Nitrous Oxide from Beef Cattle Manure: Effects of Temperature, Water Addition and Manure Properties on Denitrification and Nitrification †
Abstract
1. Introduction
2. Experiments
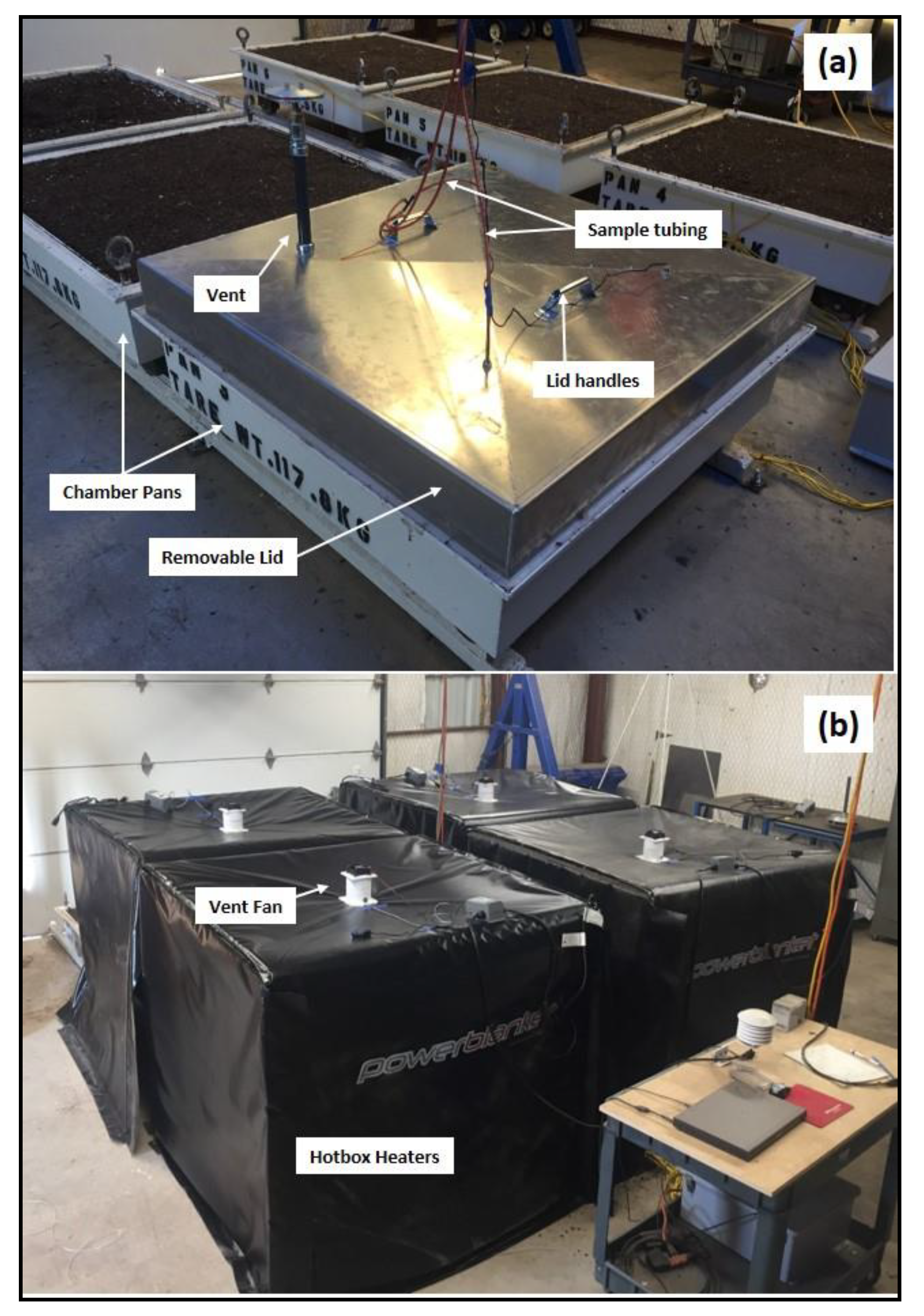
2.1. Large–Chamber Incubation Study
2.2. Manure Collection and Analyses
2.3. Potential Denitrifier and Nitrification Enzyme Activities
2.4. Statistical Analyses
3. Results and Discussion
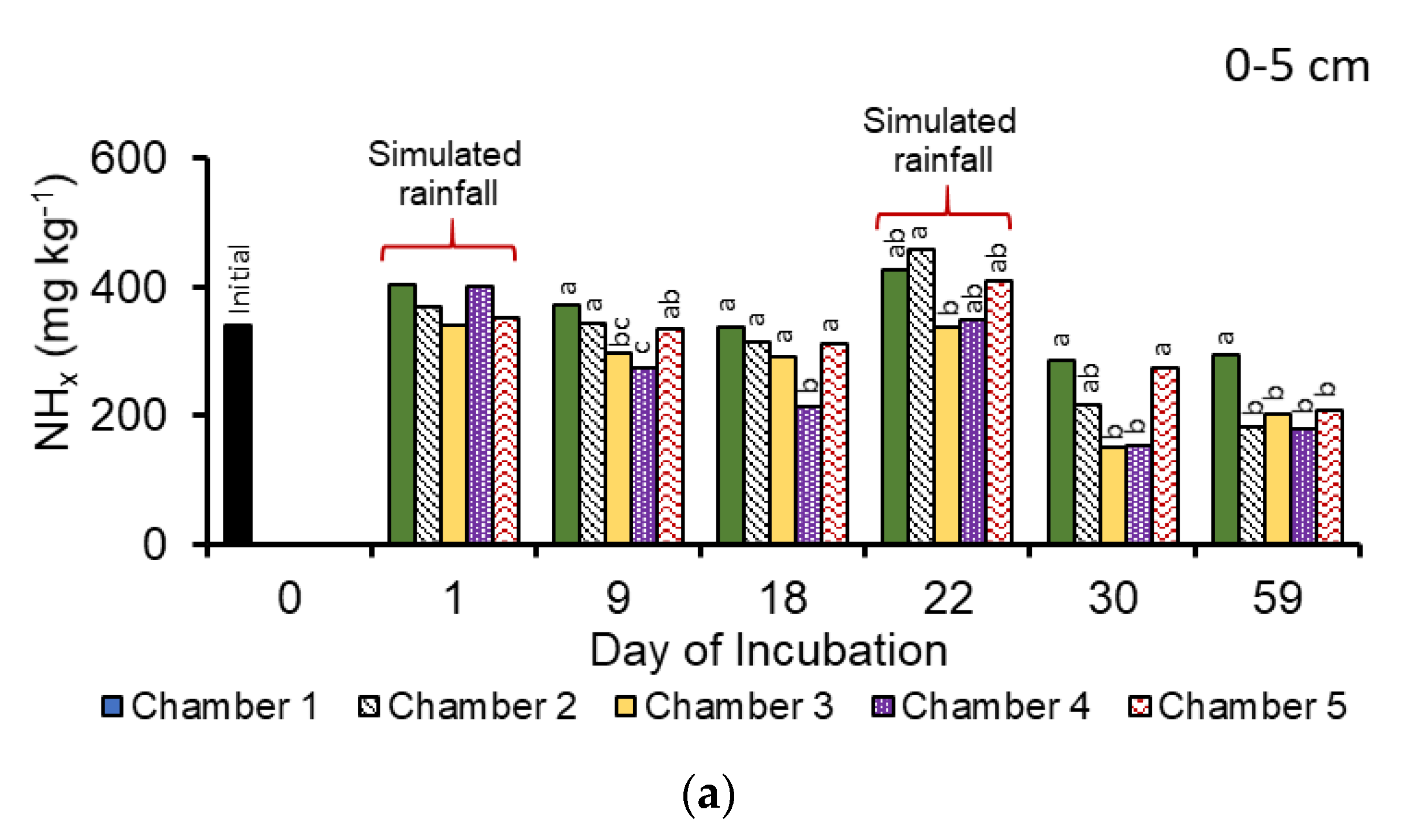
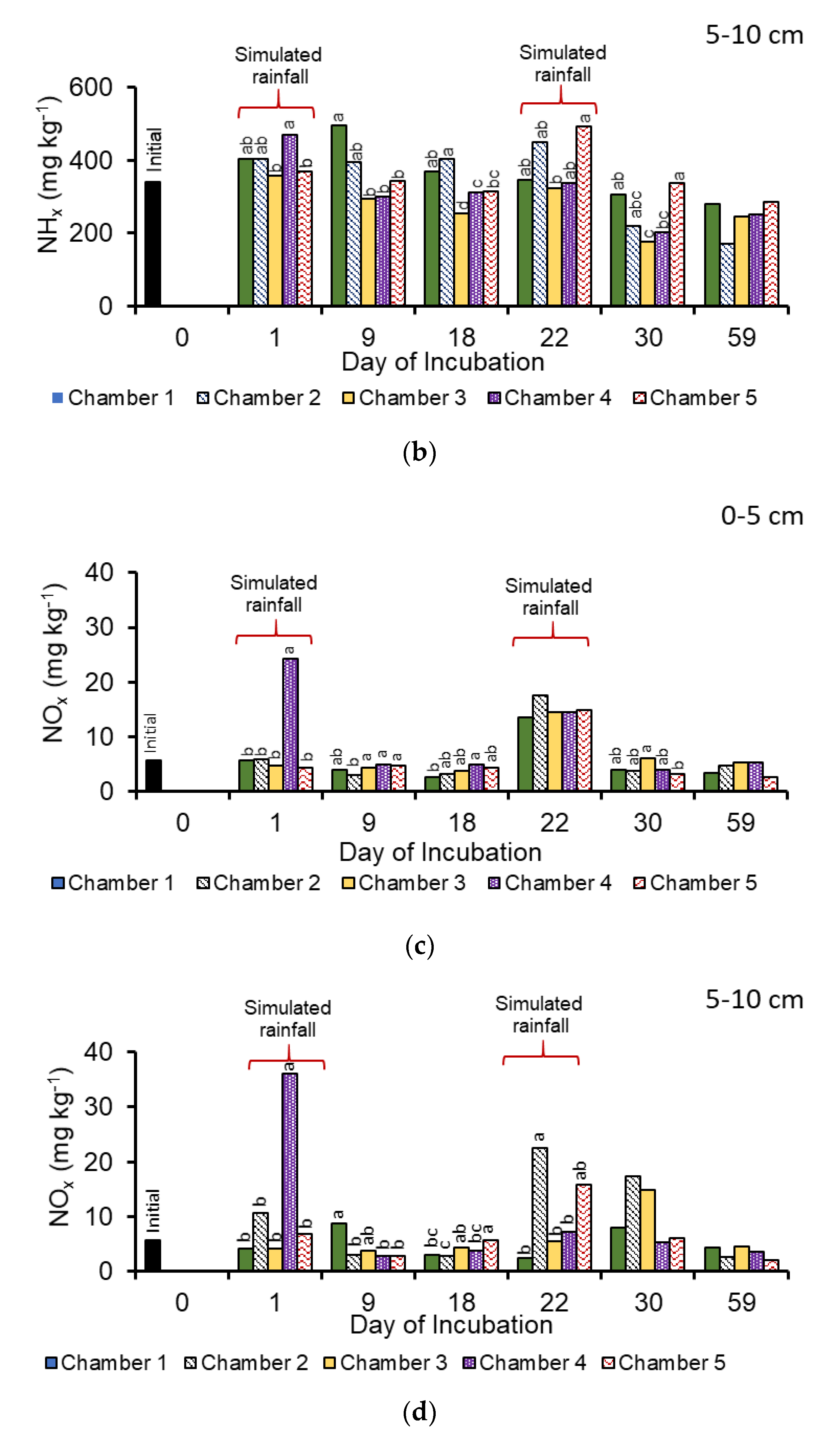
3.1. Manure Properties
3.2. Nitrous Oxide Emissions
3.3. Denitrification Enzyme Activity
3.4. Nitrification Enzyme Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Redding, M.R.; Devereux, J.; Phillips, F.; Lewis, R.; Naylor, T.; Kearton, T.; Hill, C.J.; Weidemann, S. Field measurement of beef pen manure methane and nitrous oxide reveals a surprise for inventory calculations. J. Environ. Qual. 2015, 44, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.B.; Waldrip, H.M.; Casey, K.D.; Todd, R.W.; Willis, W.W.; Webb, K. Temporal nitrous oxide emissions from beef cattle feedlot manure following a simulated rainfall event. J. Environ. Qual. 2017, 46, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.B.; Casey, K.D.; Todd, R.W.; Waldrip, H.M.; Marek, G.M.; Auvermann, B.W.; Marek, T.H.; Webb, K.; Willis, W.M.; Pemberton, B.; et al. Improved chamber systems for rapid, real–time nitrous oxide emissions from manure and soil. Trans. ASABE 2017, 60, 1235–1258. [Google Scholar] [CrossRef]
- Waldrip, H.M.; Casey, K.D.; Todd, R.W.; Parker, D.B. Nitrous oxide emissions from Southern High Plains beef cattle feedyards: Measurement and modeling. Trans. ASABE 2017, 60, 1209–1221. [Google Scholar] [CrossRef]
- Waldrip, H.M.; Todd, R.W.; Parker, D.B.; Cole, N.A.; Rotz, C.A.; Casey, K.D. Nitrous oxide emissions from open–lot cattle feedyards: A review. J. Environ. Qual. 2016, 45, 17971811. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, B.L.; Gilley, J.E.; Parker, D.B.; Stromer, B.S. Greenhouse gas emissions from beef feedlot surface materials as affected by diet, moisture, temperature, and time. Trans. ASABE 2018, 61, 571–582. [Google Scholar] [CrossRef]
- Costa, C., Jr.; Li, C.; Cerri, C.E.P.; Cerri, C.C. Measuring and modeling nitrous oxide and methane emissions from beef cattle feedlot manure management: First assessments under Brazilian condition. J. Environ. Sci. Health B 2014, 49, 696–711. [Google Scholar] [CrossRef]
- Butterbach–Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister–Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Parker, D.B.; Waldrip, H.M.; Casey, K.D.; Woodbury, B.L.; Spiehs, M.J.; Webb, K.; Willis, W.M. How do temperature and rainfall affect nitrous oxide emissions from open–lot beef cattle feedyard pens? Trans. ASABE 2018, 61, 1049–1061. [Google Scholar] [CrossRef]
