Functional Factors of Biomass Burning Contribution to Spring Aerosol Composition in a Megacity: Combined FTIR-PCA Analyses
Abstract
1. Introduction
2. Experiments
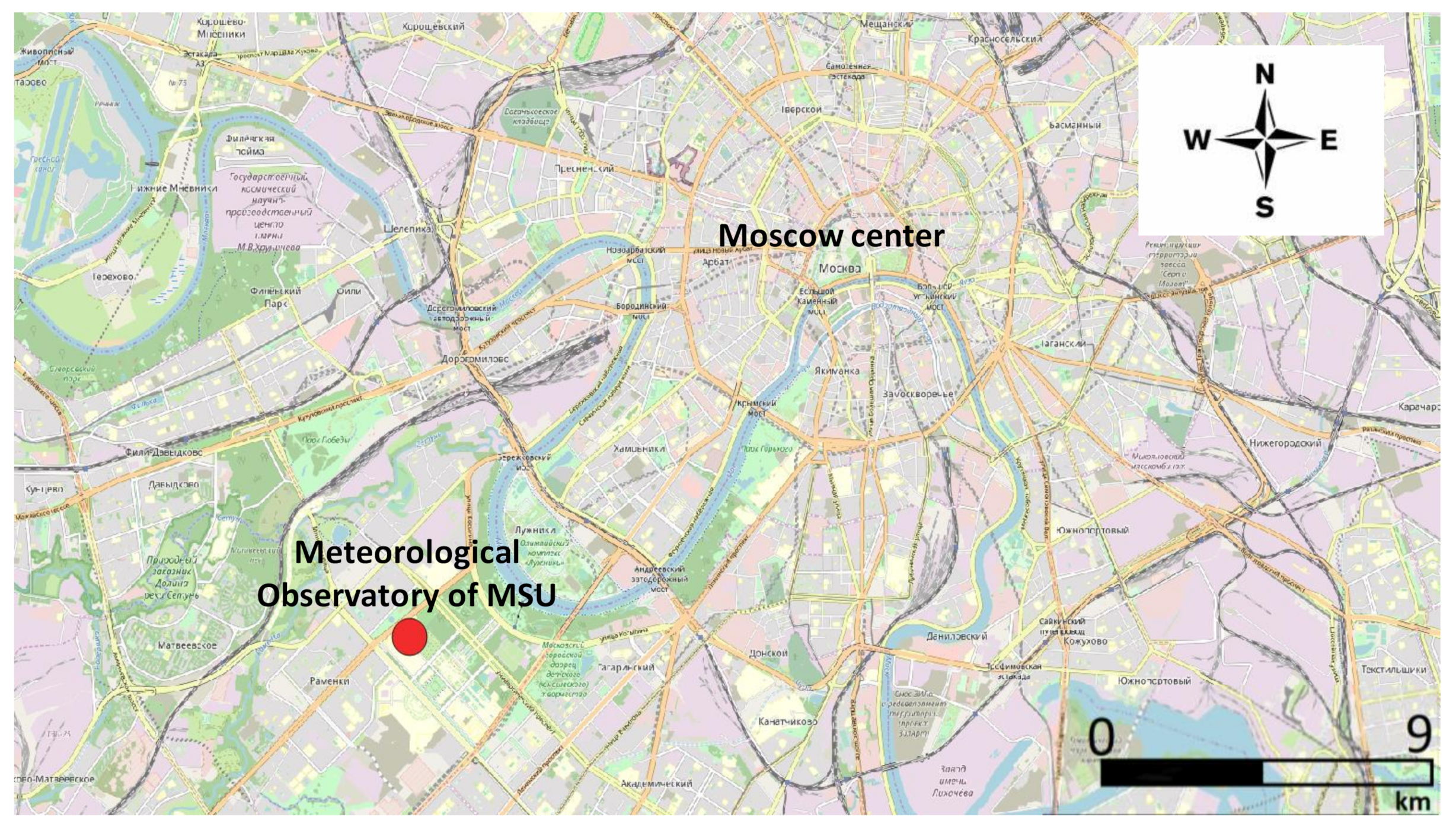
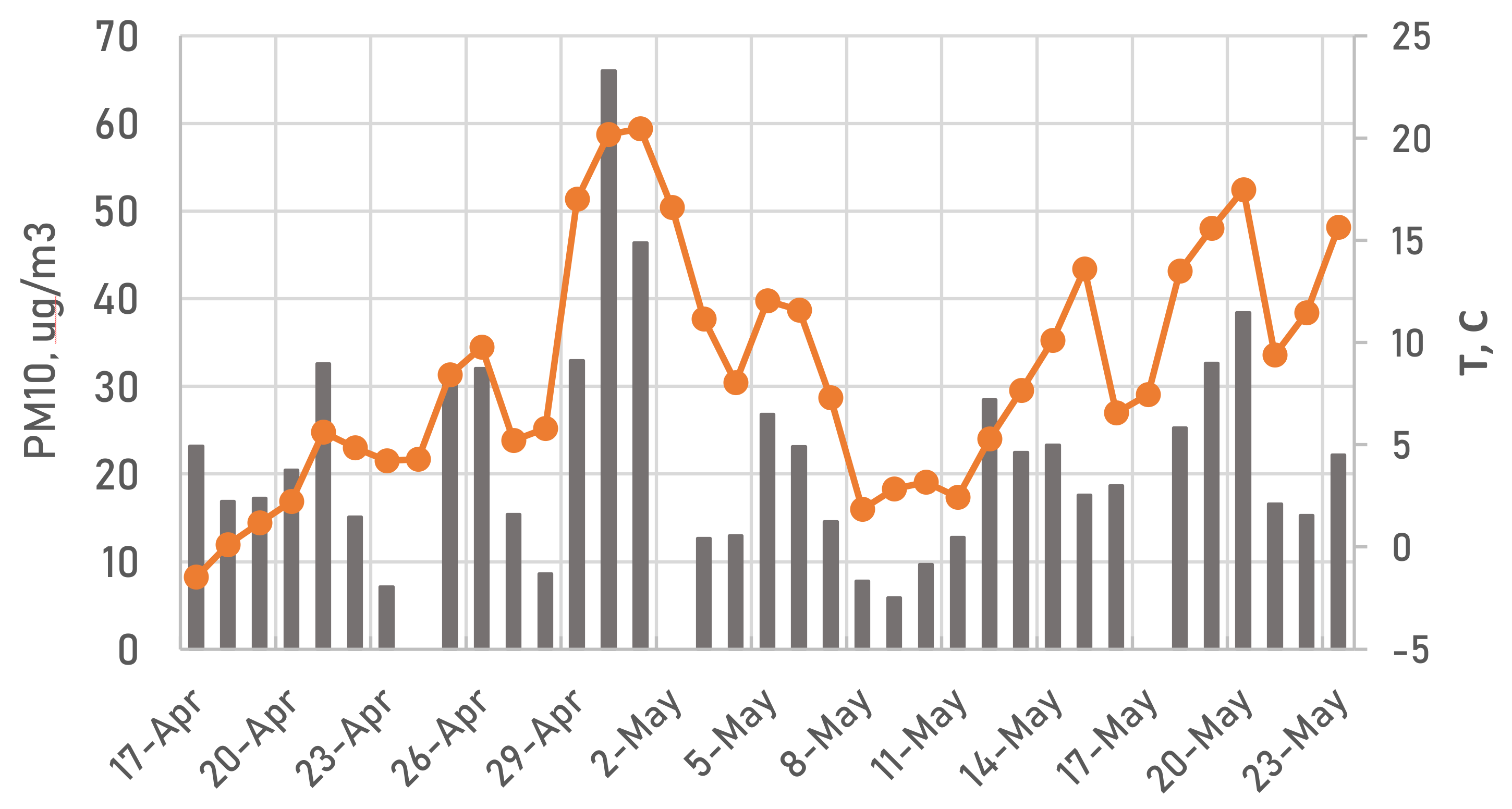
2.1. Ambient Sampling Campaign
2.2. Near-Gasoline Source Sampling Campaign
2.3. Methods and Techniques
2.4. Functional Markers
2.5. Principal Component Analyses
3. Results and Discussions
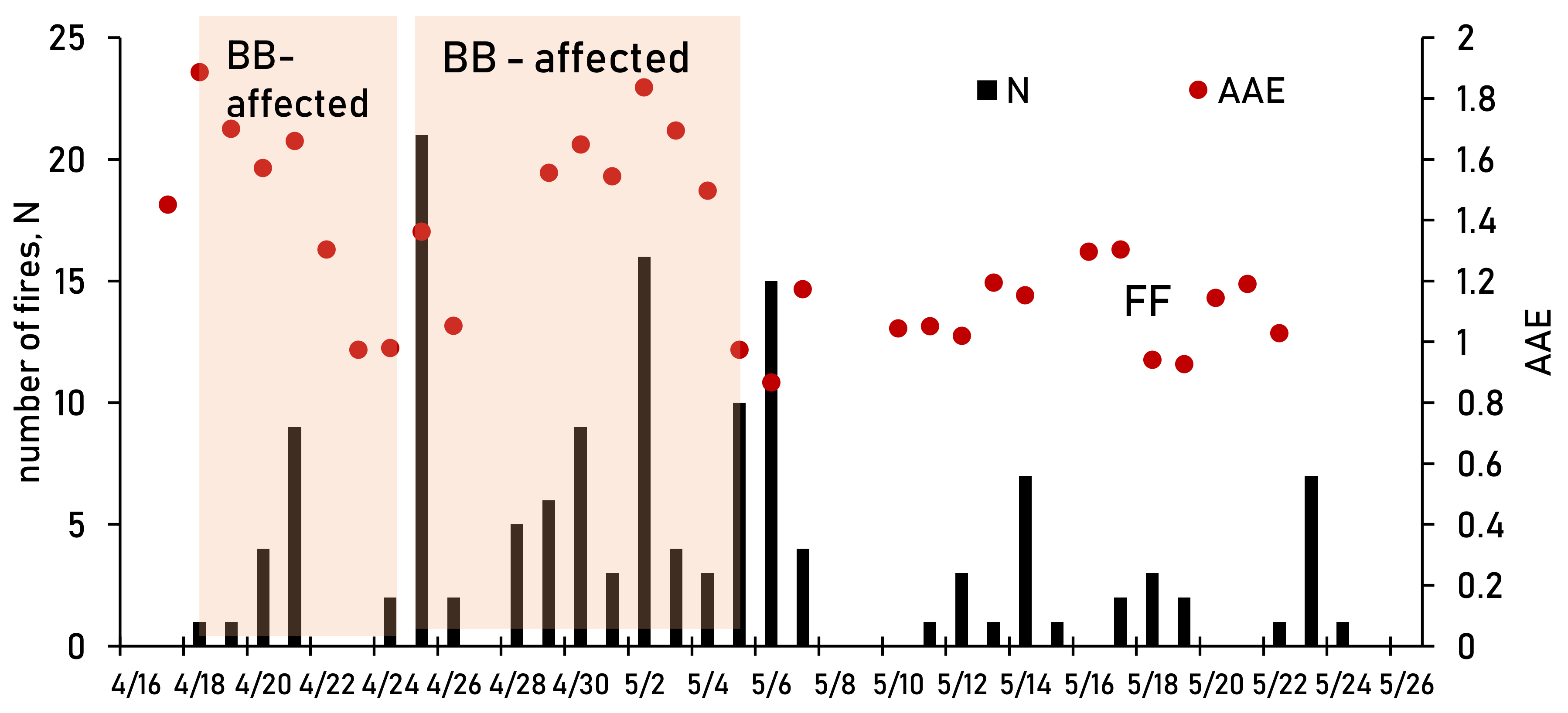
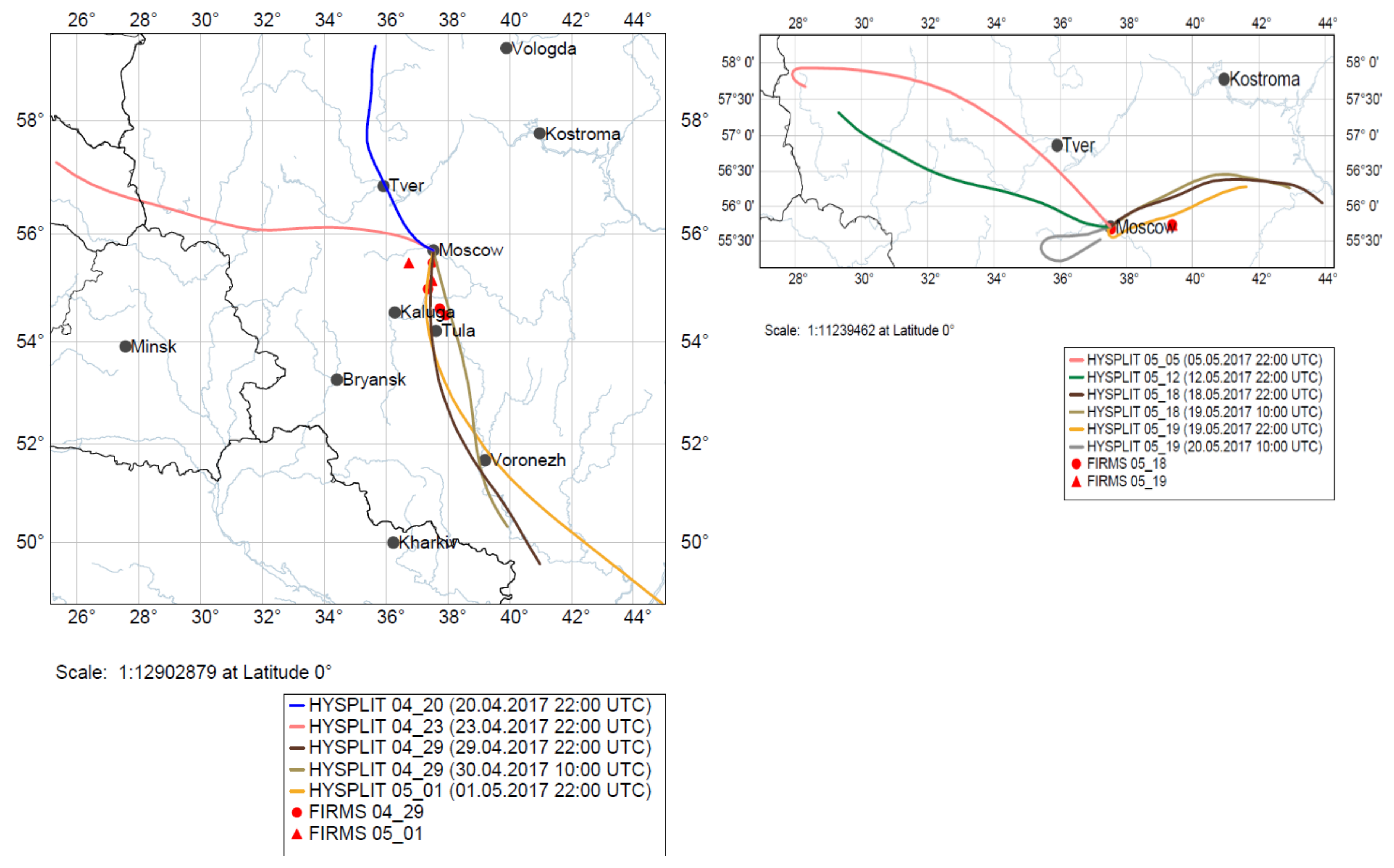
3.1. FF and BB—Affected Periods
3.2. FF-Related FTIR Spectral Features
3.3. BB-Related FTIR Spectral Features
3.4. Combined FTIR-PCA Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fuzzi, S.; Baltensperger, U.; Carslaw, K.; Decesari, S.; Denier Van Der Gon, H.; Facchini, M.; Fowler, D.; Koren, I.; Langford, B.; Lohmann, U. Part. matter, air quality and climate: Lessons learned and fure needs. Atmos. Chem. Phys. 2015, 15, 8217–8299. [Google Scholar] [CrossRef]
- Pope III, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.M.; Bahadur, R.; Hawkins, L.N.; Allan, J.; Baumgardner, D.; Quinn, P.K.; Bates, T.S. Organic aerosol characterization by complementary measurements of chemical bonds and molecular fragments. Atmos. Environ. 2009, 43, 6100–6105. [Google Scholar] [CrossRef]
- Russell, L.M.; Bahadur, R.; Ziemann, P.J. Identifying organic aerosol sources by comparing functional group composition in chamber and atmospheric particles. Proc. Natl. Acad. Sci. USA 2011, 108, 3516–3521. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, G.; Takahama, S. Development of chemoinformatic tools to enumerate functional groups in molecules for organic aerosol characterization. Atmos. Chem. Phys. 2016, 16, 4401–4422. [Google Scholar] [CrossRef]
- Liu, S.; Takahama, S.; Russell, L.; Gilardoni, S.; Baumgardner, D. Oxygenated organic functional groups and their sources in single and submicron organic particles in MILAGRO 2006 campaign. Atmos. Chem. Phys. Discuss. 2009, 9, 4567–4607. [Google Scholar] [CrossRef]
- Coury, C.; Dillner, A.M. ATR-FTIR characterization of organic functional groups and inorganic ions in ambient aerosols at a rural site. Atmos. Environ. 2009, 43, 940–948. [Google Scholar] [CrossRef]
- Steiner, S.; Czerwinski, J.; Comte, P.; Popovicheva, O.; Kireeva, E.; Müller, L.; Heeb, N.; Mayer, A.; Fink, A.; Rothen-Rutishauser, B. Comparison of the toxicity of diesel exhaust produced by bio-and fossil diesel combustion in human lung cells in vitro. Atmos. Environ. 2013, 81, 380–388. [Google Scholar] [CrossRef]
