Short-Term Associations of Nitrogen Dioxide (NO2) on Mortality in 18 French Cities, 2010–2014
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Period and Study Area
2.2. Data Sources
2.2.1. Mortality Data
2.2.2. Pollution Data
2.2.3. Meteorological Data
2.2.4. Statistical Analyses
3. Results
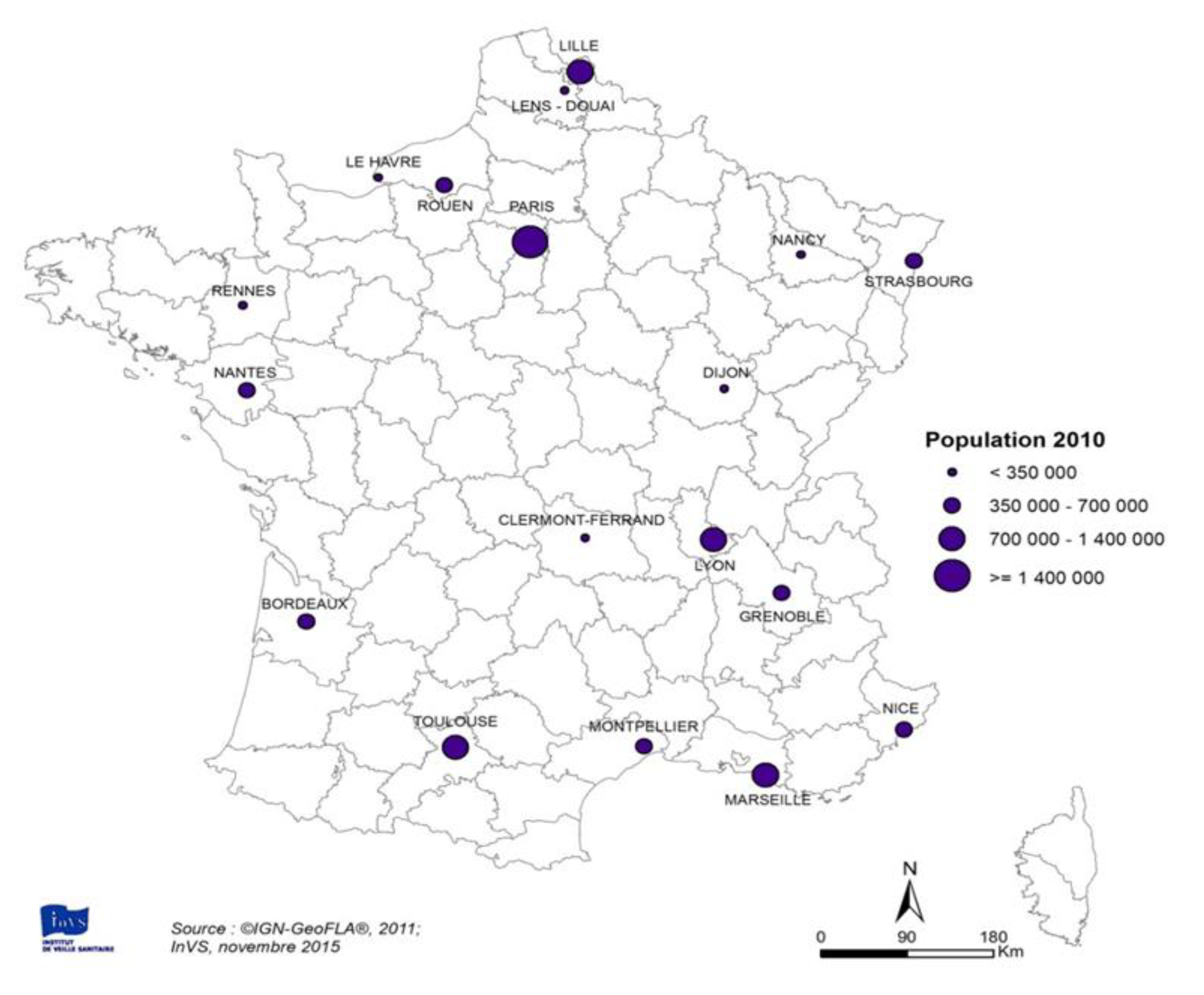
3.1. Main Characteristics of the Study Areas
3.1.1. Population Data
3.1.2. Mortality Data
3.1.3. Pollution Data
3.1.4. Temperature Data
3.2. Concentration-Response Functions
3.2.1. NO2 Analysis of the Same Day and the Day before (lag 0–1)
3.2.2. NO2 Analysis—Over the First Six Days (Lag 0–5)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

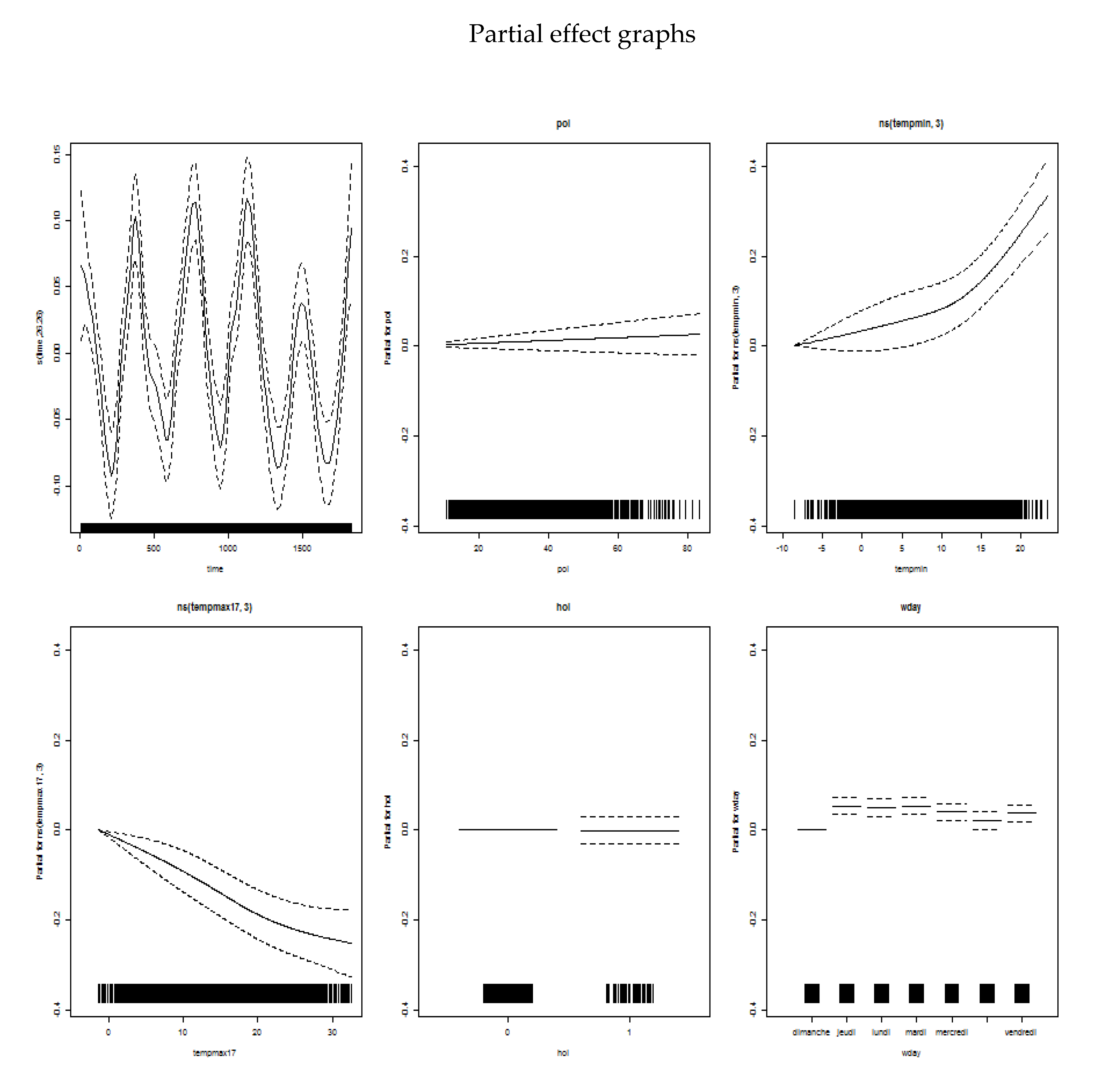
