Bacteria as Cloud Condensation Nuclei (CCN) in the Atmosphere
Abstract
1. Introduction
2. Kinetic Model of Heterogeneous Nucleation
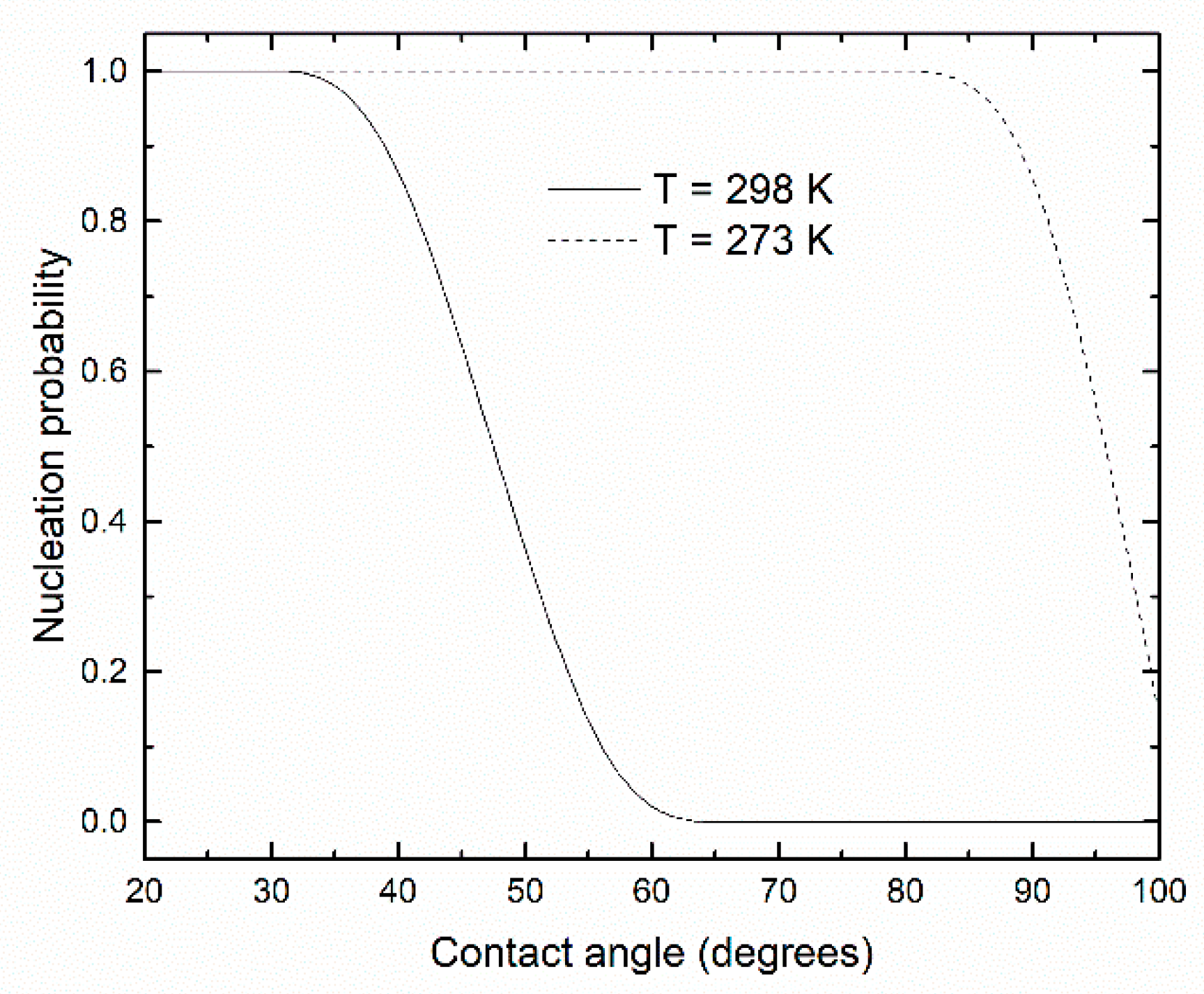
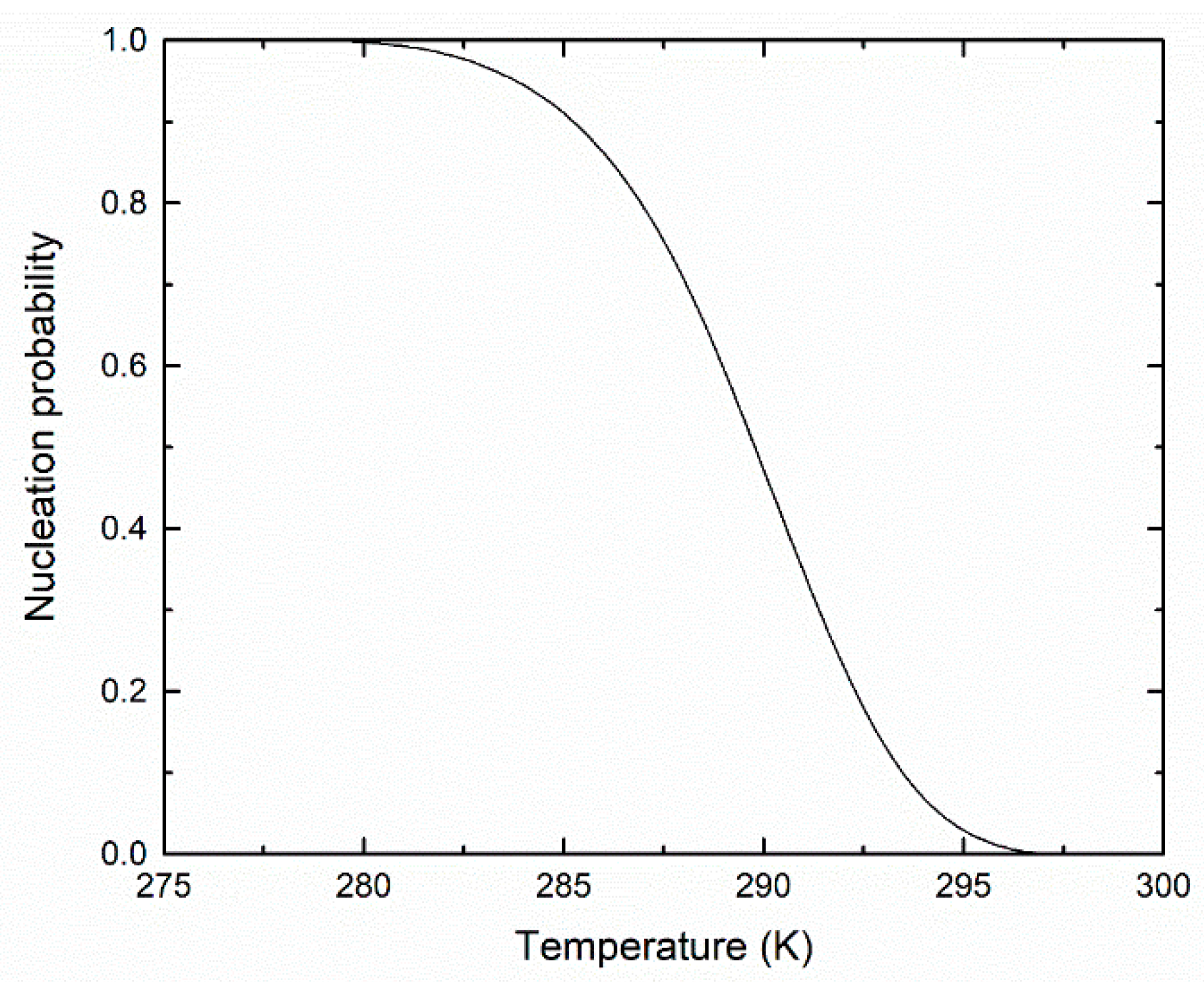
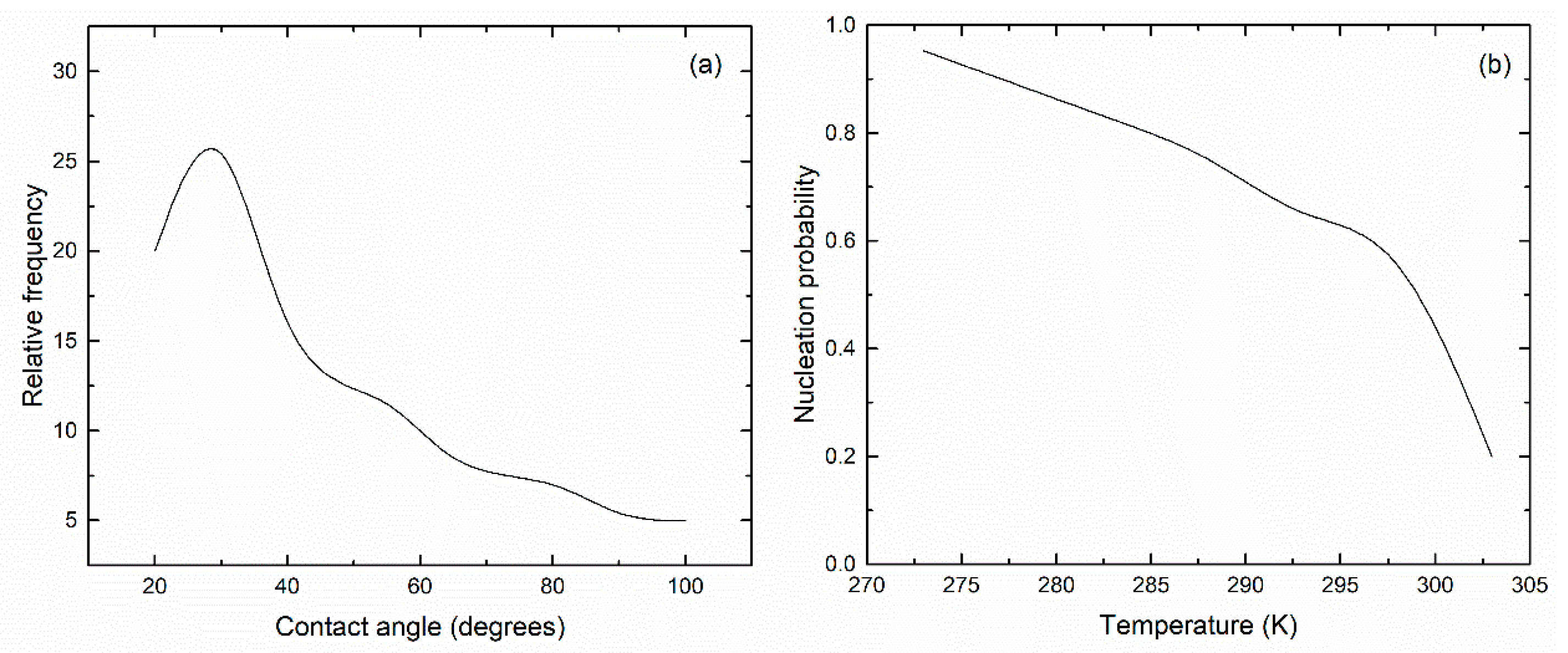
3. Results and Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Després, V.R.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.O.; Pöschl, U.; et al. Primary Biological Aerosol Particles in the Atmosphere: A Review. Tellus B 2012, 64, 15598. [Google Scholar] [CrossRef]
- Sun, J.; Ariya, P.A. Atmospheric organic and bio-aerosols as cloud condensation nuclei (CCN): A review. Atmos. Environ. 2006, 40, 795–820. [Google Scholar] [CrossRef]
- Katsivela, E.; Latos, E.; Raisi, L.; Aleksandropoulou, V.; Lazaridis, M. Particle size distribution of cultivable airborne microbes and inhalable particulate matter in a wastewater treatment plant facility. Aerobiologia 2017, 33, 297–314. [Google Scholar] [CrossRef]
- Korzeniewska, E. Emission of bacteria and fungi in the air from wastewater treatment plants—A review. Front. Biosci. 2011, 3, 303–407. [Google Scholar] [CrossRef] [PubMed]
- Ziembra, L.D.; Beyersdorf, A.J.; Chen, G.; Corr, C.A.; Crumeyrolle, S.N.; Diskin, G.; Hudgins, C.; Martin, R.; Mikoviny, T.; Moore, R.; et al. Airborne observations of bioaerosol over the Southeast United States using a wideband integrated bioaerosol sensor. J. Geophys. Res. 2016, 121, 8506–8524. [Google Scholar]