- Guzman-Morales, J.; Frossard, A.; Corrigan, A.; Russell, L.; Liu, S.; Takahama, S.; Taylor, J.; Allan, J.; Coe, H.; Zhao, Y. Estimated contributions of primary and secondary organic aerosol from fossil fuel combustion during the CalNex and Cal-Mex campaigns. Atmos. Environ. 2014, 88, 330–340. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Kireeva, E.D.; Shonija, N.K.; Vojtisek-Lom, M.; Schwarz, J. FTIR analysis of surface functionalities on particulate matter produced by off-road diesel engines operating on diesel and biofuel. Environ. Sci. Pollut. Res. 2015, 22, 4534–4544. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Irimiea, C.; Carpentier, Y.; Ortega, I.K.; Kireeva, E.D.; Shonija, N.K.; Schwarz, J.; Vojtíšek-Lom, M.; Focsa, C. Chemical composition of diesel/biodiesel particulate exhaust by ftir spectroscopy and mass spectrometry: Impact of fuel and driving cycle. Aerosol Air Qual. Res. 2017, 17, 1717–1734. [Google Scholar] [CrossRef]
- Weingartner, E.; Keller, C.; Stahel, W.; Burtscher, H.; Baltensperger, U. Aerosol emission in a road tunnel. Atmos. Environ. 1997, 31, 451–462. [Google Scholar] [CrossRef]
- Platt, S.M.; El Haddad, I.; Pieber, S.; Zardini, A.; Suarez-Bertoa, R.; Clairotte, M.; Daellenbach, K.; Huang, R.-J.; Slowik, J.; Hellebust, S. Gasoline cars produce more carbonaceous particulate matter than modern filter-equipped diesel cars. Sci. Rep. 2017, 7, 4926. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.; Arbuckle-Keil, G.; Dighton, J. FT-IR study of the changes in carbohydrate chemistry of three New Jersey pine barrens leaf litters during simulated control burning. Soil Biol. Biochem. 2009, 41, 340–347. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Ross, A.; Bates, J.; Andrews, G.; Jones, J.; Phylaktou, H.; Pourkashanian, M.; Williams, A. Emission of oxygenated species from the combustion of pine wood and its relation to soot formation. Process Saf. Environ. Prot. 2007, 85, 430–440. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Kozlov, V.S.; Rakhimov, R.F.; Shmargunov, V.P.; Kireeva, E.D.; Persiantseva, N.M.; Timofeev, M.A.; Engling, G.; Elephteriadis, K.; Diapouli, L.; et al. Optical-microphysical and physical-chemical characteristics of Siberian biomass burning: Small-scale fires in an aerosol chamber. Atmos. Ocean. Opt. 2016, 29, 492–500. [Google Scholar] [CrossRef]