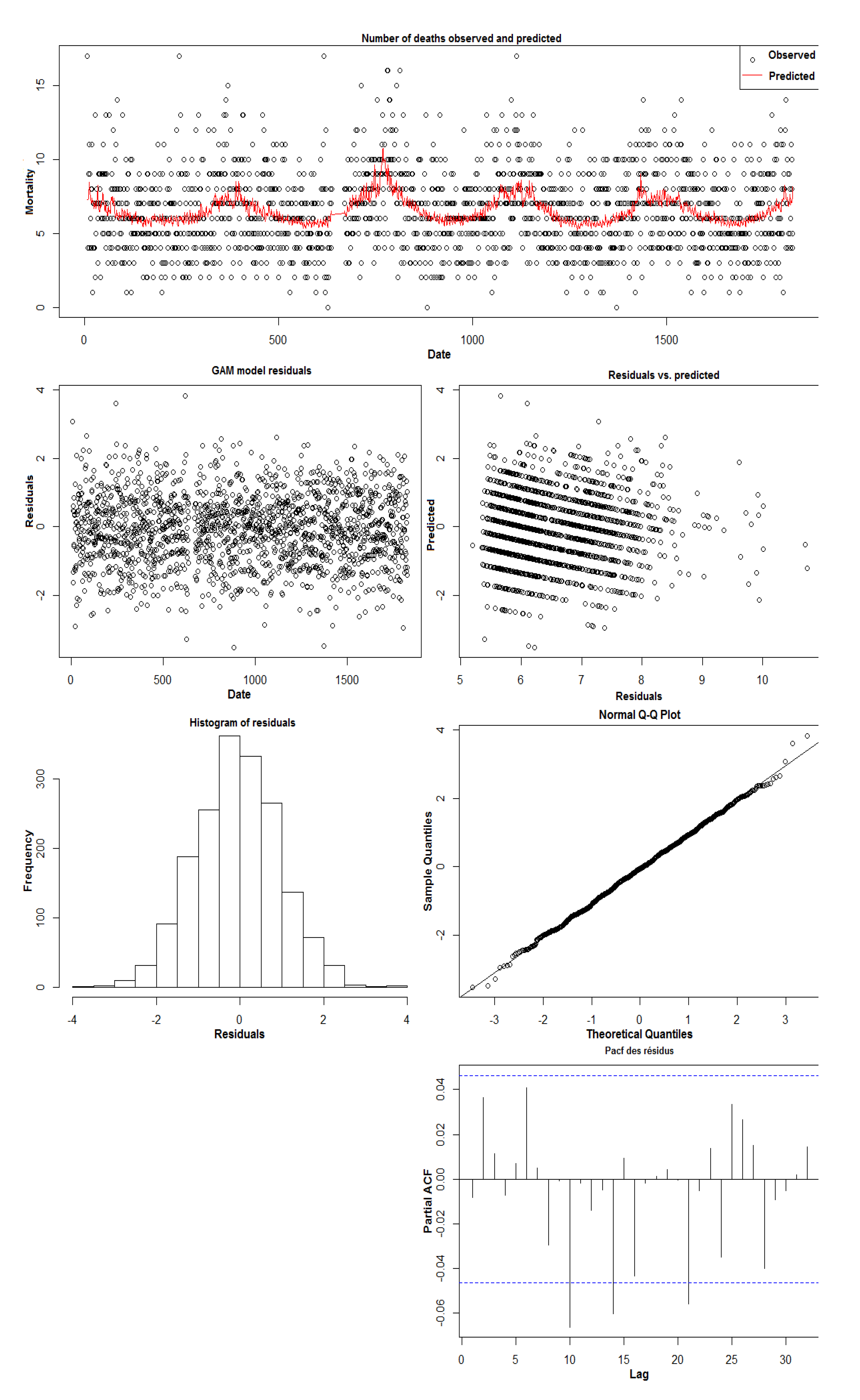
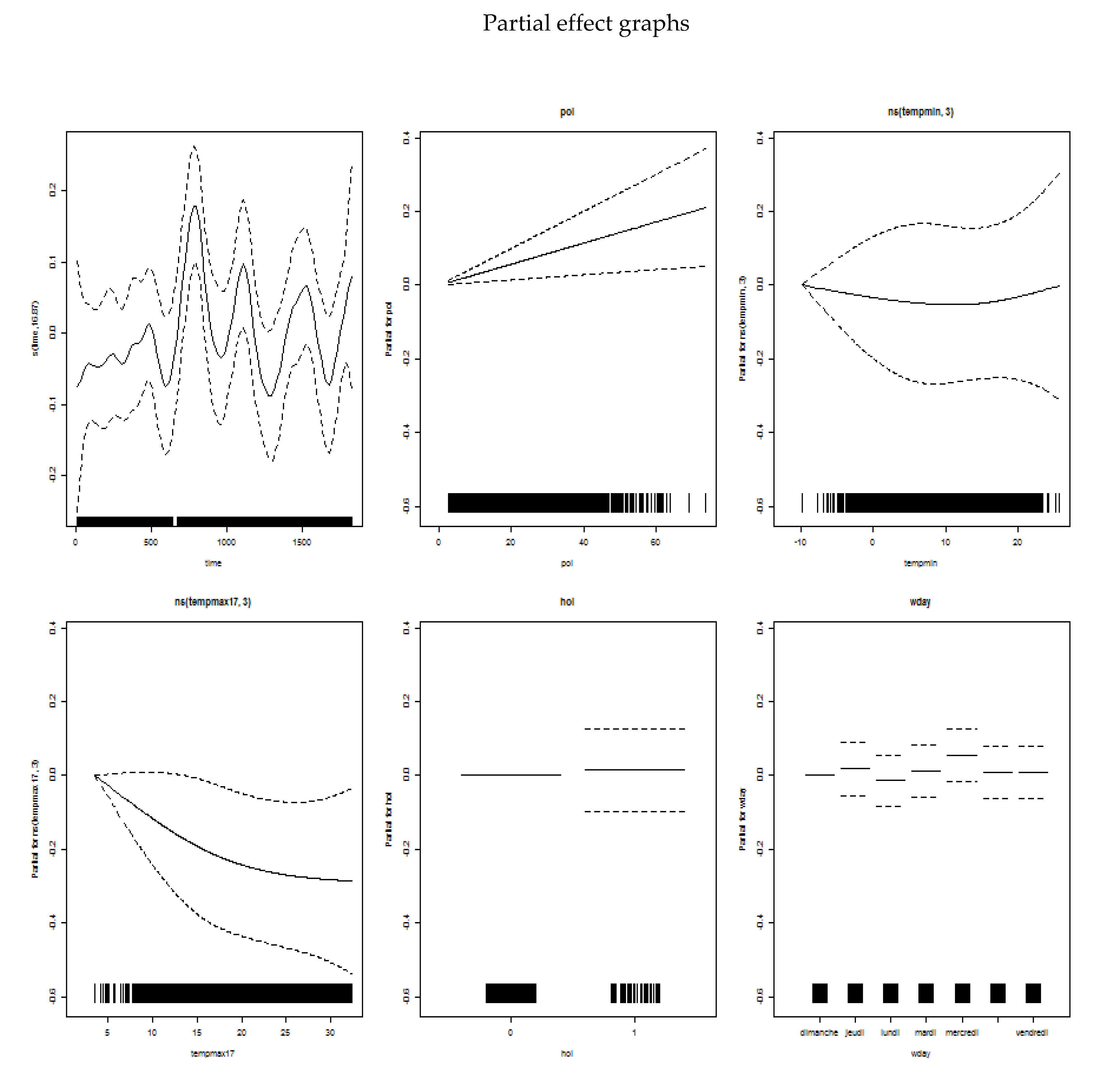
Appendix B
| Urban Area | Population Density (Inhabitants/km2) (2014 census) | Number of Municipalities |
|---|---|---|
| Bordeaux | 2056 | 22 |
| Clermont-Ferrand | 1950 | 16 |
| Dijon | 1455 | 15 |
| Grenoble | 1013 | 46 |
| Le Havre | 1287 | 16 |
| Lens-Douai | 1417 | 32 |
| Lille | 1854 | 85 |
| Lyon | 4679 | 19 |
| Marseille | 2214 | 8 |
| Montpellier | 1360 | 22 |
| Nancy | 1010 | 38 |
| Nantes | 1058 | 27 |
| Nice | 3634 | 4 |
| Paris | 8861 | 124 |
| Rennes | 2240 | 4 |
| Rouen | 1262 | 43 |
| Strasbourg | 2016 | 20 |
| Toulouse | 1537 | 51 |
References