- Bauer, H.; Giebl, H.; Hitzenberger, R.; Kasper-Giebl, A.; Reichl, G.; Zibuschka, F.; Puxbaum, H. Airborne bacteria as cloud condensation nuclei. J. Geophys. Res. 2003, 108, 4658. [Google Scholar] [CrossRef]
- Frohlich-Nowoisky, J.; Kampf, J.; Weber, B.; Huffman, J.A.; Pohlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Murray, B.J.; O’Sullivan, D.; Atkinson, J.D.; Webb, M.E. Ice nucleation by particles immersed in supercooled cloud droplets. Chem. Soc. Rev. 2012, 41, 6519–6554. [Google Scholar] [CrossRef]
- Franc, G.D.; DeMott, P.J. Cloud activation characteristics of airborne Ervinia carotova cells. J. Appl. Meteorol. 1998, 37, 1293–1300. [Google Scholar] [CrossRef]
- Chernoff, D.I.; Bertram, A.K. Effects of surface coatings on the ice nucleation properties of a biological ice nucleus and several types of minerals. J. Geophys. Res. 2010, 115, 20205. [Google Scholar] [CrossRef]
- Prisle, N.L.; Lin, J.J.; Purdue, S.; Lin, H.; Meredith, J.C.; Nenes, A. Cloud condensation nuclei activity of six pollenkitts and the influence of their surface activity. Atmos. Chem. Phys. 2019, 19, 4741–4761. