Hormonal and Molecular Regulation of Phallus Differentiation in a Marsupial Tammar Wallaby
Abstract
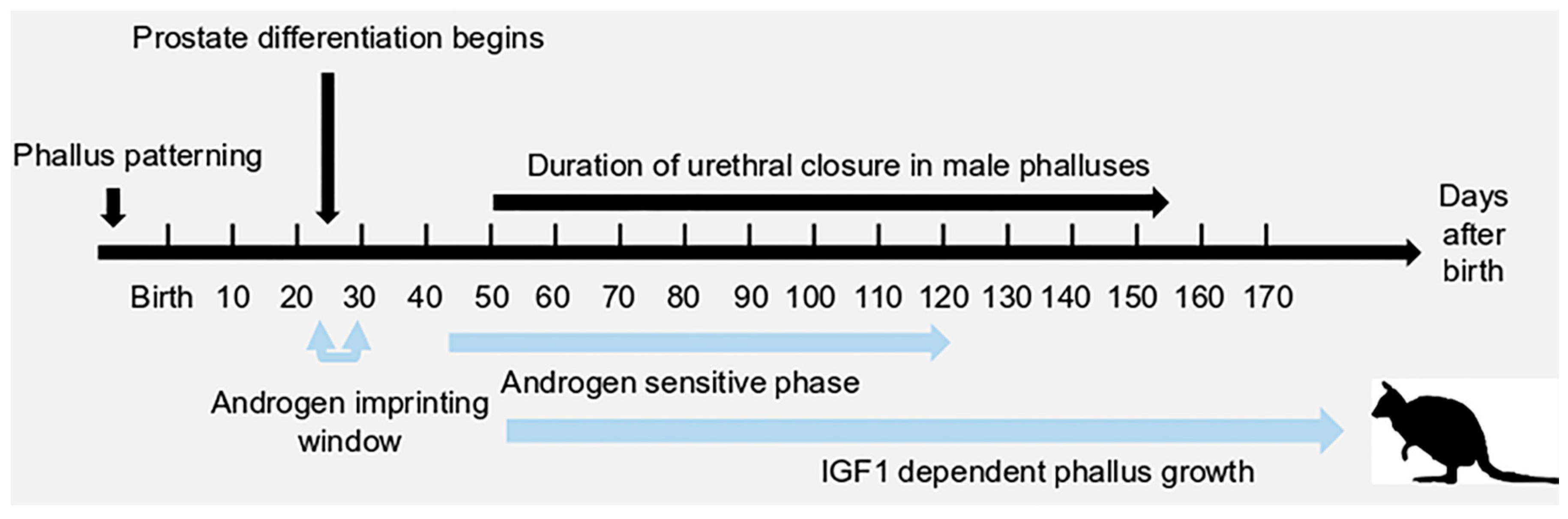
1. Introduction
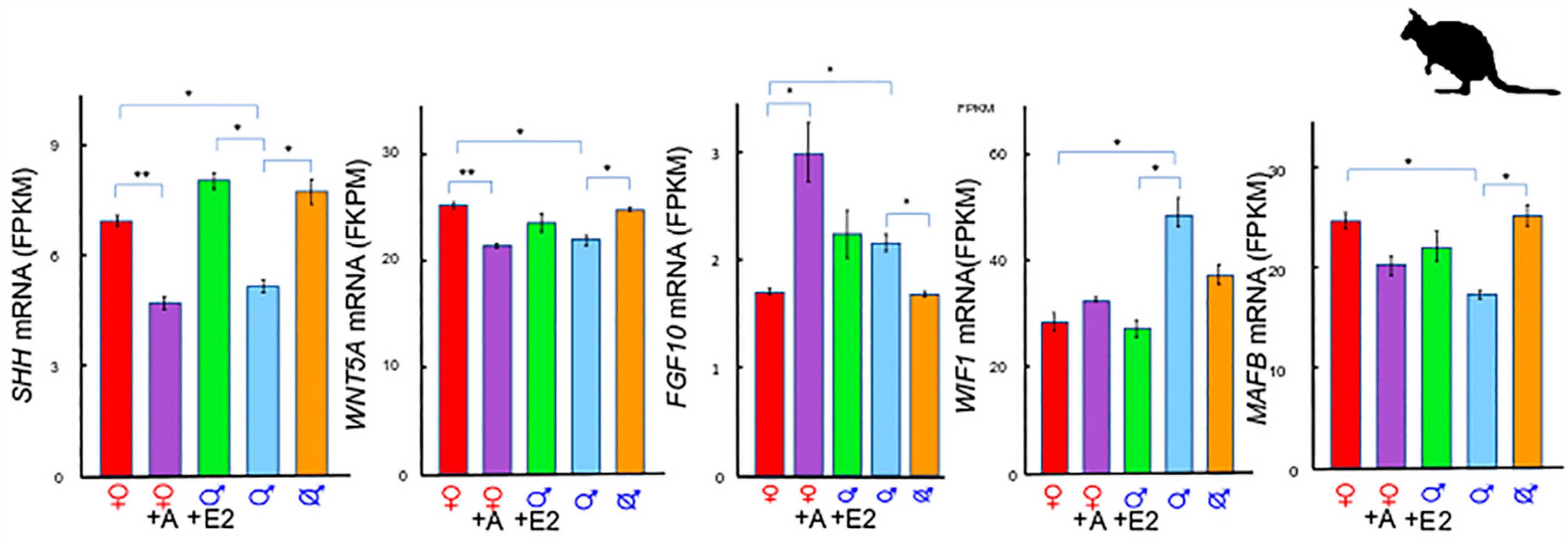
2. A Unique Androgen-Sensitive Regulation Network of Sonic Hedgehog (SHH)
2.1. SHH and WNTs
2.2. SHH and MAF BZIP Transcription Factor B (MAFB)
2.3. SHH and Fibroblast Growth Factor 10 (FGF10)
2.4. The SHH Switch
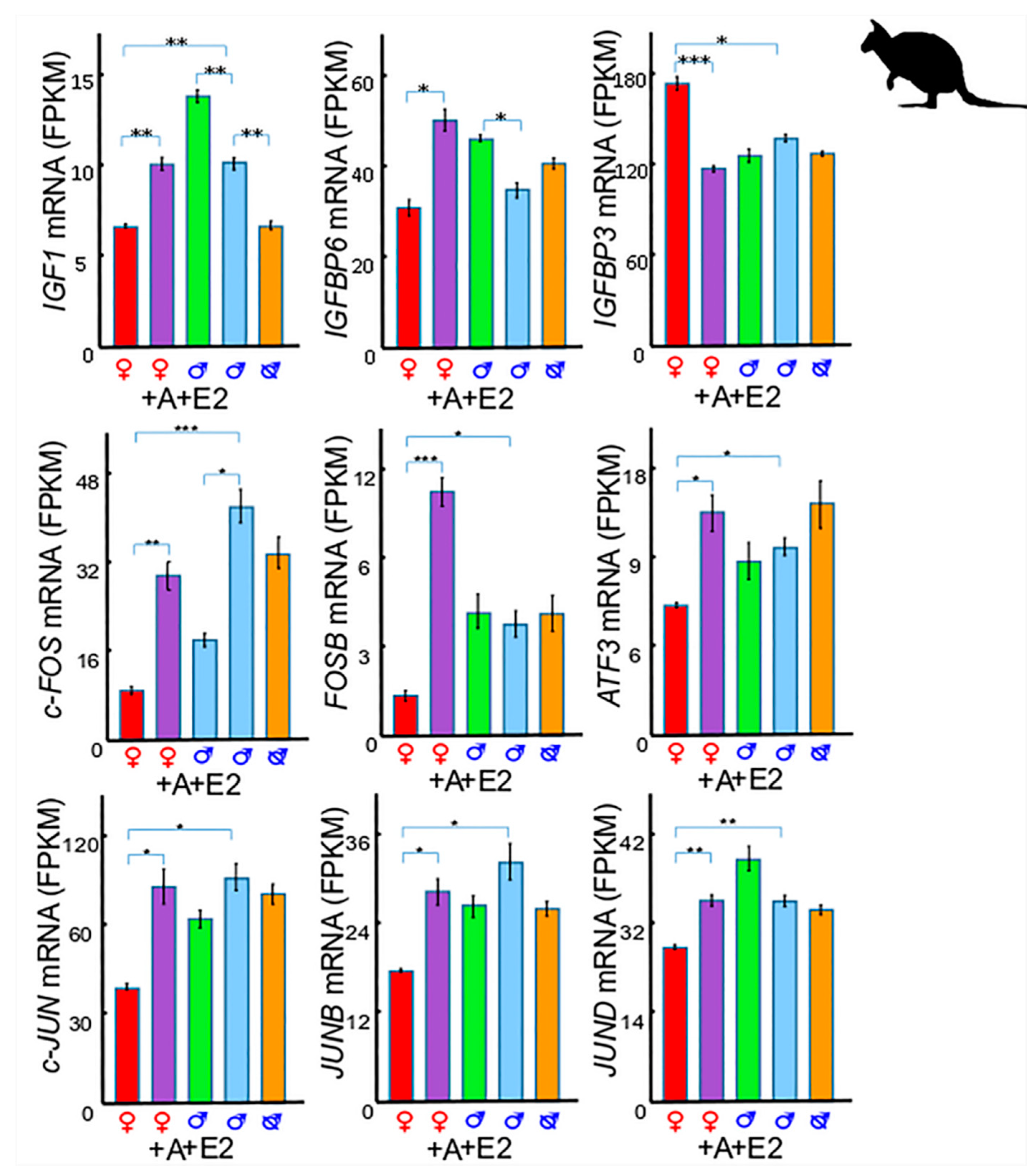
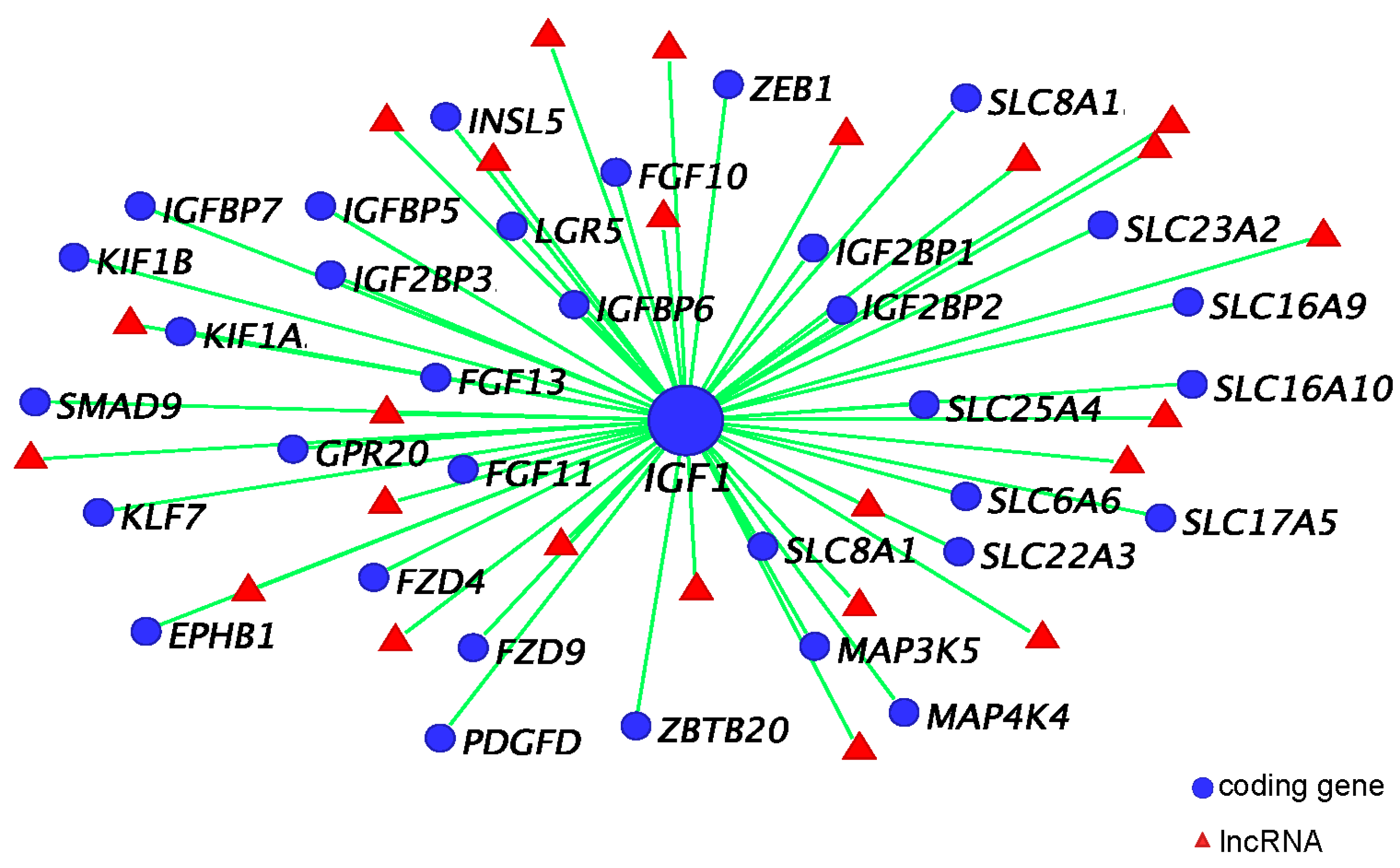
3. Insulin-Like Growth Factor 1 (IGF1) in Phallus Growth and Urethral Closure
3.1. IGF1 and Insulin-Like Growth Factor Binding Protein 6 (IGFBP6)
3.2. IGF1 and Insulin-Like Growth Factor Binding Protein 3 (IGFBP3)
3.3. IGF1 and Activator Protein 1 (AP-1)
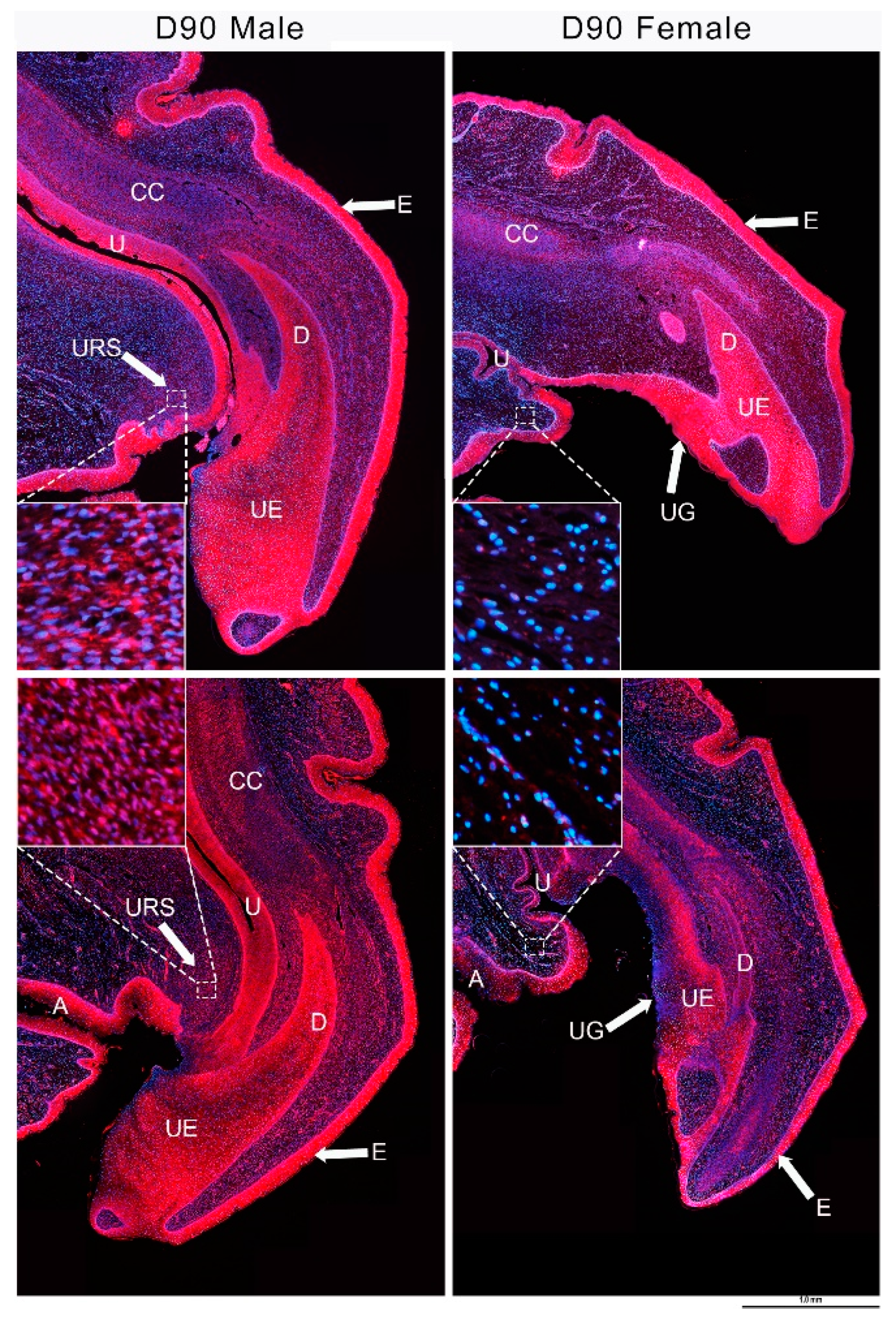
3.4. IGF1 and Urethral Closure
3.5. IGF1 Dependent Phallus Growth
4. Co-Expression Network and Hormonally Responsive Long Non-Coding RNAs
4.1. IGF1, Androgen Receptor (AR), and ESR1 Co-Expression Network
4.2. lnc-RSPO4, lnc-BMP5, and lnc-ZBTB16
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Renfree, M.B.; Pask, A.J.; Shaw, G. Sexual development of a model marsupial male. Aust. J. Zool. 2006, 54, 151–158. [Google Scholar] [CrossRef]
- Renfree, M.B.; Short, R.V.; Shaw, G. Sexual differentiation of the urogenital system of the fetal and neonatal tammar wallaby, Macropus eugenii. Anat. Embryol. 1996, 194, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Leihy, M.W.; Shaw, G.; Wilson, J.D.; Renfree, M.B. Development of the penile urethra in the tammar wallaby. Sex. Dev. 2011, 5, 241–249. [Google Scholar] [CrossRef]
- Butler, C.M.; Shaw, G.; Renfree, M.B. Development of the penis and clitoris in the tammar wallaby, Macropus eugenii. Anat. Embryol. 1999, 199, 451–457. [Google Scholar] [CrossRef]
