentrations were slightly higher, i.e., 15.2 and 19.3 μg m−3. The simultaneous determination of PM1 and PM2.5 in these two preschools highlighted that majority of PM2.5 fraction was composed by PM1, these particles accounting in indoor air for 86% and 87% of PM2.5, respectively [15]. Outdoors, PM1 contributed to only 62 to 80% of PM2.5 [15].
The indoor and outdoor concentrations of PM2.5 observed in our work correspond to the minimum values found in the previous studies [13,15] showing that both indoor and ambient air were not very polluted since no specific source of PMs was identified in this classroom in the absence of occupants. In addition, the school holidays presumably affected the potential effect of indoor sources, e.g., combustion sources from cooking in cafeteria, staff activities such as smoking, etc. It can be assumed that the usual near-site and on-site outdoor sources were also significantly reduced in the absence of school-transport traffic near school, in the absence of vehicle traffic from drop-off of students in morning and afternoon. Likewise, the traffic in the parking lot reserved for school staff, which is immediately adjacent to the location of the outdoor sampler and the chosen classroom, was also greatly reduced during holidays.
Besides, these values agree with those of 8.7 ± 3.6 μg m−3 and 5.3 ± 2.3 μg m−3 measured inside the Portuguese schools located in a residential and suburban areas, respectively [13]. In addition, the major fraction of PM1 found in this work either indoors or outdoors is supported by the observation made by Oliveira et al. in Portugal [15].
The PM10 values reported in the present work in the range 3.6–9.6 μg m−3 in the absence of pupils are also of the same order of magnitude as those measured by Lim et al. at five preschools in the presence of schoolchildren in Stockholm (Sweden) [19]. Indeed, these authors reported average PM10 concentrations of 23.5 and 13.8 μg m−3, for indoors and outdoors, respectively. However, such levels are considerably lower than PM10 indoor and outdoor median values measured in school environments from more than 20 studies in a large number of European studies, i.e., 105 and 46.6 μg m−3, respectively [19].
In a school classroom of a low-energy building, Liaud et al. (2014) reported that the indoor collected particle masses, were very limited for big particles causing large uncertainties on PM2.5 and PM10 concentrations which were often found very close to the blank values [31]. Similar behavior is observed in our study confirming the great efficiency of the filtration system implemented in the low-energy school building studied here and more generally in low-energy school buildings where efficient programmable ventilation systems are usually installed.
3.2. Average Particle-bound PAH Concentrations Over the Field Campaign Duration
3.2.1. Results
If the temporal variation of the PAH concentration can give certain information (see Section 3.3), it is also interesting to determine the average concentrations of the different PAHs measured individually over the full campaign duration in order to compare them with the literature ones. These values are reported in Table 2 for PM1, PM2.5, and PM10.

Table 2.
Average indoor and outdoor concentrations of individual PAH over the full campaign duration. The quoted errors correspond to the standard deviation calculated from the six measurements performed on the investigated period.
Among the 16 PAHs studied, 13 PAHs were detected in all the samples, unlike naphthalene, acenaphthene, and acenaphthylene. B[a]P, classified as a group 1 carcinogen, has been quantified at very low concentration in the ranges 14–38 pg m−3 indoors and 10–49 pg m−3 outdoors. This seems to indicate that B[a]P is not emitted by indoor sources. This point will be discussed in more detail in Section 3.4.
For a given number of aromatic rings, the proportion of PAHs bound to the finest particles (PM1) was determined. This calculation showed that most of the high molecular weight PAHs were preferentially linked to the finest particles. Indeed, 80% and 87% of PAHs with five and six aromatic rings are linked to PM1 for outdoor and indoor air, respectively. Similar percentages between indoors and outdoors were again observed for four-ring PAHs but only 63% and 60% of four-ring PAHs were adsorbed on the finest particles in outdoor and indoor air, respectively. Conversely, it was surprising to note that 91% of PAHs having three aromatic rings, namely fluorene (FLU), phenanthrene (PHE) and anthracene (ANT) were linked to PM1 outdoors against only 30% indoors. This observation could be explained by supposing that the ambient temperature being lower outdoors, a condensation of these molecules on existing particles is favored.
Indeed, when such semi-volatile organic compounds (SVOCs) enter indoors in a warmer environment, they can tend to partition in favor of the gas phase [43]. In addition, previous works performed by INERIS report that diesel vehicles emit PAHs having three or four aromatic rings, namely FLN, PHE, and PYR [21]; thus the emission of these molecules in large quantities could favor their adsorption on particles of smaller diameter, also emitted by diesel vehicles. However, it is not excluded that this result is at least partially due to a quantification artefact where the larger particles would be entrained towards the lower plateaus resulting in a bias in our measurements.
3.2.2. Comparison with Literature
The total PM10-bound PAH concentration of 414.6 pg m−3 (see Table 2) found in this low-energy labelled building is very close to that of 435 pg m−3 measured in the positive energy school investigated by Liaud et al. using the same methodology for particle sampling and PAH analysis [31]. The average indoor B[a]P level of 25.2 pg m−3 measured in this work on PM10 is also in agreement with the values found in other studies [13,23,44,45,46,47] as well as with the B[a]P concentration of 19 pg m−3 found by Liaud et al. in another low-energy school building [31].
As already mentioned in the introduction, only a limited number of European studies were focused on PAH measurements in schools [11,12,13,14,15,16], most of them being very recent.
One of them was carried out in Lithuania in five primary schools during the winter of 2011/2012 [11]. The sum of the total PAHs (15 PAHs) quantified in indoor air both in the gas and particulate phases (PM2.5), varies from 20.3 to 131.1 ng m−3 depending on the school, and the emissions were found to originate outdoors because the indoor-to-outdoor concentration ratios were significantly lower than 1. Indeed, Krugly et al. point out that the concentrations of PAHs determined in outdoor air are much higher than the average in Western Europe, which could be due to the use of older vehicles [11]. In addition, high proportions of fluorene (FLU) and phenanthrene (PHE) of the order of 10 and 5 ng m−3 were detected in PM2.5 similarly to our study even if our concentration were much smaller and varied in the ranges 1.3–29.0 and 18.5–141.5 pg m−3 for FLU and PHE, respectively. According to Krugly et al., such high proportions of fluorene and phenanthrene could be explained by indoor emissions related to cooking or evaporation from materials.
