1. Introduction
Exceptionally high ozone concentrations occur during many winters in the Uinta basin of eastern Utah [
1,
2,
3,
4,
5]. The physics and chemistry of winter ozone formation given the prevailing emissions and meteorology are not completely understood. For example, models routinely underestimate ozone concentrations. Some models suggest that there is a missing source of formaldehyde and other carbonyls [
6,
7,
8]. This has led us to consider other possible carbonyl sources [
9], including produced water evaporation ponds [
10,
11,
12] and the snowpack [
13,
14,
15], and to an examination of models of the transport of aldehydes across the air–water interface. Ice crystals in the environment have a surface liquid-like layer. To an initial approximation, transport between snow and the atmosphere resembles transport across the air–liquid water interface [
16,
17,
18]. This paper is a result of that study.
The theory of mass transport of volatile gases between a liquid and a vapor phase is well developed but becomes more complex when the gas undergoes a reaction in the aqueous phase. Examples include the following reactions:
I will represent all these reactions as 1 ↔ 2, where 1 represents the compound on the left that dominates in the vapor phase. Based on the assumption that the diols, carbonic acid, bicarbonate, carbonate, and chloride do not exist in the vapor phase, it has always been assumed that when form 1 reacts in the aqueous phase to give form 2, then 2 must convert back to 1 while still in the aqueous phase before it can cross into the vapor phase. The primary purpose of this paper is to examine models that challenge this assumption. Of course, the ions do not exist in the vapor phase, but the diols do. Many measurements have detected methanediol vapor in equilibrium with aqueous formaldehyde solutions [
19,
20]. Methanediol hydrolysis is catalyzed by water and other small molecules, and methanediol has extreme stability in isolation [