- Aguilar, O.A.; Maghirang, R.; Rice, C.W.; Trabue, S.L.; Erickson, L.E. Nitrous oxide fluxes from a commercial beef cattle feedlot in Kansas. Air Soil Water Res. 2014, 7, 35–45. [Google Scholar] [CrossRef]
- Woodbury, B.L.; Miller, D.N.; Nienaber, J.A.; Eigenberg, R.A. Seasonal and spatial variations of denitrifying enzyme activity in feedlot soil. Trans. ASABE 2001, 44, 1635–1642. [Google Scholar] [CrossRef]
- Ayadi, F.Y.; Spiehs, M.J.; Cortus, E.L.; Miller, D.N.; Djira, G.D. Physical, chemical, and biological properties of simulated beef cattle bedded manure packs. Trans. ASABE 2015, 58, 797–811. [Google Scholar] [CrossRef]
- Liao, W.; Liu, C.; Gao, Z. Impacts of feedlot floor condition, deposition frequency, and inhibitors on N2O and CH4 emissions from feedlot dung and urine patches. J. Air Waste Manag. Assoc. 2018, 68, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.B.; Casey, K.D.; Cortus, E.L.; Min, B.R.; Waldrip, H.M.; Woodbury, B.; Spiehs, M. Empirical model of annual nitrous oxide emissions from open–lot beef cattle feedyard pens (version 2). In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019. Paper No. 1900299. [Google Scholar] [CrossRef]
- Giles, M.; Morley, N.; Baggs, E.M.; Daniell, T.J. Soil nitrate reducing processes–drivers, mechanisms for spatial variation, and significance for nitrous oxide production. Front. Microbiol. 2012, 3, 407. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.C.; Mason, A.M.; Cole, N.A.; Clark, R.N. The Influence of Feedlot Pen Surface Layers on Microbial Community Structure and Diversity. In Proceedings of the International Symposium on Air Quality and Waste Management for Agriculture, Broomfield, CO, USA, 16–19 September 2017. [Google Scholar]
- Cole, N.A.; Mason, A.M.; Todd, R.W.; Rhoades, M.; Parker, D.B. Chemical composition of pen surface layers of beef cattle feedyards. Prof. Anim. Sci. 2009, 25, 541–552. [Google Scholar] [CrossRef]
- Hart, S.C.; Stark, J.M.; Davidson, E.A.; Firestone, M.K. Nitrogen mineralization, immobilization and nitrification. In Methods of Soil Analysis. Part. 2—Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 985–1018. [Google Scholar]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Stres, B.; Rosenquist, M.; Hallin, S. Phylogenetic analysis of nitrite, nitric oxide, and nitrous oxide respiratory enzymes reveal a complex evolutionary history for denitrification. Mol. Biol. Evol. 2008, 25, 1955–1966. [Google Scholar] [CrossRef]
- Firestone, M.K.; Firestone, R.B.; Tiedje, J.M. Nitrous oxide from soil denitrification: Factors controlling its biological production. Science 1980, 208, 749–751. [Google Scholar] [CrossRef]
- Tiedje, J.M. Denitrifiers. In Methods of Soil Analysis: Part 2. Microbial and Biochemical Properties; Chapter 14; Bigham, J.M., Ed.; SSSA: Madison, WI, USA, 1994. [Google Scholar]
- Azam, F.; Müller, C.; Weiske, A.; Benckiser, G.; Ottow, J. Nitrification and denitrification as sources of atmospheric nitrous oxide– Role of oxidizable carbon and applied nitrogen. Biol. Fertil. Soils 2002, 35, 54–61. [Google Scholar] [CrossRef]
