Air Pollution and Mortality: Timing Is Everything
Abstract
1. Introduction
Landmark Studies of Air Pollution and Mortality
- Well-defined end point.
- Identified victims, since 90% of deaths were “excess”.
- Autopsies showing relevant lung deposits.
- Identified pollutants and sources.
- Exposures widely distributed across the city including indoors.
- Reliable air quality monitoring.
2. Structures of Air Pollution Health Studies
2.1. Multiple Pollutant Modeling
2.2. Dose–Response Functions
3. Exposure Issues
3.1. Accuracy and Precision of Measurements
- Instrument errors include bias from instrument design and variability from external influences that may include the height of the sampler above the locations of personal exposure such as rooftops.
- Spatial bias may result from the distributions of emission sources surrounding the monitoring station. New York City deployed a dense network in the 1970s that reported the following ranges of variability within approximately 800 km2: SO2, 15–49 ppb; TSP, 61–106 μg/m3; SO42−, 13–20 μg/m3 [20]. These ranges were well within the variability of annual averages among cities at that time, such that a single reading should not be used in a long-term study. However, it would not be unreasonable to assume that they would all vary similarly in response to weather fluctuations.
- Temporal variability includes averaging time, diurnal and seasonal variability, and historical trends. Concentrations of all air pollutants are likely to fluctuate in concert from local weather fluctuations.
- Indoor air quality factors are perhaps the most important as I discussed below.
- Physical and temporal modeling may have the advantage of precision and widespread coverage at the expense of unknown biases.
3.2. Indoor Air Quality
3.3. Exposure Issues in Time-Series Modeling
3.4. Exposure Issues with Long-Term Studies
4. Comparisons between Short- and Long-Term Effects
5. Aging and Progression of Chronic Diseases
5.1. Cohort Subsets
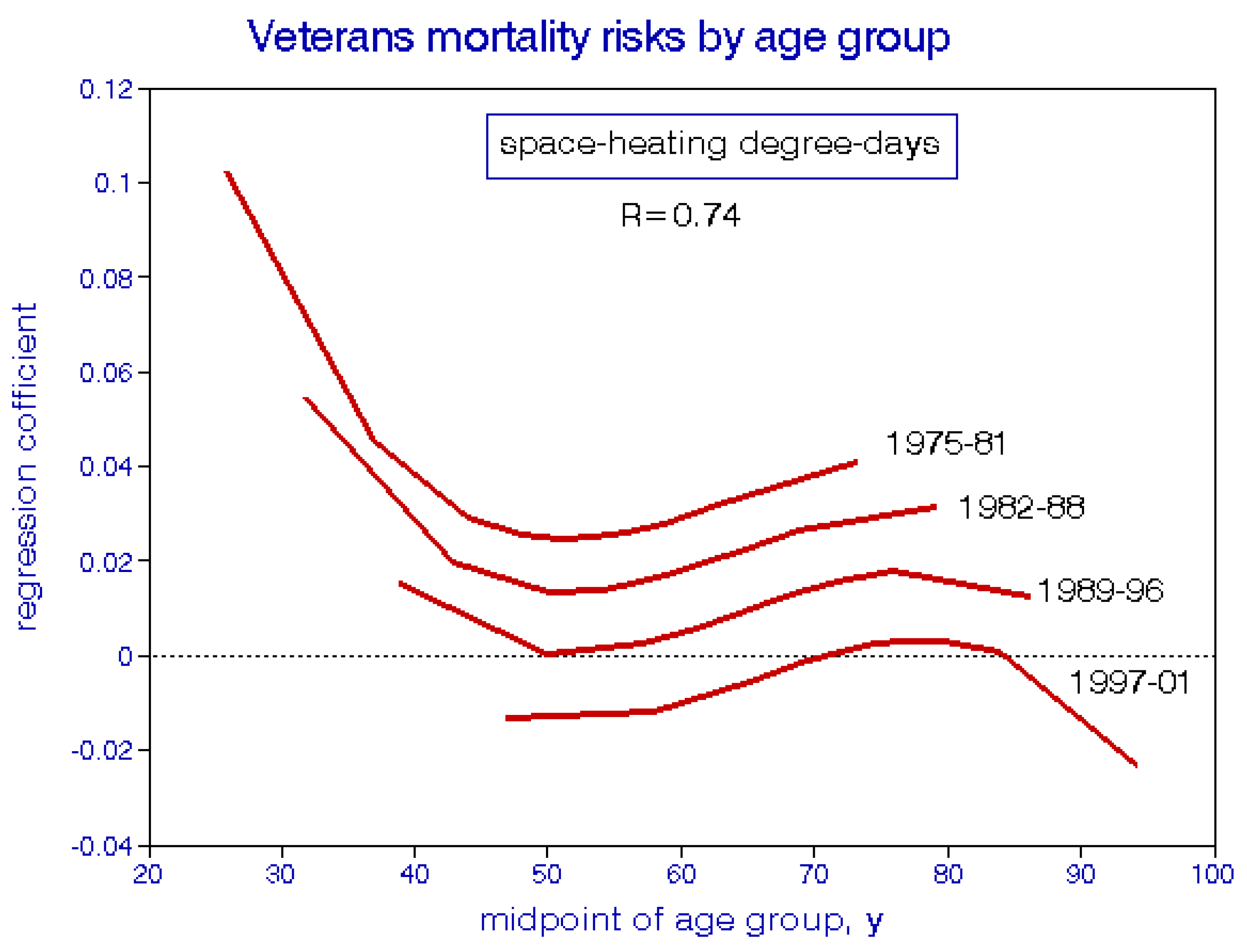
5.2. Age-Specific Mortality Analyses
6. Concluding Discussion
Funding
Conflicts of Interest
References
- Logan, W. Mortality in the London Fog Incident. Lancet 1953, 261, 336–338. [Google Scholar] [CrossRef]
- Lave, L.B.; Seskin, E.P. Air Pollution and Human Health; Johns Hopkins University Press: Baltimore, MD, USA, 1977. [Google Scholar]