21,
22,
23,
24]. 1,1-Ethanediol in the gas phase has not been studied experimentally, but its reactions are probably similar to methanediol. Once we accept the gas-phase existence of these molecules, we also have to assume that they cross the air–water interface in the diol form. In this paper, I generalize existing models to the case in which both compounds are assumed to exist in both phases and are also assumed to cross the interface. I apply these models to formaldehyde and acetaldehyde transport across the air–water interface.
Interestingly, the existence of carbonic acid molecules in the gas phase has also been established, and they are also predicted to be highly stable in isolation [
25,
26,
27,
28,
29,
30,
31,
32]; thus, it is also safe to assume that they cross the air–water interface. However, at the acidities expected in the environment, only a small fraction of dissolved carbon dioxide is found in the carbonic acid form; much more is present as free carbon dioxide, bicarbonate, or carbonate. Therefore, when we write 1 ↔ 2 for the carbon dioxide system, we can assume that form 1 is free carbon dioxide, while form 2 is bicarbonate plus carbonate, and we can ignore carbonic acid altogether. Whenever form 2 is ionic, it is appropriate to assume that it does not cross the interface and that existing models suffice. New models are needed for the aldehydes, but not for carbon dioxide or hydrogen chloride.
Mass-transfer theory of transport across the air–water interface has been under development for nearly a century, beginning with Whitman [
33] and his “two-film” model. Later decades saw the development of “surface renewal” and “penetration” models [
34,
35,
36,
37,
38,
39] and arguments based on the Schmidt number [
40,
41,
42]. The Whitman model is relatively simple and today is used mainly only for pedagogy [