- Maria, S.F.; Russell, L.M.; Turpin, B.J.; Porcja, R.J. FTIR measurements of functional groups and organic mass in aerosol samples over the Caribbean. Atmos. Environ. 2002, 36, 5185–5196. [Google Scholar] [CrossRef]
- Coury, C.; Dillner, A.M. A method to quantify organic functional groups and inorganic compounds in ambient aerosols using attenuated total reflectance FTIR spectroscopy and multivariate chemometric techniques. Atmos. Environ. 2008, 42, 5923–5932. [Google Scholar] [CrossRef]
- Kirchstetter, T.W.; Novakov, T.; Hobbs, P.V. Evidence that the spectral dependence of light absorption by aerosols is affected by organic carbon. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Healy, R.; Sofowote, U.; Su, Y.; Debosz, J.; Noble, M.; Jeong, C.-H.; Wang, J.; Hilker, N.; Evans, G.; Doerksen, G. Ambient measurements and source apportionment of fossil fuel and biomass burning black carbon in Ontario. Atmos. Environ. 2017, 161, 34–47. [Google Scholar] [CrossRef]
- Diapouli, E.; Kalogridis, A.-C.; Markantonaki, C.; Vratolis, S.; Fetfatzis, P.; Colombi, C.; Eleftheriadis, K. Annual variability of black carbon concentrations originating from biomass and fossil fuel combustion for the suburban aerosol in Athens, Greece. Atmosphere 2017, 8, 234. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Shonija, N.K.; Persiantseva, N.; Timofeev, M.; Diapouli, E.; Eleftheriadis, K.; Borgese, L.; Nguyen, X.A. Aerosol Pollutants during Agricultural Biomass Burning: A Case Study in Ba Vi Region in Hanoi, Vietnam. Aerosol Air Qual. Res. 2017, 17, 2762–2779. [Google Scholar] [CrossRef]
- Elliott, G.N.; Worgan, H.; Broadhurst, D.; Draper, J.; Scullion, J. Soil differentiation using fingerprint Fourier transform infrared spectroscopy, chemometrics and genetic algorithm-based feature selection. Soil Biol. Biochem. 2007, 39, 2888–2896. [Google Scholar] [CrossRef]
- Cadet, F.; Robert, C.; Offmann, B. Simultaneous determination of sugars by multivariate analysis applied to mid-infrared spectra of biological samples. Appl. Spectrosc. 1997, 51, 369–375. [Google Scholar] [CrossRef]
- Hori, R.; Sugiyama, J. A combined FT-IR microscopy and principal component analysis on softwood cell walls. Carbohydr. Polym. 2003, 52, 449–453. [Google Scholar] [CrossRef]
- Kulbachevsky, A.O. About environment state. In Moscow City in 2017; Department of Environmental Management and Protection: Moscow, Russia, 2018; 358 pages. [Google Scholar]