- Lipfert, F.W. Statistical studies of mortality and air pollution multiple regression analyses by cause of death. Sci. Total. Environ. 1980, 16, 165–183. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An Association between Air Pollution and Mortality in Six U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Lipfert, F.W.; Perry, H.M., Jr.; Miller, J.P.; Baty, J.D.; Wyzga, R.E.; Carmody, S.E.; Perry, H.M. The Washington University–Epri Veterans’ Cohort Mortality Study: Preliminary Results. Inhal. Toxicol. 2000, 12, 41–73. [Google Scholar] [CrossRef]
- Lipfert, F.W.; Wyzga, R.E.; Baty, J.D.; Miller, J.P. Vehicular Traffic Effects on Survival within the Washington University–EPRI Veterans Cohort: New Estimates and Sensitivity Studies. Inhal. Toxicol. 2008, 20, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Lipfert, F.W.; Wyzga, R.E.; Baty, J.D.; Miller, J.P. Air pollution and survival within the Washington University-EPRI veterans cohort: Risks based on modeled estimates of ambient levels of hazardous and criteria air pollutants. J. Air Waste Manag. Assoc. 2009, 59, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Lipfert, F.W.; Wyzga, R.E. Environmental predictors of survival in a cohort of U.S. military veterans: A multi-level spatio-temporal analysis stratified by race. Environ. Res. 2020, 183, 108842. [Google Scholar] [CrossRef]
- Lipfert, F.W.; Wyzga, R.E. Revisiting the Veterans Cohort Mortality Study: New results and synthesis. J. Air Waste Manag. Assoc. 2018, 68, 1248–1268. [Google Scholar] [CrossRef]
- Christidis, T.; Erickson, A.C.; Pappin, A.J.; Crouse, D.L.; Pinault, L.L.; Weichenthal, S.A.; Brook, J.R.; Van Donkelaar, A.; Hystad, P.; Martin, R.V.; et al. Low concentrations of fine particle air pollution and mortality in the Canadian Community Health Survey cohort. Environ. Health 2019, 18, 1–16. [Google Scholar] [CrossRef]
- Weichenthal, S.; Crouse, D.L.; Pinault, L.; Godri-Pollitt, K.; Lavigne, E.; Evans, G.; Van Donkelaar, A.; Martin, R.V.; Burnett, R.T. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC). Environ. Res. 2016, 146, 92–99. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 6, 1132–1141. [Google Scholar] [CrossRef]