43]. However, it was the first model to postulate that to cross the interface, solute molecules must traverse two separate, stagnant films, one on each side of the interface, under conditions in which transport only occurs by molecular diffusivity. It predicts mass-transfer coefficients that scale with molecular diffusivity
D to the first power. Later models are more in line with experiments with
D1/2 or
D2/3 scaling laws [
44,
45,
46,
47,
48,
49,
50,
51,
52]. There have also been generalizations that allow both for irreversible [
37,
53] and reversible [
54,
55] aqueous phase reactions. However, to the best of my knowledge, no one has ever studied models that allow for reactions in both phases and for both forms to cross the interface.
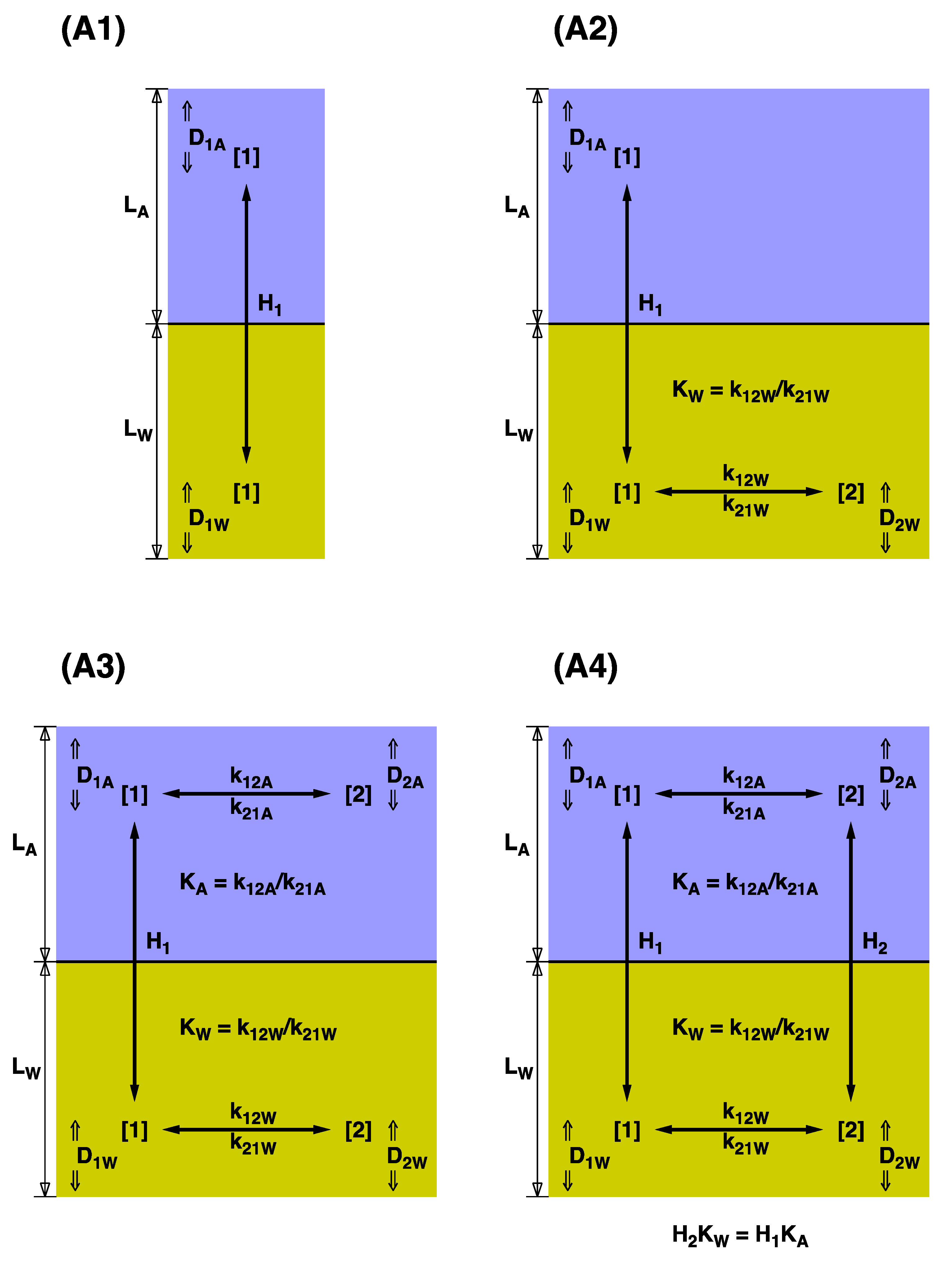
2. Description of the Models
In this paper, I generalize the Whitman model [
33] to the case of two reacting species. Five separate models, represented in
Figure 1 and
Figure 2, will be discussed in this paper. Whitman’s model [
33] is denoted A1. It models a single compound able to move between the water and air phases. Model A2 was first introduced by Hoover and Berkshire [
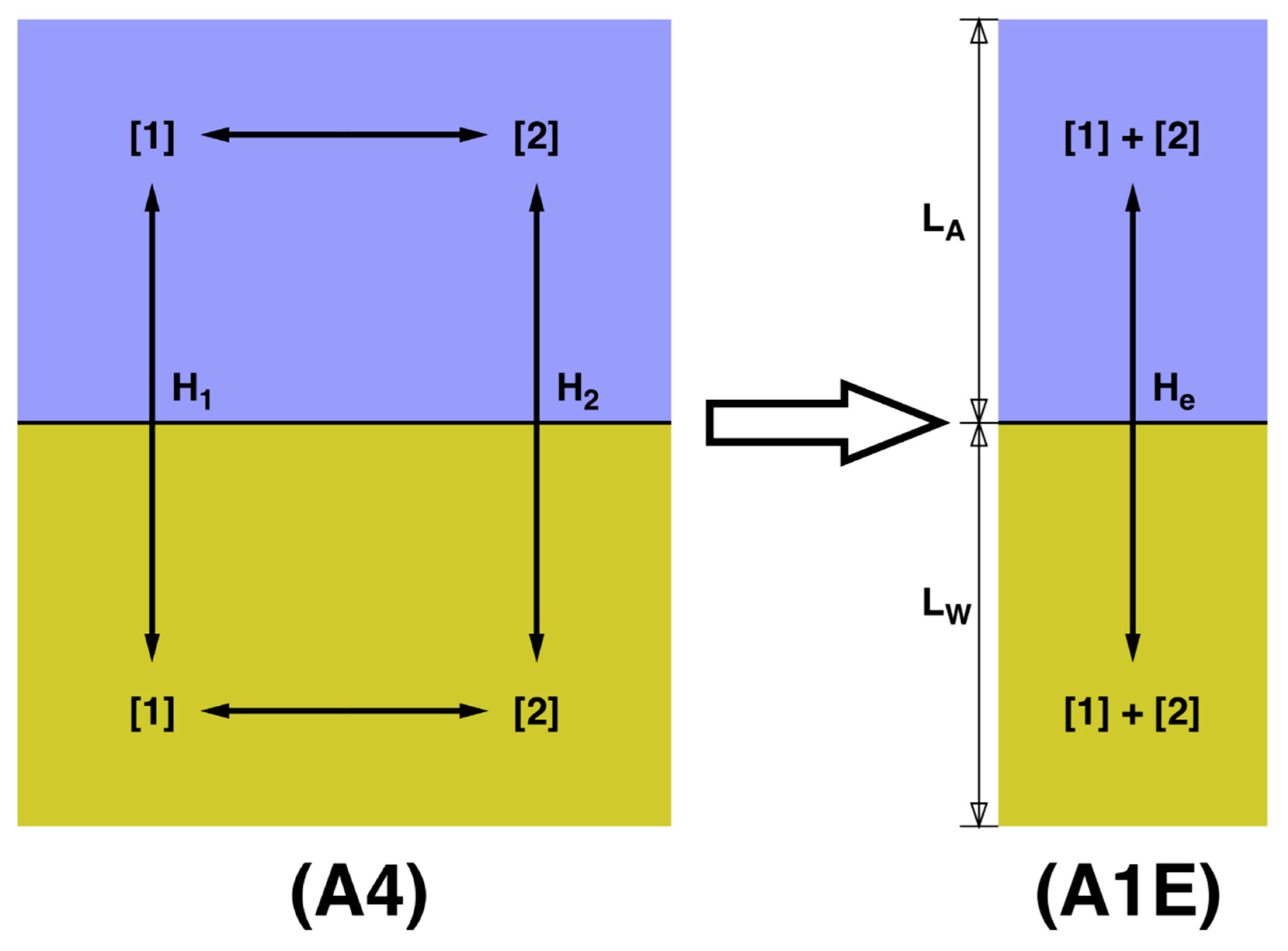
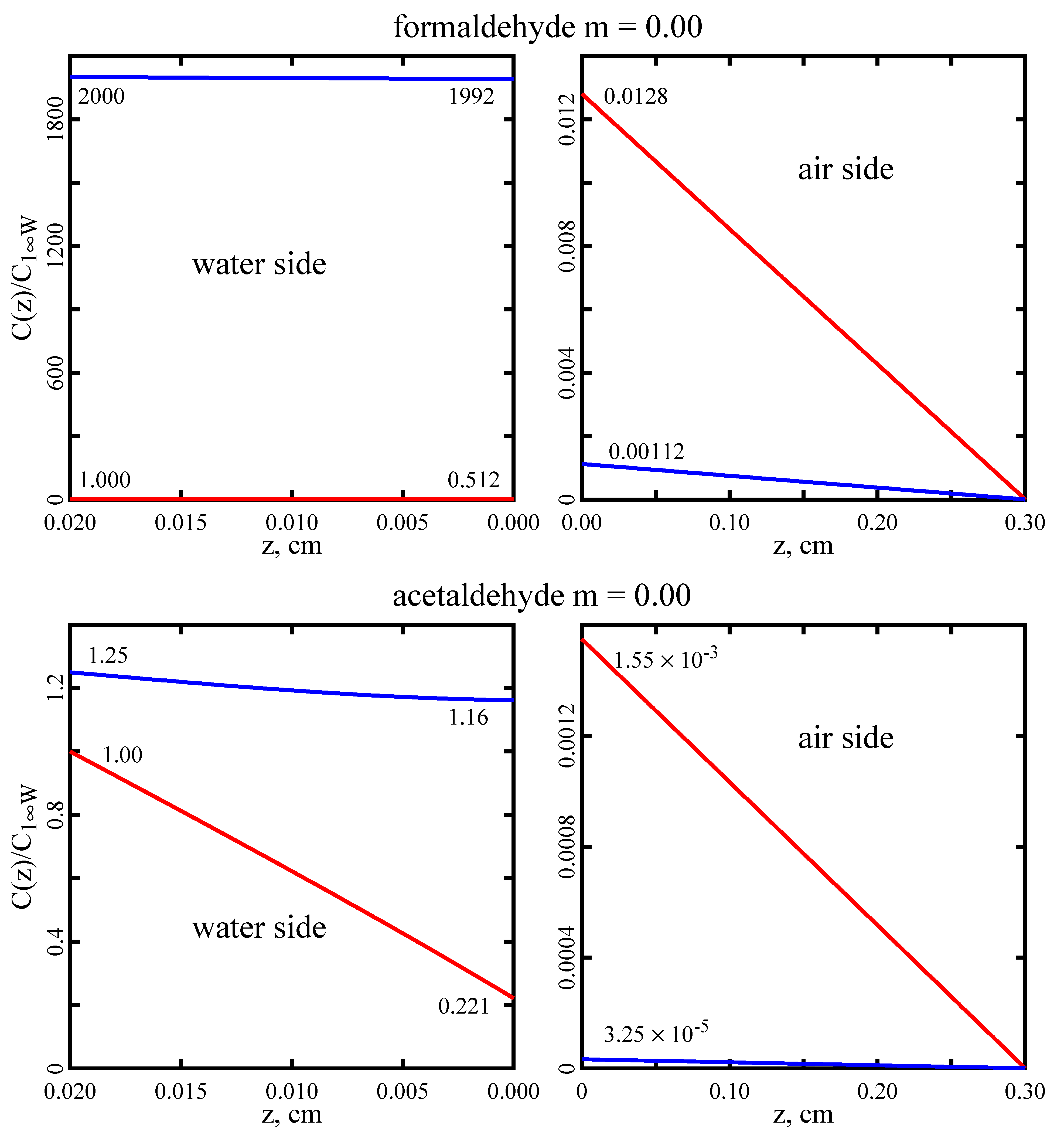
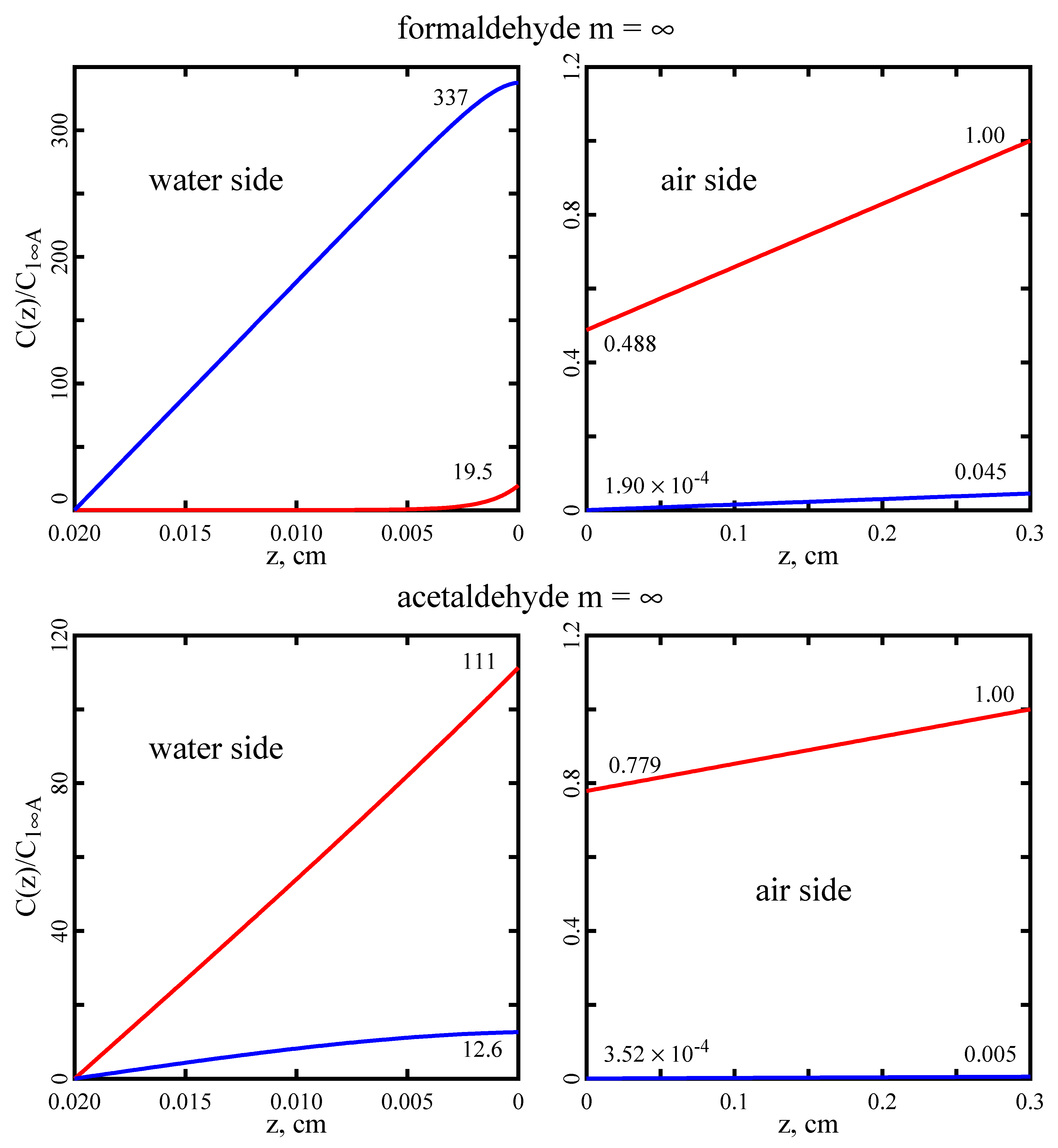
54]. It permits the reversible reaction 1 ↔ 2 only in the water phase, and only form 1 is able to move across the barrier and exist in the vapor phase. Model A3 assumes that 1 and 2 react reversibly in both phases, but still, only form 1 is able to cross the barrier. Model A4 allows for the reaction in both phases, and it allows for both compounds to cross the barrier. To the best of my knowledge, models A3 and A4 have never been presented in the literature. A fifth model is considered when, without ignoring the existence of both forms of the compound, our analytical techniques do not permit us to distinguish them, and we treat 1 + 2 as a blended compound. Let A1E refer to the model designed to be a single-compound effective representation of A4. Below, I give best techniques for designing an appropriate A1E model.
