EHBP1L1 Frameshift Deletion in English Springer Spaniel Dogs with Dyserythropoietic Anemia and Myopathy Syndrome (DAMS) or Neonatal Losses
Abstract
:1. Introduction
2. Animals, Materials, and Methods
2.1. Animal Selection
2.2. Samples and Data Collection
2.3. Laboratory Diagnostics
2.4. Gross Pathology and Histopathology
2.5. Statistical Analysis
2.6. DNA Extraction and SNV Genotyping
2.7. Linkage Analysis and Homozygosity Mapping
2.8. Whole Genome Sequencing
2.9. Variant Calling
2.10. Gene Analysis, PCR, and Sanger Sequencing
3. Results
3.1. Animals, Pedigree, and Family History
3.2. Clinical Examination and Imaging Findings
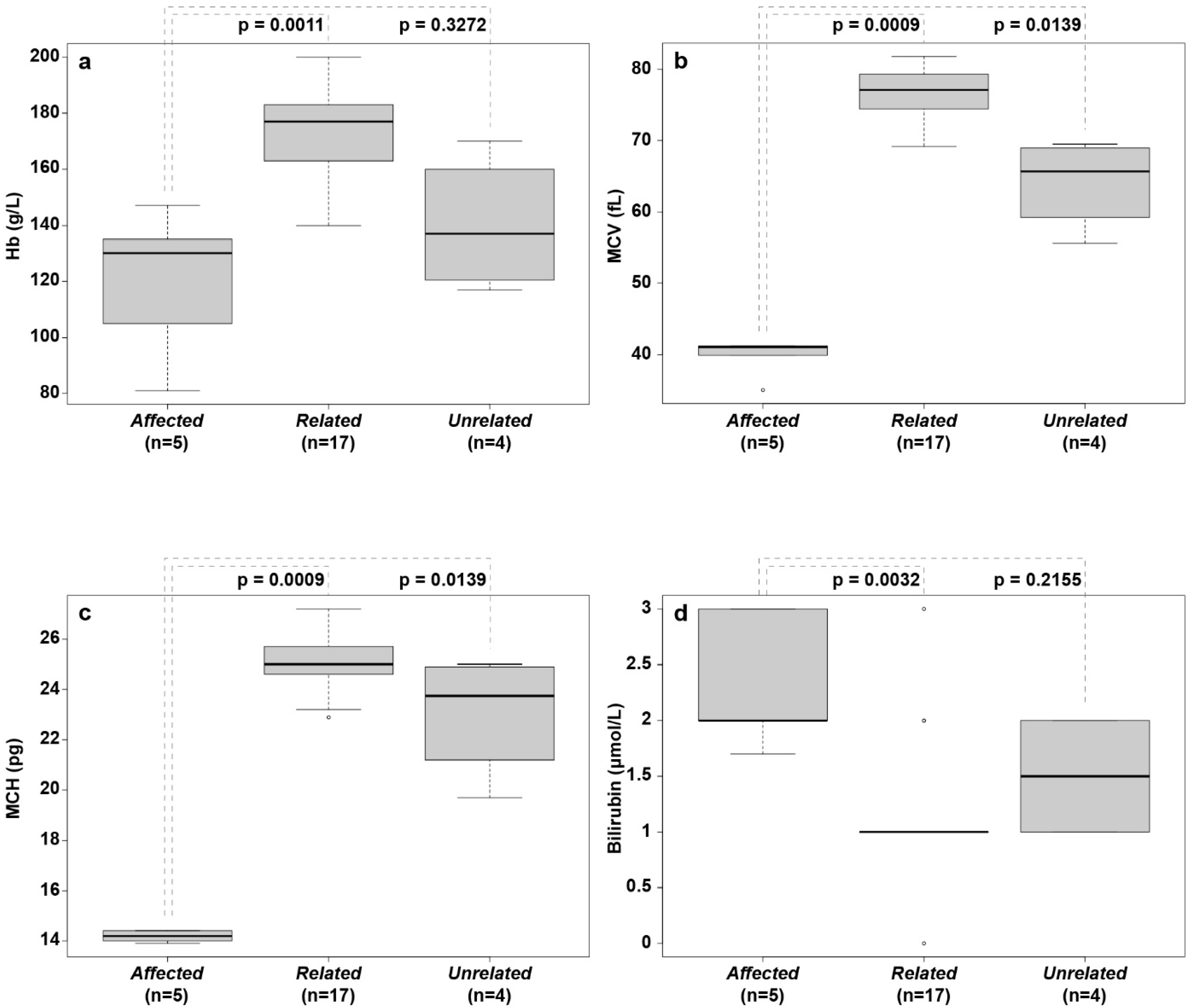
3.3. Hematological Abnormalities
3.4. Serum Chemistry
3.5. Other Serum Tests
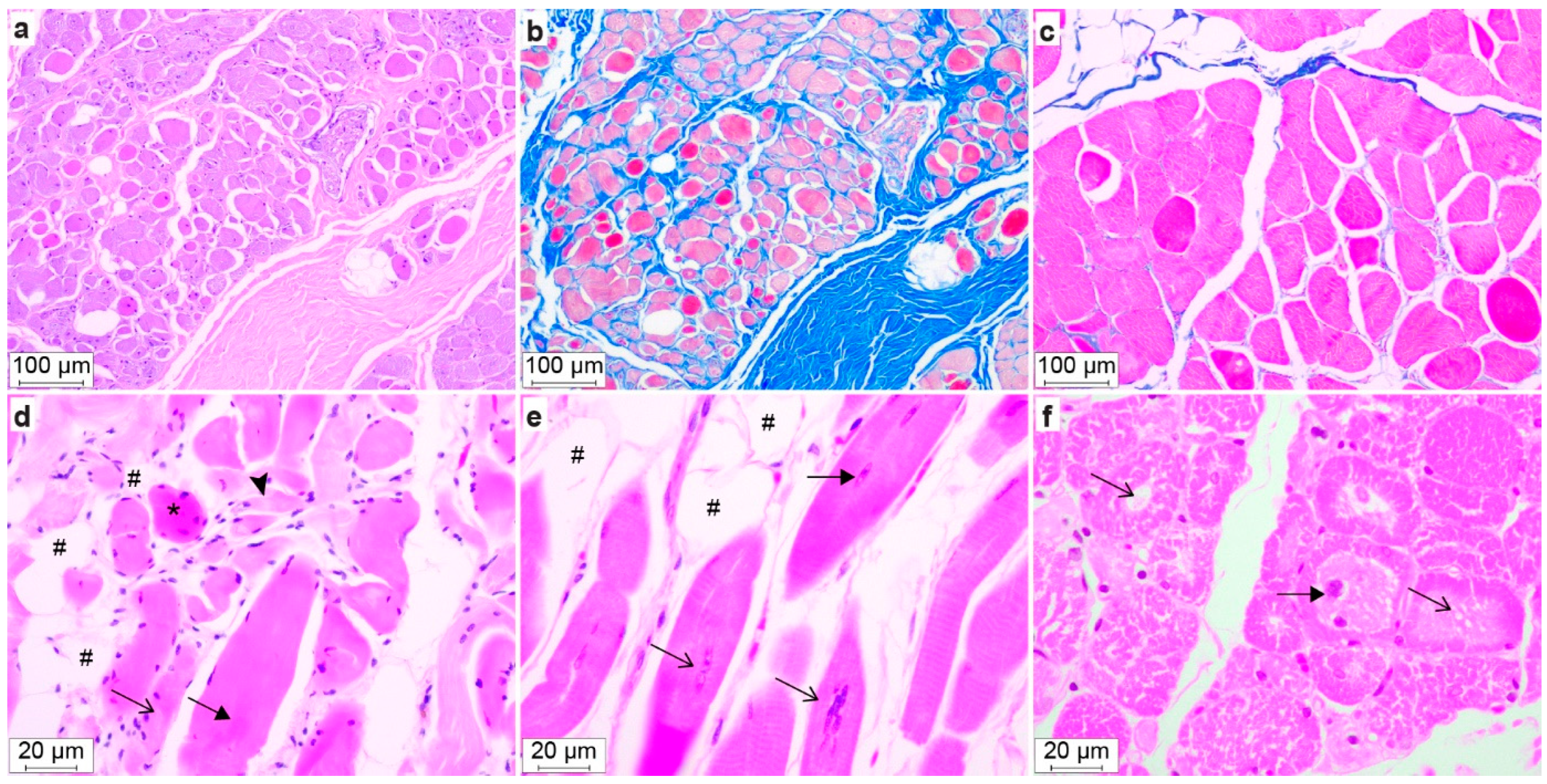
3.6. Gross Pathology and Histopathology
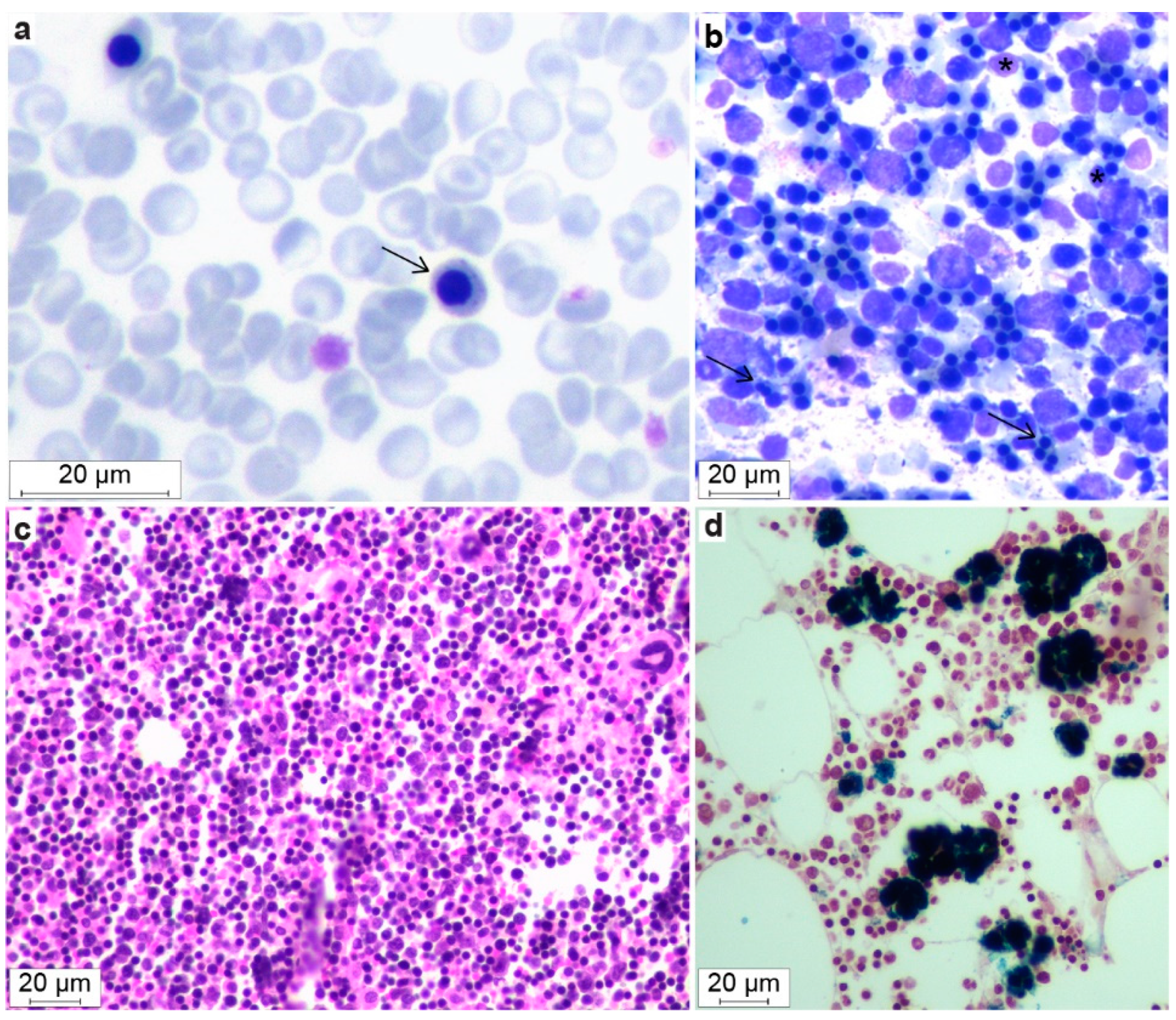
3.7. Bone Marrow Evaluation
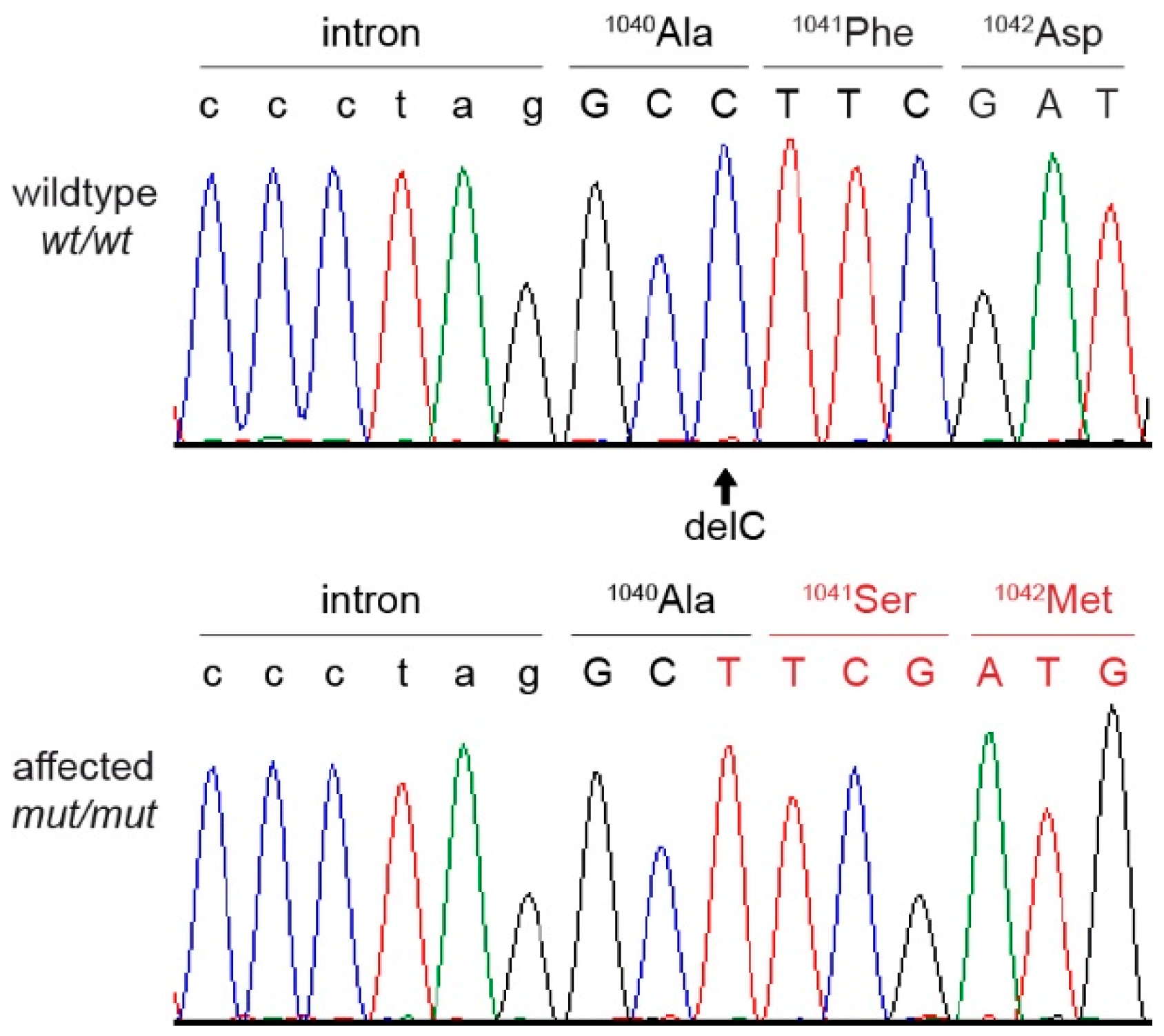
3.8. Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nussbaum, R.; McInnes, R.; Willard, H. Thompson & Thompson Genetics in Medicine, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Nicholas, F.W. Introduction to Veterinary Genetics, 3rd ed.; Wiley: Hoboken, NJ, USA; Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Online Mendelian Inheritance in Animals, OMIA. Sydney School of Veterinary Science. Available online: https://omia.org/ (accessed on 22 June 2022).