The second study was carried out in winter and spring 2011/2012 in six schools located in Roma to observe seasonal variations in the concentrations of PM2.5-bound PAHs [12]. It was shown that winter PAH concentrations were higher than those determined in spring and summer. This study was carried out as part of a European project called EXPAH (Population EXposure to PAHs) to assess the exposure of children and seniors to PAHs adsorbed on particles in highly urbanized spaces. The PAHs targeted are those supposed to be carcinogenic, namely Benzo[a]anthracene (B[a]A), Benzo[b]fluoranthene (B[b]F), Benzo[k]fluoranthene (B[k]F), Benzo[j]fluoranthene (B[j]F), Benzo[a]pyrene (B[a]P), Dibenzo[a,h]anthracene (DB[a,h]A), Benzo[g,h,i]perylene (B[g,h,i]P) and Indeno[1,2,3-c,d]pyrene (INP) in individual dwellings, schools, and offices. In order to compare the values obtained in our work with those obtained during this study, Table 3 presents the values of the concentrations obtained in indoor and outdoor air for the Maubeuge’s school (this work) and for the six schools of the Italian study over the spring period [12], a period comparable to ours. Note that B[j]F was not measured in our study.

Table 3.
Comparison of PM2.5 bound PAHs concentrations (in ng m−3) measured indoors in this work and during the Italian study EXPAH [12]. The bold numbers correspond to the sum of the concentrations indoors or outdoors.
The indoor PAH concentrations determined in our work are of the same order of magnitude as the values measured in the six Italian schools [12]. Indeed, the concentration of ∑8PAHs is about 0.22 ng m−3 in our study whereas they varied from 0.30 to 1.02 ng m−3 in Italy. Regarding our study, the total outdoor concentration of ∑8PAHs is equal to that determined in indoor air while the outdoor Italian ones ranging from 0.48 to 1.21 ng m−3 were slightly higher than those measured indoors in Roma. The I/O ratios are close to or less than one for our study and the EXPAH project [12], which confirms the importance of external sources and in particular the influence of car traffic (see Table 3). On the other hand, the values of PAH concentrations in outdoor air are significantly lower in the North of France, which is easily explained by the difference in population density between Roma and Maubeuge. As mentioned above, the sampling and measurement period chosen in the present work, i.e., during school holydays, has presumably affected and minimized both indoor and outdoor PAH concentrations.
In North of Portugal, Oliveira et al. reported inside 10 schools, with mean total levels of PM2.5-bound PAHs varying usually in the range 2.8–20 ng m−3 These PM2.5-bound PAHs values appear much higher than those found in our study and in Roma (Italy) [12] but are still lower than those of 20.3 to 131.1 ng m−3 reported by Krugly et al. [11]. In majority of the investigated Portuguese schools, acenaphthylene and dibenzo[a,h]anthracene were the most indoor particulate–bound PAH [13] with contributions in the ranges 29–68% and 5–25% of Σ18PAHs for acenaphthylene and dibenzo[a,h]anthracene, respectively. Such observations disagree with our results since acenaphthylene was never detected in our study according to its relative high volatility leading mainly to its presence in the gas phase. In our study, the relative contribution of dibenzo[a,h]anthracene to Σ16PAHs was found to be only 4.2 and 5.9% for indoors and outdoors, respectively. In our study, the major PM2.5-bound PAHs were benzo[g,h,i]perylene, phenanthrene and indeno[1,2,3-c,d]pyrene with contribution of 18.0, 17.2 and 14.9% for indoor air, these three PAHs contributing to the half amount of PAHs indoors. Outdoors, benzo[g,h,i]perylene, benzo[b]fluoranthene, fluoranthene and indeno[1,2,3-c,d]pyrene represent 15.8, 15.0, 12.1, and 11.6% of Σ16PAHs.
The same research group measured indoor and outdoor gaseous and particulate (PM1 and PM2.5) PAHs concentrations over two months in two Portuguese preschools PS1 and PS2 [15]. The total airborne concentrations of 18 PAHs, i.e., including both particulate and gaseous phases, were in the ranges 25.1–149 ng m−3 and 51.4–84.1 ng m−3 at indoor air of PS1 and PS2, respectively. When considering only PM2.5-bound PAHs, the total concentration of Σ18PAHs varied from 0.74 to 4.27 ng m−3 at PS1 and from 2.70 to 10.5 ng m−3 at PS2. These particle-bound PAH values are again much higher than that of 0.37 ng m−3 determined in our work (PM2.5, Σ16PAHs). These authors reported that lighter PAHs came mainly from indoor sources whereas PAHs with four to six rings originated mostly from outdoor emissions penetration (motor vehicle, fuel burning) [15]. Particulate PAHs were predominantly associated with PM1 (54% and 74% of particulate PAHs at PS1 and PS2, respectively), five-ring PAHs being the most abundant compounds. They reported that dibenzo[a,h]anthracene emitted by motor light-duty gasoline vehicles [48] was the most abundant indoor particulate-bound PAH at both preschools and accounted (in both PM fractions) for 37 to 40% and 21% of particulate PAHs at PS1 and PS2, respectively. Indoors, the three main PAHs in gas phase were naphthalene, phenanthrene and fluorene, these three PAHs accounting in total for 87 to 94% of indoor gaseous PAHs at PS1 and PS2, respectively [15].
Regarding the extensive French study performed by Wei et al., they measured simultaneously 52 SVOCs including PAHs in air and the settled dust. Four PAHs, namely acenaphthene, fluoranthene, fluorene, and phenanthrene, were detected in the air of more than 95% of the classrooms [14]. Forty-six SVOCs including 11 PAHs, were measured in the dust samples. The median total particulate-bound PAHs calculated by Wei et al. was then 63 pg m−3, which is quite consistent with our value of 414.6 pg m−3 if we consider that Wei et al. took into account only seven light PAHs and did not consider the heaviest ones while their contributions to PM are expected to be higher. Besides, the median PAHs concentrations in the settled dust of the 564 classrooms were the following (in ng/g): Anthracene, 198; Benzo[a]pyrene, 78.1; Fluoranthene, 311; Fluorene, 300; Phenanthrene, 782; Pyrene, 320; Benzo[a]anthracene, 115; Benzo[b]fluoranthene, 189; Benzo[k]fluoranthene, 38; Dibenzo[a,h]anthracene, 17; Indeno[1,2,3-c,d]pyrene, 70. The resulting median total PAH concentrations in dust was 2418 ng/g [14].