The steady-state reaction–diffusion equations can be solved exactly for all models A1 through A4 if we restrict ourselves to generalizations of the Whitman model [
33]. Numerical treatments of model A2 in the context of surface renewal and other more modern models have been considered by Glasscock and Rochelle [
55]. Despite yielding exact solutions, each succeeding model is algebraically and numerically more complex than the previous. Therefore, the exact solutions will only be summarized here; details and derivations are given in the
Supplementary Material.
3. Definition of Variables
Table 1 defines the variables employed. We distinguish between “system” variables that define the physical and chemical properties of the system, “concentration” variables that represent concentrations of the two compounds at specific depths, and “derived” variables, used for notational convenience. The subscripts “1” and “2” identify variables that are specific to compounds 1 and 2, respectively. The subscripts “A” and “W” identify variables specific to the air and water phases, respectively. The subscript “t” for total represents a sum of properties over both compounds, e.g.,
Ft =
F1 +
F2 is the total flux. The subscripts “0” and “∞” attached to concentration variables indicate interfacial and far-field concentrations, respectively. A generic expression valid for either phase or for either compound can appear in this paper without the relevant subscripts.
The reaction rate constants
k12 and
k21 are pseudo-first order, since variations in water concentration are negligible.
k12 refers to the rate of 1 → 2 and vice versa. Enhancement factors appear in models A2 and A3 as multipliers of certain transfer coefficients. As defined, enhancement factors are always greater than 1, so these models predict that reactions can accelerate transfer. Because diffusivities of small molecules agree better than an order of magnitude, we can usually expect that
. Because compounds 1 and 2 usually do not have the same mass, molar concentrations are used throughout. The equilibrium constants are defined as
K = [diol]/[aldehyde]. I use the dimensionless air-over-water form of Henry’s constant.
with the subscript “eq” denoting equilibrium concentrations. It is important to distinguish between “intrinsic” and “effective” Henry’s constants.
H1 and
H2 as they are used here are intrinsic.
H1 is the ratio of aldehyde concentrations only, excluding the gem-diol form.
H2 is the ratio of only the gem-diol concentrations. Effective Henry’s constants blur the distinction between the aldehyde and the gem-diol forms:
Effective Henry’s constants appear commonly in the literature, especially when measurement techniques are unable to distinguish the two forms of the molecule. Not all the variables given in
Table 1 are independent. Interrelations between variables are listed in
Table 2. These interrelations are results of physical laws or of fundamental assumptions in the models.
4. Justification of the Whitman Model
Because the Whitman model [
33] has fallen into disuse, I now justify using generalizations of it in this study. The essential argument is that when Whitman’s film thickness is chosen to duplicate the flux of any of the more recent models, we can expect that it gives the same qualitative dynamics, the difference in
D-scaling notwithstanding. Fundamentally, the difference between the Whitman model and, for example, the Schmidt number models is the assumed mathematical form of the eddy diffusivity. For Whitman, we have
The eddy diffusivity for the Schmidt number models can be written as
L′ is a lumped quantity with units of length. Physical arguments require
p = 2 or 3 [
40,
41,