- Leihy, M.W.; Shaw, G.; Wilson, J.D.; Renfree, M.B. Penile development is initiated in the tammar wallaby pouch young during the period when 5α-androstane-3α, 17β-diol is secreted by the testes. Endocrinology 2004, 145, 3346–3352. [Google Scholar] [CrossRef]
- Renfree, M.B.; Chew, K.Y.; Shaw, G. Inducing sex reversal of the urogenital system of marsupials. Differentiation 2014, 87, 23–31. [Google Scholar] [CrossRef]
- Renfree, M.B.; Wilson, J.D.; Short, R.V.; Shaw, G.; George, F.W. Steroid hormone content of the gonads of the tammar wallaby during sexual differentiation. Biol. Reprod. 1992, 47, 644–647. [Google Scholar] [CrossRef]
- Wilson, J.D.; George, F.W.; Shaw, G.; Renfree, M.B. Virilization of the male pouch young of the tammar wallaby does not appear to be mediated by plasma testosterone or dihydrotestosterone. Biol. Reprod. 1999, 61, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.Y.; Pask, A.J.; Hickford, D.; Shaw, G.; Renfree, M.B. A dual role for SHH during phallus development in a marsupial. Sex. Dev. 2014, 8, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.; Saunders, P.T.; Fisken, M.; Scott, H.M.; Hutchison, G.R.; Smith, L.B.; Sharpe, R.M. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Investig. 2008, 118, 1479–1490. [Google Scholar] [CrossRef]
- Welsh, M.; Suzuki, H.; Yamada, G. The masculinization programming window. Endocr. Dev. 2014, 27, 17–27. [Google Scholar] [PubMed]
- Welsh, M.; MacLeod, D.J.; Walker, M.; Smith, L.B.; Sharpe, R.M. Critical androgen-sensitive periods of rat penis and clitoris development. Int. J. Androl. 