- Hansell, A.; Ghosh, R.E.; Blangiardo, M.; Perkins, C.; Vienneau, D.; Goffe, K.; Briggs, D.; Gulliver, J. Historic air pollution exposure and long-term mortality risks in England and Wales: Prospective longitudinal cohort study. Thorax 2016, 71, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Jerrett, M.; Burnett, R.T.; Ma, R.; Hughes, E.; Shi, Y.; Turner, M.C.; Pope, C.A., 3rd; Thurston, G.; Calle, E.E.; et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res. Rep. Health Eff. Inst. 2009, 140, 5–114; discussion 115–136. [Google Scholar]
- Lipfert, F.W.; Wyzga, R.E. Air Pollution and Mortality: Issues and Uncertainties. J. Air Waste Manag. Assoc. 1995, 45, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M. A Systematic Review of the Relation between Long-term Exposure to Ambient Air Pollution and Chronic Diseases. Rev. Environ. Health 2008, 23, 243–297. [Google Scholar] [CrossRef]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A.; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef]
- U.S Public Health Service. Air Pollution Measurements of the National Air Sampling Network, Analysis of Suspended Particulate Samples Collected 1953–1957; Public Health Service Publication, U.S. Government Printing Office: Washington, DC, USA, 1958.
- Lipfert, F.W.; Wyzga, R.E. Air pollution and mortality: The implications of uncertainties in regression modeling and exposure measurement. J. Air Waste Manag. Assoc. 1997, 47, 517–523. [Google Scholar] [CrossRef]
- Lipfert, F.W. The Association of Human Mortality with Air Pollution: Statistical Analyses by Region, by Age, and by Cause of Death. Ph.D. Thesis, Union Graduate School, Cincinnati, OH, USA, 1978. [Google Scholar]
- Mitsakou, C.; Dimitroulopoulou, S.; Heaviside, C.; Katsouyanni, K.; Samoli, E.; Rodopoulou, S.; Costa, C.; Almendra, R.; Santana, P.; Dell’Olmo, M.M.; et al. Environmental public health risks in European metropolitan areas within the EURO-HEALTHY project. Sci. Total. Environ. 2019, 658, 1630–1639. [Google Scholar] [CrossRef]
- Jerrett, M.; Newbold, K.B.; Burnett, R.T.; Thurston, G.; Lall, R.; Pope, C.A.; Ma, R.; De Luca, P.; Thun, M.; Calle, J.; et al. Geographies of uncertainty in the health benefits of air quality improvements. Stoch. Environ. Res. Risk Assess. 2007, 21, 511–522. [Google Scholar] [CrossRef]
- Lipfert, F.W. An Assessment of Air Pollution Exposure Information for Health Studies. Atmosphere 2015, 6, 1736–1752. [Google Scholar] [CrossRef]