- Ministry of Health. Bilan de la Qualité de l’air en France en 2016; French Ministry of Health: Paris, France, 2017; pp. 1–28.
- Mills, I.C.; Atkinson, R.W.; Kang, S.; Walton, H.; Anderson, H.R. Quantitative systematic review of the associations between short-term exposure to nitrogen dioxide and mortality and hospital admissions. BMJ Open 2015, 5, e006946. [Google Scholar] [CrossRef]
- WHO. Review of Evidence on Health Aspectes of Air Pollution—Revihaap Project; World Health Organisation: Copenhague, Danemark, 2013; pp. 1–309. [Google Scholar]
- Anderson, H.R.; Atkinson, R.W.; Bremner, S.A.; Carrington, J.; Peacock, J. Quantitative Systematic Review of Short Term Associations Between Ambient Air Pollution (Particulate Matter, Ozone, Nitrogen Dioxide, Sulphur Dioxide and Carbon Monoxide), and Mortality and Morbidity; Department of Health: London, UK, 2007; p. 706.
- Blanchard, M.; Borrelli, D.; Chardon, B.; Chatignoux, E.; Declercq, C.; Fabre, P.; Host, S.; Jusot, J.F.; Larrieu, S.; Lefranc, A.; et al. Programme de surveillance air et santé. In Analyse des Liens à Court Terme Entre Pollution Atmosphérique Urbaine et Mortalité Dans Neuf Villes Françaises; Institut de Veille Sanitaire: Saint-Maurice, France, 2008; p. 41. [Google Scholar]
- EPA. U.S. Integrated Science Assessment (ISA) for Oxides of Nitrogen-Health Criteria (Final Report, 2016); EPA/600/R-15/068; U.S. Environmental Protection Agency: Washington, DC, USA, 2016.