In Sweden, Lim et al. analysed 46 PAHs and alkylated PAHs, in indoor and outdoor air particulate matter (PM10) and indoor dust at preschools in Stockholm [16]. The total PM10-bound PAH concentrations were in the ranges 48–970 and 151–1730 pg m−3 for indoors and outdoors, respectively. The corresponding indoor and outdoor mean levels were 239 and 381 pg m−3 which is close to those obtained in our study. The quantity of PAHs in dust ranged between 748 and 5440 pg/g. Among PAHs, Fluoranthene, pyrene, triphenylene + chrysene, benzo[b]fluoranthene, benzo[e]pyrene, B[a]P, indeno[1,2,3-c,d]pyrene and benzo[g,h,i]perylene were the most abundant ones, representing about 60% of ΣPAH, in both indoor and outdoor PM10.
3.3. Temporal Variation of PAHs on Particles
To our knowledge, only one previous study in schools or preschools was carried out over a long period to monitor particle-bound PAHs as a function of particle size and time. In Portugal, Oliveira et al. (2016) measured gaseous and particulate (PM1 and PM2.5) PAHs indoors and outdoors over about two months [15]. However, their publication mentions only mean, minimum, and maximum values of PAHs and do not show the temporal evolution of the distribution of PAHs as a function of particle size so that no comparison can be done. The next sections present the temporal variation of either total PAH concentrations or individual ones as a function of the particle size obtained in this work.
3.3.1. Temporal Variation of Total PAH Concentrations According to the Particle Size
In a first approach, the total PAH concentrations were obtained by adding the PAH quantities determined on all the four sampling plates. Whether in indoor or outdoor air, PAHs associated with airborne particles were quantified in all samples. However, the total PAH concentrations remain very low, i.e., of the order of tens or hundreds pg m−3 depending on the considered particle fraction.
In indoor air, the total PAH concentrations vary from 215 to 720 pg m−3 for the periods from April 28th to May 2nd and from April 14th to 17th, respectively. In outdoor air, the total concentrations of PAHs are, surprisingly, also very low and vary from 174 to 600 pg m−3 for the periods of 24 to 28 April and 21 to 24 April, respectively.
In a second approach, the temporal evolution of the total PAH concentration was plotted according to the particle sizes, i.e., for aerodynamic diameters Dae >10 µm, 2.5 µm < Dae < 10 µm, 1 µm < Dae < 2.5 µm and Dae < 1µm (PM1). The results are presented in Figure 4 and clearly show that PAHs are mainly associated with PM1 (Dae < 1µm) which agrees with the results reported by Liaud et al. [31] and Oliveira et al. [15]. It is remarkable to observe that the outdoor PAH concentrations are higher during the first three weeks of the campaign compared to the second period of three weeks (from 24 April). In addition, the indoor and outdoor air concentrations do not show significant differences, suggesting that the sources of PAHs are exclusively external. This result is therefore in line with our expectations since no specific source of PAHs was identified in this junior high school classroom in the absence of occupants.
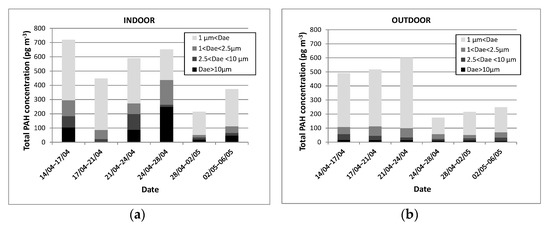
Figure 4.
Indoor (a) and outdoor (b) total PAH concentrations (µg m−3) measured in 2014 vs. time and particle size.
However, a notable exception over the period from 24 to 28 April 2014 highlights PAH concentrations in indoor air higher than outdoor values whatever the particle size, even if these differences remain very low. To explain this remarkable fact, we can assume that the numerous rainy episodes occurring in this period entrained the decrease in PM (see Figure 3) and then outdoor PAH concentrations (see Figure 4). Indeed, the weather was particularly good during the first period of three weeks, i.e., from 14 to 24 April 2014, while many rainy episodes occurred thereafter. Thus, the rain caused particles precipitation on the ground, resulting in particles decrease as observed in Figure 3 and therefore also in decrease of total PAH level (see Figure 4). Concerning the highest indoor PAH concentrations observed from April 24th to 28th, 2014, it could be supposed that PAHs most likely remained trapped inside the classroom because the ventilation system was switched off at the start of the sampling period corresponding to the weekend, i.e., for about 50% of the sampling time. The fresh air ventilation was deactivated for several days during this sampling period, and this implied that the observed indoor PAH levels remained high despite outdoor levels decreasing over this period. Such observation confirms the importance of active ventilation in management of indoor air quality, demonstrating the need of an efficient ventilation system for low-energy buildings. This ventilation system must be smartly controlled and scheduled to avoid the accumulation of pollutants indoors.
3.3.2. Temporal Variation of Individual PAH Concentrations According to the Particle Size
Figure 5 shows the temporal variations obtained for some PAHs with three and four aromatic rings from 24 April to 6 May 2014, where the concentrations are represented as a function of the aerodynamic diameter.
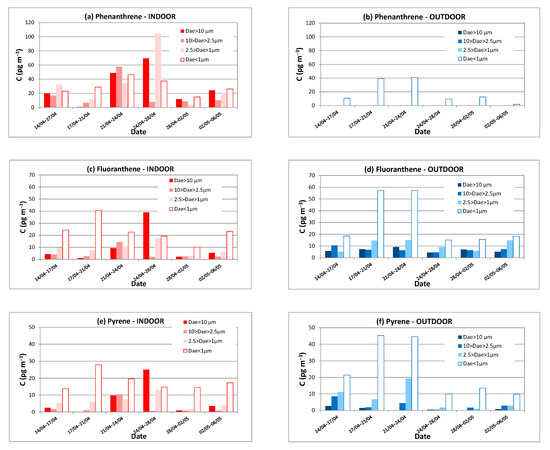
Figure 5.
Indoor and outdoor concentrations (pg m−3) of PAHs with three or four rings measured in 2014 vs. time and particle size. (a) Phenanthrene, indoors; (b) Phenanthrene, outdoors; (c) Fluoranthene, indoors; (d) Fluoranthene, outdoors; (e) Pyrene, indoors; (f) Pyrene, outdoors.