42]. Interestingly, Equation (3) is the large-
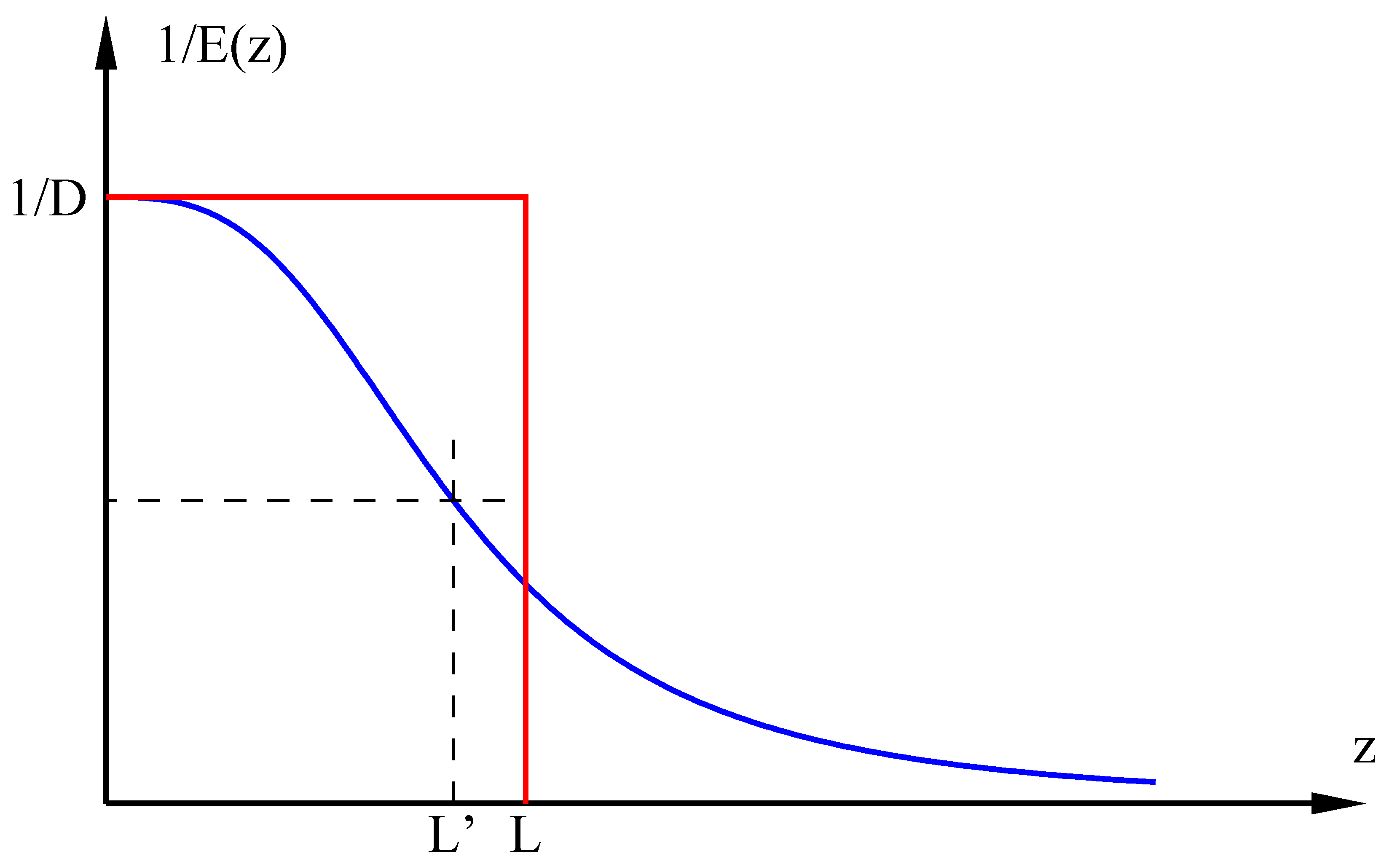
p version of Equation (4). Calculation of the transfer coefficient involves the integral
The integrands 1/
E(
z) of Equations (3) and (4) are both plotted in
Figure 3.
L′ and 1/
D are measures, respectively, of the breadth and height of the area under the blue curve, meaning that Equation (4) gives this scaling law:
. Equation (3) implies
. Therefore, when properly chosen, there is a value of
for which both models give the same
k. Because the Whitman model has the same effective film thickness as the Schmidt number model, and because its diffusivity is approximately equal to the eddy diffusivity in the film, we expect that the two models give the same qualitative dynamics.
However, the scaling law
does not seem to be appropriate for the Schmidt number models, which give
or
. The explanation of the discrepancy is that
L′ is indeed a measure of film thickness, but as a lumped variable, it has its own
D dependence. The scaling law for the Schmidt number models emerges from the assumptions (1) that
E(0) =
D, and (2) that the term in
zp is determined only by fluid-dynamic properties of the solvent; no property of the solute, including
D, is assumed to enter. Rather, the coefficient of the term in
zp is assumed to depend only on three parameters, solvent viscosity
η, solvent density
ρ, and friction velocity at the interface,
u* [
42]. For any given
p, there is only one combination of these three parameters that yields the correct units. The resultant scaling laws for
L′ and
k are:
Here, is the Schmidt number of the solvent; u* is the friction velocity; and n is 1/2 and 2/3 respectively, when p = 2 or 3. Dependence on wind speed enters L′ through the parameter u*.
6. Aldehyde ↔ Gem-Diol Reactions
We consider the hydration reaction of formaldehyde to form methanediol:
and of acetaldehyde to form 1,1-ethanediol:
In aqueous solution, the diols can continue to add aldehyde units to form higher oligomers [
56], but at the aldehyde and diol concentrations expected in the environment, those compounds can be neglected [
57].
According to theoretical estimates [
21,
23] the unimolecular decay rate of methanediol is very low, giving a half-life at 300 K in an extreme vacuum longer than the age of the universe. However, in the atmosphere and in the aqueous phase, the reaction is catalyzed by H
2O, by organic and inorganic acids, by bases, and by the hydroperoxyl radical. The reaction is probably also autocatalytic [
22,
58,
59,
60]. Calculations also indicate that the acid and radical catalysts may be more efficient than water, although the relative abundance of water still means that it is the most important catalyst.
A half-century ago, Eigen [
61] suggested that water catalysis proceeds via a concerted exchange of protons involving two water molecules. That view has been confirmed theoretically, along with the suggestion that three-water catalysis is even faster [
21,
23,
62,
63]. These findings imply that the absolute rate constants are probably second or third order in water. The gas-phase pseudo-first-order reaction rates
k12A and
k21A are then expected to be strong functions of the concentrations of any catalysts and to be slower than the equivalent reactions in the aqueous phase where the catalyst concentration is larger. The air film at the interface is expected to be water saturated, which translates into a strong temperature dependence for
k12A and
k21A arising from the dependence of absolute humidity on temperature. The aqueous reactions are also catalyzed by H
3O
+ and OH
− so that
k12W and
k21W are pH dependent, and their values are lowest at about pH 7.