2010, 33, e144–e152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Pask, A.J.; Fujiyama, A.; Suzuki, Y.; Sugano, S.; Shaw, G.; Renfree, M.B. Hormone-responsive genes in the SHH and WNT/β-catenin signaling pathways influence urethral closure and phallus growth. Biol. Reprod. 2018, 99, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Sirab, N.; Terry, S.; Giton, F.; Caradec, J.; Chimingqi, M.; Moutereau, S.; Vacherot, F.; Taille, A.D.L.; Kouyoumdjian, J.C.; Loric, S. Androgens regulate hedgehog signalling and proliferation in androgen-dependent prostate cells. Int. J. Cancer 2012, 131, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Pask, A.J.; Fujiyama, A.; Suzuki, Y.; Sugano, S.; Shaw, G.; Renfree, M.B. Effects of androgen and oestrogen on the IGF pathways controlling phallus growth. Reproduction 2019, 157, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Menzies, B.R.; Shaw, G.; Fletcher, T.P.; Pask, A.J.; Renfree, M.B. Maturation of the growth axis in marsupials occurs gradually during post-natal life and over an equivalent developmental stage relative to eutherian species. Mol. Cell. Endocrinol. 2012, 349, 189–194. [Google Scholar] [CrossRef]
- Miyagawa, S.; Satoh, Y.; Haraguchi, R.; Suzuki, K.; Iguchi, T.; Taketo, M.M.; Nakagata, N.; Matsumoto, T.; Takeyama, K.-I.; Kato, S. Genetic interactions of the androgen and Wnt/β-catenin pathways for the masculinization of external genitalia. Mol. Endocrinol. 2009, 23, 871–880. [Google Scholar] [CrossRef]
- Miyagawa, S.; Moon, A.; Haraguchi, R.; Inoue, C.; Harada, M.; Nakahara, C.; Suzuki, K.; Matsumaru, D.; Kaneko, T.; Matsuo, I. Dosage-dependent hedgehog signals integrated with Wnt/β-catenin signaling regulate external genitalia formation as an appendicular program. Development 2009, 136, 3969–3978. [Google Scholar] [CrossRef]