Phenanthrene was chosen as being representative of PAHs having three aromatic rings (see Figure 5a,b). This PAH is usually present in gas phase as reported in the literature [15]. However, it has been quantified in this study on indoor air particles of all classes of diameters. Moreover, Figure 5a indicates that the higher total PAH concentrations observed above from 24 to 28 April in indoor air compared to outdoor air (see Figure 4) could be partly due to the increase in PAH concentrations of low molecular weight, especially phenanthrene. In outdoor air, as illustrated in Figure 5b, phenanthrene seems to be present only on the finest particles. Thus, it can be assumed that a certain proportion of outdoor fine particles have coagulated once inside. In general, it should be noted that the total outdoor phenanthrene concentration is lower than that found indoors.
Concerning PAHs with four rings such as pyrene and fluoranthene (see Figure 5c–f), the indoor concentrations are slightly lower than outdoor ones. In outdoor air, these two molecules are clearly adsorbed on PM1 while the distribution is less marked in indoor air, confirming the hypothesis of a partial coagulation process occurring in indoor air. Furthermore, as these molecules are being adsorbed at the gas/particle interface, it is likely that the ambient temperature increase (approximately 24 °C indoors) causes their partial volatilization, resulting in a different distribution over the particle sizes. In addition, Figure 5c–f indicates that pyrene and fluoranthene exhibit strictly identical profiles and size distribution over time for both indoor and outdoor air. To a lesser extent, the outdoor phenanthrene concentration also shows a behaviour such as those observed for outdoor pyrene and fluoranthene concentrations (see Figure 5b), suggesting that external emission sources are common to all these PAHs and that the behaviour of these three PAHs under the same environmental conditions is similar.
This observation is even more remarkable on the PAHs with five to six rings in Figure 6 where a certain number of indoor and outdoor partitioning profiles are presented as a function of the particle size. Indeed, the profile obtained for each PAH taken individually is almost identical to the concentration profile shown in Figure 4 for the total PAHs concentration. This observation confirms that high molecular weight PAHs are essentially adsorbed on the finest particles as already reported by other studies [15,31].
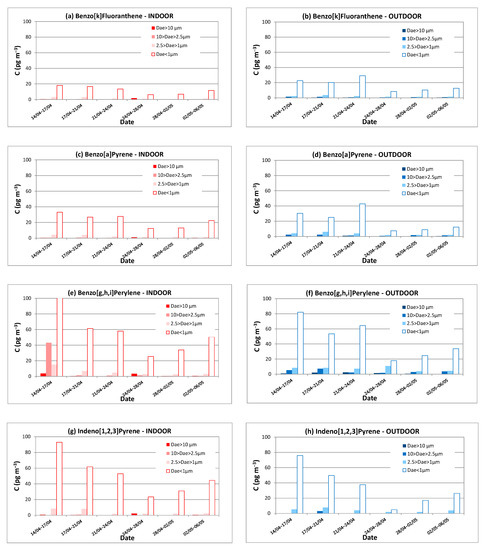
Figure 6.
Indoor and outdoor concentrations (pg m−3) of PAHs with five or six rings measured in 2014 vs. time and particle size. (a) Benzo[k]Fluoranthene, indoors; (b) Benzo[k]Fluoranthene, outdoors; (c) Benzo[a]Pyrene, indoors; (d) Benzo[a]Pyrene, indoors; (e) Benzo[g,h,i]Perylene, indoors; (f) Benzo[g,h,i]Perylene, outdoors; (g) Indeno[1,2,3]Pyrene, indoors; (h) Indeno[1,2,3]Pyrene, outdoors.
Benzo[k]fluoranthene and benzo[a]pyrene, two PAHs with five aromatic rings, have rigorously identical concentration profiles in indoor and outdoor air, which are slightly different from the profiles previously highlighted for PAHs with three and four aromatic rings. Their concentrations are also slightly lower than those found for fluoranthene and pyrene (Figure 5c–f).
The PAHs with six aromatic rings, i.e., Benzo[g,h,i]perylene (B[g,h,i]P) and indeno[1,2,3]pyrene (INP), also have a profile very close to those of five ring-PAHs but their concentrations are approximately two times higher. Contrary to the observations made for light PAHs for the period from 24th to 28th April, the concentrations of PAHs with five and six aromatic rings measured in indoor air are usually slightly higher than the outdoors ones, this phenomenon being less marked for the heaviest PAHs.
All the above-mentioned results highlight that it is possible and of interest to determine the temporal variations of the PAHs with a cascade impactor, which also makes it possible to obtain the temporal evolution of PAH concentration distribution as a function of their particle size.
3.4. Indoor-to-Outdoor Concentration Ratios (I/O) of Individual PAHs
The chosen spring holidays sampling period makes it possible to dispense with the opening/closing of doors or windows throughout the school. Thus, the only way of infiltration of external pollutants towards the interior of the classroom is linked to controlled mechanical ventilation. This operation mode is not very far from a normal operation of a low energy building, where it is often recommended to avoid opening windows as much as possible when the heating operates for reasons of energy saving. The mechanical ventilation is then supposed to provide the necessary fresh air. Therefore, the indoor-to-outdoor concentration ratios of individual PAHs are only driven by the efficiency of mechanical ventilation in our experimental conditions which greatly simplifies the data interpretations.
3.4.1. Results
The indoor-to-outdoor concentration ratios (I/O) were calculated for each PAH on the basis of the total PAH concentration obtained by summing the PAH quantity collected on all the plateaus of cascade impactor for a given sampling period. These results are shown in Figure 7 for PAHs with four to six aromatic rings for the whole campaign duration, i.e., from 14 April to 6 May 2014.
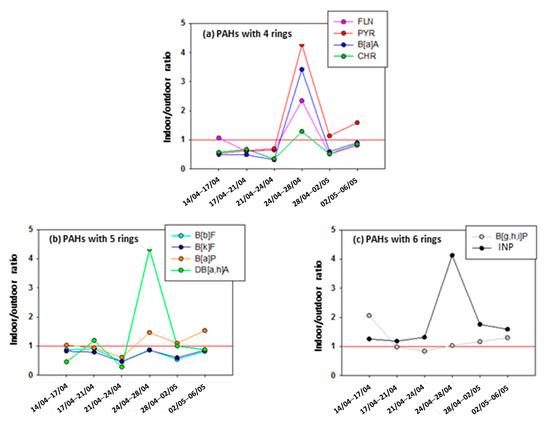
Figure 7.
Indoor-to-outdoor concentration ratios (I/O) vs. time in 2014 for (a) PAHs with four rings, (b) PAHs with five rings and (c) PAHs with six rings. The PAH concentrations are calculated by summing the PAH quantity collected on all the plateaus of cascade impactor for a given sampling period. The red line corresponds to I/O = 1.