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, X.; Wang, T.; Wang, B.; Li, C.; Zeng, G. A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ. Int. 2018, 113, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Rao, K.H. Analysis of different approaches for evaluation of surface energy of microbial cells by contact angle goniometry. Adv. Colloid Interface Sci. 2002, 98, 341–463. [Google Scholar] [CrossRef]
- Möhler, O.; De Mott, P.J.; Vali, G.; Levin, Z. Microbiology and atmospheric processes: The role of biological particles in cloud physics. Biogeosciences 2007, 4, 1059–1071. [Google Scholar] [CrossRef]
- Guarany de Araujo, G.; Rodrigues, F.; Texeira Goncalves, L.T.; Galante, D. Survival and ice nucleation activity of Pseudomonas syringae strains exposed to simulated high-altitude atmospheric conditions. Sci. Rep. 2019, 9, 7768. [Google Scholar] [CrossRef]
- Pruppacher, H.R.; Klett, J.D. Microphysics of Clouds and Precipitation; Kluwer Academic: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Fletcher, N.H. The Physics of Rainclouds; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Lazaridis, M. A theoretical study on the activation of insoluble particles in atmospheric conditions. Atmos. Res. 2019, 218, 306–314. [Google Scholar] [CrossRef]
- Ickes, L.; Welti, A.; Lohmann, U. Classical nucleation theory of immersion freezing: Sensitivity of contact angle schemes to thermodynamic and kinetic parameters. Atmos. Chem. Phys. 2017, 17, 1713–1739. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Bretherton, C.; Carslaw, K.S.; Coe, H.; DeMott, P.J.; Dunlea, E.J.; Feingold, G.; Ghan, S.; Guenther, A.B.; Kahn, R.; et al. Improving our fundamental understanding of the role of aerosol−cloud interactions in the climate system. Proc. Natl. Acad. Sci. USA 2016, 113, 5781–5790. [Google Scholar] [CrossRef]
- Hummel, M.; Hoose, C.; Pummer, B.; Schaupp, C.; Frohlich-Nowoisky, J.; Mohler, O. Simulating the influence of primary biological aerosol particles on clouds by heterogeneous ice nucleation. Atmos. Chem. Phys. 2018, 18, 15437–15450. [Google Scholar] [CrossRef]
- Wilson, T.W.; Ladino, L.A.; Alpert, P.A.; Breckels, M.N.; Brooks, I.M.; Browse, J.; Burrows, S.M.; Carslaw, K.S.; Huffman, J.A.; Judd, C.; et al. A marine biogenic source of atmospheric ice-nucleating particles. Nature 2015. [Google Scholar] [CrossRef]
- Sullivan, D.O.; Murray, B.J.; Ross, J.F.; Whale, T.F.; Price, H.C.; Atkinson, J.D.; Umo, N.S.; Webb, M.E. The relevance of nanoscale biological fragments for ice nucleation in clouds. Sci. Rep. 2015, 5, 8082. [Google Scholar] [CrossRef] [PubMed]
- Kevrekidis, P.G.; Lazaridis, M.; Drossinos, Y.; Georgopoulos, P.G. A unified kinetic approach to binary nucleation. J. Chem. Phys. 1999, 111, 8010–8012. [Google Scholar] [CrossRef]
- Lazaridis, M.; Kulmala, M.; Laaksonen, A. Binary heterogeneous nucleation of a water-sulphuric acid system: The effect of hydrate interaction. J. Aerosol Sci. 1991, 22, 823–830. [Google Scholar] [CrossRef]