- Wu, D.; Cárdenas, L.M.; Calvet, S.; Brüggemann, N.; Loick, N.; Liu, S.; Bol, R. The effect of nitrification inhibitor on N2O, NO and N2 emissions under different soil moisture levels in a permanent grassland soil. Soil Biol. Biochem. 2017, 113, 153–160. [Google Scholar] [CrossRef]
- Lai, T.V.; Denton, M.D. N2O and N2 emissions from denitrification respond differently to temperature and nitrogen supply. J. Soils Sediments 2018, 18, 1548–1557. [Google Scholar] [CrossRef]
- USEPA. Methods for the Determination of Inorganic Substances in Environmental Samples; USEPA 600/R–93/100; United States Environmental Protection Agency: Cincinnati, OH, USA, 1993. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/30002U3P.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1991+Thru+1994&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C91thru94%5CTxt%5C00000008%5C30002U3P.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C–&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 18 September 2020).
- Mielke, L.N.; Swanson, N.P.; McCalla, T.M. Soil profile conditions of cattle feedlots. J. Environ. Qual. 1974, 3, 14–17. [Google Scholar] [CrossRef]
- Miller, J.J.; Curtis, T.; Larney, F.J.; McAllister, T.A. Physical and chemical properties of feedlot pen surfaces located on moderately coarse– and moderately fine–textured soils in Southern Alberta. J. Environ. Qual. 2008, 37, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Toyoda, S.; Hanajima, D.; Yoshida, N. Denitrifiers in the surface zone are primarily responsible for the nitrous oxide emission of dairy manure compost. J. Hazard. Mat. 2013, 248–249, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Baral, K.R.; Arthur, E.; Olesen, J.E.; Peterson, S.O. Predicting nitrous oxide emissions from manure properties and soil moisture: An incubation experiment. Soil Biol. Biochem. 2016, 97, 112–120. [Google Scholar] [CrossRef]
- Rudaz, A.O.; Davidson, E.A.; Firestone, M.K. Sources of nitrous oxide production following wetting of dry soil. FEMS Microbiol. Lett. 1991, 85, 117–124. [Google Scholar] [CrossRef]
- Butterly, C.R.; Baldock, J.A.; Tang, C.X. The contribution of crop residues to changes in soil pH under field conditions. Plant Soil 2013, 366, 185–198. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified soil acidification from chemical N fertilization and prevention by manure in an 18–year field experiment in the red soil of southern China. J. Soils Sediments 2015, 15, 260–270. [Google Scholar] [CrossRef]
- Shi, R.-Y.; Liu, Z.-D.; Li, Y.; Jiant, T.; Xu, M.; Li, J.-Y.; Xu, R.-K. Mechanisms for increasing soil resistance to acidification by long–term manure application. Soil Tillage Res. 2019, 185, 77–84. [Google Scholar] [CrossRef]