The rigorously defined gas-phase equilibrium constant for the reaction aldehyde + H
2O ↔ diol is written in terms of the partial pressures of each gas as
where
P0 is a reference pressure of 1 atm. The expression we use for
KA is
meaning that literature values of
must be corrected using the partial pressure of water vapor (0.039 atm at 298.15 K). The rigorous equilibrium constant has strong temperature dependence [
64,
65], but so does the partial pressure of water vapor [
66]. Interestingly, the two effects largely compensate, and
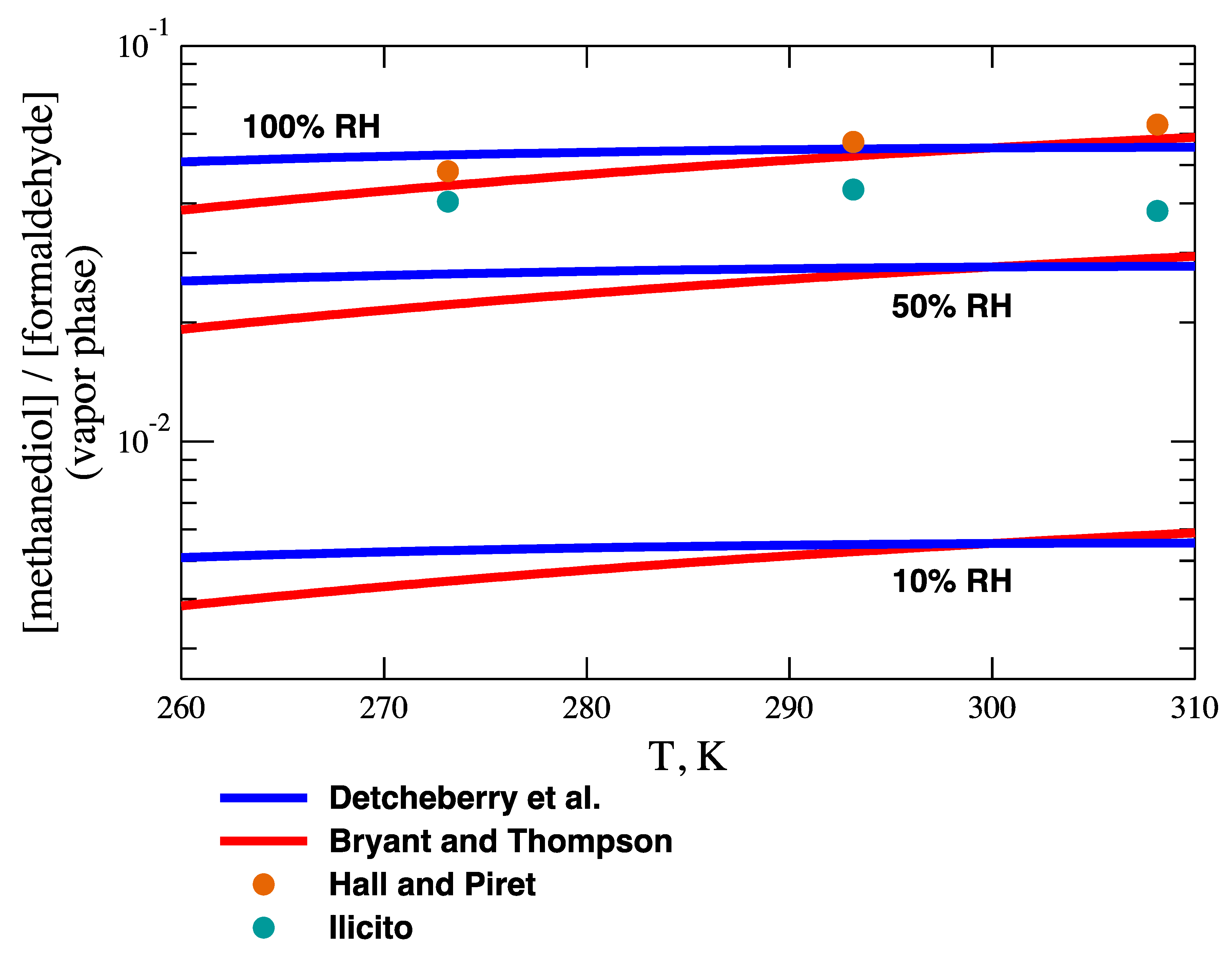
KA for the formaldehyde reaction is essentially independent of temperature, as shown in
Figure 4. The effect of relative humidity on
KA is also displayed in
Figure 4. Of course, in the air film at the air–water interface, 100% relative humidity can be assumed.
Table 4 and
Table 5 contain literature data on each aldehyde reaction. Henry’s constants
H1 for the aldehydes have been retrieved from Sander’s compilation [
67].
H2 for the gem-diols does not appear in his compilation, but it is constrained by the values of
H1,
KA, and
KW. Henry’s constants are often difficult to measure, and there is usually considerable scatter in Sander’s compilation for any one compound, often by more than an order of magnitude. Here, I take the median Henry’s constants from the compilation, which appear to be good consensus values. Sander tabulates effective Henry’s constants,
He, for formaldehyde and acetaldehyde. Therefore, his entries must also be converted to intrinsic constants.
Molecular diffusivities were calculated using several different empirical correlations [
68,
69,
70,
71]. The quantities
KW,
k12W and
k21W have been extensively measured and calculated theoretically. For these quantities,
Table 4 and
Table 5 report ranges of values equal to the middle half of datasets of independently measured or computed values. The value of the formaldehyde
KA is taken from
Figure 4 at 100% relative humidity.
Apparently, the only publication considering the equilibrium constant of the vapor-phase hydration of acetaldehyde is a theoretical calculation by Rayne and Forest [
83] obtaining
(geometric mean of three separate estimates) at 298.15 K. For formaldehyde at 298.15 K, Rayne and Forest obtain
(geometric mean of three separate estimates), whereas experimental values are around 1.75 [
64], i.e., the Rayne–Forest formaldehyde estimate is a factor of about 25 too low. Applying the same ratio to acetaldehyde yields
and
. Therefore, in the following, we will estimate
for acetaldehyde, all the while considering this to be a poorly constrained value.
The rates k12A and k21A have never been measured for either system, but their ratio is constrained by KA. Therefore, the only available constraints on k12A and k21A are and , as explained above. In calculations reported below, I have allowed k12A and k21A to vary by about four orders of magnitude while enforcing these constraints.
8. Summary
Volatile gases such as formaldehyde and acetaldehyde undergo hydration reactions in aqueous solution, forming methanediol and 1,1 ethanediol, respectively. Conventional wisdom has dictated that the hydrated forms do not exist in the vapor phase. Therefore, models designed to treat the transport of these compounds between an aqueous phase (oceans, lakes, wastewater lagoons, aqueous aerosols, etc.) and the atmosphere have always assumed that molecules traverse the interface only in the anhydrous form [
37,
43,
53,
54,
55]. We now know that the conventional wisdom is wrong—the diols exit in the vapor phase [
19,
20,
21,
22,
23,
24]. The hydrated molecules are inherently very stable, but catalysts are readily available in the atmosphere leading to chemical equilibrium between both forms. These equilibria strongly favor the anhydrous form [
21,
22,
23,
61,
62].