Concerning the three-ring PAHs, the I/O values are difficult to interpret in terms of source determination since it is not usual to quantify them in the particulate phase. Therefore, I/O values were not calculated for these species.
For PAHs with four to six rings (Figure 7a–c), all the indoor-to-outdoor concentration ratios are usually around 1, confirming that their presence in this indoor environment is explained by their infiltration from outside the building. However, the period from 24 to 28 April appears again as an exception since the I/O values increases approximately by a factor of four for pyrene, three for benzo[a]anthracene and fluoranthene, two for chrysene, five for dibenzo[a,h]anthracene, and two for benzo[a]pyrene as well as a factor of three for indeno[1,2,3]pyrene. This behaviour was much less marked for the final period from 2 to 6 May including the weekend of 3 to 4 May where the ventilation was also switched off. This tends to confirm that the weather change combined with the stop of ventilation for the weekend of 26 to 27 April explains the high I/O values found for 24 to 28t of April.
This finding corroborates the explanation given above in the section dedicated to the temporal variation of total PAH concentrations, where it was assumed that the total PAH level decreases outdoors due to rain events while total indoor PAH concentration remained constant for half of the sampling period in the absence of ventilation during the weekend. However, it is surprising to observe almost no variation over time for I/O values corresponding to certain PAH molecules with five rings, e.g., benzo[b]fluoranthene and benzo[k]fluoranthene.
3.4.2. Comparison with Literature
Even if this study reports the first temporal monitoring of indoor-to-outdoor concentration ratios (I/O) of individual PAHs in an European School, the comparison can be made with the mean values of I/O reported in previous studies performed in European schools [11,12,13,15] or other indoor environments [43,49,50].
Concerning the individual indoor-to-outdoor concentration ratios reported in the literature for PAHs in schools, Romagnoli et al. showed that all the I/O values calculated for the six Italian schools are lower or close to 1, in both winter and summer [12]. The results obtained by Krugly et al. are also in agreement with this behavior except for the I/O values found for the light PAHs, which sometimes exceeded 1 [11] as observed in this work (see Figure 7a). These authors showed that the I/O values for PAHs with four aromatic rings were close to 0.5, which is also observed in our study except for the period from 24 to 28 April 2014.
Oliveira et al. reported also I/O ratios of PM2.5-bound PAHs at two preschools [15] and at 10 primary schools [13] in Portugal. At both preschools, I/O ratios of lighter PAH congeners (naphthalene, acenaphthene, fluorene, and phenanthrene) exceeded unity, suggesting potential contribution of indoor sources. Indeed, they can originate from evaporation from building materials, occupants’ activities including children artistic activities, and classroom cleaning [11]. In the 10 investigated primary schools, this behavior was less remarkable since PAHs with two to three aromatic rings (fluorene, phenanthrene, anthracene, and fluoranthene) showed a mixed trend: I/O > 1 at S2, S6, S10, and I/O < 1 at S7, S8, S9 [13]. In preschools and schools, the PAHs with four to six aromatic rings showed I/O ratios lower than 1 at all locations (with the exception of one school), confirming the outdoor origin of these compounds [13,15]. Moreover, ratios of PAHs with four rings (namely pyrene, chrysene, benzo[a]anthracene) were lower than 0.5 at PS1 and varied between 0.4 and 0.7 at PS2, which agrees with our values.
These comparisons have been extended to different indoor environments, namely offices and private dwellings, which are more studied in terms of PAH measurements. The indoor-to-outdoor concentration ratios determined in our study are again in agreement with the following studies: 0.31–0.78 in winter and 0.73–0.98 in summer in offices in Italy [43]; close to 1 for PAHs considered as non-volatile compounds (MW > 228 g mol−1) and between 1.2 and 3.7 for volatile PAHs (MW < 206 g mol−1) in individual dwellings in USA [49], the highest values being reached during the summer period. Likewise, I/O values between 0.05 and 0.36 were reported in Lithuanian dwellings in the absence of combustion sources [50]. In addition, this later study highlighted very low ratio values in a newly constructed building with thermal insulation criteria, the indoor-to-outdoor concentration ratios ranging from 0.05 to 0.25 for PAHs.
3.5. Risk Assessment
To express the carcinogenic risk for both indoors and outdoors due to PAHs exposure, the B[a]P equivalent factor (BaPeq) was evaluated, and results are reported in Table 4. This factor is based on the PAHs concentrations determined with sampling followed by analysis and on a toxic equivalency factor (TEF), which estimates the carcinogenic potency of PAHs to relative potency of B[a]P. These factors have been determined by numerous studies and we chose the most used approach to assess PAHs carcinogenic potential, i.e., the method proposed by Nisbet and LaGoy (1992) [51] improved later by Malcom and Dobson [52]. This method used all the TEFs values from Nisbet and LaGoy except for DB[a,h]A which TEF value is 1 instead of 5. The TEFs values are reported in Table S3 in the Supplementary Materials. The BaPeq is derived from equation (1) in order to express the level of carcinogenicity of the mixture relative to B[a]P:
where i is one of the 16 priority PAHs. This formula is built on two assumptions: (1) the impact of individual PAHs on animals is the same on humans, (2) the risk that individual PAHs is additive and so the sum of the individual risks is representative of the risk of the exposure to the entire PAHs mixture.

Table 4.
Evaluation of BaPeq indicator during the whole field campaign.
The evaluated BaPeq varies in the ranges 0.03–0.08 and 0.02–0.12 ng m−3 for indoors and outdoors, respectively. These values are all below the European guideline n°2004/107/CE fixing a threshold of 1 ng m−3 [53] confirming that the French investigated school is in a low contaminated area in terms of PAH levels. These values are consistent but slightly lower than those reported by Liaud et al. [31] who used the same methodology and the same TEF values, and where the evaluated BaPeq ranged between 0.038 and 0.374 ng m−3 for eight indoor sampling locations in France. All these data can be contrasted with the Lithuanian study in five schools located in various environments where the BaPeq indicators, calculated again with the same assumptions, varied between 1.20 and 50.8 ng m−3 [11], i.e., much higher than the threshold value set by Europe. Our BaPeq values are also lower than those found in others studies: 0.2–10.7 ng m−3 with candle burning emissions [28]; 0.48 ng m−3 indoors and 2.83 ng m−3 for Environment Tobacco Smoking (ETS) [44]; 1.70 ng m−3 in Japan [54].