- Samoli, E.; Aga, E.; Touloumi, G.; Nisiotis, K.; Forsberg, B.; Lefranc, A.; Pekkanen, J.; Wojtyniak, B.; Schindler, C.; Niciu, E.; et al. Short-term effects of nitrogen dioxide on mortality: An analysis within the APHEA project. Eur. Respir. J. 2006, 27, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Faustini, A.; Rognoni, M.; Tessari, R.; Cadum, E.; Pacelli, B.; Pandolfi, P.; Miglio, R.; Mallone, S.; Vigotti, M.A.; et al. Air pollution and mortality in ten Italian cities. Results of the EpiAir Project. Epidemiol. Prev. 2009, 33 (Suppl. 1), 65–76. [Google Scholar] [PubMed]
- Perez, L.; Grize, L.; Infanger, D.; Kunzli, N.; Sommer, H.; Alt, G.M.; Schindler, C. Associations of daily levels of PM10 and NO2 with emergency hospital admissions and mortality in Switzerland: Trends and missed prevention potential over the last decade. Environ. Res. 2015, 140, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, M.; Moshammer, H.; Rabczenko, D. Acute and subacute effects of urban air pollution on cardiopulmonary emergencies and mortality: Time series studies in Austrian cities. Int. J. Environ. Res. Public Health 2013, 10, 4728–4751. [Google Scholar] [CrossRef]
- Corso, M.; Lagarrigue, R.; Medina, S. Pollution atmosphérique. In Guide Pour la Réalisation d’une Évaluation Quantitative des Impacts sur la Santé (EQIS). EQIS Avec une Exposition Mesurée; Santé Publique France: Saint-Maurice, France, 2019; p. 88. [Google Scholar]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; CRC Press: Boca Raton, FL, USA, 1990; Volume 43. [Google Scholar]
- Touloumi, G.; Samoli, E.; Pipikou, M.; Le Tertre, A.; Atkinson, R.; Katsouyanni, K. Seasonal confounding in air pollution and health time-series studies: Effect on air pollution effect estimates. Stat. Med. 2006, 25, 4164–4178. [Google Scholar] [CrossRef]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklöv, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Pascal, M.; Wagner, V.; Corso, M.; Laaidi, K.; Ung, A.; Beaudeau, P. Heat and cold related-mortality in 18 French cities. Environ. Int. 2018, 121, 189–198. [Google Scholar] [CrossRef]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Distributed lag non-linear models. Stat. Med. 2010, 29, 2224–2234. [Google Scholar] [CrossRef] [Green Version]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat. Med. 2012, 31, 3821–3839. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Prüss-Ustün, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- EPA. U.S. Final Report: Relative Toxicity of Air Pollution Mixtures U.S.; Environmental Protection Agency: Washington, DC, USA, 2016.
- Diaz, E.A.; Chung, Y.; Long, M.S.; Lawrence, J.; Papapostolou, V.; Lamoureux, D.; Mazzaro, V.; Buonfiglio, H.; Yoo, P.S.; Franco, F.; et al. Toxicological evaluation of realistic emissions source aerosols (TERESA): Mobile sources—Tunnel studies. In B52. Toxicology of Occupational and Environnemental Disease; American Thoracic Society: New York, NY, USA, 2011; p. A3231. [Google Scholar]
- Diaz, E.A.; Chung, Y.; Papapostolou, V.; Lawrence, J.; Long, M.S.; Hatakeyama, V.; Gomes, B.; Calil, Y.; Sato, R.; Koutrakis, P.; et al. Effects of fresh and aged vehicular exhaust emissions on breathing pattern and cellular responses--pilot single vehicle study. Inhal. Toxicol. 2012, 24, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Petit, P.C.; Fine, D.H.; Vásquez, G.B.; Gamero, L.; Slaughter, M.S.; Dasse, K.A. The Pathophysiology of Nitrogen Dioxide During Inhaled Nitric Oxide Therapy. ASAIO J. 2017, 63, 7–13. [Google Scholar] [CrossRef]