- Fordham, A.W.; Schwertmann, U. Composition and reactions of liquid manure (gulle), with particular reference to phosphate: III. pH buffering capacity and organic components. J. Environ. Qual. 1977, 6, 140–144. [Google Scholar] [CrossRef]
- Ni, J. Mechanistic models of ammonia release from liquid manure: A review. J. Agric. Eng. Res. 1999, 72, 1–17. [Google Scholar] [CrossRef]
- Xu, J.; Wei, Q.; Yang, S.; Liao, L.; Qi, Z.; Wang, W. Soil degassing during watering: An overlooked soil N2O emission process. Environ. Pollut. 2018, 242, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, H.; Montes, F.; Rotz, C.A.; Richard, T.L. Volatile ammonia fraction and flux from thin layers of buffered ammonium solution and dairy cattle manure. Trans. ASABE 2009, 52, 1695–1706. [Google Scholar] [CrossRef]
- Harrison–Kirk, T.; Beare, M.H.; Meenken, E.D.; Condron, L.M. Soil organic matter and texture affect. responses to dry/wet cycles: Effects on carbon dioxide and nitrous oxide emissions. Soil Biol. Biochem. 2013, 57, 43–55. [Google Scholar] [CrossRef]
- Manalil, S.; Riethmuller, G.; Flower, K. Rapid emissions of nitrous oxide from fallow over summer following wetting in a Mediterranean–type environment. Soil Tillage Res. 2014, 143, 130–136. [Google Scholar] [CrossRef]
- Congreves, K.A.; Wagner–Riddle, C.; Si, B.C.; Clough, T.J. Nitrous oxide emissions and biogeochemical responses to soil freezing–thawing and drying–rewetting. Soil Biol. Biochem. 2018, 117, 5–15. [Google Scholar] [CrossRef]
- Waldrip, H.M.; He, Z.; Todd, R.W.; Hunt, J.F.; Cole, N.A. Characterization of organic matter in beef feedyard manure by UV–visible and Fourier transform infrared spectroscopies. J. Environ. Qual. 2014, 43, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.S.; Tiedje, J.M. Isolation and characterization of a nitrite reductase gene and its use as a probe for denitrifying bacteria. Appl. Environ. Microbiol. 1979, 58, 376–384. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Luo, J.; Di, H.J.; Liu, D.; Fan, J.; Ding, W. Nitrospira Cluster 8a plays a predominant role in the nitrification process of a subtropical ultisol under long–term inorganic and organic fertilization. Appl. Environ. Microbiol. 2018, 84, e01031-18. [Google Scholar] [CrossRef]






| (a) | ||||||||||
| Day and Chamber | Temp (°C) | N2O–N (mg·m−2·h−1) | DM (%) | OM (% DM) | Eh (mV) | pH | NHx (mg·kg−1) | NOx (mg·kg−1) | Total N (% DM) | Total C (% DM) |
| 1 (1st H2O application) | ||||||||||
| 1 | 5.0 | 1.43 | 77.6 ab* | 54.7 | −27.0 | 9.00 | 403 | 5.68 b | 2.49 b | 26.3 bc |
| 2 | 11.2 | 4.26 | 76.3 ab | 55.3 | −29.7 | 9.05 | 368 | 5.78 b | 2.62 ab | 28.4 a |
| 3 | 21.5 | 66.1 | 80.6 a | 55.7 | −19.7 | 8.92 | 341 | 4.65 b | 2.58 ab | 26.5 bc |
| 4 | 26.8 | 152 | 68.7 b | 55.1 | −22.3 | 8.86 | 402 | 24.2 a | 2.68 a | 28.1 ab |
| 5 | 17.2 | 25.5 | 81.9 a | 56.0 | −14.0 | 8.94 | 352 | 4.27 b | 2.51 b | 26.1 c |
| 9 | ||||||||||
| 1 | 5.0 | 0.21 | 80.7 b | 56.2 | −15.3 | 8.95 a | 371 a | 3.85 ab | 2.67 a | 28.6 a |