- Avery, C.L.; Mills, K.T.; Williams, R.; McGraw, K.A.; Poole, C.; Smith, R.L.; Whitsel, E.A. Estimating Error in Using Residential Outdoor PM2.5 Concentrations as Proxies for Personal Exposures: A Meta-analysis. Environ. Health Perspect. 2010, 118, 673–678. [Google Scholar] [CrossRef]
- Jenkins, A.R.; Palausky, A.; Counts, R.W.; Bayne, C.K.; Dindal, A.B.; Guerin, M.R. Exposure to environmental tobacco smoke in sixteen cities in the United States as determined by personal breathing zone air sampling. J. Expo. Anal. Environ. Epidemiol. 1996, 6, 473–502. [Google Scholar] [PubMed]
- Murray, C.J.; Nelson, C.R. State-space modeling of the relationship between air quality and mortality. J. Air Waste Manag. Assoc. 2000, 50, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lipfert, F.W. A new time-series methodology for estimating relationships between elderly frailty, remaining life expectancy, and ambient air quality. Inhal. Toxicol. 2012, 24, 89–98. [Google Scholar] [CrossRef]
- Murray, C.J.; Lipfert, F.W. Inferring frail life expectancies in Chicago from daily fluctuations in elderly mortality. Inhal. Toxicol. 2013, 25, 461–479. [Google Scholar] [CrossRef]
- Di, Q.; Dai, L.; Wang, Y.; Zanobetti, A.; Choirat, C.; Schwartz, J.D.; Dominici, F. Association of Short-term Exposure to Air Pollution With Mortality in Older Adults. JAMA 2017, 318, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Kang, S.; Anderson, R.; Mills, I.; Walton, H. Epidemiological time series studies of PM2.5and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef]
- Zanobetti, A.; Schwartz, J.; Samoli, E.; Gryparis, A.; Touloumi, G.; Atkinson, R.; Le Tertre, A.; Bobros, J.; Celko, M.; Goren, A.; et al. The Temporal Pattern of Mortality Responses to Air Pollution: A Multicity Assessment of Mortality Displacement. Epidemiology 2002, 13, 87–93. [Google Scholar] [CrossRef]
- Lipfert, F.W.; Morris, S.C.; Wyzga, R.E. Daily mortality in the Philadelphia metropolitan area and size-classified particulate matter. J. Air Waste Manag. Assoc. 2000, 50, 1501–1513. [Google Scholar] [CrossRef][Green Version]
- Pope, C.A., 3rd; Thun, M.J.; Namboodiri, M.M.; Dockery, D.W.; Evans, J.S.; Speizer, F.E.; Heath, C.W., Jr. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am. J. Respir. Crit. Care Med. 1995, 151, 669–674. [Google Scholar] [CrossRef]
