Numerical Modeling for the Accidental Dispersion of Hazardous Air Pollutants in the Urban Metropolitan Area
Abstract
:1. Introduction
2. Experiments
2.1. Atmospheric Dispersion Model
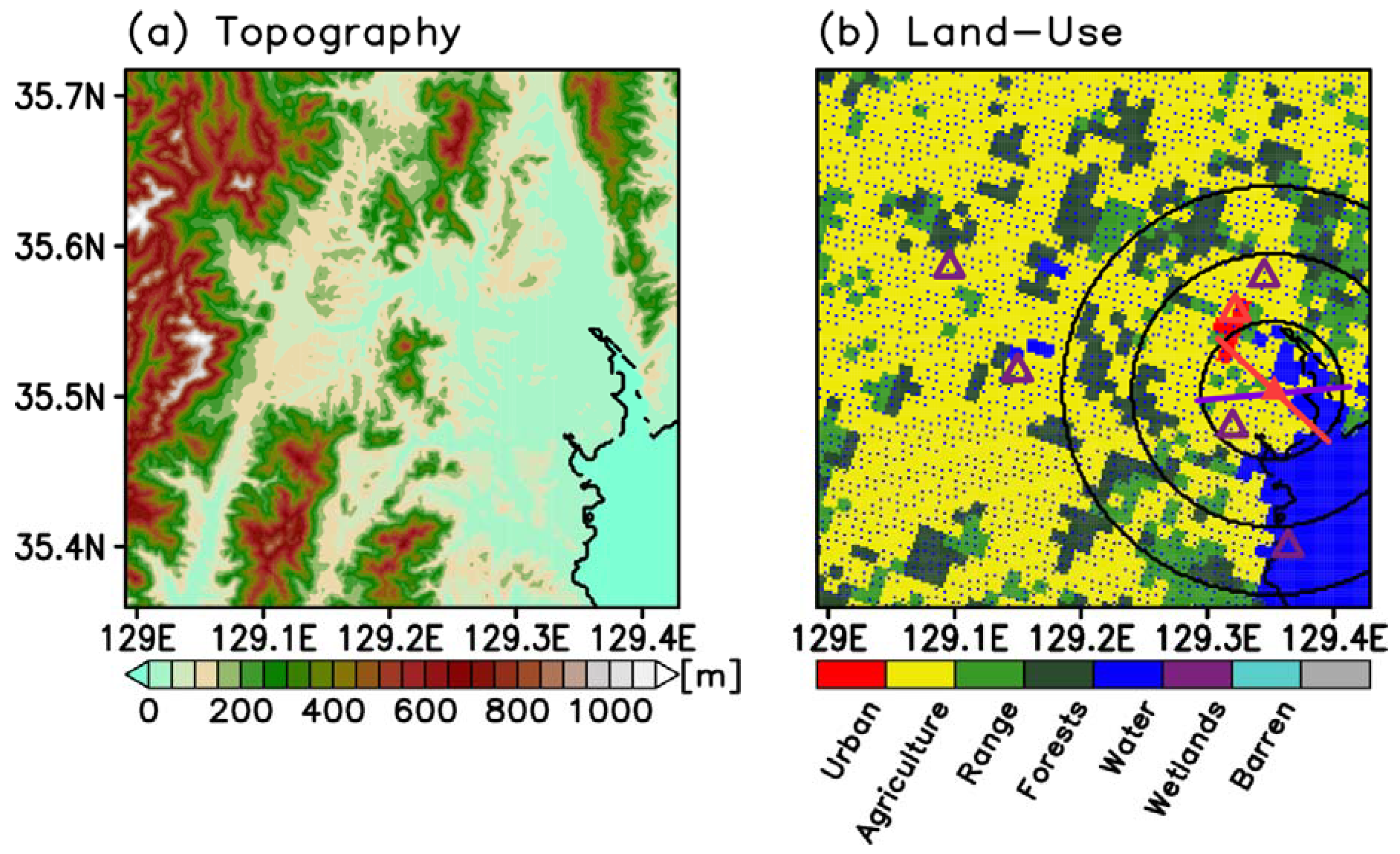
2.2. Experiment Settings
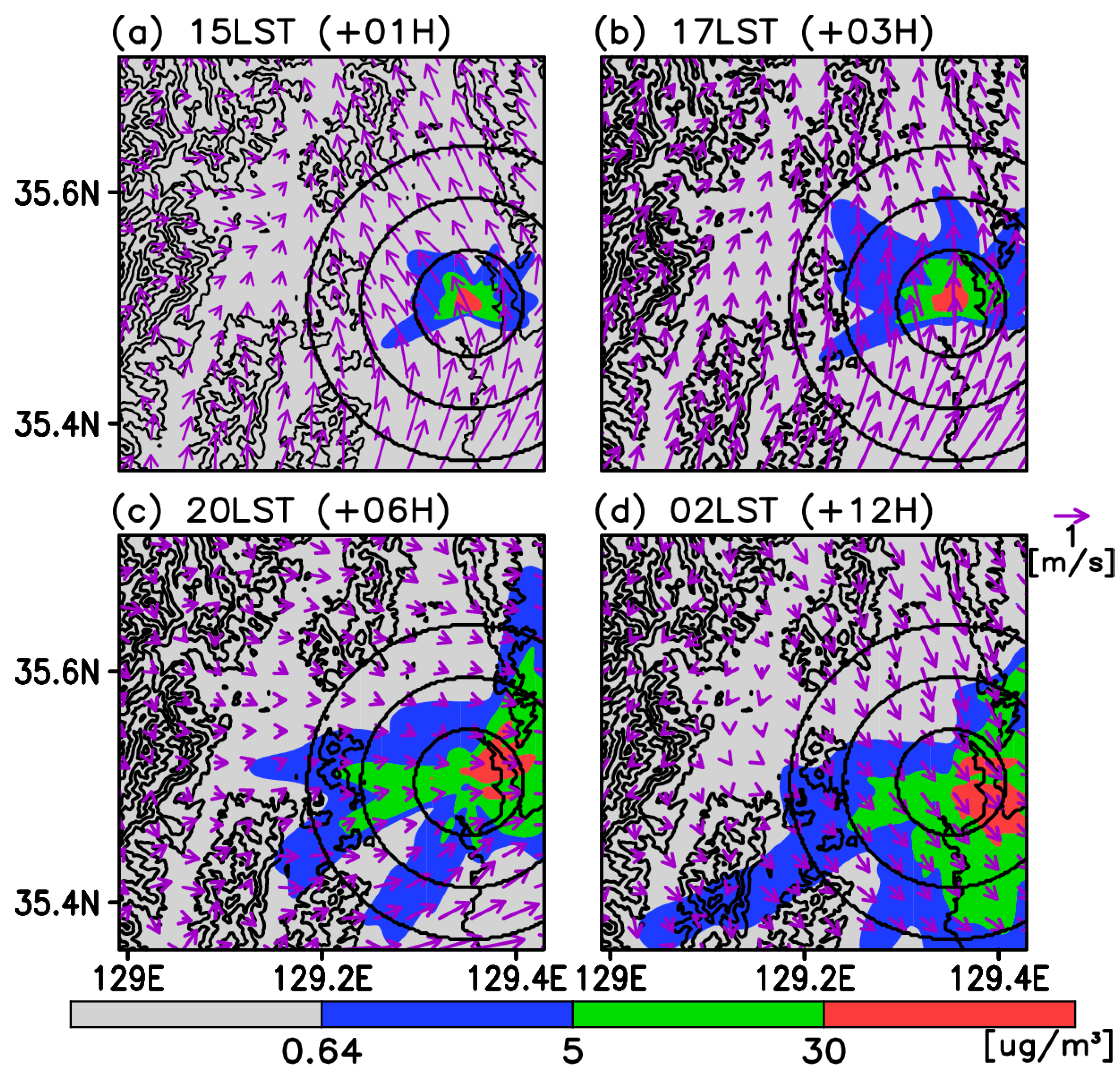
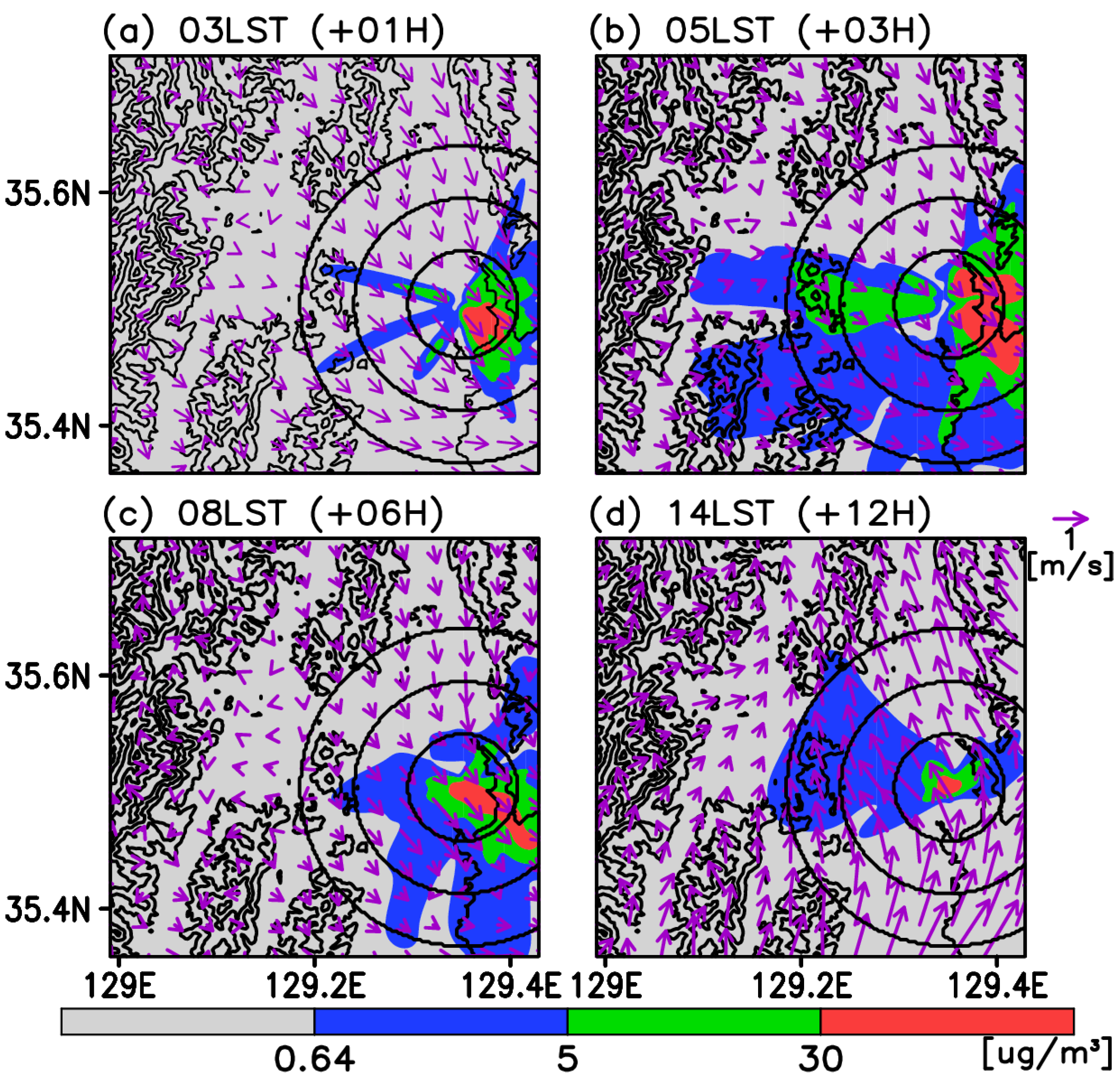
3. Results
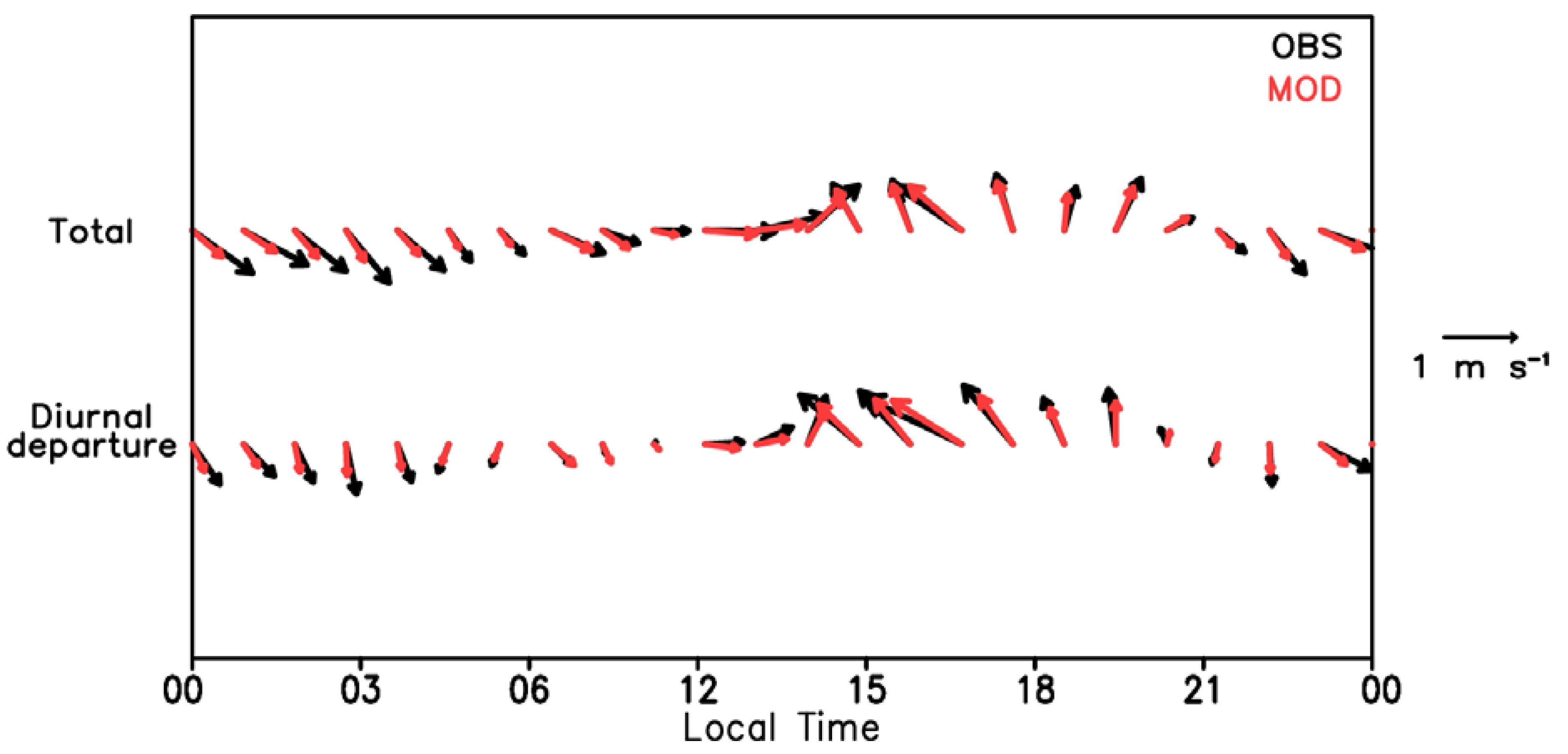