| 2 | 11.2 | 0.46 | 87.4 a | 54.3 | −32.7 | 8.82 b | 343 a | 2.93 b | 2.53 b | 26.6 b |
| 3 | 21.5 | 0.50 | 86.1 ab | 54.9 | −12.3 | 8.85 ab | 297 bc | 4.38 a | 2.62 ab | 27.6 ab |
| 4 | 26.8 | 1.58 | 87.9 a | 56.0 | −21.3 | 8.95 a | 273 c | 4.99 a | 2.68 a | 28.3 a |
| 5 | 17.2 | 0.45 | 87.6 a | 55.1 | −12.3 | 8.86 ab | 334 ab | 4.68 a | 2.66 ab | 27.6 ab |
| 18 | ||||||||||
| 1 | 5.0 | 0.01 | 88.8 b | 56.0 | −65.7 | 9.07 | 337 a | 2.65 b | 2.55 | 26.4 b |
| 2 | 11.2 | 2.13 | 91.6 a | 56.4 | −61.3 | 8.94 | 315 a | 3.10 ab | 2.63 | 28.3 a |
| 3 | 21.5 | 0.83 | 91.8 a | 55.6 | −67.7 | 9.03 | 290 a | 3.67 ab | 2.56 | 27.2 ab |
| 4 | 26.8 | 0.93 | 92.0 a | 55.3 | −66.3 | 9.04 | 215 b | 4.98 a | 2.59 | 26.6 ab |
| 5 | 17.2 | 4.34 | 90.1 ab | 57.0 | −68.3 | 9.00 | 312 a | 4.42 ab | 2.66 | 28.1 ab |
| 22 (2nd H2O application) | ||||||||||
| 1 | 15.0 | 21.3 | 70.9 | 57.4 a | −16.3 a | 9.06 a | 427 ab | 13.6 | 2.59 | 28.1 ab |
| 2 | 38.1 | 94.1 | 63.1 | 53.4 c | −98.3 b | 8.85 b | 458 a | 17.6 | 2.59 | 27.2 ab |
| 3 | 21.5 | 58.7 | 70.0 | 56.3 ab | −37.3 ab | 8.98 a | 338 b | 12.5 | 2.67 | 28.8 a |
| 4 | 26.8 | 85.2 | 63.4 | 55.5 b | −69.7 ab | 8.94 ab | 348 ab | 14.5 | 2.47 | 26.0 b |
| 5 | 46.2 | 106 | 71.5 | 54.7 bc | −62.7 ab | 8.94 ab | 408 ab | 14.9 | 2.40 | 25.6 b |
| 30 | ||||||||||
| 1 | 15.0 | 0.22 | 73.1 c | 56.1 a | −26.7 b | 9.23 a | 286 a | 3.99 ab | 2.90 a | 30.5 a |
| 2 | 38.1 | 13.1 | 89.0 ab | 52.7 b | 2.33 a | 9.03 bc | 217 ab | 3.69 ab | 2.70 ab | 28.0 ab |
| 3 | 21.5 | 3.33 | 82.3 b | 55.4 a | −16.3 ab | 9.22 ab | 150 b | 5.99 a | 2.75 ab | 28.0 ab |
| 4 | 26.8 | 4.50 | 86.9 ab | 53.7 ab | −16.3 ab | 9.16 ab | 153 b | 3.89 ab | 2.65 b | 26.8 bc |
| 5 | 46.2 | 6.71 | 92.1 a | 55.5 a | −23.0 b | 8.89 c | 275 a | 3.25 b | 2.43 c | 25.1 c |
| 59 | ||||||||||
| 1 | 15.0 | 2.08 | 87.9 c | 56.5 | 52.3 a | 8.95 ab | 294 a | 3.45 bc | 2.66 a | 28.1 |
| 2 | 38.1 | 0.89 | 91.7 ab | 54.9 | 16.0 b | 8.96 ab | 183 b | 3.83 abc | 2.66 a | 27.7 |
| 3 | 21.5 | 0.41 | 90.4 b | 55.1 | −3.33 c | 9.09 ab | 201 b | 4.79 ab | 2.56 ab | 26.9 |
| 4 | 26.8 | 0.62 | 91.1 b | 55.0 | −11.3 c | 9.13 a | 179 b | 5.32 a | 2.61 ab | 27.4 |
| 5 | 46.2 | 0.55 | 93.4 a | 53.8 | 0.00 bc | 8.94 b | 209 b | 2.66 c | 2.50 b | 27.0 |
| (b) | ||||||||||
| Day and Chamber | Temp (°C) | N2O–N (mg·m−2·h−1) | DM (%) | OM (% DM) | Eh (mV) | pH | NHx (mg·kg−1) | NOx (mg·kg−1) | Total N (% DM) | Total C (% DM) |
| 1 (1st H2O application) | ||||||||||
| 1 | 5.0 | 1.43 | 75.1 | 55.6 | −32.7 b | 9.03 a | 404 ab | 4.19 b | 2.57 | 28.4 |
| 2 | 11.2 | 4.26 | 71.6 | 55.1 | −30.7 b | 8.97 ab | 405 ab | 10.7 b | 2.64 | 28.0 |
| 3 | 21.5 | 66.1 | 83.0 | 54.3 | −32.0 b | 8.94 ab | 357 b | 4.12 b | 2.55 | 26.0 |
| 4 | 26.8 | 152 | 65.6 | 54.7 | −20.7 ab | 8.83 b | 469 a | 36.2 a | 2.69 | 27.2 |
| 5 | 17.2 | 25.5 | 77.7 | 55.7 | −13.0 a | 8.96 ab | 370 b | 6.74 b | 2.57 | 26.0 |
| 9 | ||||||||||