- Seifert, A.W.; Zheng, Z.; Ormerod, B.K.; Cohn, M.J. Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat. Commun. 2010, 1, 23. [Google Scholar] [CrossRef]
- Kawano, Y.; Kypta, R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003, 116, 2627–2634. [Google Scholar] [CrossRef]
- Hsieh, J.-C.; Kodjabachian, L.; Rebbert, M.L.; Rattner, A.; Smallwood, P.M.; Samos, C.H.; Nusse, R.; Dawid, I.B.; Nathans, J. A new secreted protein that binds to Wnt proteins and inhibits their activites. Nature 1999, 398, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.; Matsumaru, D.; Ho, A.S.; Garcia-Barceló, M.; Yuan, Z.; Smith, D.; Kodjabachian, L.; Tam, P.K.; Yamada, G.; Lui, V.C. Dysregulation of Wnt inhibitory factor 1 (Wif1) expression resulted in aberrant wnt-β-catenin signaling and cell death of the cloaca endoderm, and anorectal malformations. Cell Death Differ. 2014, 21, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Numata, T.; Suzuki, H.; Raga, D.D.; Ipulan, L.A.; Yokoyama, C.; Matsushita, S.; Hamada, M.; Nakagata, N.; Nishinakamura, R.; et al. Sexually dimorphic expression of Mafb regulates masculinization of the embryonic urethral formation. Proc. Natl. Acad. Sci. USA 2014, 111, 16407–16412. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, S.; Suzuki, K.; Ogino, Y.; Hino, S.; Sato, T.; Suyama, M.; Matsumoto, T.; Omori, A.; Inoue, S.; Yamada, G. Androgen regulates Mafb expression through its 3’ UTR during mouse urethral masculinization. Endocrinology 2015, 157, 844–857. [Google Scholar] [CrossRef]
- Matsushita, S.; Suzuki, K.; Murashima, A.; Kajioka, D.; Acebedo, A.R.; Miyagawa, S.; Haraguchi, R.; Ogino, Y.; Yamada, G. Regulation of masculinization: Androgen signalling for external genitalia development. Nat. Rev. Urol. 2018, 15, 358. [Google Scholar] [CrossRef]