- Elansky, N.F.; Ponomarev, N.A.; Verevkin, Y.M. Air quality and pollutant emissions in the Moscow megacity in 2005–2014. Atmos. Environ. 2018, 175, 54–64. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, L.; Wang, S.; Wang, Y.; Sharma, S.; Shimadera, H.; Wang, X.; Bressi, M.; de Miranda, R.M.; Jiang, J.; et al. Status and characteristics of ambient PM2.5 pollution in global megacities. Environ. Int. 2016, 89–90, 212–221. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Kistler, M.; Kireeva, E.; Persiantseva, N.; Timofeev, M.; Kopeikin, V.; Kasper-Giebl, A. Physicochemical characterization of smoke aerosol during large-scale wildfires: Extreme event of August 2010 in Moscow. Atmos. Environ. 2014, 96, 405–414. [Google Scholar] [CrossRef]
- Chubarova, N.E.; Androsova, E.E.; Kirsanov, A.A.; Vogel, B.; Vogel, H.; Popovicheva, O.B.; Rivin, G.S. Aerosol and its radiative effects during the aeroradcity 2018 moscow experiment. Geogr. Environ. Sustain. 2019, 12, 114–131. [Google Scholar] [CrossRef][Green Version]
- Stein, A.; Draxler, R.; Rolph, G.; Stunder, B.; Cohen, M.; Ngan, F. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Vojtisek-Lom, M.; Pechout, M.; Dittrich, L.; Beránek, V.; Kotek, M.; Schwarz, J.; Vodička, P.; Milcová, A.; Rossnerová, A.; Ambrož, A. Polycyclic aromatic hydrocarbons (PAH) and their genotoxicity in exhaust emissions from a diesel engine during extended low-load operation on diesel and biodiesel fuels. Atmos. Environ. 2015, 109, 9–18. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. Appl. Theory Instrum. 2006. [Google Scholar] [CrossRef]
- Maria, S.; Russell, L.; Turpin, B.; Porcja, R.; Campos, T.; Weber, R.; Huebert, B. Source signatures of carbon monoxide and organic functional groups in Asian Pacific Regional Aerosol Characterization Experiment (ACE-Asia) submicron aerosol types. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Cain, J.P.; Gassman, P.L.; Wang, H.; Laskin, A. Micro-FTIR study of soot chemical composition—Evidence of aliphatic hydrocarbons on nascent soot surfaces. Phys. Chem. Chem. Phys. 2010, 12, 5206–5218. [Google Scholar] [CrossRef]
- Bladt, H.; Schmid, J.; Kireeva, E.D.; Popovicheva, O.B.; Perseantseva, N.M.; Timofeev, M.A.; Heister, K.; Uihlein, J.; Ivleva, N.P.; Niessner, R. Impact of Fe content in laboratory-produced soot aerosol on its composition, structure, and thermo-chemical properties. Aerosol Sci. Technol. 2012, 46, 1337–1348. [Google Scholar] [CrossRef]