| 1 | 5.0 | 0.21 | 74.1 b | 54.9 | −21.0 | 8.87 | 497 a | 8.65 a | 2.61 | 27.5 |
| 2 | 11.2 | 0.46 | 81.4 ab | 52.9 | −30.3 | 8.84 | 394 ab | 2.99 b | 2.57 | 26.6 |
| 3 | 21.5 | 0.50 | 82.1 ab | 53.1 | −18.3 | 8.83 | 294 b | 3.78 ab | 2.45 | 25.5 |
| 4 | 26.8 | 1.58 | 84.9 a | 54.2 | −21.7 | 8.85 | 301 b | 2.76 b | 2.60 | 27.2 |
| 5 | 17.2 | 0.45 | 84.3 a | 52.7 | −19.3 | 8.81 | 343 b | 2.86 b | 2.59 | 26.9 |
| 18 | ||||||||||
| 1 | 5.0 | 0.008 | 84.3 | 55.6 | −59.0 | 9.00 | 369 ab | 2.96 bc | 2.58 | 27.1 |
| 2 | 11.2 | 2.13 | 86.7 | 55.0 | −60.0 | 8.99 | 404 a | 2.72 c | 2.58 | 26.9 |
| 3 | 21.5 | 0.830 | 88.0 | 55.2 | −62.7 | 9.02 | 255 d | 4.40 ab | 2.60 | 27.1 |
| 4 | 26.8 | 0.930 | 87.1 | 55.2 | −62.0 | 9.07 | 313 c | 3.67 bc | 2.61 | 26.7 |
| 5 | 17.2 | 4.34 | 82.7 | 56.2 | −67.3 | 9.00 | 316 bc | 5.68 a | 2.65 | 28.1 |
| 22 (2nd H2O application) | ||||||||||
| 1 | 15.0 | 21.3 | 85.4 a | 55.5 ab | −25.3 a | 9.02 a | 347 ab | 2.48 b | 2.55 b | 25.7 |
| 2 | 38.1 | 94.1 | 68.1 ab | 53.6 bc | −146 b | 8.79 b | 450 ab | 22.5 a | 2.58 b | 26.5 |
| 3 | 21.5 | 58.7 | 81.3 ab | 56.5 a | −67.0 a | 8.99 a | 323 b | 5.40 b | 2.65 ab | 27.3 |
| 4 | 26.8 | 85.2 | 66.7 b | 52.3 c | −80.7 ab | 8.94 a | 339 ab | 7.20 b | 2.87 a | 28.9 |
| 5 | 46.2 | 106 | 70.6 ab | 54.9 abc | −67.0 a | 8.92 ab | 492 a | 15.8 ab | 2.66 ab | 27.2 |
| 30 | ||||||||||
| 1 | 15.0 | 0.22 | 63.8 b | 55.3 | −22.7 b | 9.05 a | 307 ab | 8.03 | 2.71 | 26.9 |
| 2 | 38.1 | 13.1 | 79.3 a | 52.5 | −6.67 a | 8.97 a | 220 abc | 17.4 | 2.69 | 26.1 |
| 3 | 21.5 | 3.33 | 66.2 b | 52.4 | −14.3 ab | 9.04 a | 176 c | 14.8 | 2.77 | 26.2 |
| 4 | 26.8 | 4.50 | 78.8 a | 52.1 | −22.3 b | 9.00 a | 203 bc | 5.24 | 2.63 | 26.2 |
| 5 | 46.2 | 6.71 | 84.3 a | 53.6 | −26.3 b | 8.82 b | 337 a | 6.10 | 2.73 | 27.7 |
| 59 | ||||||||||
| 1 | 15.0 | 2.08 | 83.6 b | 53.2 | 35.0 a | 8.97 | 279 | 4.41 | 2.60 ab | 27.2 |
| 2 | 38.1 | 0.89 | 87.1 ab | 50.6 | 15.7 ab | 8.96 | 170 | 2.66 | 2.59 ab | 25.8 |
| 3 | 21.5 | 0.41 | 86.0 ab | 52.9 | 0.33 bc | 8.92 | 247 | 4.58 | 2.65 a | 26.4 |
| 4 | 26.8 | 0.62 | 89.3 ab | 55.0 | −9.67 c | 8.93 | 253 | 3.51 | 2.48 b | 25.4 |
| 5 | 46.2 | 0.55 | 91.1 a | 53.8 | −12.3 c | 8.88 | 285 | 2.06 | 2.53 ab | 26.1 |
| Day and Chamber | Temp (°C) | DEA (μmol·g−1·h−1) | DEAs (μmol·g−1·h−1) | DEAl (μmol·g−1·h−1) | NA (μmol·g−1·h−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0–5 cm | 5–10 cm | 0–5 cm | 5–10 cm | 0–5 cm | 5–10 cm | 0–5 cm | 5–10 cm | ||
| 1 | |||||||||
| 1 | 5.0 | 0.34 b | 0.46 b | 0.24 | 0.23 | 1.14 ab | 0.79 b | 0.18 a | 0.17 |
| 2 | 11.2 | 0.69 ab | 0.74 ab | 0.25 | 0.17 | 1.24 ab | 1.45 a | 0.10 b | 0.12 |
| 3 | 21.5 | 0.67 b | 0.80 ab | 0.34 | 0.24 | 0.94 b | 1.40 ab | 0.14 ab | 0.16 |
| 4 | 26.8 | 0.90 ab | 1.05 a | 0.44 | 0.29 | 1.69 a | 1.96 a | 0.11 ab | 0.11 |
| 5 | 17.2 | 0.95 a | 0.94 a | 0.80 | 0.15 | 1.58 a | 1.79 a | 0.11 ab | 0.13 |
| 9 | |||||||||