4. Conclusions
During this field campaign, two cascade impactors were deployed simultaneously indoors and outdoors to study the temporal variations of PAH concentrations on particle matter according to their size distribution.
This methodology exhibited a drawback because it was not always possible to accurately weigh the particles impacted on the plateaus of both cascade impactors regarding the small quantities collected for large particles. However, this work highlights that fine particles (<1 µm) are often the majority. Despite the small quantities collected over three to four days of sampling, it was possible to determine precise PAH concentrations and to show that indoor PAH exposure levels are comparable to outdoor ones. These exposure levels were of the same of magnitude to those found in other French and Italian schools [14,31] but significantly lower than those measured in other European countries such as Portugal [13,15] and Lithuania [11]. For instance, the BAPeq values derived indoors for the six periods in this work are 15 to 1660 times lower than the corresponding ones reported by Krugly et al. (2014) in five schools of Lithuania [11]. However, our measurements conducted during the spring holidays most likely minimize the PAH concentrations in this period of low car traffic and in the absence of indoor sources from occupant’s activities.
The temporal variations in the PAH concentrations as a function of the particle size distribution show that the behavior of certain HAPs was identical, suggesting a common source of emission. The indoor-to-outdoor concentration ratios were usually found close to or less than ine over the field campaign duration, except for air sampled between 24 and 28 April, where the ratios are between two and five times higher depending on PAHs. Indeed, indoor levels remained high despite outdoor levels decreasing due to the fresh air ventilation being deactivated for several days. This remarkable fact demonstrates that outdoor PAH sources appear to dominate indoor PAH exposure levels. This observation confirms the importance of an efficient ventilation ensuring a high air exchange rate in buildings and more particularly in low-energy buildings which are very insulated.
The methodology used in this work is thus suitable for determining the PAH concentrations adsorbed on particles according to their size and to monitor their changes over time, even in environments with very little pollution such as that monitored in this work. Particle sampling with a shorter sampling time could be carried out by means of cascade impactor in more polluted environments to either help the identification of emission sources or identify specific tracers and fingerprints for a given emission source.
Finally, this method could also be used to measure the temporal variations in concentrations of other semi-volatile organic compounds adsorbed on the particles as a function of the particle size.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4433/12/1/108/s1, Table S1: Indoor and outdoor sample Volumes (in units of m3) for each sampling period during the field campaign, Table S2: Calibration parameters for the PAHs quantification with LOD and LOQ for the analytical instrument (LC for Liquid Chromatography) in µg L-1, converted in injected mass (pg) and considering Airborne Concentrations (AC) in (pg m−3), Table S3: Toxic Equivalency Factor (TEF) values extracted from the works reported by Malcolm and Dobson (1994) and by Nisbet and LaGoy (1992).
Author Contributions
Conceptualization, S.L.C., and C.L.; methodology, C.L., and S.L.C.; validation, C.L., S.C., and S.L.C.; formal analysis, C.L., S.C., and S.L.C.; resources, S.L.C.; writing—original draft preparation, S.L.C. and C.L.; writing—review and editing, S.L.C.; visualization, C.L., and S.L.C.; supervision, S.L.C.; project administration, S.L.C.; funding acquisition, S.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ADEME (Agence De l’Environnement et de la Maîtrise de l’Energie), grant number 1262C0023.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because the authors want to keep priority for conference presentations.
Acknowledgments
The authors would like to thank all the people working at the junior high school and the school administration for their support. The authors also thank all the participants of the MERMAID field campaign coordinated by Coralie Schoemaecker.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weschler, C.J.; Shields, H.C. Potential reactions among indoor pollutants. Atmos. Environ. 1997, 31, 3487–3495. [Google Scholar] [CrossRef]
- Carslaw, N. Chemical Reactions in the Indoor Atmosphere. In Indoor Air Pollution; The Royal Society of Chemistry: London, UK, 2019; pp. 105–126. ISBN 978-1-78801-514-1. [Google Scholar]
- Poggi, P. Réglementation thermique 2012, une rupture de méthode, mais de fortes modulations des exigences de performance. 1er volet: Nouveaux mécanismes et différences par rapport à la RT 2005. Qual. Constr. 2011, 1269, 124. [Google Scholar]
- Jovanović, M.; Vučićević, B.; Turanjanin, V.; Živković, M.; Spasojević, V. Investigation of indoor and outdoor air quality of the classrooms at a school in Serbia. Energy 2014, 77, 42–48. [Google Scholar] [CrossRef]
- Wargocki, P.; Wyon, D.P. Providing better thermal and air quality conditions in school classrooms would be cost-effective. Build. Environ. 2013, 59, 581–589. [Google Scholar] [CrossRef]
- Becker, R.; Goldberger, I.; Paciuk, M. Improving energy performance of school buildings while ensuring indoor air quality ventilation. Build. Environ. 2007, 42, 3261–3276. [Google Scholar] [CrossRef]
- Mumovic, D.; Palmer, J.; Davies, M.; Orme, M.; Ridley, I.; Oreszczyn, T.; Judd, C.; Critchlow, R.; Medina, H.A.; Pilmoor, G.; et al. Winter indoor air quality, thermal comfort and acoustic performance of newly built secondary schools in England. Build. Environ. 2009, 44, 1466–1477. [Google Scholar] [CrossRef]