- Rokhsar, J.L.; Canino, J.; Raj, K.; Yuhnke, S.; Slutsky, J.; Giger, U. Web resource on available DNA variant tests for hereditary diseases and genetic predispositions in dogs and cats: An Update. Hum. Genet. 2021, 140, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Cavanagh, K.; Tilley, L.; Smith, F.W.K. Veterinary Medical Guide to Dog and Cat Breeds, 1st ed.; Teton: Driggs, ID, USA, 2012. [Google Scholar]
- Giger, U. Update on WSAVA’s work on hereditary diseases. Companion 2017, 2017, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Giger, U. Hereditary Erythroenzymopathies. In Schalm’s Veterinary Hematology, 7th ed.; Brooks, M., Ed.; Wiley: Hoboken, NJ, USA; Blackwell: Hoboken, NJ, USA, 2022; Chapter 29; pp. 229–237. [Google Scholar]
- Inaba, M.; Messick, J.B. Erythrocyte Membrane Defects. In Schalm’s Veterinary Hematology, 7th ed.; Brooks, M., Ed.; Wiley: Hoboken, NJ, USA; Blackwell: Hoboken, NJ, USA, 2022; Chapter 30; pp. 238–247. [Google Scholar]
- Weiss, D.J. Congenital Dyserythropoeisis. In Schalm’s Veterinary Hematology, 7th ed.; Brooks, M., Ed.; Wiley: Hoboken, NJ, USA; Blackwell: Hoboken, NJ, USA, 2022; Chapter 31; pp. 248–251. [Google Scholar]
- Shelton, G.D. Muscular Disorders. In Textbook of Veterinary Internal Medicine. Diseases of the Dog and Cat, 8th ed.; Ettinger, S.J., Feldman, E.C., Côté, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 354; pp. 2146–2150. ISBN 9780-3233-1211-0. [Google Scholar]
- Dewey, D.W.; Talarico, L.R. Myopathies: Disorders of Skeletal Muscle. In Practical Guide to Canine and Feline Neurology, 3rd ed.; Dewey, C.W., Da Costa, R.C., Eds.; Wiley: Hoboken, NJ, USA; Blackwell: Hoboken, NJ, USA, 2016; Chapter 18; pp. 481–520. [Google Scholar]
- Pelé, M.; Tiret, L.; Kessler, J.L.; Blot, S.; Panthier, J.J. SINE exonic insertion in the PTPLA gene leads to multiple splicing defects and segregates with the autosomal recessive centronuclear myopathy in dogs. Hum. Mol. Genet. 2005, 14, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Mary, J.; Guillaud, L.; Fender, M.; Pelé, M.; Bilzer, T.; Olby, N.; Penderis, J.; Shelton, G.D.; Panthier, J.-J.; et al. Centronuclear myopathy in Labrador Retrievers: A recent founder mutation in the PTPLA gene has rapidly disseminated worldwide. PLoS ONE 2012, 7, e46408. [Google Scholar] [CrossRef]
- Böhm, J.; Vasli, N.; Maurer, M.; Cowling, B.S.; Shelton, G.D.; Kress, W.; Toussaint, A.; Prokic, I.; Schara, U.; Anderson, T.J.; et al. Altered splicing of the BIN1 muscle-specific exon in humans and dogs with highly progressive centronuclear myopathy. PLoS Genet. 2013, 9, e1003430. [Google Scholar] [CrossRef]
- Smith, B.F.; Stedman, H.; Rajpurohit, Y.; Henthorn, P.S.; Wolfe, J.H.; Patterson, D.F.; Giger, U. Molecular basis of canine muscle type phosphofructokinase deficiency. J. Biol. Chem. 1996, 271, 20070–20074. [Google Scholar] [CrossRef]
- Giger, U.; Argov, Z.; Schnall, M.; Bank, W.J.; Chance, B. Metabolic myopathy in canine muscle-type phosphofructokinase deficiency. Muscle Nerve 1988, 11, 1260–1265. [Google Scholar] [CrossRef]
- Holland, C.T.; Canfield, P.J.; Watson, A.D.J.; Allan, G.S. Dyserythropoiesis, polymyopathy, and cardiac disease in three related English springer spaniels. J. Vet. Intern. Med. 1991, 5, 151–159. [Google Scholar] [CrossRef]
- Thomas-Hollands, A.; Shelton, G.D.; Guo, L.T.; Loughran, K.; Kaiman, G.; Hutton, T.; Walsh, K.A. Congenital dyserythropoiesis and polymyopathy without cardiac disease in male Labrador retriever littermates. J. Vet. Intern. Med. 2021, 35, 2409–2414. [Google Scholar] [CrossRef]
- The English Springer Spaniel Club. Available online: http://www.englishspringer.org/about-the-breed (accessed on 12 July 2022).