- Lazaridis, M.; Kulmala, M.; Gorbunov, B. Heterogeneous Nucleation at a Non Uniform Surface. J. Aerosol Sci. 1992, 23, 457–466. [Google Scholar] [CrossRef]
- Lazaridis, M. A Statistical Mechanical Model of Heterogeneous Nucleation Including the Effects of line Tension and Surface Diffusion. J. Colloid Interface Sci. 1994, 162, 431–436. [Google Scholar] [CrossRef]
- Lazaridis, M. The Effects of Surface Diffusion and Line Tension on the Mechanism of Heterogeneous Nucleation. J. Colloid Interface Sci. 1993, 155, 386–391. [Google Scholar] [CrossRef]
- Hamill, P.; Turco, R.P.; Kiang, C.S.; Toon, O.B.; Whitten, R.C. An analysis of various nucleation mechanisms for sulfate particles in the stratosphere. J. Aerosol Sci. 1982, 13, 561–585. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, S.; Peng, Z.; Chen, J.; Su, M.; Moghtaderi, B.; Doroodchi, E. Numerical investigation of heterogeneous nucleation of water vapour on PM2019, 10 for particulate abatement. Can. J. Chem. Eng. 2019, 97, 930–939. [Google Scholar] [CrossRef]
- Wagner, P.E.; Kaller, D.; Vrtala, A.; Lauri, A.; Kulmala, M.; Laaksonen, A. Nucleation probability in binary heterogeneous nucleation of water–n-propanol vapor mixtures on insoluble and soluble nanoparticles. Phys. Rev. E 2005, 67, 021605. [Google Scholar] [CrossRef]
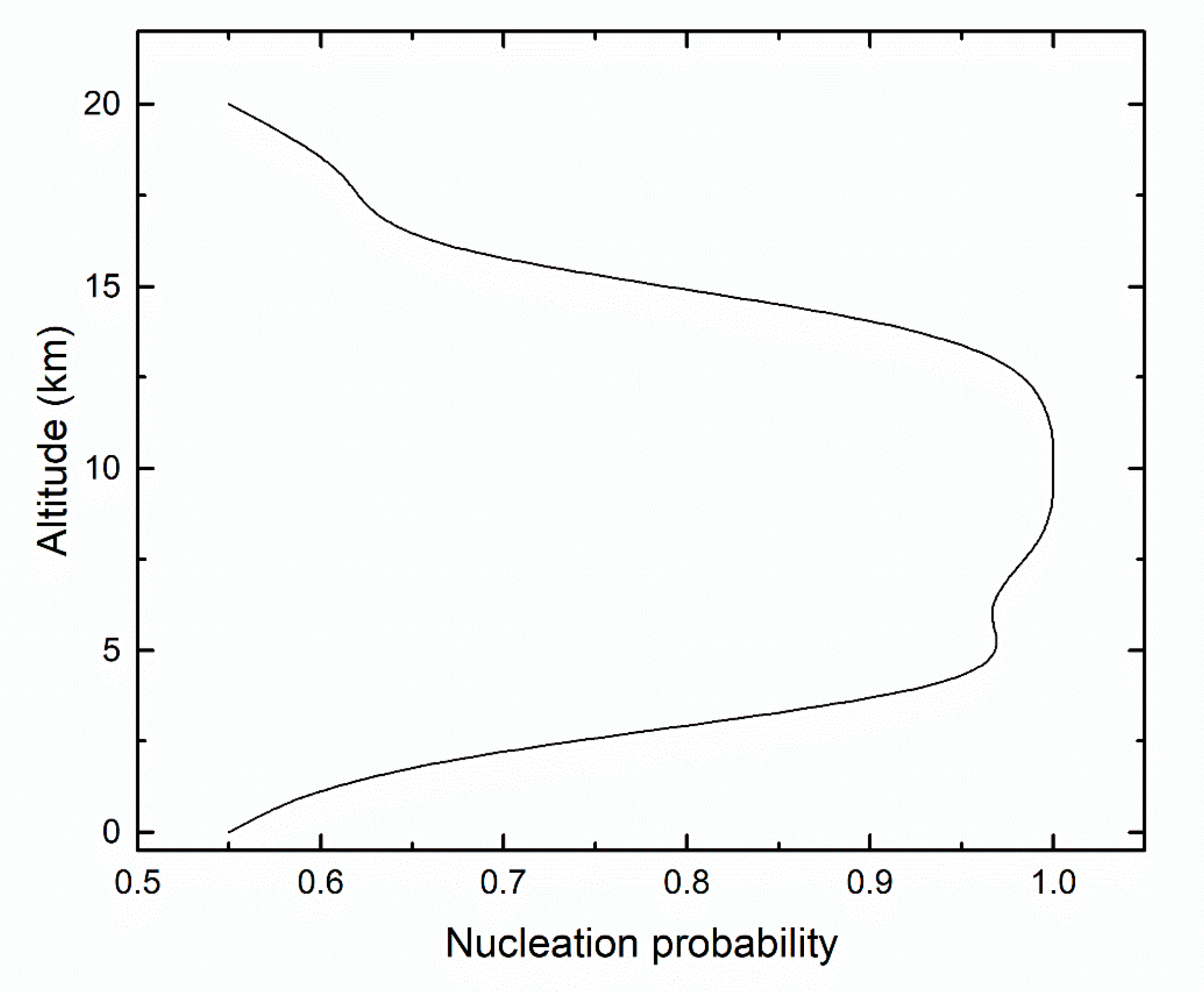