- Bellusci, S.; Grindley, J.; Emoto, H.; Itoh, N.; Hogan, B. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 1997, 124, 4867–4878. [Google Scholar]
- Haraguchi, R.; Suzuki, K.; Murakami, R.; Sakai, M.; Kamikawa, M.; Kengaku, M.; Sekine, K.; Kawano, H.; Kato, S.; Ueno, N. Molecular analysis of external genitalia formation: The role of fibroblast growth factor (FGF) genes during genital tubercle formation. Development 2000, 127, 2471–2479. [Google Scholar]
- Petiot, A.; Perriton, C.L.; Dickson, C.; Cohn, M.J. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development 2005, 132, 2441–2450. [Google Scholar] [CrossRef]
- Satoh, Y.; Haraguchi, R.; Wright, T.J.; Mansour, S.L.; Partanen, J.; Hajihosseini, M.K.; Eswarakumar, V.P.; Lonai, P.; Yamada, G. Regulation of external genitalia development by concerted actions of FGF ligands and FGF receptors. Anat. Embryol. 2004, 208, 479–486. [Google Scholar] [CrossRef]
- Shaw, G.; Renfree, M.B.; Leihy, M.W.; Shackleton, C.H.; Roitman, E.; Wilson, J.D. Prostate formation in a marsupial is mediated by the testicular androgen 5α-androstane-3α, 17β-diol. Proc. Natl. Acad. Sci. USA 2000, 97, 12256–12259. [Google Scholar] [CrossRef]
- Shaw, G.; Renfree, M.B.; Short, R.V. Experimental manipulation of sexual differentiation in wallaby pouch young treated with exogenous steroids. Development 1988, 104, 689–701. [Google Scholar] [PubMed]
- Kurzrock, E.A.; Baskin, L.S.; Cunha, G.R. Ontogeny of the male urethra: Theory of endodermal differentiation. Differentiation 1999, 64, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Baskin, L.; Lee, Y.; Cunha, G. Neuroanatomical ontogeny of the human fetal penis. Br. J. Urol. 1997, 79, 628–640. [Google Scholar] [CrossRef]