3.1. Validations for Wind and the Test Cases
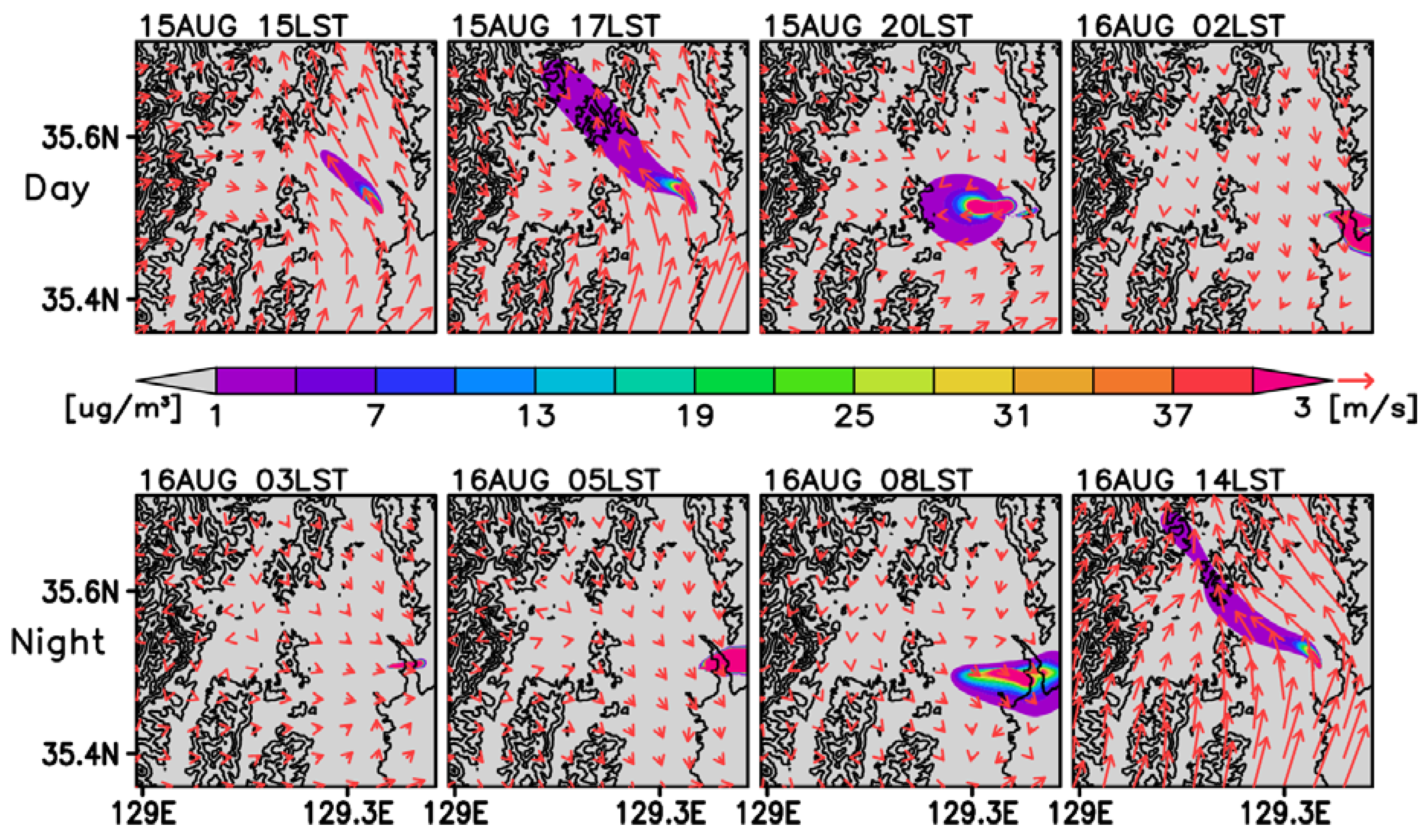
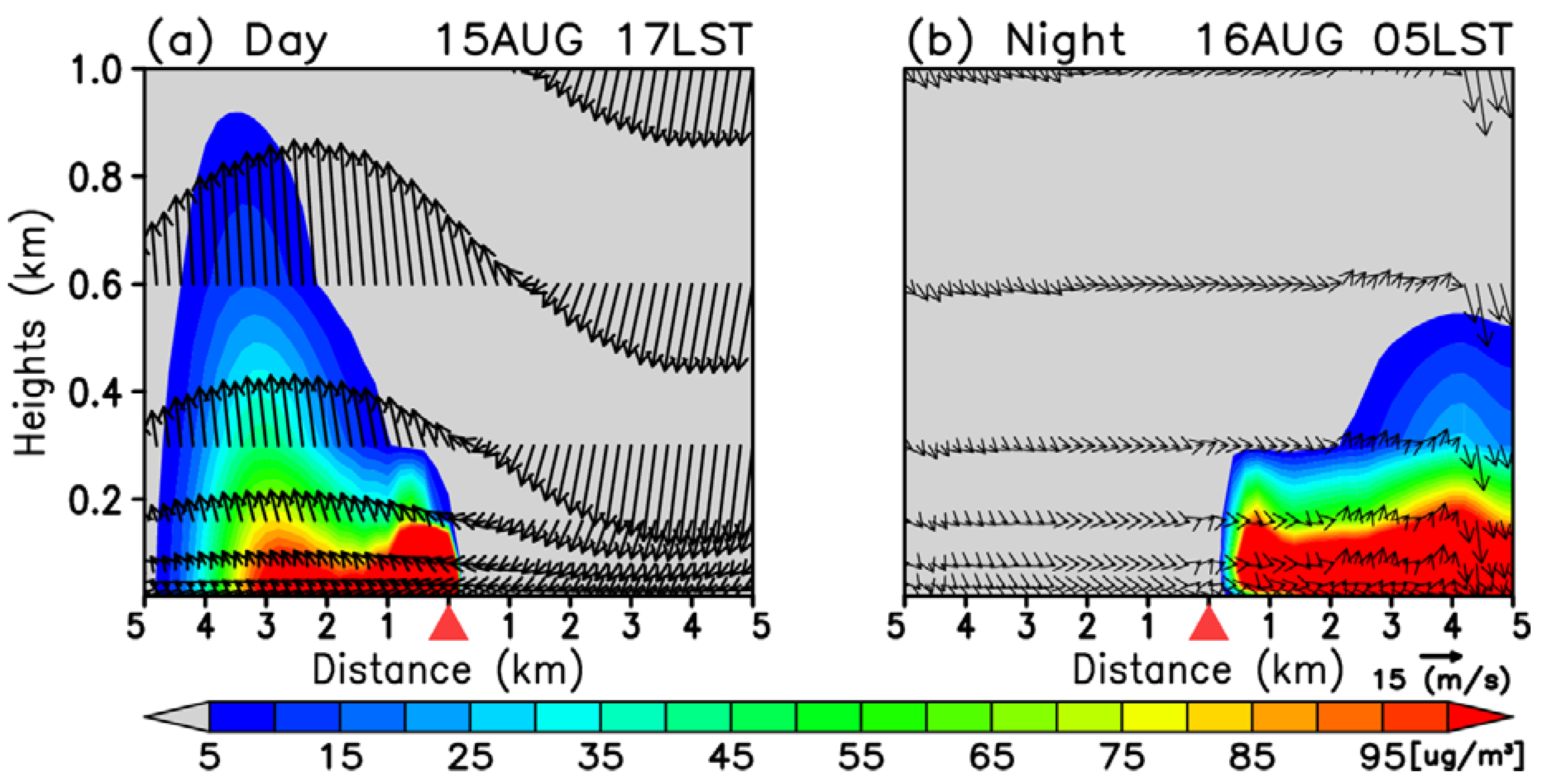
3.2. Time-Averaged Dispersion Pattern
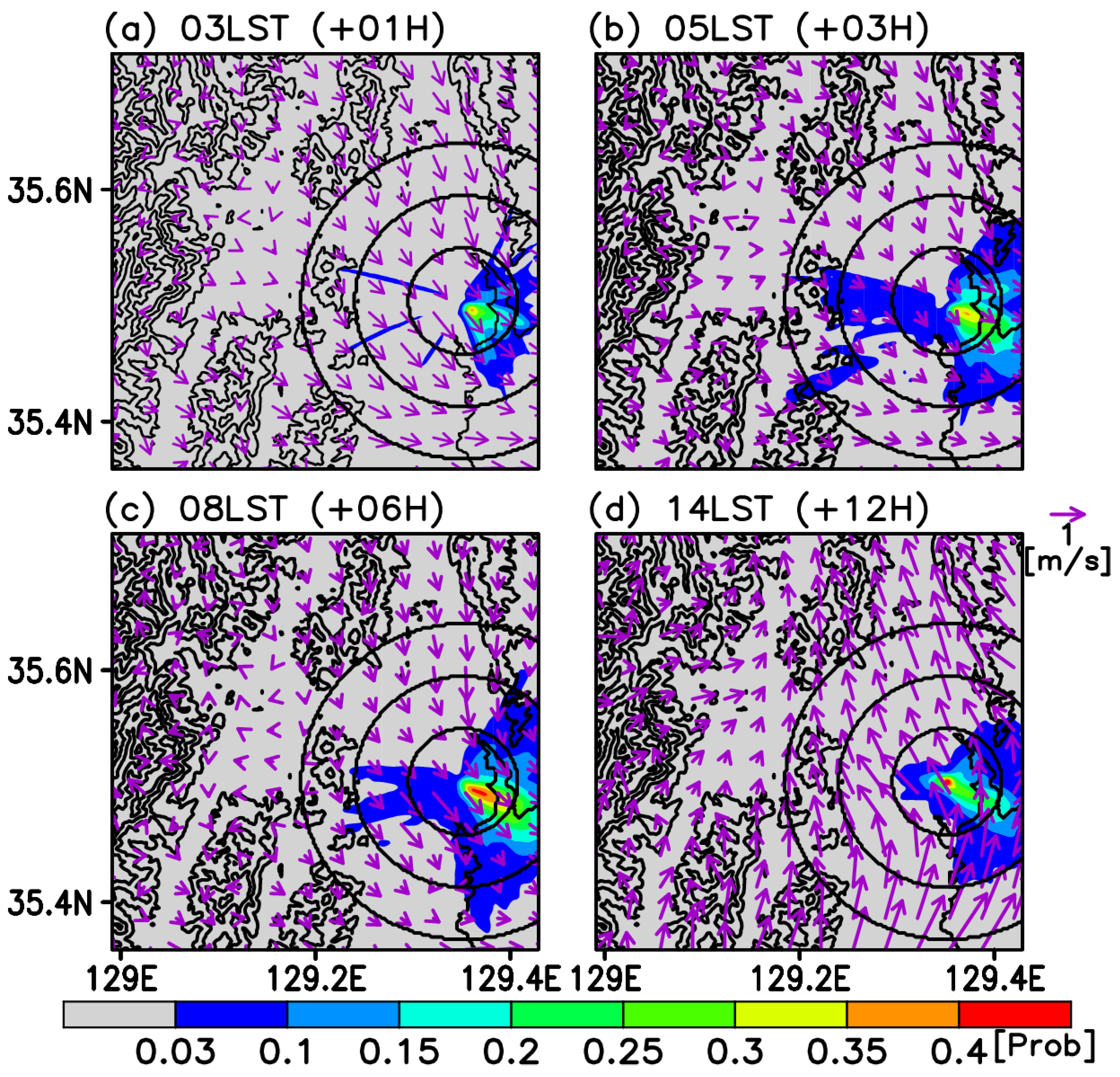