- Carugno, M.; Consonni, D.; Randi, G.; Catelan, D.; Grisotto, L.; Bertazzi, P.A.; Biggeri, A.; Baccini, M. Air pollution exposure, cause-specific deaths and hospitalizations in a highly polluted Italian region. Environ. Res. 2016, 147, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Renzi, M.; Stafoggia, M.; Faustini, A.; Cesaroni, G.; Cattani, G.; Forastiere, F. Analysis of Temporal Variability in the Short-term Effects of Ambient Air Pollutants on Nonaccidental Mortality in Rome, Italy (1998–2014). Environ. Health Perspect. 2017, 125, 067019. [Google Scholar] [CrossRef] [Green Version]
- Crouse, D.L.; Peters, P.A.; Hystad, P.; Brook, J.R.; Van Donkelaar, A.; Martin, R.V.; Villeneuve, P.J.; Jerrett, M.; Goldberg, M.S.; Pope, C.A., III; et al. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the Canadian Census Health and Environment Cohort (CanCHEC). Environ. Health Perspect. 2015, 123, 1180–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Ding, R.; Xiao, C.; Xu, Y.; Cheng, H.; Zhu, F.; Lei, R.; Di, D.; Zhao, Q.; Cao, J. Association between air pollution and cardiovascular mortality in Hefei, China: A time-series analysis. Environ. Pollut. 2017, 229, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhou, L.; Xu, Y.; Zheng, T.; Guo, Y.; Wellenius, G.A.; Bassig, B.A.; Chen, X.; Wang, H.; Zheng, X. Short-term effects of air pollution on daily mortality and years of life lost in Nanjing, China. Sci. Total Environ. 2015, 536, 123–129. [Google Scholar] [CrossRef]
- Chiusolo, M.; Cadum, E.; Stafoggia, M.; Galassi, C.; Berti, G.; Faustini, A.; Bisanti, L.; Vigotti, M.A.; Dessi, M.P.; Cernigliaro, A.; et al. Short-Term Effects of Nitrogen Dioxide on Mortality and Susceptibility Factors in 10 Italian Cities: The EpiAir Study. Environ. Health Perspect. 2011, 119, 1233–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichmann, J.; Voyi, K. Ambient air pollution exposure and respiratory, cardiovascular and cerebrovascular mortality in Cape Town, South Africa: 2001–2006. Int. J. Environ. Res. Public Health 2012, 9, 3978–4016. [Google Scholar] [CrossRef]
- Shin, H.H.; Stieb, D.; Burnett, R.; Takahara, G.; Jessiman, B. Tracking national and regional spatial-temporal mortality risk associated with NO2 concentrations in Canada: A Bayesian hierarchical two-level model. Risk Anal. 2012, 32, 513–530. [Google Scholar] [CrossRef]
- Chen, R.; Samoli, E.; Wong, C.M.; Huang, W.; Wang, Z.; Chen, B.; Kan, H. Associations between short-term exposure to nitrogen dioxide and mortality in 17 Chinese cities: The China Air Pollution and Health Effects Study (CAPES). Environ. Int. 2012, 45, 32–38. [Google Scholar] [CrossRef]
- Park, A.K.; Hong, Y.C.; Kim, H. Effect of changes in season and temperature on mortality associated with air pollution in Seoul, Korea. J. Epidemiol. Community Health 2011, 65, 368–375. [Google Scholar] [CrossRef]
- Wong, C.M.; Vichit-Vadakan, N.; Vajanapoom, N.; Ostro, B.; Thach, T.Q.; Chau, P.Y.; Chan, E.K.; Chung, R.Y.; Ou, C.Q.; Yang, L.; et al. Part 5. Public health and air pollution in Asia (PAPA): A combined analysis of four studies of air pollution and mortality. Res. Rep. Health Eff. Inst. 2010, 377–418. [Google Scholar] [PubMed]
- Costa, A.F.; Hoek, G.; Brunekreef, B.; Ponce de Leon, A.C.M. Effects of NO2 exposure on daily mortality in Sao Paulo, Brazil. Environ. Res. 2017, 159, 539–544. [Google Scholar] [CrossRef]