- Baskin, L.; Shen, J.; Sinclair, A.; Cao, M.; Liu, X.; Liu, G.; Isaacson, D.; Overland, M.; Li, Y.; Cunha, G.R. Development of the human penis and clitoris. Differentiation 2018, 103, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lowsley, O.S. The development of the human prostate gland with reference to the development of other structures at the neck of the urinary bladder. Am. J. Anat. 1912, 13, 299–349. [Google Scholar] [CrossRef]
- Kellokumpu-Lehtinen, P.; Santti, R.; Pelliniemi, L. Correlation of early cytodifferentiation of the human fetal prostate and leydig cells. Anat. Rec. 1980, 196, 263–273. [Google Scholar] [CrossRef]
- Perriton, C.L.; Powles, N.; Chiang, C.; Maconochie, M.K.; Cohn, M.J. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev. Biol. 2002, 247, 26–46. [Google Scholar] [CrossRef]
- Haraguchi, R.; Mo, R.; Hui, C.; Motoyama, J.; Makino, S.; Shiroishi, T.; Gaffield, W.; Yamada, G. Unique functions of sonic hedgehog signaling during external genitalia development. Development 2001, 128, 4241–4250. [Google Scholar]
- Liu, G.; Liu, X.; Shen, J.; Sinclair, A.; Baskin, L.; Cunha, G.R. Contrasting mechanisms of penile urethral formation in mouse and human. Differentiation 2018, 101, 46–64. [Google Scholar] [CrossRef]
- Cunha, G.R.; Donjacour, A.A.; Cooke, P.S.; Mee, H.; Bigsby, R.M.; Higgins, S.J.; Sugimura, Y. The endocrinology and developmental biology of the prostate. Endocr. Rev. 1987, 8, 338–362. [Google Scholar] [CrossRef]
- Timms, B.G.; Mohs, T.J.; Didio, L.J. Ductal budding and branching patterns in the developing prostate. J. Urol. 1994, 151, 1427–1432. [Google Scholar] [CrossRef]
- Price, D. Normal development of the prostate and seminal vesicles of the rat with a study of experimental postnatal modifications. Am. J. Anat. 1936, 60, 79–127. [Google Scholar] [CrossRef]