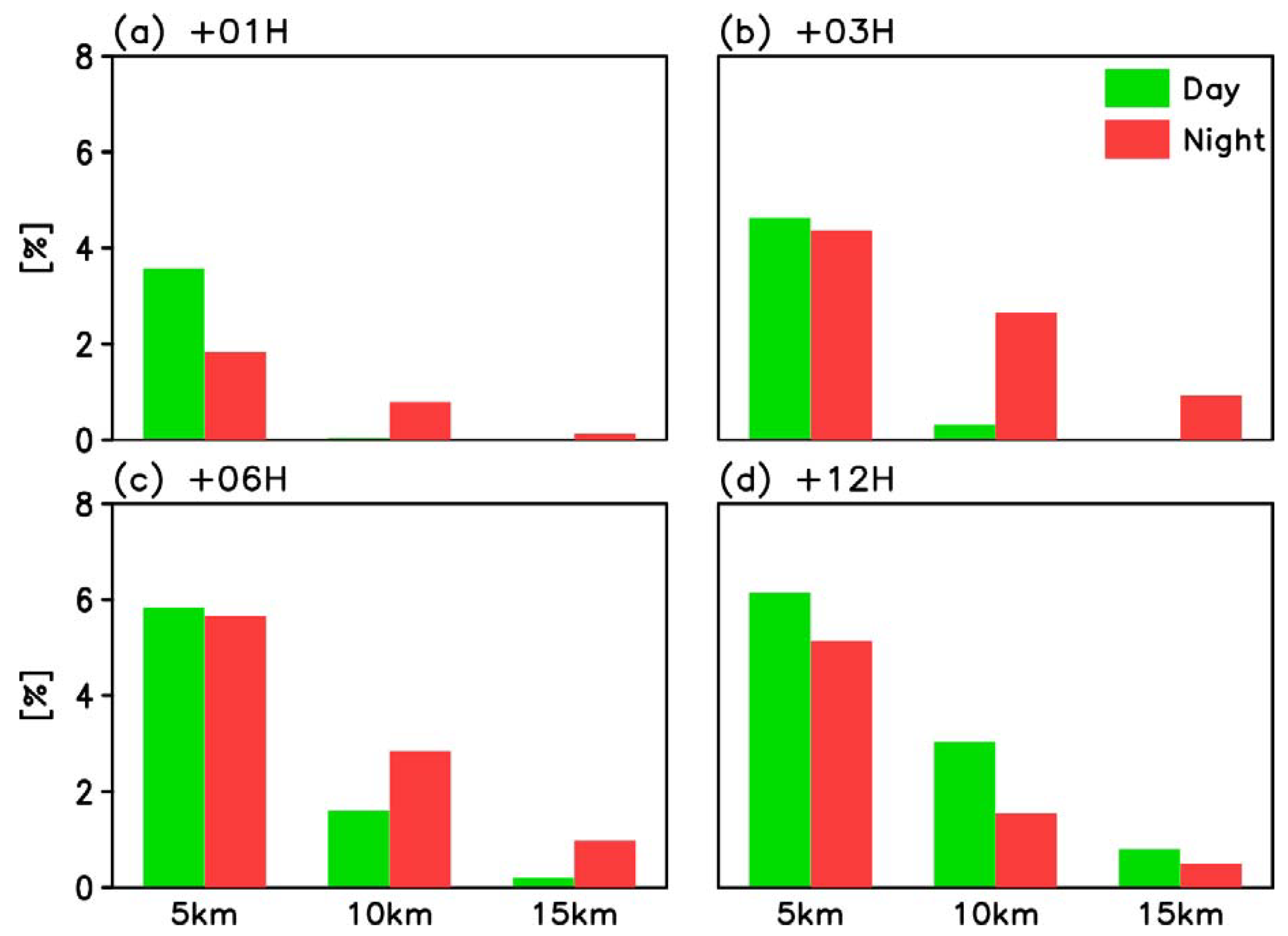
3.3. Risk Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grimm, N.B.; Foster, D.; Groffman, P.; Grove, J.M.; Hopkinson, C.S.; Nadelhoffer, K.J.; Pataki, D.E.; Peters, D.P. The changing landscape: Ecosystem responses to urbanization and pollution across climatic and societal gradients. Front. Ecol. Environ. 2008, 6, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Statistics Korea. Available online: http://www.index.go.kr/potal/stts/idxMain/selectPoSttsIdxSearch.do?idx_cd=4088&stts_cd=408802&freq=Y (accessed on 24 March 2020).