- Rosen, H.; Novakov, T. Optical transmission through aerosol deposits on diffusely reflective filters: A method for measuring the absorbing component of aerosol particles. Appl. Opt. 1983, 22, 1265–1267. [Google Scholar] [CrossRef]
- Bond, T.C.; Bergstrom, R.W. Light absorption by carbonaceous particles: An investigative review. Aerosol Sci. Technol. 2006, 40, 27–67. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Engling, G.; Ku, I.-T.; Timofeev, M.A.; Shonija, N.K. Aerosol emissions from long-lasting smoldering of boreal peatlands: Chemical composition, markers, and microstructure. Aerosol Air Qual. Res. 2019, 19, 484–503. [Google Scholar] [CrossRef]
- Popovicheva, O.; Timofeev, M.; Persiantseva, N.; Jefferson, M.A.; Johnson, M.; Rogak, S.N.; Baldelli, A. Microstructure and chemical composition of particles from small-scale gas flaring. Aerosol Air Qual. Res. 2019, 19, 2205–2221. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Kireeva, E.D.; Steiner, S.; Rothen-Rutishauser, B.; Persiantseva, N.M.; Timofeev, M.A.; Shonija, N.K.; Comte, P.; Czerwinski, J. Microstructure and chemical composition of diesel and biodiesel particle exhaust. Aerosol Air Qual. Res. 2014, 14, 1392–1401. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Engling, G.; Lin, K.-T.; Persiantseva, N.; Timofeev, M.; Kireeva, E.; Voelk, P.; Hubert, A.; Wachtmeister, G. Diesel/biofuel exhaust particles from modern internal combustion engines: Microstructure, composition, and hygroscopicity. Fuel 2015, 157, 232–239. [Google Scholar] [CrossRef]
- Gentner, D.R.; Isaacman, G.; Worton, D.R.; Chan, A.W.; Dallmann, T.R.; Davis, L.; Liu, S.; Day, D.A.; Russell, L.M.; Wilson, K.R. Elucidating secondary organic aerosol from diesel and gasoline vehicles through detailed characterization of organic carbon emissions. Proc. Natl. Acad. Sci. USA 2012, 109, 18318–18323. [Google Scholar] [CrossRef] [PubMed]
- Giakoumis, E.G.; Rakopoulos, C.D.; Dimaratos, A.M.; Rakopoulos, D.C. Exhaust emissions of diesel engines operating under transient conditions with biodiesel fuel blends. Prog. Energy Combust. Sci. 2012, 38, 691–715. [Google Scholar] [CrossRef]