- Inomata, T.; Eguchi, Y.; Nakamura, T. Development of the external genitalia in rat fetuses. Exp. Anim. 1985, 34, 439–444. [Google Scholar] [CrossRef]
- Fernandez, C.; Tatard, V.M.; Bertrand, N.; Dahmane, N. Differential modulation of sonic-hedgehog-induced cerebellar granule cell precursor proliferation by the IGF signaling network. Dev. Neurosci. 2010, 32, 59–70. [Google Scholar] [CrossRef]
- Rao, G.; Pedone, C.A.; del Valle, L.; Reiss, K.; Holland, E.C.; Fults, D.W. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene 2004, 23, 6156. [Google Scholar] [CrossRef] [PubMed]
- Pirskanen, A.; Kiefer, J.C.; Hauschka, S.D. IGFs, insulin, Shh, bFGF, and TFG-β1 interact synergistically to promote somite myogenesis in vitro. Dev. Biol. 2000, 224, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.; Nørgaard-Pedersen, D.; Brandt, J.; Pettersson, I.; Slaaby, R. IGF1 and IGF2 specificities to the two insulin receptor isoforms are determined by insulin receptor amino acid 718. PLoS ONE 2017, 12, e0178885. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z.; Pertzelan, A.; Mannheimer, S. Genetic pituitary dwarfism with high serum concentation of growth hormone—A new inborn error of metabolism? Isr. J. Med. Sci. 1966, 2, 152–155. [Google Scholar]
- Laron, Z.; Klinger, B. Effect of insulin-like growth factor-I treatment on serum androgens and testicular and penile size in males with Laron syndrome (primary growth hormone resistance). Eur. J. Endocrinol. 1998, 138, 176–180. [Google Scholar] [CrossRef]
- Levy, J.; Husmann, D. Micropenis secondary to growth hormone deficiency: Does treatment with growth hormone alone result in adequate penile growth? J. Urol. 1996, 156, 214–216. [Google Scholar] [CrossRef]