| 1 | 5.0 | 0.94 | 3.76 | 0.32 | 0.52 a* | 1.56 | 1.46 | 0.14 b | 0.11 b |
| 2 | 11.2 | 0.93 | 0.97 | 0.64 | 0.33 ab | 1.07 | 1.52 | 0.17 ab | 0.15 ab |
| 3 | 21.5 | 0.96 | 0.97 | 0.61 | 0.22 ab | 1.16 | 1.66 | 0.20 ab | 0.20 a |
| 4 | 26.8 | 0.96 | 0.92 | 0.81 | 0.26 ab | 0.95 | 1.53 | 0.24 a | 0.15 ab |
| 5 | 17.2 | 0.89 | 0.88 | 0.27 | 0.14 b | 1.44 | 1.70 | 0.18 ab | 0.12 b |
| 18 | |||||||||
| 1 | 5.0 | 0.81 | 0.85 a | 0.17 b | 0.23 | 1.50a | 1.33 | 0.14 | 0.11 c |
| 2 | 11.2 | 0.85 | 0.88 a | 0.17 b | 0.30 | 1.32a | 1.33 | 0.14 | 0.13 bc |
| 3 | 21.5 | 0.87 | 0.88 a | 0.56 ab | 0.33 | 1.07ab | 1.28 | 0.18 | 0.16 b |
| 4 | 26.8 | 0.84 | 0.43 b | 0.84 a | 0.43 | 0.79b | 1.30 | 0.16 | 0.23 a |
| 5 | 17.2 | 0.78 | 0.61 ab | 0.27 b | 0.43 | 1.36a | 1.09 | 0.18 | 0.13 bc |
| 22 | |||||||||
| 1 | 15.0 | 0.63 | 0.74 | 0.25 | 0.16 b | 1.08 | 1.06 | 0.09 abc | 0.13 ab |
| 2 | 38.1 | 0.68 | 0.94 | 0.52 | 0.65 a | 1.01 | 1.54 | 0.10 ab | 0.10 ab |
| 3 | 21.5 | 0.57 | 0.64 | 0.31 | 0.32 b | 0.90 | 1.03 | 0.12 a | 0.16 a |
| 4 | 26.8 | 0.84 | 0.66 | 0.54 | 0.47 ab | 1.22 | 0.95 | 0.05 c | 0.09 b |
| 5 | 46.2 | 0.64 | 0.57 | 0.21 | 0.18 b | 1.16 | 1.07 | 0.06 bc | 0.08 b |
| 30 | |||||||||
| 1 | 15.0 | 0.74 | 1.03 a | 0.33 b | 0.74 | 1.20 | 0.96 ab | 0.10 b | 0.05 b |
| 2 | 38.1 | 0.85 | 0.66 b | 0.72 ab | 0.65 | 0.89 | 0.75 b | 0.08 b | 0.14 a |
| 3 | 21.5 | 0.82 | 1.02 a | 0.66 ab | 0.82 | 0.83 | 1.04 ab | 0.14 ab | 0.08 b |
| 4 | 26.8 | 0.96 | 1.12 a | 0.82 a | 0.90 | 0.87 | 0.90 ab | 0.07 b | 0.16 a |
| 5 | 46.2 | 0.85 | 0.94 a | 0.40 ab | 0.35 | 1.02 | 1.24 a | 0.24 a | 0.16 a |
| 59 | |||||||||
| 1 | 15.0 | 0.90 a | 1.02 a | 0.31 b | 0.51 | 1.29 a | 1.16 | 0.17 ab | 0.14 ab |
| 2 | 38.1 | 0.85 a | 0.83 a | 0.63 ab | 0.56 | 0.84 ab | 1.01 | 0.09 b | 0.18 a |
| 3 | 21.5 | 0.86 a | 0.92 a | 0.66 ab | 0.52 | 1.09 ab | 1.35 | 0.14 ab | 0.14 ab |
| 4 | 26.8 | 0.93 a | 0.83 a | 0.88 a | 0.24 | 0.97 ab | 1.48 | 0.12 ab | 0.13 ab |
| 5 | 46.2 | 0.45 b | 0.40 b | 0.64 ab | 0.40 | 0.60 b | 0.71 | 0.20 a | 0.09 b |
| (a) | ||||
| 0–5 cm depth | DEA | DEAs | DEAl | NA |
| N2O † emissions | −0.156 (0.137) | −0.083 (0.428) | 0.155 (0.139) | −0.369 (0.0003) *** |
| Temperature | −0.139 (0.191) | 0.169 (0.112) | −0.359 (0.0005) *** | −0.038 (0.722) |
| Dry matter | 0.171 (0.103) | 0.114 (0.275) | −0.183 (0.079) | 0.471 (<0.0001) *** |
| Eh | 0.065 (0.540) | −0.018 (0.862) | −0.056 (0.591) | 0.129 (0.218) |
| pH | 0.047 (0.659) | 0.143 (0.180) | −0.152 (0.152) | −0.278 (0.008) ** |
| Total nitrogen | 0.098 (0.352) | 0.082 (0.436) | 0.022 (0.835) | −0.060 (0.570) |
| Total carbon | −0.041 (0.700) | −0.079 (0.452) | 0.088 (0.400) | −0.023 (0.823) |
| Ammonium/ammonia | −0.176 (0.094) | −0.442 (<0.0001) *** | 0.464 (<0.0001) *** | −0.026 (0.802) |
| Nitrate/nitrite | −0.289 (0.005) ** | −0.098 (0.350) | 0.042 (0.689) | −0.320 (0.002) ** |
| TC:TN | −0.266 (0.010) ** | −0.308 (0.003) ** | 0.134 (0.202) | 0.053 (0.614) |