- Kalogridis, A.C.; Popovicheva, O.B.; Engling, G.; Diapouli, E.; Kawamura, K.; Tachibana, E.; Ono, K.; Kozlov, V.S.; Eleftheriadis, K. Smoke aerosol chemistry and aging of Siberian biomass burning emissions in a large aerosol chamber. Atmos. Environ. 2018, 185, 15–28. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Engling, G.; Diapouli, E.; Saraga, D.; Persiantseva, N.M.; Timofeev, M.A.; Kireeva, E.D.; Shonija, N.K.; Chen, S.-H.; Nguyen, D.-L.; et al. Impact of smoke intensity on size-resolved aerosol composition and microstructure during the biomass burning season in northwest Vietnam. Aerosol Air Qual. Res. 2016, 16, 2635–3654. [Google Scholar] [CrossRef]
- Anıl, I.; Golcuk, K.; Karaca, F. ATR-FTIR spectroscopic study of functional groups in aerosols: The contribution of a Saharan dust transport to urban atmosphere in Istanbul, Turkey. Water Air Soil Pollut. 2014, 225, 1898. [Google Scholar] [CrossRef]
- Ravisankar, R.; Kiruba, S.; Eswaran, P.; Senthilkumar, G.; Chandrasekaran, A. Mineralogical characterization studies of ancient potteries of Tamilnadu, India by FT-IR spectroscopic technique. J. Chem. 2010, 7, S185–S190. [Google Scholar] [CrossRef]
- Martınez, J.; Ruiz, F.; Vorobiev, Y.V.; Pérez-Robles, F.; González-Hernández, J. Infrared spectroscopy analysis of the local atomic structure in silica prepared by sol-gel. J. Chem. Phys. 1998, 109, 7511–7514. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Olson, M.R.; Victoria Garcia, M.; Robinson, M.A.; Van Rooy, P.; Dietenberger, M.A.; Bergin, M.; Schauer, J.J. Investigation of black and brown carbon multiple-wavelength-dependent light absorption from biomass and fossil fuel combustion source emissions. J. Geophys. Res. Atmos. 2015, 120, 6682–6697. [Google Scholar] [CrossRef]
- Fanning, P.E.; Vannice, M.A. A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon 1993, 31, 721–730. [Google Scholar] [CrossRef]
- Akhter, M.; Chughtai, A.; Smith, D. The structure of hexane soot I: Spectroscopic studies. Appl. Spectrosc. 1985, 39, 143–153. [Google Scholar] [CrossRef]
- Sauvain, J.-J.; Rossi, M.J. Quantitative aspects of the interfacial catalytic oxidation of dithiothreitol by dissolved oxygen in the presence of carbon nanoparticles. Environ. Sci. Technol. 2016, 50, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumdjian, H.; Saliba, N. Ion concentrations of PM10 and PM2.5 aerosols over the eastern Mediterranean region: Seasonal variation and source identification. Atmos. Chem. Phys. Discuss. Eur. Geosci. Union 2005, 5, 13053–13073. [Google Scholar] [CrossRef]
- Bauer, H.; Claeys, M.; Vermeylen, R.; Schueller, E.; Weinke, G.; Berger, A.; Puxbaum, H. Arabitol and mannitol as tracers for the quantification of airborne fungal spores. Atmos. Environ. 2008, 42, 588–593. [Google Scholar] [CrossRef]