- SKK Hunddata Online Database of Swedish Purebred Dogs. Available online: https://hundar.skk.se/hunddata/ (accessed on 12 July 2022).
- Fleckenbase Online Spaniel Database. Available online: https://www.fleckenbase.de/db1/index.php?breed=ESS (accessed on 12 July 2022).
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Abecasis, G.R.; Cherny, S.S.; Cookson, W.O.; Cardon, L.R. Merlin—Rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 2002, 30, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, V.; Drögemüller, C.; Leeb, T.; Aguirre, G.; André, C.; Bannasch, D.; Becker, D.; Davis, B.; Ekenstedt, K.; Faller, K.; et al. A comprehensive biomedical variant catalogue based on whole genome sequences of 582 dogs and eight wolves. Anim. Genet. 2019, 50, 695–704. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.V.; Shorter, R.G. Structure of the canine eosphagus and its sphincters. J. Surg. Res. 1964, 4, 160–163. [Google Scholar] [CrossRef]
- McCourt, M.R.; Rizzi, T.E. Normal Hematology of Dogs. In Schalm’s Veterinary Hematology, 7th ed.; Brooks, M., Ed.; Wiley: Hoboken, NJ, USA; Blackwell: Hoboken, NJ, USA, 2022; Chapter 108; pp. 799–809. [Google Scholar]
- Iolascon, A.; Andolfo, I.; Russo, R. Congenital dyserythropoietic anemias. Blood 2020, 136, 1274–1283. [Google Scholar] [CrossRef]
- Gambale, A.; Iolascon, A.; Andolfo, I.; Russo, R. Diagnosis and management of congenital dyserythropoietic anemias. Expert Rev. Hematol. 2016, 9, 283–296. [Google Scholar] [CrossRef]
- Ogasawara, M.; Nishino, I. A review of major causative genes in congenital myopathies. J. Hum. Genet. 2022. [Google Scholar] [CrossRef]
- Radke, J.; Stenzel, W.; Goebel, H.W. Recently identified congenital myopathies. Pediatr. Neurol. 2019, 29, 83–90. [Google Scholar] [CrossRef]
- Beecher, G.; Fleming, M.D.; Liewluck, T. Hereditary myopathies associated with hematological abnormalities. Muscle Nerve 2022, 65, 374–390. [Google Scholar] [CrossRef]
- Nakajo, A.; Yoshimura, S.; Togawa, H.; Kunii, M.; Iwano, T.; Izumi, A.; Noguchi, Y.; Watanabe, A.; Goto, A.; Sato, T.; et al. EHBP1L1 coordinates Rab8 and Bin1 to regulate apical-directed transport in polarized epithelial cells. J. Cell Biol. 2016, 212, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Shamseldin, H.E.; AlAbdi, L.; Maddirevula, S.; Alsaif, H.S.; Alzahrani, F.; Ewida, N.; Hashem, M.; Abdulwahab, F.; Abuyousef, O.; Kuwahara, H.; et al. Lethal variants in humans: Lessons learned from a large molecular autopsy cohort. Genome Med. 2021, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, S.; Di Fiore, P.P. The Eps15 homology (EH) domain. FEBS Lett. 2002, 513, 24–29. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, Y.K. When a ribosome encounters a premature termination codon. BMB Rep. 2013, 46, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tønnessen, R.; Borge, K.S.; Nødtvedt, A.; Indrebø, A. Canine perinatal mortality: A cohort study of 224 breeds. Theriogenology 2012, 77, 1788–1801. [Google Scholar] [CrossRef]
- Pereira, K.H.N.P.; dos Santos Correia, L.E.C.; Oliveira, E.L.R.; Bernardo, R.B.; Jorge, M.L.N.; Gobato, M.L.M.; de Souza, F.F.; Rocha, N.S.; Chiacchio, S.B.; Lourenço, M.L.G. Incidence of congenital malformations and impact on the mortality of neonatal canines. Theriogenology 2019, 140, 52–57. [Google Scholar] [CrossRef]
- Hildick-Smith, G.J.; Cooney, J.D.; Garone, C.; Kremer, L.S.; Haack, T.B.; Thon, J.N.; Miyata, N.; Lieber, D.S.; Calvo, S.E.; Akman, H.O.; et al. Macrocytic anemia and mitochondriopathy resulting from a defect in sideroflexin 4. Am. J. Hum. Genet. 2013, 93, 906–914. [Google Scholar] [CrossRef]