- Mills, I.C.; Atkinson, R.W.; Anderson, H.R.; Maynard, R.L.; Strachan, D.P. Distinguishing the associations between daily mortality and hospital admissions and nitrogen dioxide from those of particulate matter: A systematic review and meta-analysis. BMJ Open 2016, 6, e010751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Chen, R.; Meng, X.; Zhao, Z.; Cai, J.; Wang, C.; Yang, C.; Kan, H. Short-term exposure to ambient air pollution and coronary heart disease mortality in 8 Chinese cities. Int. J. Cardiol. 2015, 197, 265–270. [Google Scholar] [CrossRef]


| Study Area | Daily Mean NO2 Levels | Daily Mean PM10 Levels | Daily Maximum O3 from 8 h Running Means Levels | |||
|---|---|---|---|---|---|---|
| Mean (Min–Max) (µg m−3) | Number of Monitoring Stations | Mean (Min–Max) (µg m−3) | Number of Monitoring Stations | Mean (Min–Max) (µg m−3) | Number of Monitoring Stations | |
| Bordeaux | 19.6 (2.0; 67.9) | 3 | 21.9 (6.2; 88.3) | 3 | 81.3 (3.7; 175.3) | 3 |
| Clermont-Ferrand | 24.0 (2.1; 104.1) | 2 | 19.9 (2.4; 86.5) | 2 | 72.6 (1.7; 153.5) | 2 |
| Dijon | 22.5 (2.4; 73.1) | 4 | 18.9 (2.9; 81.3) | 1 | 69.6 (4.0; 156.0) | 4 |
| Grenoble | 23.8 (2.7; 80.8) | 3 | 24.1 (3.5; 90.7) | 3 | 63.0 (1.2; 157.1) | 6 |
| Le Havre | 22.4 (2.0; 79.0) | 1 | 22.4 (4.0; 96.0) | 1 | 70.8 (3.8; 178.1) | 1 |
| Lens-Douai | 21.7 (1.0; 81.0) | 2 | 24.2 (3.0; 97.6) | 2 | 55.5 (0.8; 175.5) | 2 |
| Lille | 25.3 (3.5; 80.3) | 3 | 23.6 (4.9; 100.8) | 3 | 57.5 (1.0; 193.6) | 5 |
| Lyon | 31.1 (4.3; 96.3) | 4 | 25.2 (5.0; 98.3) | 3 | 64.8 (0.5; 184.4) | 4 |
| Marseille | 32.7 (4.5; 82.3) | 4 | 31.2 (6.0; 105.5) | 2 | 77.6 (4.5; 178.0) | 2 |
| Montpellier | 25.9 (1.1; 79.5) | 2 | 20.5 (3.7; 77.1) | 1 | 80.0 (13.3; 156.9) | 3 |
| Nancy | 24.4 (4.7; 67.1) | 3 | 24.9 (4.6; 91.4) | 2 | 68.6 (1.9; 164.9) | 3 |
| Nantes | 17.9 (1.8; 73.4) | 2 | 20.2 (5.7; 89.2) | 2 | 75.8 (7.6; 172.8) | 2 |
| Nice | 24.9 (5.0; 55.0) | 1 | 25.3 (4.0; 59.0) | 1 | 85.0 (14.6; 176.5) | 2 |
| Paris | 36.0 (8.3; 93.0) | 15 | 25.1 (5.3; 111.8) | 7 | 58.7 (1.5; 179.8) | 13 |
| Rennes | 18.7 (0.7; 84.45) | 1 | 19.6 (3.2; 89.0) | 1 | 63.8 (4.3; 154.4) | 1 |
| Rouen | 25.7 (3.7; 75.0) | 3 | 24.6 (5.5; 112.5) | 2 | 65.1 (3.8; 173.8) | 4 |
| Strasbourg | 27.0 (4.7; 73.5) | 2 | 20.9 (3.0; 78.7) | 2 | 46.2 (0.3; 196.1) | 2 |
| Toulouse | 21.1 (2.3; 76.8) | 3 | 21.2 (4.1; 85.3) | 3 | 77.1 (5.1; 158.5) | 5 |
| Demographic Characteristics | Daily Mean Number of Deaths | ||||
|---|---|---|---|---|---|
| Non-Accidental | Cardiovascular | ||||
| Area | Total Population | All Ages | 75 Years and Over | All Ages | 75 Years and Over |
| Bordeaux | 686,824 | 12.6 | 8.9 | 3.3 | 2.7 |
| Clermont-Ferrand | 284,672 | 5.8 | 4 | 1.5 | 1.2 |
| Dijon | 241,591 | 4.7 | 3.3 | 1.2 | 1 |
| Grenoble | 484,122 | 8 | 5.7 | 2.1 | 1.8 |
| Le Havre | 235,56 | 5.8 | 3.6 | 1.5 | 1.1 |
| Lens-Douai | 324,286 | 8.6 | 5.3 | 2.1 | 1.6 |
| Lille | 1,133,920 | 20.7 | 12.9 | 5.2 | 3.9 |
| Lyon | 1,082,180 | 18.1 | 12.4 | 4.5 | 3.7 |
| Marseille | 979,05 | 21.6 | 15.3 | 5.8 | 4.7 |
| Montpellier | 421,647 | 6.6 | 4.6 | 1.8 | 1.4 |
| Nancy | 328,919 | 6.9 | 4.7 | 1.7 | 1.3 |
| Nantes | 633,391 | 10.6 | 7.2 | 2.8 | 2.3 |
| Nice | 434,581 | 11.7 | 8.6 | 3.1 | 2.6 |
| Paris | 6,754,282 | 104.5 | 66.8 | 23.8 | 18.7 |
| Rennes | 250,458 | 4.0 | 2.8 | 1.2 | 1 |
| Rouen | 449,687 | 9.9 | 6.6 | 2.7 | 2.1 |
| Strasbourg | 448,424 | 8.6 | 5.7 | 2.3 | 1.8 |
| Toulouse | 814,162 | 12.2 | 8.3 | 2.9 | 2.4 |
| Urban Area | Average Temperatures (°C) by Period | |||||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | ||||
| Cold * | Warm ** | Cold * | Warm ** | Cold * | Warm ** | |
| Bordeaux | 5.3 | 13.6 | 13.4 | 24.1 | 9.4 | 18.9 |
| Clermont-Ferrand | 2.3 | 11.6 | 11.2 | 22.8 | 6.8 | 17.2 |
| Dijon | 2.1 | 11.5 | 9.6 | 22.1 | 5.9 | 16.8 |