- Pey, J.; Querol, X.; De la Rosa, J.; González-Castanedo, Y.; Alastuey, A.; Gangoiti, G.; de la Campa, A.S.; Alados-Arboledas, L.; Sorribas, M.; Pio, C. Characterization of a long range transport pollution episode affecting PM in SW Spain. J. Environ. Monit. 2008, 10, 1158–1171. [Google Scholar] [CrossRef]
- Agarwal, S.; Aggarwal, S.G.; Okuzawa, K.; Kawamura, K. Size distributions of dicarboxylic acids, ketoacids, α-dicarbonyls, sugars, WSOC, OC, EC and inorganic ions in atmospheric particles over Northern Japan: Implication for long-range transport of Siberian biomass burning and East Asian polluted aerosols. Atmos. Chem. Phys. 2010, 10, 5839–5858. [Google Scholar] [CrossRef]
- Cuccia, E.; Piazzalunga, A.; Bernardoni, V.; Brambilla, L.; Fermo, P.; Massabò, D.; Molteni, U.; Prati, P.; Valli, G.; Vecchi, R. Carbonate measurements in PM10 near the marble quarries of Carrara (Italy) by infrared spectroscopy (FT-IR) and source apportionment by positive matrix factorization (PMF). Atmos. Environ. 2011, 45, 6481–6487. [Google Scholar] [CrossRef]
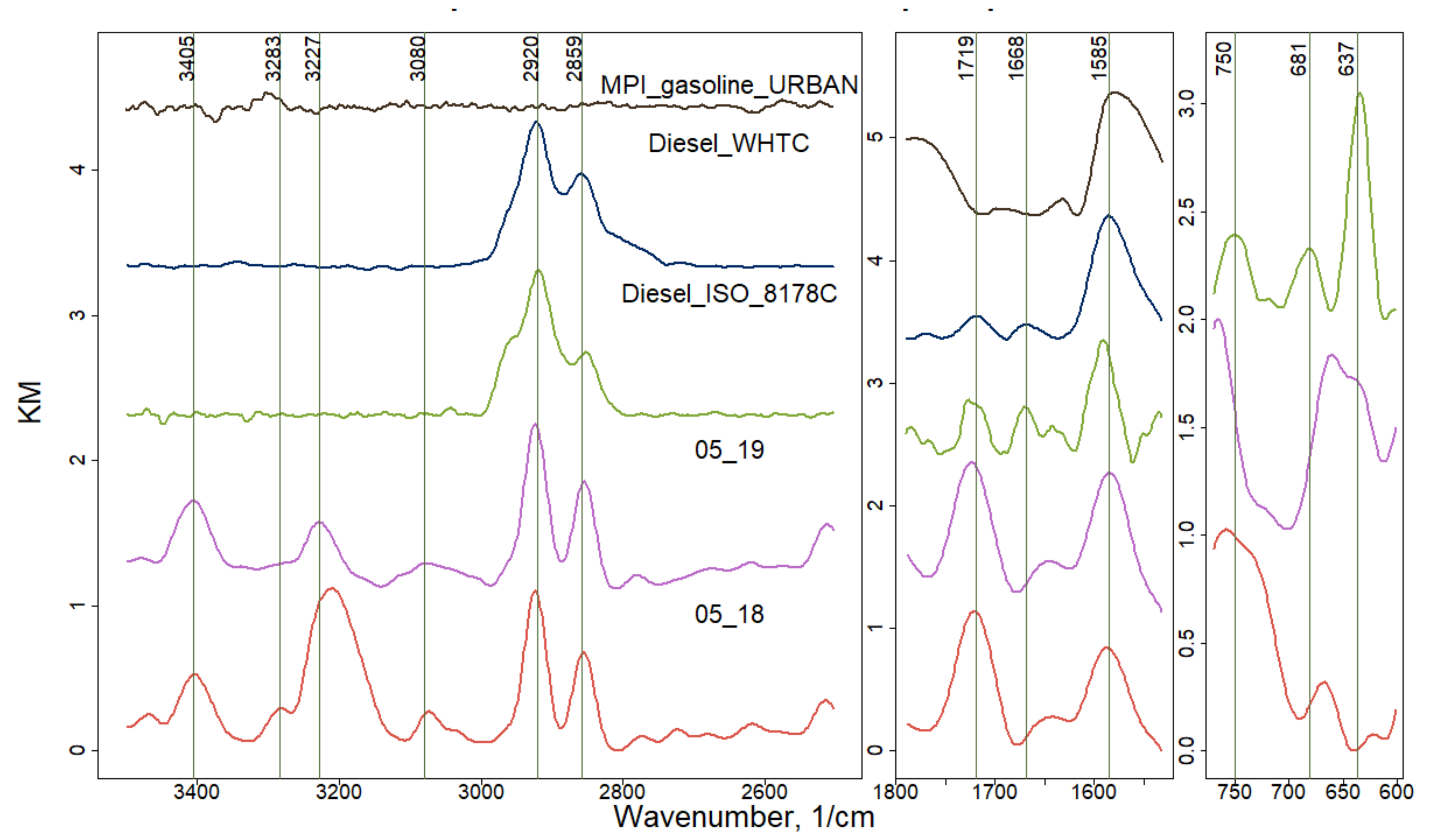
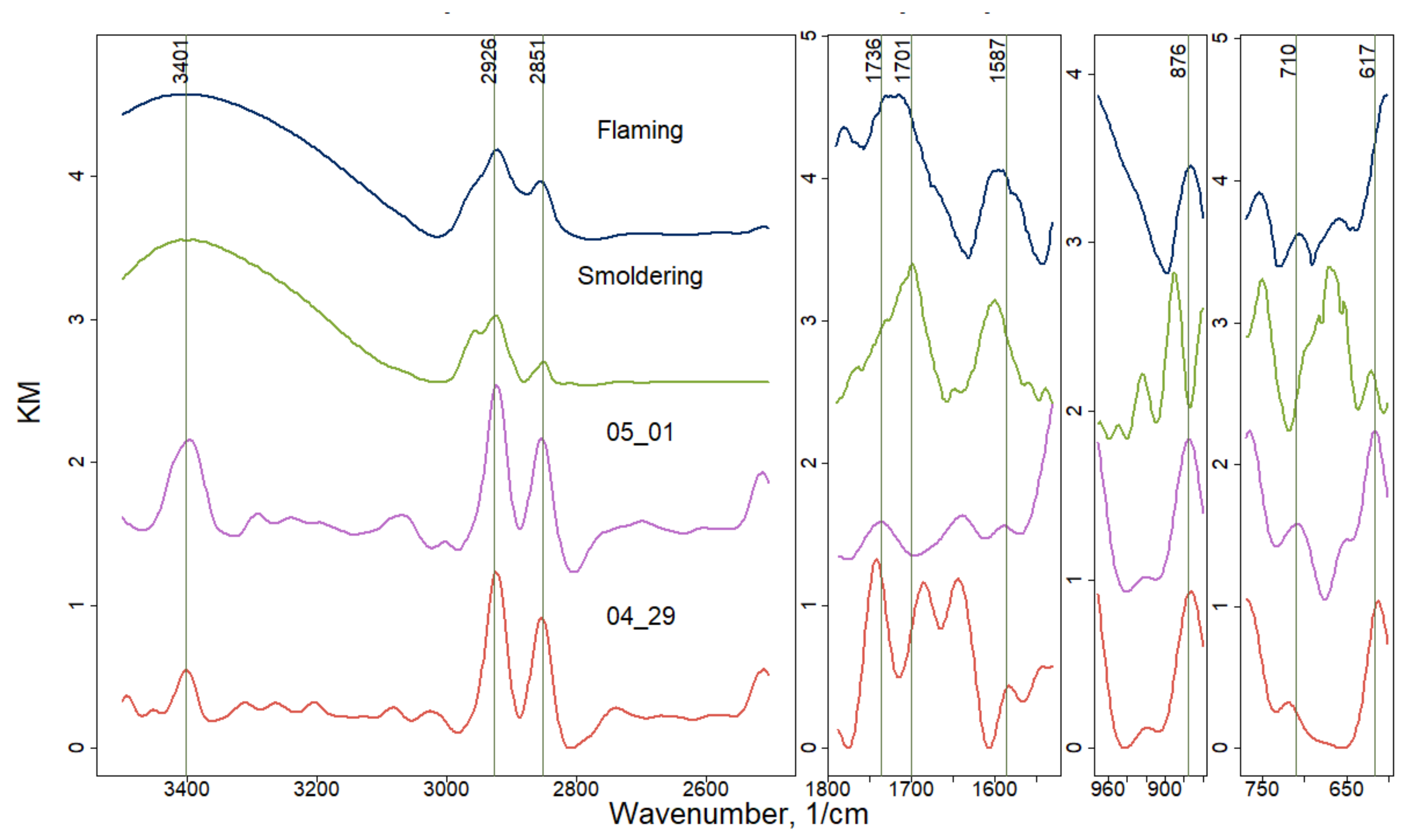
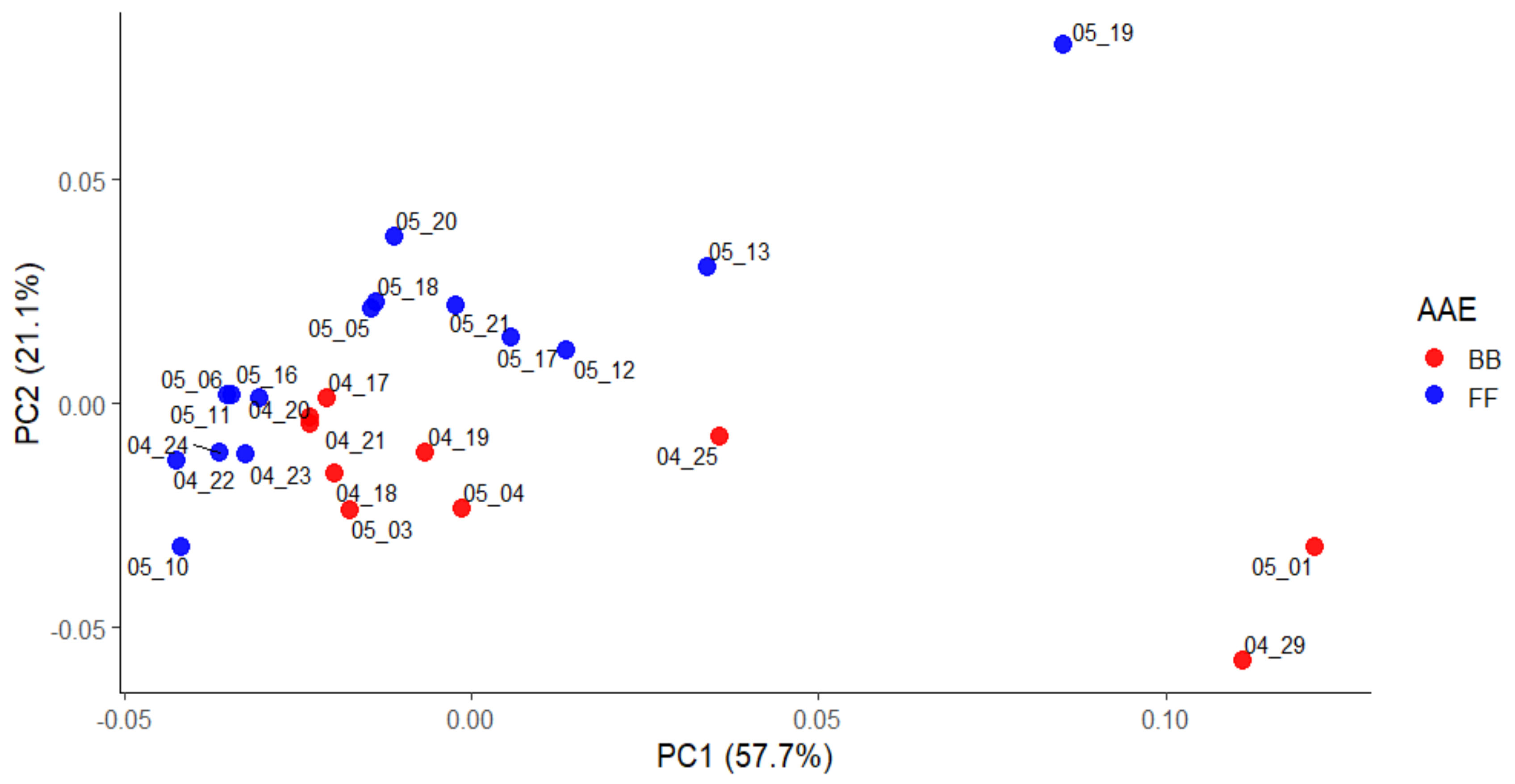
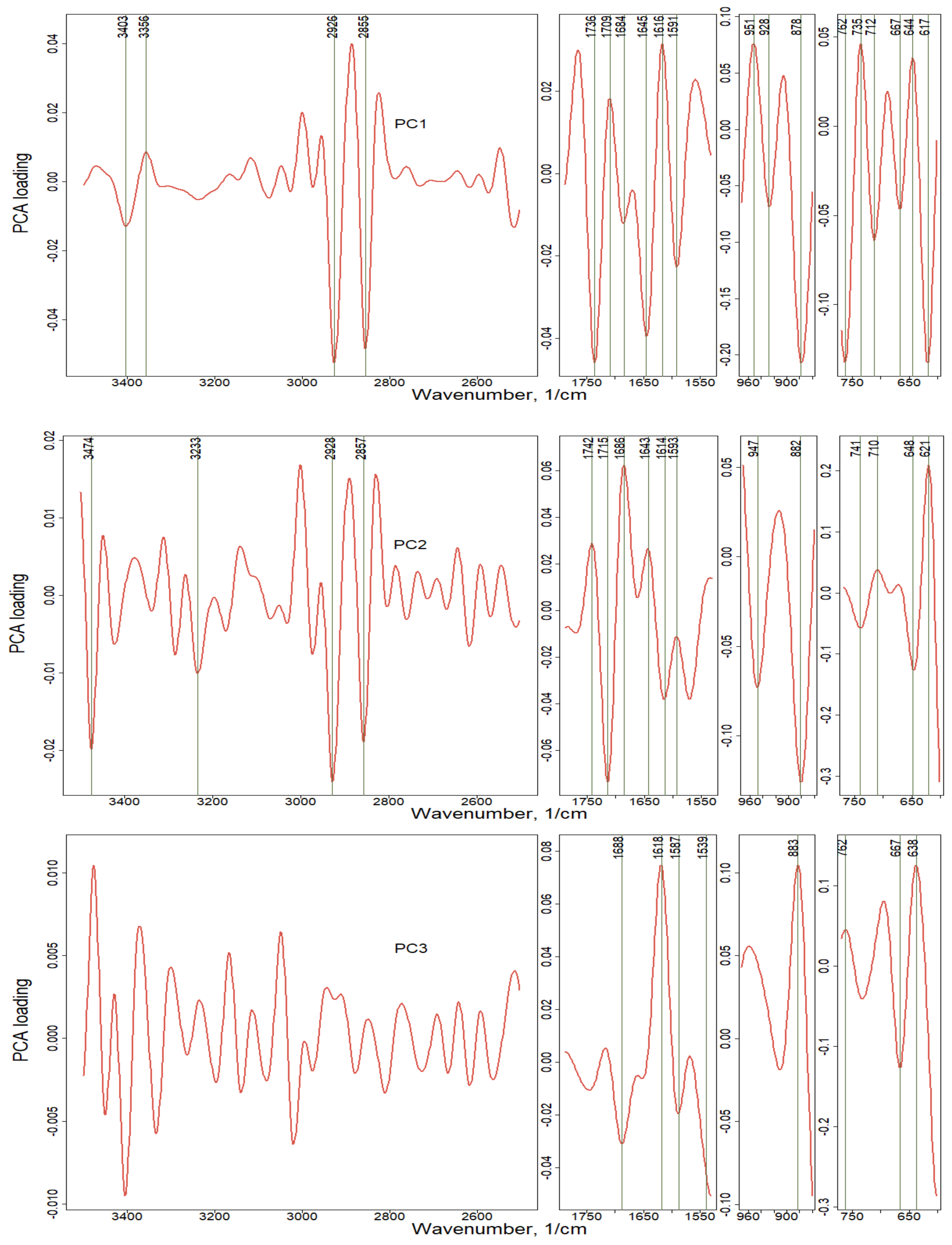
| Wavenumbers, cm−1 | Diesel/Gasoline Transport | Wavenumbers, cm−1 | Smoldering/Flaming |
|---|---|---|---|
| 637 | SO42− | 617-621 | SO42− |
| 750 | C=C-H | 762 | C=C-H |
| 1585 | C=C | 1530 | -NO2 |
| 1668 | C=O | 1618 | C=C |
| 1719 | C=O | 1730–1680 | C=O |
| 2920–2859 | C-C-H | 2926–2851 | C-C-H |
| ~3401 | O-H,N-H |
| Wavenumber, 1/cm | PC1 Loading (58%) | PC2 Loading (21%) | PC3 Loading (11%) | Functional Groups |
|---|---|---|---|---|
| 617 | −0.133 | SO42− sulphates | ||
| 621 | 0.21 | SO42− sulphates | ||
| 638 | 0.126 | SO42− sulfuric acid, sulphates | ||
| 644 | 0.038 | SO42− sulfuric acid | ||
| 648 | −0.125 | SO42− sulfuric acid, sulphates | ||
| 667 | −0.046 | −0.126 | SO42− sulphates, sulfuric acid | |
| 710 | 0.038 | CO32− carbonates; SO42− sulphates; C=C-H polyaromatics | ||
| 712 | −0.064 | CO32− carbonates; C=C-H polyaromatics | ||
| 735 | 0.046 | C=C-H polyaromatics | ||
| 741 | −0.058 | C=C-H polyaromatics | ||
| 762 | −0.132 | 0.046 | C=C-H polyaromatics | |
| 878 | −0.207 | CO32− carbonates; C=C-H polyaromatics | ||
| 882 | −0.126 | CO32− carbonates; C=C-H polyaromatics | ||
| 883 | 0.105 | CO32− carbonates; C=C-H polyaromatics | ||