- Sur, L.; Floca, E.; Samasca, G.; Lupan, I.; Aldea, C.; Sur, G. Pearson Syndrome, A Medical Diagnosis Difficult to Sustain without Genetic Testing. Clin. Lab. 2018, 64, 375–377. [Google Scholar] [CrossRef]
- Ferguson, P.J.; El-Shanti, H. Majeed Syndrome: A review of the clinical, genetic and immunologic features. Biomolecules 2021, 11, 367. [Google Scholar] [CrossRef]
- Abu-Zeinah, G.; DeSancho, M.T. Understanding Sideroblastic Anemia: An overview of genetics, epidemiology, pathophysiology and current therapeutic options. J. Blood Med. 2020, 11, 305–318. [Google Scholar] [CrossRef]
- Hohendahl, A.; Roux, A.; Galli, V. Structural insights into the centronuclear myopathy-associated functions of BIN1 and dynamin 2. J. Struct. Biol. 2016, 196, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Fujise, K.; Okubo, M.; Abe, T.; Yamada, H.; Nishino, I.; Noguchi, S.; Takei, K.; Takeda, T. Mutant BIN1-Dynamin 2 complexes dysregulate membrane remodeling in the pathogenesis of centronuclear myopathy. J. Biol. Chem. 2021, 296, 100077. [Google Scholar] [CrossRef] [PubMed]
- Fujise, K.; Noguchi, S.; Takeda, T. Centronuclear myopathy caused by defective membrane remodelling of dynamin 2 and BIN1 Variants. Int. J. Mol. Sci. 2022, 23, 6274. [Google Scholar] [CrossRef]
- Böhm, J.; Barthélémy, I.; Landwerlin, C.; Blanchard-Gutton, N.; Relaix, F.; Blot, S.; Laporte, J.; Tiret, L. A dog model for centronuclear myopathy carrying the most common DNM2 mutation. Dis. Model. Mech. 2022, 15, dmm049219. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.C.; Chadt, A.; Heesom, K.J.; Kishida, S.; Al-Hasani, H.; Tavaré, J.M.; Thomas, E.C. TBC1D1 interacting proteins, VPS13A and VPS13C, regulate GLUT4 homeostasis in C2C12 myotubes. Sci. Rep. 2020, 10, 17953. [Google Scholar] [CrossRef] [PubMed]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Hu, X.; Huang, X.J.; Chen, G.X. Current understanding of glucose transporter 4 expression and functional mechanisms. World J. Biol. Chem. 2020, 11, 76–98. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, E.; Zhao, H.T.; Cole, T.; West, A.B. LRRK2 and Rab10 coordinate macropinocytosis to mediate immunological responses in phagocytes. EMBO J. 2020, 39, e104862. [Google Scholar] [CrossRef]
- González-Jamett, A.M.; Baez-Matus, X.; Olivares, M.J.; Hinostroza, F.; Guerra-Fernández, M.J.; Vasquez-Navarrete, J.; Bui, M.T.; Guicheney, P.; Romero, N.B.; Bevilacqua, J.A.; et al. Dynamin-2 mutations linked to Centronuclear Myopathy impair actin-dependent trafficking in muscle cells. Sci. Rep. 2019, 7, 4580. [Google Scholar] [CrossRef]
- Fongy, A.; Falcone, S.; Lainé, J.; Prudhon, B.; Martins-Bach, A.; Bitoun, M. Nuclear defects in skeletal muscle from a Dynamin 2-linked centronuclear myopathy mouse model. Sci. Rep. 2019, 9, 1580. [Google Scholar] [CrossRef] [Green Version]
- Falcone, S.; Roman, W.; Hnia, K.; Gache, V.; Didier, N.; Lainé, J.; Auradé, F.; Marty, I.; Nishino, I.; Charlet-Berguerand, N.; et al. N-WASP is required for Amphiphysin-2/BIN1-dependent nuclear positioning and triad organization in skeletal muscle and is involved in the pathophysiology of centronuclear myopathy. EMBO Mol. Med. 2014, 6, 1455–7145. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Jagannathan, V.; Högler, S.; Richter, B.; McEwan, N.A.; Thomas, A.; Cadieu, E.; André, C.; Hytönen, M.K.; Lohi, H.; et al. MKLN1 splicing defect in dogs with lethal acrodermatitis. PLoS Genet. 2018, 14, e1007264. [Google Scholar] [CrossRef] [PubMed]