| Altitude (km) | Temperature (K) | [H2SO4] (Molecules/cm3) | [H2O] (Molecules/cm3) |
|---|---|---|---|
| 0 | 288 | 3.58 × 108 | 7.1 × 1016 |
| 2 | 275 | 2.03 × 108 | 3.6 × 1016 |
| 4 | 262 | 9.29 × 108 | 1.3 × 1016 |
| 6 | 249 | 3.85 × 107 | 4.6 × 1015 |
| 8 | 236 | 1.73 × 107 | 1.3 × 1015 |
| 10 | 223 | 8.83 × 106 | 2.2 × 1014 |
| 12 | 219 | 5.50 × 106 | 4.3 × 1013 |
| 14 | 216 | 1.01 × 106 | 9.3 × 1012 |
| 16 | 216 | 1.12 × 105 | 7.0 × 1012 |
| 18 | 216 | 3.67 × 104 | 5.2 × 1012 |
| 20 | 217 | 4.20 × 104 | 4.0 × 1012 |
| 22 | 219 | 8.68 × 104 | 2.9 × 1012 |
| 24 | 221 | 1.93 × 105 | 2.1 × 1012 |
| 26 | 223 | 4.59 × 105 | 1.5 × 1012 |
| 28 | 225 | 1.23 × 106 | 1.2 × 1012 |
| 30 | 227 | 2.88 × 106 | 9.8 × 1012 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaridis, M. Bacteria as Cloud Condensation Nuclei (CCN) in the Atmosphere. Atmosphere 2019, 10, 786. https://doi.org/10.3390/atmos10120786
Lazaridis M. Bacteria as Cloud Condensation Nuclei (CCN) in the Atmosphere. Atmosphere. 2019; 10(12):786. https://doi.org/10.3390/atmos10120786
Chicago/Turabian StyleLazaridis, Mihalis. 2019. "Bacteria as Cloud Condensation Nuclei (CCN) in the Atmosphere" Atmosphere 10, no. 12: 786. https://doi.org/10.3390/atmos10120786
APA StyleLazaridis, M. (2019). Bacteria as Cloud Condensation Nuclei (CCN) in the Atmosphere. Atmosphere, 10(12), 786. https://doi.org/10.3390/atmos10120786