- Schwartz, J.; Dockery, D.W.; Neas, L.M. Is Daily Mortality Associated Specifically with Fine Particles? J. Air Waste Manag. Assoc. 1996, 46, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Greven, S.; Dominici, F.; Zeger, S.L. An Approach to the Estimation of Chronic Air Pollution Effects Using Spatio-Temporal Information. J. Am. Stat. Assoc. 2011, 106, 396–406. [Google Scholar] [CrossRef]
- Huangfu, P.; Atkinson, R. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 2020, 144, 105998. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Negri, E.; Gallus, S.; Boffetta, P.; Tramacere, I.; La Vecchia, C. Long-term particulate matter exposure and mortality: A review of European epidemiological studies. BMC Public Health 2009, 9, 453. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Butland, B.K.; Dimitroulopoulou, C.; Heal, M.R.; Stedman, J.R.; Carslaw, N.; Jarvis, D.; Heaviside, C.; Vardoulakis, S.; Walton, H.; et al. Long-term exposure to ambient ozone and mortality: A quantitative systematic review and meta-analysis of evidence from cohort studies. BMJ Open 2016, 6, e009493. [Google Scholar] [CrossRef] [PubMed]
- Lipfert, F.W.; Wyzga, R.E. Longitudinal relationships between lung cancer mortality rates, smoking, and ambient air quality: A comprehensive review and analysis. Crit. Rev. Toxicol. 2019, 49, 790–818. [Google Scholar] [CrossRef]
- Lipfert, F.W. Long-term associations of morbidity with air pollution: A catalog and synthesis. J. Air Waste Manag. Assoc. 2017, 68, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Lipfert, F.W. Temporal and spatial relations between age specific mortality and ambient air quality in the United States: Regression results for counties, 1960-97. Occup. Environ. Med. 2002, 59, 156–174. [Google Scholar] [CrossRef] [PubMed]

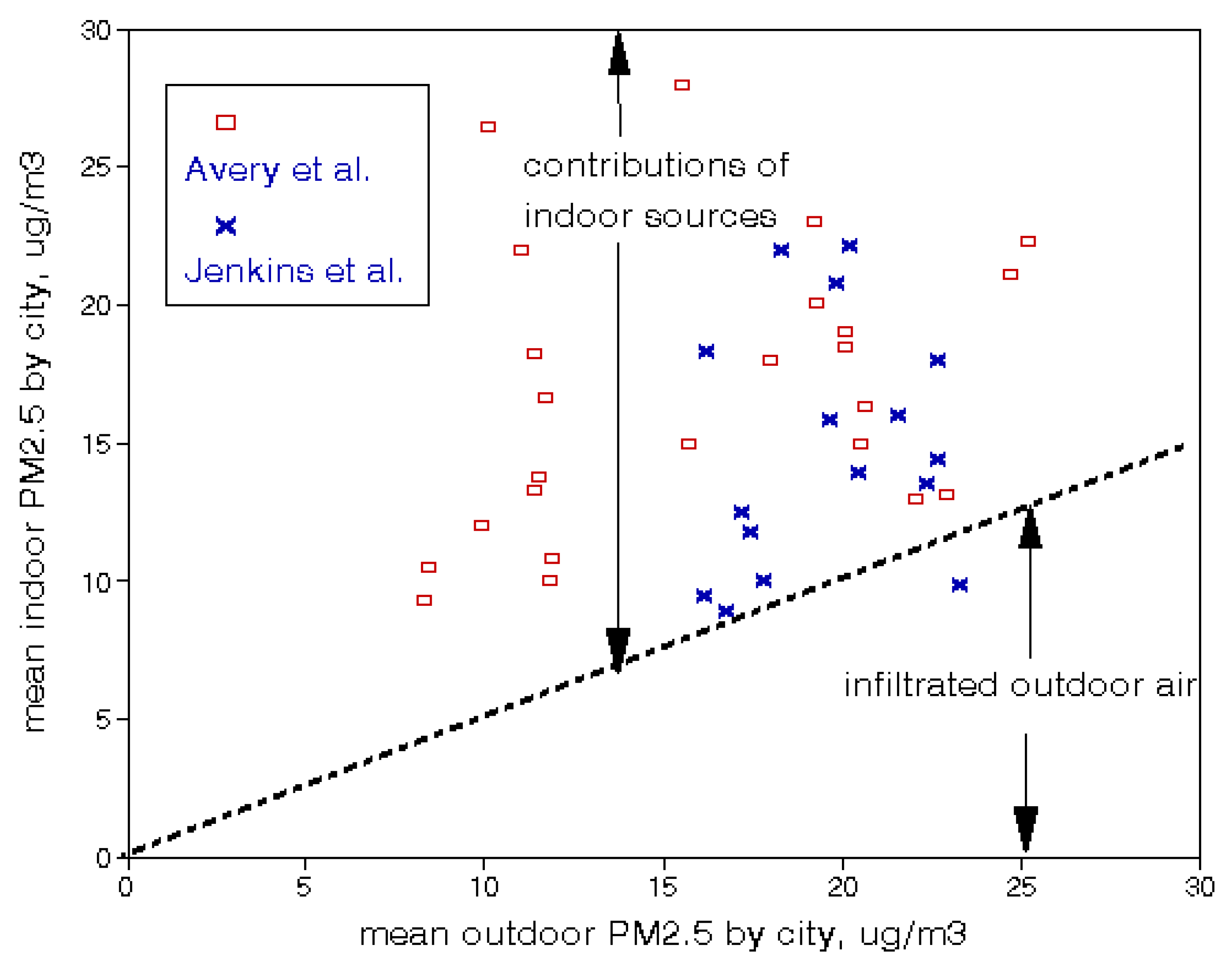

| PM2.5 | 8 μg/m3 |
| TSP | 25 μg/m3 |
| SO42− | 4 μg/m3 |
| H+ | 0 nmol/m3 |
| NO2 | 6 ppb |
| SO2 | 0 ppb |
| O3 | not determined |
| Authors | Location | Pollutants | RR (95% CIs) |
|---|---|---|---|
| Murray, Lipfert | Chicago | sum of PM10, O3, NO2, SO2, and CO | 1.024 (0.99–1.06) |
| Di et al. | entire US | PM2.5, O3 | 1.06 (1.04–1.07) |
| Atkinson et al. | various | PM2.5 | 1.06 (1.01–1.10) |
| Zanobetti et al. | European cities | PM10 | 1.048 (1.03–1.06) |
| Lipfert, Wyzga | Philadelphia | 45 different PM metrics | 1.058 (1.05–1.07) |
| Dockery | six cities | PM2.5 | 1.056 (1.041–1.072) |