| Grenoble | 1.7 | 12.3 | 11.2 | 24.2 | 6.5 | 18.3 |
| Le Havre | 5.4 | 13.0 | 9.7 | 18.4 | 7.6 | 15.7 |
| Lens-Douai | 3.1 | 11.4 | 9.5 | 20.3 | 6.3 | 15.9 |
| Lille | 3.1 | 11.4 | 9.5 | 20.3 | 6.3 | 15.9 |
| Lyon | 3.6 | 13.6 | 11.1 | 23.9 | 7.4 | 18.7 |
| Marseille | 5.8 | 16.1 | 14.5 | 26.7 | 10.2 | 21.4 |
| Montpellier | 5.7 | 15.9 | 14.6 | 25.8 | 10.2 | 20.9 |
| Nancy | 1.9 | 10.9 | 9.3 | 21.6 | 5.6 | 16.3 |
| Nantes | 4.4 | 11.9 | 12.2 | 22.2 | 8.3 | 17.0 |
| Nice | 8.1 | 18.1 | 14.8 | 24.2 | 11.5 | 21.2 |
| Paris | 5.0 | 13.4 | 10.8 | 22.0 | 7.9 | 17.7 |
| Rennes | 4.0 | 11.1 | 11.8 | 21.6 | 7.9 | 16.4 |
| Rouen | 2.9 | 10.6 | 9.8 | 20.2 | 6.4 | 15.4 |
| Strasbourg | 2.1 | 11.6 | 9.6 | 22.5 | 5.9 | 17.0 |
| Toulouse | 4.9 | 14.3 | 12.8 | 24.8 | 8.9 | 19.6 |
| Pollutant | Non-Accidental Deaths | Cardiovascular Deaths | ||
|---|---|---|---|---|
| Total Population | ≥75 Years | Total Population | ≥75 Years | |
| Annual | ||||
| NO2 | 0.75 (0.40; 1.10) | 1.14 (0.63; 1.66) | 1.13 (0.37; 1.90) | 1.15 (0.40; 1.91) |
| +PM10 | 0.58 (0.15; 1.01) | 0.79 (0.27; 1.33) | 0.96 (−0.10; 2.04) | 1.11 (0.08; 2.16) |
| +O3 | 1.12 (0.63; 1.61) | 1.47 (0.85; 2.10) | 1.39 (0.53; 2.25) | 1.39 (0.58; 2.21) |
| Cold period | ||||
| NO2 | 0.01 (−0.41; 0.42) | 0.20 (−0.30; 0.71) | 0.23 (−0.61; 1.08) | 0.25 (−0.65; 1.16) |
| +PM10 | 0.09 (−0.42; 0.60) | 0.28 (-0.35; 0.90) | 0.46 (−0.74; 1.67) | 0.73 (−0.64; 2.11) |
| +O3 | 0.30 (−0.17; 0.77) | 0.59 (-0.03; 1.21) | 0.48 (−0.54; 1.51) | 0.55 (−0.52; 1.63) |
| Warm period | ||||
| NO2 | 2.65 (1.82; 3.48) | 3.07 (1.97; 4.18) | 3.05 (1.38; 4.74) | 3.17 (1.53; 4.84) |
| +PM10 | 1.33 (0.37; 2.29) | 1.61 (0.23; 3.00) | 1.48 (0.08; 2.90) | 1.68 (0.20; 3.18) |
| +O3 | 2.54 (1.62; 3.47) | 2.98 (1.82; 4.17) | 3.16 (1.32; 5.03) | 3.41 (1.57; 5.29) |
| Non-Accidental Deaths | Cardiovascular Deaths | |||||
|---|---|---|---|---|---|---|
| References | Years | Areas | All Ages (%) | 75 Years and Over (%) | All Ages (%) | 75 Years and Over (%) |
| Perez et al. | 2015 | Switzerland | 0.7 (0.1; 1.3) | 0.6 (0.0; 1.2) | 0.4 (−0.1; 0.8) | 0.3 (−0.3; 0.8) |
| Carugno et al. | 2016 | Italy | 0.7 (0.13; 1.27) | - | 1.12 (0.14; 2.11) | 1.17 (0.1; 2.26) |
| Mills et al. | 2015 | World | 0.71 (0.43; 1.00) | - | 0.88 (0.63; 1.13) | - |
| European | 0.9 (0.45; 1.35) | - | 1.03 (0.70; 1.36) | - | ||
| Renzi et al. | 2017 | Rome | 1.8 (1.35; 2.25) | - | - | - |
| Neuberger et al. | 2013 | Vienne | 0.8 (0.0; 1.6) | - | - | - |
| Crouse et al. | 2015 | Canada | 0.5 (0.4; 0.6) | - | 0.4 (0.3; 0.5) | - |
| This study | 2020 | France | 0.75 (0.40; 1.106) | 1.14 (0.63; 1.66) | 1.13 (0.37; 1.90) | 1.15 (0.40; 1.91) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corso, M.; Blanchard, M.; Medina, S.; Wagner, V. Short-Term Associations of Nitrogen Dioxide (NO2) on Mortality in 18 French Cities, 2010–2014. Atmosphere 2020, 11, 1198. https://doi.org/10.3390/atmos11111198
Corso M, Blanchard M, Medina S, Wagner V. Short-Term Associations of Nitrogen Dioxide (NO2) on Mortality in 18 French Cities, 2010–2014. Atmosphere. 2020; 11(11):1198. https://doi.org/10.3390/atmos11111198
Chicago/Turabian StyleCorso, Magali, Myriam Blanchard, Sylvia Medina, and Vérène Wagner. 2020. "Short-Term Associations of Nitrogen Dioxide (NO2) on Mortality in 18 French Cities, 2010–2014" Atmosphere 11, no. 11: 1198. https://doi.org/10.3390/atmos11111198
APA StyleCorso, M., Blanchard, M., Medina, S., & Wagner, V. (2020). Short-Term Associations of Nitrogen Dioxide (NO2) on Mortality in 18 French Cities, 2010–2014. Atmosphere, 11(11), 1198. https://doi.org/10.3390/atmos11111198