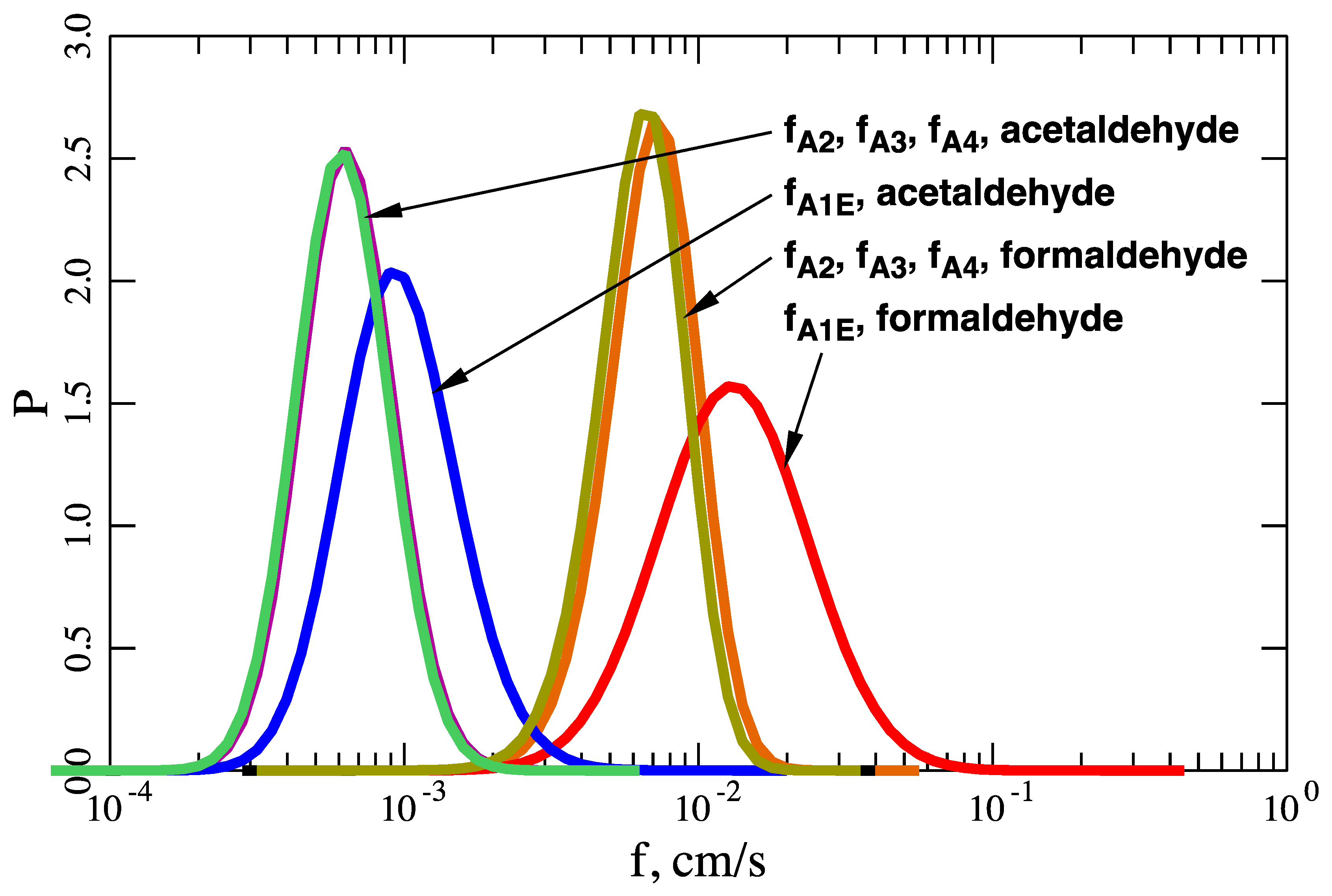
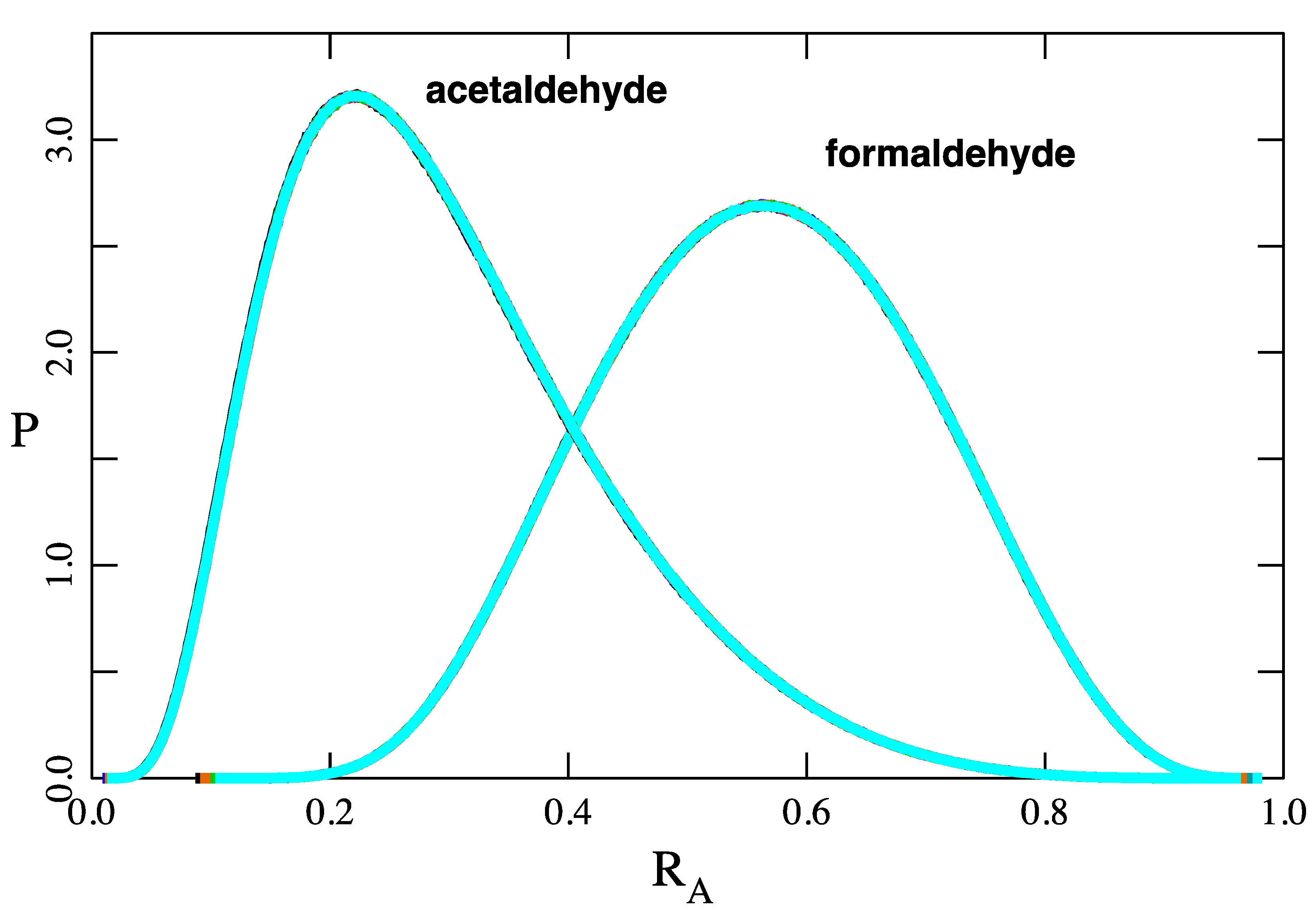
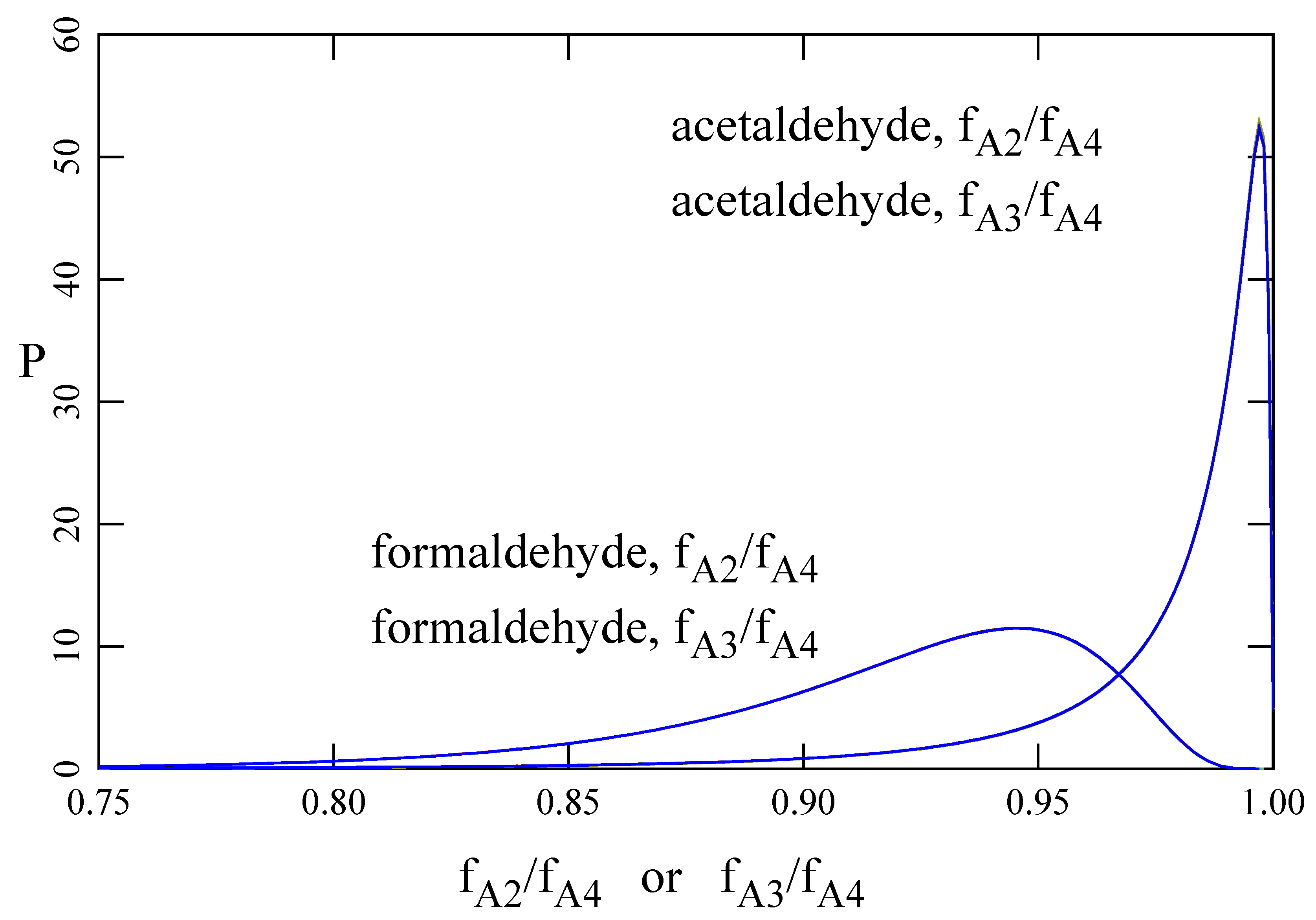
This paper introduces a model of the transport of molecules across the air–water interface that exist in two interconvertible forms, 1 ↔ 2, in both phases and in which both forms are assumed to cross the interface. For formaldehyde and acetaldehyde, the former model (A3) predicts fluxes a few percent less that the new one (A4). However, there are conditions for which the differences between the two models may be much greater.
It is also important to emphasize the difference between intrinsic and effective Henry’s constants. For formaldehyde and acetaldehyde, the model employing an effective Henry’s constant (A1E) overpredicts fluxes by about 90% and 60%, respectively. This is significant because most literature citations report the effective Henry’s constant [
67].