| Exposure | Mortality Period | ||||||
|---|---|---|---|---|---|---|---|
| Period | Mean | 1976–81 | 1982–88 | 1989–96 | 1997–01 | 1976+ | |
| TSP | <1975 | 96 | 0.971 | 0.993 | 0.881 | 0.983 | |
| 1976–81 | 74 | 1.005 | 0.96 | 0.861 | 1.031 | ||
| 1982–88 | 59 | 1.052 | 0.991 | 1.057 | |||
| PM10 | 1989–96 | 39 | 1.101 | 1.165 | |||
| 1997–01 | 30 | 1.121 | |||||
| average (std dev) | 0.988 (0.024) | 1.002 (0.47) | 0.959 (0.11) | 1.059 (0.077) | |||
| NO2 | <1975 | 30 | 1.062 | 1.057 | 1.062 | 0.990 | |
| 1976–81 | 27 | 1.094 | 1.069 | 1.074 | 1.040 | ||
| 1982–88 | 24 | 1.032 | 1.035 | 0.995 | |||
| 1989–96 | 21 | 1.052 | 1.002 | ||||
| 1997–01 | 20 | 1.120 | 1.084 | ||||
| average (std dev) | 1.092 (0.029) | 1.071 (0.039) | 1.059 (0.016) | 1.049 (0.074) | |||
| peak O3 | <1975 | 132 | 0.95 | 0.981 | 0.978 | 0.931 | |
| 1976–81 | 140 | 1.086 | 1.084 | 0.992 | 1.082 | ||
| 1982–88 | 94 | 1.186 | 1.072 | 1.125 | |||
| 1989–96 | 85 | 1.210 | 1.225 | ||||
| 1997–01 | 83 | 1.247 | 1.058 | ||||
| average (std dev) | 1.018 (0.096) | 1.083 (0.10) | 1.070 (0.033) | 1.084 (0.053) | |||
| ln(traffic dens) | 1997 | 1.90 | 1.051 | 1.054 | 1.052 | 1.055 | 1.060 |
| Attribute | Short-Term Studies | Long-Term Studies |
|---|---|---|
| Identification of individuals | London, 1952 | no |
| Confirmation by autopsy | London, 1952 | no |
| Accounting for preconditions | frailty | no |
| Accounting for lag effects | yes | limited |
| Accounting for cumulative effects | yes | no |
| Support from morbidity studies | yes | limited |
| Control for confounders | yes (weather) | partial |
| Multi-pollutant analyses | limited | limited |
| Control for indoor exposures | infiltration only | no |
| Spatial exposure uncertainties | n/a | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipfert, F.W. Air Pollution and Mortality: Timing Is Everything. Atmosphere 2020, 11, 1274. https://doi.org/10.3390/atmos11121274
Lipfert FW. Air Pollution and Mortality: Timing Is Everything. Atmosphere. 2020; 11(12):1274. https://doi.org/10.3390/atmos11121274
Chicago/Turabian StyleLipfert, Frederick W. 2020. "Air Pollution and Mortality: Timing Is Everything" Atmosphere 11, no. 12: 1274. https://doi.org/10.3390/atmos11121274
APA StyleLipfert, F. W. (2020). Air Pollution and Mortality: Timing Is Everything. Atmosphere, 11(12), 1274. https://doi.org/10.3390/atmos11121274