| DEA | ––– | 0.405 (<0.0001) *** | 0.426 (<0.0001) *** | 0.001 (0.991) |
| DEAs | 0.405 (<0.0001) *** | ––– | −0.408 (<0.0001) *** | −0.118 (0.268) |
| DEAl | 0.426 (<0.0001) *** | −0.408 (<0.0001) *** | ––– | −0.039 (0.712) |
| NA | 0.001 (0.991) | −0.118 (0.268) | −0.039 (0.712) | ––– |
| (b) | ||||
| 5–10 cm depth | DEA | DEAs | DEAl | NA |
| N2O † emissions | −0.065 (0.545) | −0.055 (0.608) | 0.156 (0.142) | −0.244 (0.020) * |
| Temperature | −0.196 (0.064) | 0.069 (0.522) | −0.181 (0.087) | −0.022 (0.834) |
| Dry matter | −0.025 (0.817) | −0.378 (0.0003) *** | −0.108 (0.310) | 0.516 (<0.0001) *** |
| Eh | 0.134 (0.208) | −0.028 (0.792) | −0.008 (0.939) | 0.146 (0.170) |
| pH | −0.228 (0.031) * | 0.183 (0.086) | −0.372 (0.0003) ** | 0.125 (0.242) |
| Total nitrogen | 0.003 (0.978) | 0.239 (0.024) * | −0.102 (0.340) | −0.180 (0.090) |
| Total carbon | −0.044 (0.683) | −0.166 (0.121) | −0.025 (0.815) | −0.055 (0.609) |
| Ammonium/ammonia | 0.021(0.846) | −0.387 (0.0002) *** | 0.339 (0.0011) ** | −0.241 (0.022) * |
| Nitrate/nitrite | −0.001 (0.992) | −0.255 (0.016) * | 0.193 (0.068) | −0.265 (0.012) * |
| TC:TN | −0.060 (0.573) | −0.482 (<0.0001) *** | 0.084 (0.429) | 0.136 (0.202) |
| DEA | ––– | 0.060 (0.574) | 0.219 (0.038) * | −0.075 (0.483) |
| DEAs | 0.060 (0.574) | ––– | −0.291 (0.006) ** | −0.189 (0.076) |
| DEAl | 0.219 (0.038) * | −0.291 (0.006) ** | ––– | 0.0004 (1.00) |
| NA | −0.075 (0.483) | −0.189 (0.076) | 0.0004 (1.00) | ––– |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldrip, H.M.; Parker, D.B.; Miller, S.; Miller, D.N.; Casey, K.D.; Todd, R.W.; Min, B.R.; Spiehs, M.J.; Woodbury, B. Nitrous Oxide from Beef Cattle Manure: Effects of Temperature, Water Addition and Manure Properties on Denitrification and Nitrification. Atmosphere 2020, 11, 1056. https://doi.org/10.3390/atmos11101056
Waldrip HM, Parker DB, Miller S, Miller DN, Casey KD, Todd RW, Min BR, Spiehs MJ, Woodbury B. Nitrous Oxide from Beef Cattle Manure: Effects of Temperature, Water Addition and Manure Properties on Denitrification and Nitrification. Atmosphere. 2020; 11(10):1056. https://doi.org/10.3390/atmos11101056
Chicago/Turabian StyleWaldrip, Heidi M., David B. Parker, Sierra Miller, Daniel N. Miller, Kenneth D. Casey, Richard W. Todd, Byeng R. Min, Mindy J. Spiehs, and Bryan Woodbury. 2020. "Nitrous Oxide from Beef Cattle Manure: Effects of Temperature, Water Addition and Manure Properties on Denitrification and Nitrification" Atmosphere 11, no. 10: 1056. https://doi.org/10.3390/atmos11101056
APA StyleWaldrip, H. M., Parker, D. B., Miller, S., Miller, D. N., Casey, K. D., Todd, R. W., Min, B. R., Spiehs, M. J., & Woodbury, B. (2020). Nitrous Oxide from Beef Cattle Manure: Effects of Temperature, Water Addition and Manure Properties on Denitrification and Nitrification. Atmosphere, 11(10), 1056. https://doi.org/10.3390/atmos11101056