- Leeb, T.; Leuthard, F.; Jagannathan, V.; Kiener, S.; Letko, A.; Roosje, P.; Welle, M.M.; Gailbreath, K.L.; Cannon, A.; Linek, M.; et al. A missense variant affecting the C-terminal tail of UNC93B1 in dogs with exfoliative cutaneous lupus erythematosus (ECLE). Genes 2020, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Shelton, G.D.; Minor, K.M.; Guo, L.T.; Thomas-Hollands, A.; Walsh, K.A.; Friedenberg, S.G.; Cullen, J.N.; Mickelson, J.R. An EHPB1L1 nonsense mutation associated with congenital dyserythropoietic anemia and polymyopathy in Labrador Retriever littermates. Genes 2022, 13, 1427. [Google Scholar] [CrossRef]


| Analytes | Affected (n = 5) Median (Range) | Related (n = 17) Median (Range) | Unrelated (n = 4) Median (Range) | Reference Intervals a |
|---|---|---|---|---|
| RBCs (1012/L) | 9.16 (5.64–10.60) | 6.84 (5.93–8.62) | 6.28 (5.01–9.80) | 6.2–8.9 |
| HCT (%) | 37.3 (23.1–38.0) b | 53.4 (45.0–62.1) | 38.0 (33.0–47.2) | 41–57 |
| Hb (g/L) | 130 (81–147) b | 177 (140–200) | 137 (117–170) | 145–200 |
| MCV (fL) | 41.0 (35.0–41.2) b | 77.10 (69.2–81.8) | 65.65 (55.6–69.5) | 57–73 |
| MCH (pg) | 14.2 (13.9–14.4) b | 25.0 (22.9–27.2) | 23.8 (19.7–25.0) | 21–25 |
| MCHC (g/L) | 351 (339–391) | 332 (297–349) | 361 (355–362) | 335–380 |
| RET (109/L) | 68 (45–150) | 56 (32–139) | 60 (13–130) | 60–150 |
| PLT (109/L) | 248 (148–380) | 199 (105–321) | 273 (164–505) | 108–562 |
| nRBC/100 leukocytes | 79 (4–297) b | 0 (0–0) | 0 (0–0) | <1 |
| WBC (109/L) | 7.8 (4.4–17.0) | 9.5 (5.7–20.0) | 6.9 (3.6–21.3) | 4.9–14.7 |
| Seg. neutrophils (109/L) | 5.9 (3.5–9.8) | 6.0 (4.1–16.9) | 4.7 (2.1–11.0) | 2.3–11.3 |
| Band neutrophils (109/L) | 0.5 (0.1–1.0) | 0.2 (0.0–2.0) | 0.1 (0.0–1.3) | 0–0.3 |
| Lymphocytes (109/L) | 2.2 (0.3–4.8) | 2.4 (0.6–4.8) | 1.60 (0.6–3.6) | 1.2–5.7 |
| Monocytes (109/L) | 0.2 (0.10–0.6) | 0.3 (0.1–0.6) | 0.40 (0.0–1.7) | 0.1–1.3 |
| Eosinophils (109/L) | 0.2 (0.1–1.7) | 0.3 (0.0–1.1) | 0.3 (0.1–0.6) | 0–1.7 |
| Basophils (109/L) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0–0.1 |
| Serum Parameters | Affected (n = 5) Median (Range) | Related (n = 17) Median (Range) | Unrelated (n = 4) Median (Range) | Reference Intervals a |
|---|---|---|---|---|
| Bilirubin (μmol/L) | 2.0 (1.7–3.0) b | 1.0 (0.0–3.0) | 1.5 (1.0–2.0) | <2.8 |
| Cholesterol (mmol/L) | 6.8 (6.7–7.5) | 5.6 (4.2–8.8) | 5.8 (5.4–11.0) | 3.0–10.3 |
| Triglycerides (mmol/L) | 1.0 (0.7–1.7) | 1.6 (0.5–5.6) | 1.1 (0.7–1.4) | 0.5–2.7 |
| Glucose (mmol/L) | 4.9 (4.5–6.3) | 3.8 (3.1–5.2) | 4.2 (3.4–5.3) | 3.7–6.6 |
| Urea (mmol/L) | 5.0 (4.0–33.0) | 6.0 (4.0–10.0) | 4.0 (3.0–5.0) | 3.0–9.0 |
| ALP (U/L) | 1.2 (0.5–4.8) | 0.8 (0.3–2.1) | 0.8 (0.4–4.1) | <1.4 |
| ALT (U/L) | 0.5 (0.3–0.8) | 0.6 (0.3–1.2) | 1.4 (0.6–11.7) | <1.2 |
| AST (U/L) | 0.5 (0.-1.20) | 0.4 (0.2–2.0) | 0.5 (0.4–17.1) | <1.0 |
| Creatine kinase (U/L) | 2.2 (0.9–2.6) | 1.9 (1.0–38.0) | 2.3 (1.0–8.5) | <3.7 |
| Creatinine (μmol/L) | 44 (35–246) | 71 (42–125) | 65 (43–81) | <135 |
| Albumin (g/L) | 38 (30–44) | 34 (28–42) | 29 (21–33.3) | 30–45 |
| Total protein (g/L) | 63.0 (60.0–73.0) | 46 (58.5–63.6) | 53.0 (41.0–81.0) | 49.0–71.0 |
| Calcium (mmol/L) | 2.8 (2.6–3.0) | 2.6 (2.0–2.8) c | 2.5 (2.0–2.8) | 2.4–3.0 |
| Phosphate (mmol/L) | 1.5 (1.3–2.5) b | 1.3 (0.9–1.6) | 1.2 (0.8–1.4) | 0.7–1.9 |
| Sodium (mmol/L) | 145 (137–150) | 141 (127–150) | 134 (121–145) | 138–149 |