- Dabberdt, W.; Frederick, G.; Hardesty, R.; Lee, W.C.; Underwood, K. Advances in meteorological instrumentation for air quality and emergency response. Meteorol. Atmos. Phys. 2004, 87, 57–88. [Google Scholar] [CrossRef]
- Chen, W.K. Managing Emergency Response of Air Pollution by the Expert System. In Air Pollution–A Comprehensive Perspective; Hariyanto, B., Ed.; InTech: Rijeka, Croatia, 2012; p. 319. [Google Scholar]
- Jones, R.; Lehr, W.; Simecek-Beatty, D.; Reynolds, R.M. ALOHA® (Areal Locations of Harzadous Atmospheres) 5.4.4; Technical Documentation; NOAA Technical Memorandum NOS OR&R 43; U.S. Deptartment of Commerce: Washington, DC, USA, 2013; p. 96.
- Bruckner, J.V.; Keys, D.A.; Fisher, J.W. The Acute Exposure Guideline Level (AEGL) program: Applications of physiologically based pharmacokinetic modeling. J. Toxicol. Environ. Health Part A 2004, 67, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Elbir, T. A GIS based decision support system for estimation, visualization and analysis of air pollution for large Turkish cities. Atmos. Environ. 2004, 38, 4509–4517. [Google Scholar] [CrossRef]
- Levy, J.I.; Spengler, J.D.; Hlinka, D.; Sullivan, D.; Moon, D. Using CALPUFF to evaluate the impacts of power plant emissions in Illinois: Model sensitivity and implications. Atmos. Environ. 2002, 36, 1063–1075. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.; Sappurd, A.; Al-Damkhi, A. Application of California Puff (CALPUFF) model: A case study for Oman. Clean Tech. Environ. Policy 2011, 13, 177–189. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.A.; Ali, S.; Sardar, S.; Irfan, N.; Al-Damkhi, A. Evaluating the performance of an integrated CALPUFF-MM5 modeling system for predicting SO2 emission from a refinery. Clean Tech. Environ. Policy 2011, 13, 841–854. [Google Scholar] [CrossRef]
- Tayanç, M.; Berçin, A. SO2 modeling in Izmit Gulf, Turkey during the winter of 1997: 3 cases. Environ. Model. Assess. 2007, 12, 119–129. [Google Scholar] [CrossRef]
- Dehghani, M.; Taghizadeh, M.M.; Hashemi, H.; Rastgoo, E. A Preliminary Assessment of Dispersion Level of SO2 in Fars Industrial Region, South of Iran, by GIS. J. Environ. Public Health 2013, 2013, 670590. [Google Scholar] [CrossRef]
- Ozkurt, N.; Sari, D.; Akalin, N.; Hilmioglu, B. Evaluation of the impact of SO2 and NO2 emissions on the ambient air-quality in the Çan–Bayramiç region of northwest Turkey during 2007–2008. Sci. Total Environ. 2013, 456, 254–266. [Google Scholar] [CrossRef]
- Prueksakorn, K.; Kim, T.H.; Vongmahadlek, C. Applications of WRF/CALPUFF modeling system and multi-monitoring methods to investigate the effect of seasonal variations on odor dispersion: A case study of Changwon City, South Korea. Air Qual. Atmos. Health 2014, 7, 13–27. [Google Scholar] [CrossRef]
- Ranzato, L.; Barausse, A.; Mantovani, A.; Pittarello, A.; Benzo, M.; Palmeri, L. A comparison of methods for the assessment of odor impacts on air quality: Field inspection (VDI 3940) and the air dispersion model CALPUFF. Atmos. Environ. 2012, 61, 570–579. [Google Scholar] [CrossRef]
- Venturini, E.; Vassura, I.; Ferroni, L.; Raffo, S.; Passarini, F.; Beddows, D.; Harrison, R.M. Bulk deposition close to a Municipal Solid Waste incinerator: One source among many. Sci. Total Environ. 2013, 456, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Tchepel, O. Modelling of human exposure to air pollution in the urban environment: A GPS-based approach. Environ. Sci. Pollut. Res. 2014, 21, 3558–3571. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, D.; Broday, D.M.; Stern, E. Evaluation of AERMOD and CALPUFF for predicting ambient concentrations of total suspended particulate matter (TSP) emissions from a quarry in complex terrain. Environ. Pollut. 2013, 179, 138–145. [Google Scholar] [CrossRef]
- Barsotti, S.; Neri, A.; Scire, J. The VOL-CALPUFF model for atmospheric ash dispersal: 1. Approach and physical formulation. J. Geophys. Res. Solid Earth 2008, 113. [Google Scholar] [CrossRef]
- Scire, J.S.; Strimaitis, D.G.; Yamartino, R.J. Model Formulation and User’s Guide for the CALPUFF Dispersion Model; Sigma Research Corporation: Concord, MA, USA, 1990. [Google Scholar]
- Ghannam, K.; El-Fadel, M. Emissions characterization and regulatory compliance at an industrial complex: An integrated MM5/CALPUFF approach. Atmos. Environ. 2013, 69, 156–169. [Google Scholar] [CrossRef]
- Vieira de Melo, A.M.; Santos, J.M.; Mavroidis, I.; Reis Junior, N.C. Modelling of odour dispersion around a pig farm building complex using AERMOD and CALPUFF. Comparison with wind tunnel results. Build. Environ. 2012, 56, 8–20. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.A.; Chan, K.; Elkamel, A.; Ahmadi, L. Effects of meteorological conditions on the concentration and dispersion of an accidental release of H2S in Canada. Atmos. Environ. 2014, 82, 316–326. [Google Scholar] [CrossRef]
- Indumati, S.; Oza, R.; Mayya, Y.; Puranik, V.; Kushwaha, H. Dispersion of pollutants over land–water–land interface: Study using CALPUFF model. Atmos. Environ. 2009, 43, 473–478. [Google Scholar] [CrossRef]
- Scire, J.S.; Strimaitis, D.G.; Yamartino, R.J. A User’s Guide for the CALPUFF Dispersion Model; Earth Tech, Inc.: Concord, MA, USA, 2000; pp. 1–521. [Google Scholar]
- Scire, J.S.; Robe, F.R.; Fernau, M.E.; Yamartino, R.J. A User’s Guide for the CALMET Meteorological Model; Version 5; Earth Tech, Inc.: Concord, MA, USA, 2000; p. 37. [Google Scholar]
- Korean Ministry of Environment. Guidelines for Initial Risk Assessment Using Chemical Emission Information; Ministry of Environment: Sejong, Korea; National Institute of Environmental Research: Inchon, Korea; pp. 1–111. (In Korean)
- PRTR Information System. Available online: https://icis.me.go.kr/prtr/main.do (accessed on 24 March 2020).