| 928 | −0.068 | O-H organic acids | ||
| 947 | −0.073 | O-H organic acids | ||
| 951 | 0.076 | O-H organic acids | ||
| 1539 | −0.043 | -NO2 nitrocompounds | ||
| 1587 | −0.019 | N-H amino acid; -NO2 nitrocompounds | ||
| 1591 | −0.023 | N-H amino acid; C=C polyaromatics | ||
| 1593 | −0.011 | N-H amino acid; C=C polyaromatics | ||
| 1614 | −0.038 | C=C polyaromatics; N-H amino acid | ||
| 1616 | 0.032 | C=C polyaromatics; N-H amino acid | ||
| 1618 | 0.075 | C=C polyaromatics; N-H amino acid | ||
| 1643 | 0.027 | C=C alkenes | ||
| 1645 | −0.04 | C=C alkenes | ||
| 1684 | −0.012 | C=O aldehydes | ||
| 1686 | 0.063 | C=O aldehydes | ||
| 1688 | −0.031 | C=O aldehydes, carboxylic acid | ||
| 1709 | 0.018 | C=O carboxylic acid, ketones, aldehydes | ||
| 1715 | −0.074 | C=O carboxylic acid, ketones, aldehydes | ||
| 1736 | −0.046 | C=O esters, carboxylic acid | ||
| 1742 | 0.029 | C=O esters, carboxylic acid | ||
| 2855 | −0.049 | C-C-H aliphatic hydrocarbons; O-H organic acids | ||
| 2857 | −0.019 | C-C-H aliphatic hydrocarbons; O-H organic acids | ||
| 2926 | −0.053 | C-C-H aliphatic hydrocarbons; C=C alkenes | ||
| 2928 | −0.024 | C=C alkenes | ||
| 3233 | −0.01 | NH4+ ammonium | ||
| 3356 | 0.009 | N-H amino acid; O-H carbohydrates and alcohols | ||
| 3403 | −0.013 | N-H amines and amino acid; O-H alcohols | ||
| 3474 | −0.02 | O-H sugar, alcohols and carbohydrates |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovicheva, O.; Ivanov, A.; Vojtisek, M. Functional Factors of Biomass Burning Contribution to Spring Aerosol Composition in a Megacity: Combined FTIR-PCA Analyses. Atmosphere 2020, 11, 319. https://doi.org/10.3390/atmos11040319
Popovicheva O, Ivanov A, Vojtisek M. Functional Factors of Biomass Burning Contribution to Spring Aerosol Composition in a Megacity: Combined FTIR-PCA Analyses. Atmosphere. 2020; 11(4):319. https://doi.org/10.3390/atmos11040319
Chicago/Turabian StylePopovicheva, Olga, Alexey Ivanov, and Michal Vojtisek. 2020. "Functional Factors of Biomass Burning Contribution to Spring Aerosol Composition in a Megacity: Combined FTIR-PCA Analyses" Atmosphere 11, no. 4: 319. https://doi.org/10.3390/atmos11040319
APA StylePopovicheva, O., Ivanov, A., & Vojtisek, M. (2020). Functional Factors of Biomass Burning Contribution to Spring Aerosol Composition in a Megacity: Combined FTIR-PCA Analyses. Atmosphere, 11(4), 319. https://doi.org/10.3390/atmos11040319