| Potassium (mmol/L) | 4.6 (3.9–5.1) | 4.5 (4.0–5.1) c | 4.1 (3.8–4.8) | 3.4–4.8 |
| Chloride (mmol/L) | 109 (101–111) | 105 (94–114) | 101 (91–110) | 98–119 |
| CRP (mg/L) | 6.9 (5.3–10.6) d | 2.3 (1.3–65.6) d | ND | <15 |
| Serum Parameters | Affected (n = 4) Median (Range) | Related (n = 4) Median (Range) | Reference Interval |
|---|---|---|---|
| Iron (µmol/L) | 39 (19–54) | 28 (18–43) | 15–45 |
| UIBC (µg/dL) | 155 (127–258) | 240 (182–327) a | NA |
| TIBC (µg/dL) | 402 (294–442) | 423 (362–522) a | 260–430 |
| Iron Saturation (%) | 58 (30–70) | 37 (34–57) a | 20–60 |
| Ferritin (µg/L) | 192 (170–212) | 180 (129–217) a | NA |
| sTFR (mg/L) | 66.5 (22.6–116.2) b | 10.1 (9.5–12.8) a | NA |
| sTFR–Ferritin Index | 28.9 (9.7–51.8) b | 4.8 (4.1–5.69) a | NA |
| Hepcidin (ng/mL) | 9.1 (8.5–30.4) a,b | 37.7 (12.9–59.8) | NA |
| Dog | Striated Muscle | Myocyte Diameter, µm a | Centralized Nuclei b | Central Pallor b | Lipocyte Infiltrate | Fibrosis |
|---|---|---|---|---|---|---|
| #2 | m. triceps brachii | 29.0 (12.5–52.9) | 19 | 3 | severe | no |
| m. biceps femoris | 41.3 (13.0–84.8) | 11 | 15 | mild | no | |
| m. masseter | 23.3 (10.1–47.4) | 23 | 3 | moderate | moderate | |
| #4 | m. triceps brachii | 26.5 (14.7–47.1) | 28 | 4 | mild | no |
| m. biceps femoris | 28.2 (12.9–55.4) | 25 | 7 | no | no | |
| m. masseter | 15.1 (7.2–42.9) | 52 | 3 | moderate | moderate | |
| #1 | m. triceps brachii | 34.5 (15.2–58.9) | 2 | 0 | no | no |
| m. biceps femoris | 28.6 (10.2–56.7) | 20 | 39 | no | no |
| Filtering Step | Homozygous Variants | |
|---|---|---|
| In Whole Genome | In Critical Interval | |
| Shared variants in two affected dogs | 1,659,097 | 14,272 |
| Private variants in two affected dogs | 112 | 15 |
| Private protein changing variants | 1 | 1 |
| Dogs and Phenotype | n | EHBP1L1 Genotype | ||
|---|---|---|---|---|
| +/+ | +/del | del/del | ||
| Family from Sweden (n = 26) | ||||
| Dogs affected by DAMS | 5 | - | - | 5 |
| Puppies with neonatal death a | 2 | - | - | 2 |
| Clinically unaffected relatives | 19 | 12 | 7 | - |
| Dogs affected with DAMS from Australia b | 2 | - | - | 2 |
| Unrelated dogs from Switzerland/Germany a | 13 | 13 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Østergård Jensen, S.; Christen, M.; Rondahl, V.; Holland, C.T.; Jagannathan, V.; Leeb, T.; Giger, U. EHBP1L1 Frameshift Deletion in English Springer Spaniel Dogs with Dyserythropoietic Anemia and Myopathy Syndrome (DAMS) or Neonatal Losses. Genes 2022, 13, 1533. https://doi.org/10.3390/genes13091533
Østergård Jensen S, Christen M, Rondahl V, Holland CT, Jagannathan V, Leeb T, Giger U. EHBP1L1 Frameshift Deletion in English Springer Spaniel Dogs with Dyserythropoietic Anemia and Myopathy Syndrome (DAMS) or Neonatal Losses. Genes. 2022; 13(9):1533. https://doi.org/10.3390/genes13091533
Chicago/Turabian StyleØstergård Jensen, Sarah, Matthias Christen, Veronica Rondahl, Christopher T. Holland, Vidhya Jagannathan, Tosso Leeb, and Urs Giger. 2022. "EHBP1L1 Frameshift Deletion in English Springer Spaniel Dogs with Dyserythropoietic Anemia and Myopathy Syndrome (DAMS) or Neonatal Losses" Genes 13, no. 9: 1533. https://doi.org/10.3390/genes13091533
APA StyleØstergård Jensen, S., Christen, M., Rondahl, V., Holland, C. T., Jagannathan, V., Leeb, T., & Giger, U. (2022). EHBP1L1 Frameshift Deletion in English Springer Spaniel Dogs with Dyserythropoietic Anemia and Myopathy Syndrome (DAMS) or Neonatal Losses. Genes, 13(9), 1533. https://doi.org/10.3390/genes13091533