- Rizk, M.; Guo, F.; Verriele, M.; Ward, M.; Dusanter, S.; Blond, N.; Locoge, N.; Schoemaecker, C. Impact of material emissions and sorption of volatile organic compounds on indoor air quality in a low energy building: Field measurements and modeling. Indoor Air 2018, 28, 924–935. [Google Scholar] [CrossRef]
- Nasreddine, R.; Person, V.; Serra, C.A.; Schoemaecker, C.; Le Calvé, S. Portable novel micro-device for BTEX real-time monitoring: Assessment during a field campaign in a low consumption energy junior high school classroom. Atmos. Environ. 2016, 126, 211–217. [Google Scholar] [CrossRef]
- Trocquet, C.; Bernhardt, P.; Guglielmino, M.; Malandain, I.; Liaud, C.; Englaro, S.; Calvé, S.L. Near Real-Time Monitoring of Formaldehyde in a Low-Energy School Building. Atmosphere 2019, 10, 763. [Google Scholar] [CrossRef]
- Krugly, E.; Martuzevicius, D.; Sidaraviciute, R.; Ciuzas, D.; Prasauskas, T.; Kauneliene, V.; Stasiulaitiene, I.; Kliucininkas, L. Characterization of particulate and vapor phase polycyclic aromatic hydrocarbons in indoor and outdoor air of primary schools. Atmos. Environ. 2014, 82, 298–306. [Google Scholar] [CrossRef]
- Romagnoli, P.; Balducci, C.; Perilli, M.; Gherardi, M.; Gordiani, A.; Gariazzo, C.; Gatto, M.P.; Cecinato, A. Indoor PAHs at schools, homes and offices in Rome, Italy. Atmos. Environ. 2014, 92, 51–59. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Madureira, J.; de Oliveira Fernandes, E.; Delerue-Matos, C.; Morais, S.; do Carmo Pereira, M. Polycyclic aromatic hydrocarbons in primary school environments: Levels and potential risks. Sci. Total Environ. 2017, 575, 1156–1167. [Google Scholar] [CrossRef]
- Wei, W.; Dassonville, C.; Sivanantham, S.; Gregoire, A.; Mercier, F.; Le Bot, B.; Malingre, L.; Ramalho, O.; Derbez, M.; Mandin, C. Semivolatile organic compounds in French schools: Partitioning between the gas phase, airborne particles and settled dust. Indoor Air 2020. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.; do Carmo Pereira, M.; Morais, S. Assessment of polycyclic aromatic hydrocarbons in indoor and outdoor air of preschool environments (3–5 years old children). Environ. Pollut. 2016, 208, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Sadiktsis, I.; de Oliveira Galvão, M.F.; Westerholm, R.; Dreij, K. Polycyclic aromatic compounds in particulate matter and indoor dust at preschools in Stockholm, Sweden: Occurrence, sources and genotoxic potential in vitro. Sci. Total Environ. 2020, 142709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Morisaki, H.; Wei, Y.; Li, Z.; Yang, L.; Zhou, Q.; Zhang, X.; Xing, W.; Hu, M.; Shima, M.; et al. PM2.5-bound polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons inside and outside a primary school classroom in Beijing: Concentration, composition, and inhalation cancer risk. Sci. Total Environ. 2020, 705, 135840. [Google Scholar] [CrossRef] [PubMed]
- Al-Hemoud, A.; Al-Awadi, L.; Al-Rashidi, M.; Rahman, K.A.; Al-Khayat, A.; Behbehani, W. Comparison of indoor air quality in schools: Urban vs. Industrial “oil & gas” zones in Kuwait. Build. Environ. 2017, 122, 50–60. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.C.; Morais, S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019, 124, 180–204. [Google Scholar] [CrossRef]
- CITEPA Centre Interprofessionnel Technique d’Etudes de la Pollution Atmosphérique 2014. Available online: http://www.citepa.org/fr (accessed on 13 January 2021).
- Leoz-Garziandia, E. Hydrocarbures Aromatiques Polycycliques dans l’air Ambiant (HAP) 2000. Available online: www.lcsqa.org/system/files/rapfin00hapvfinale.pdf (accessed on 13 January 2021).
- ATSDR Toxicological Profile: Polycyclic Aromatic Hydrocarbons (PAHs) 1995. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=122&tid=25 (accessed on 13 January 2021).
- Fromme, H.; Lahrz, T.; Piloty, M.; Gebhardt, H.; Oddoy, A.; Rüden, H. Polycyclic aromatic hydrocarbons inside and outside of apartments in an urban area. Sci. Total Environ. 2004, 326, 143–149. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J. Sources and patterns of polycyclic aromatic hydrocarbons pollution in kitchen air, China. Chemosphere 2003, 50, 611–618. [Google Scholar] [CrossRef]
- Pey, J.; Drooge, B.L.; van Ripoll, A.; Moreno, T.; Grimalt, J.O.; Querol, X.; Alastuey, A. An evaluation of mass, number concentration, chemical composition and types of particles in a cafeteria before and after the passage of an antismoking law. Particuology 2013, 11, 527–532. [Google Scholar] [CrossRef]
- Castro, D.; Slezakova, K.; Delerue-Matos, C.; Alvim-Ferraz, M.; da Conceição Alvim-Ferraz, M.; Morais, S.; Pereira, M.; do Carmo Pereira, M. Polycyclic aromatic hydrocarbons in gas and particulate phases of indoor environments influenced by tobacco smoke: Levels, phase distributions, and health risks. Atmos. Environ. 2011, 45, 1799–1808. [Google Scholar] [CrossRef]
- Slezakova, K.; Castro, D.; Pereira, M.C.; Morais, S.; Delerue-Matos, C.; Alvim-Ferraz, M.C. Influence of tobacco smoke on carcinogenic PAH composition in indoor PM10 and PM2.5. Atmos. Environ. 2009, 43, 6376–6382. [Google Scholar] [CrossRef]
- Orecchio, S. Polycyclic aromatic hydrocarbons (PAHs) in indoor emission from decorative candles. Atmos. Environ. 2011, 45, 1888–1895. [Google Scholar] [CrossRef]
- Derudi, M.; Gelosa, S.; Sliepcevich, A.; Cattaneo, A.; Rota, R.; Cavallo, D.; Nano, G. Emissions of air pollutants from scented candles burning in a test chamber. Atmos. Environ. 2012, 55, 257–262. [Google Scholar] [CrossRef]
- Liaud, C.; Millet, M.; Calvé, S.L. An analytical method coupling accelerated solvent extraction and HPLC-fluorescence for the quantification of particle-bound PAHs in indoor air sampled with a 3-stages cascade impactor. Talanta 2015, 131, 386–394. [Google Scholar] [CrossRef]