- Chemical Information System. Available online: https://icis.me.go.kr/main.do (accessed on 24 March 2020).
- Huang, G.; Gao, L.; Duncan, J.; Harper, J.D.; Sanders, N.L.; Ouyang, Z.; Cooks, R.G. Direct detection of benzene, toluene, and ethylbenzene at trace levels in ambient air by atmospheric pressure chemical ionization using a handheld mass spectrometer. J. Am. Soc. Mass Spectrom. 2010, 21, 132–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Integrated Risk Information System. Available online: https://www.epa.gov/iris (accessed on 24 March 2020).
- IRIS Benzene Chemical Assessment Summary. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0276_summary.pdf (accessed on 24 March 2020).
- Deardorff, J.W. Three-dimensional numerical study of the height and mean structure of a heated planetary boundary layer. Bound. Layer Meteorol. 1974, 7, 81–106. [Google Scholar] [CrossRef]
- U.S. Department of Transportation. 2016 Emergency Response Guidebook. Available online: https://www.phmsa.dot.gov/sites/phmsa.dot.gov/files/docs/ERG2016.pdf (accessed on 24 March 2020).

| Diffusivity (cm2 s−1) | 0.1509 |
| Aqueous Dissociation Constant | 1.0 |
| Reactivity | 8.0 |
| Mesophyll Resistance (s cm−1) | 0.0 |
| Henry’s Law | 4.0 × 10−2 |
| Date | Time of Accident (LST in hh mm) | Duration | Pollutant | Emission Amount (kg) | Approx. Emission Rate (kg h−1) |
|---|---|---|---|---|---|
| 25 February 2014 | 14:47 | 30 min | Hydrofluoric Acid | 115 | 230 |
| 13 February 2014 | 13:00 | 2 h 50 min | Ammonia | 1500 | 530 |
| 3 January 2014 | 04:00 | 17 h | Propane | 40,000 | 2350 |
| 13 October 2013 | 21:00 | 20 min | Benzene | 438 | 1300 |
| 16 July 2013 | 05:04 | 20 min | Chlorosurfonic Acid | 3.5 | 10.5 |
| 9 June 2013 | 22:30 | 5 h | Hydrochloric Acid | 236 | 47.2 |
| 27 September 2012 | 15:43 | 12 h | Hydrofluoric Acid | 8000 | 667 |
| 4 July 2008 | 12:50 | 2 h 30 min | Benzene | 22,000 | 8800 |
| 16 October 2007 | 10:00 | At least 3 h | Benzene | Unknown | Unknown |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Lee, M.-I.; Lee, S.; Choi, S.-D.; Kim, S.-J.; Song, C.-K. Numerical Modeling for the Accidental Dispersion of Hazardous Air Pollutants in the Urban Metropolitan Area. Atmosphere 2020, 11, 477. https://doi.org/10.3390/atmos11050477
Kim G, Lee M-I, Lee S, Choi S-D, Kim S-J, Song C-K. Numerical Modeling for the Accidental Dispersion of Hazardous Air Pollutants in the Urban Metropolitan Area. Atmosphere. 2020; 11(5):477. https://doi.org/10.3390/atmos11050477
Chicago/Turabian StyleKim, Ganghan, Myong-In Lee, Seunghee Lee, Sung-Deuk Choi, Sung-Joon Kim, and Chang-Keun Song. 2020. "Numerical Modeling for the Accidental Dispersion of Hazardous Air Pollutants in the Urban Metropolitan Area" Atmosphere 11, no. 5: 477. https://doi.org/10.3390/atmos11050477
APA StyleKim, G., Lee, M.-I., Lee, S., Choi, S.-D., Kim, S.-J., & Song, C.-K. (2020). Numerical Modeling for the Accidental Dispersion of Hazardous Air Pollutants in the Urban Metropolitan Area. Atmosphere, 11(5), 477. https://doi.org/10.3390/atmos11050477