- Liaud, C.; Dintzer, T.; Tschamber, V.; Trouve, G.; Le Calvé, S. Particle-bound PAHs quantification using a 3-stages cascade impactor in French indoor environments. Environ. Pollut. 2014, 195, 64–72. [Google Scholar] [CrossRef]
- Verriele, M.; Schoemaecker, C.; Hanoune, B.; Leclerc, N.; Germain, S.; Gaudion, V.; Locoge, N. The MERMAID study: Indoor and outdoor average pollutant concentrations in 10 low-energy school buildings in France. Indoor Air 2016, 26, 702–713. [Google Scholar] [CrossRef]
- Schoemaecker, C.; Fèvre-Nollet, V.; Verriele, M.; Dusanter, S.; Le Calvé, S.; Trocquet, C.; Hanoune, B.; Locoge, N. Effect of occupancy controlled ventilation in schools on the exposure quantification. In Proceedings of the 14th International Conference of Indoor Air Quality and Climate-Indoor Air, Ghent, Belgium, 3–8 July 2016. [Google Scholar]
- Schoemaecker, C.; Blocquet, M.; Ward, M.; Hanoune, B.; Petitprez, D.; Lebègue, P.; Dusanter, S.; Locoge, N.; Rizk, M.; Verriele, M.; et al. Experimental and Modeling Characterizations of Indoor Air Quality in Low Energy Public Buildings in France—The MERMAID program. Proc. Indoor Air 2014, 6, 573–580. [Google Scholar]
- Buonanno, G.; Fuoco, F.C.; Morawska, L.; Stabile, L. Airborne particle concentrations at schools measured at different spatial scales. Atmos. Environ. 2013, 67, 38–45. [Google Scholar] [CrossRef]
- Chithra, V.S.; Nagendra, S.M.S. Indoor air quality investigations in a naturally ventilated school building located close to an urban roadway in Chennai, India. Build. Environ. 2012, 54, 159–167. [Google Scholar] [CrossRef]
- Canha, N.; Almeida, S.M.; Freitas, M.; do Carmo Freitas, M.; Wolterbeek, H.T.; Cardoso, J.; Pio, C.; Caseiro, A. Impact of wood burning on indoor PM2.5 in a primary school in rural Portugal. Atmos. Environ. 2014, 94, 663–670. [Google Scholar] [CrossRef]
- Rivas, I.; Viana, M.; Moreno, T.; Pandolfi, M.; Amato, F.; Reche, C.; Bouso, L.; Àlvarez-Pedrerol, M.; Alastuey, A.; Sunyer, J.; et al. Child exposure to indoor and outdoor air pollutants in schools in Barcelona, Spain. Environ. Int. 2014, 69, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Laiman, R.; He, C.; Mazaheri, M.; Clifford, S.; Salimi, F.; Crilley, L.R.; Mokhtar, M.A.M.; Morawska, L. Characteristics of ultrafine particle sources and deposition rates in primary school classrooms. Atmos. Environ. 2014, 94, 28–35. [Google Scholar] [CrossRef]
- Almeida, S.M.; Canha, N.; Silva, A.; do Carmo Freitas, M.; Pegas, P.; Alves, C.; Evtyugina, M.; Pio, C.A. Children exposure to atmospheric particles in indoor of Lisbon primary schools. Atmos. Environ. 2011, 45, 7594–7599. [Google Scholar] [CrossRef]
- Stranger, M.; Potgieter-Vermaak, S.S.; Van Grieken, R. Characterization of indoor air quality in primary schools in Antwerp, Belgium. Indoor Air 2008, 18, 454–463. [Google Scholar] [CrossRef]
- Fromme, H.; Twardella, D.; Dietrich, S.; Heitmann, D.; Schierl, R.; Liebl, B.; Rüden, H. Particulate matter in the indoor air of classrooms—exploratory results from Munich and surrounding area. Atmos. Environ. 2007, 41, 854–866. [Google Scholar] [CrossRef]
- Sangiorgi, G.; Ferrero, L.; Ferrini, B.S.; Porto, C.L.; Perrone, M.G.; Zangrando, R.; Gambaro, A.; Lazzati, Z.; Bolzacchini, E. Indoor airborne particle sources and semi-volatile partitioning effect of outdoor fine PM in offices. Atmos. Environ. 2013, 65, 205–214. [Google Scholar] [CrossRef]
- Delgado-Saborit, J.M.; Stark, C.; Harrison, R.M. Carcinogenic potential, levels and sources of polycyclic aromatic hydrocarbon mixtures in indoor and outdoor environments and their implications for air quality standards. Environ. Int 2011, 37, 383–392. [Google Scholar] [CrossRef]
- Fischer, P.H.; Hoek, G.; Reeuwijk, H.; van Briggs, D.J.; Lebret, E.; Wijnen, J.H.; van Kingham, S.; Elliott, P.E. Traffic-related differences in outdoor and indoor concentrations of particles and volatile organic compounds in Amsterdam. Atmos. Environ. 2000, 34, 3713–3722. [Google Scholar] [CrossRef]
- Naumova, Y.Y.; Offenberg, J.H.; Eisenreich, S.J.; Meng, Q.; Polidori, A.; Turpin, B.J.; Weisel, C.P.; Morandi, M.T.; Colome, S.D.; Stock, T.H.; et al. Gas/particle distribution of polycyclic aromatic hydrocarbons in coupled outdoor/indoor atmospheres. Atmos. Environ. 2003, 37, 703–719. [Google Scholar] [CrossRef]
- Naumova, Y.Y.; Eisenreich, S.J.; Turpin, B.J.; Weisel, C.P.; Morandi, M.T.; Colome, S.D.; Totten, L.A.; Stock, T.H.; Winer, A.M.; Alimokhtari, S.; et al. Polycyclic Aromatic Hydrocarbons in the Indoor and Outdoor Air of Three Cities in the U.S. Environ. Sci. Technol. 2002, 36, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Jung, K.H.; Patel, M.M.; Moors, K.; Kinney, P.L.; Chillrud, S.N.; Whyatt, R.; Hoepner, L.; Garfinkel, R.; Yan, B.; Ross, J.; et al. Effects of Heating Season on Residential Indoor and Outdoor Polycyclic Aromatic Hydrocarbons, Black Carbon, and Particulate Matter in an Urban Birth Cohort. Atmos Environ. 2010, 44, 4545–4552. [Google Scholar] [CrossRef] [PubMed]
- Kliucininkas, L.; Krugly, E.; Stasiulaitiene, I.; Radziuniene, I.; Prasauskas, T.; Jonusas, A.; Kauneliene, V.; Martuzevicius, D. Indoor–outdoor levels of size segregated particulate matter and mono/polycyclic aromatic hydrocarbons among urban areas using solid fuels for heating. Atmos. Environ. 2014, 97, 83–93. [Google Scholar] [CrossRef]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Malcolm, H.M.; Dobson, S. The Calculation of an Environmental Assessment Level (EAL) for Atmospheric PAHs Using Relative Potencies; DOE Report; Department of the Environment: London, UK, 1994. [Google Scholar]
- Directive, C. 107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. Off. J. Eur. Communities 2004, 26, 2005. [Google Scholar]
- Ohura, T.; Amagai, T.; Fusaya, M.; Matsushita, H. Polycyclic Aromatic Hydrocarbons in Indoor and Outdoor Environments and Factors Affecting Their Concentrations. Environ. Sci. Technol. 2004, 38, 77–83. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).