- Stewart, C.E.; Bates, P.C.; Calder, T.A.; Woodall, S.M.; Pell, J.M. Potentiation of insulin-like growth factor-I (IGF-I) activity by an antibody: Supportive evidence for enhancement of IGF-I bioavailability in vivo by IGF binding proteins. Endocrinology 1993, 133, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Kalus, W.; Zweckstetter, M.; Renner, C.; Sanchez, Y.; Georgescu, J.; Grol, M.; Demuth, D.; Schumacher, R.; Dony, C.; Lang, K.; et al. Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): Implications for IGF and IGF-I receptor interactions. EMBO J. 1998, 17, 6558–6572. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol. Metab. 2016, 27, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Kamanga-Sollo, E.; Pampusch, M.; Xi, G.; White, M.; Hathaway, M.; Dayton, W. IGF-I mRNA levels in bovine satellite cell cultures: Effects of fusion and anabolic steroid treatment. J. Cell. Physiol. 2004, 201, 181–189. [Google Scholar] [CrossRef]
- Sahlin, L.; Norstedt, G.; Eriksson, H. Androgen regulation of the insulin-like growth factor-I and the estrogen receptor in rat uterus and liver. J. Steroid Biochem. Mol. Biol. 1994, 51, 57–66. [Google Scholar] [CrossRef]
- Arnold, J.T.; Le, H.; McFann, K.K.; Blackman, M.R. Comparative effects of DHEA vs. testosterone, dihydrotestosterone, and estradiol on proliferation and gene expression in human LNCaP prostate cancer cells. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E573–E584. [Google Scholar] [CrossRef]
- Le, H.; Arnold, J.T.; McFann, K.K.; Blackman, M.R. DHT and testosterone, but not DHEA or E2, differentially modulate IGF-I, IGFBP-2, and IGFBP-3 in human prostatic stromal cells. Am. J. Physiol.-Endocrinol. Metab. 2006, 290, E952–E960. [Google Scholar] [CrossRef]
- Cheng, C.M.; Cohen, M.; Wang, J.; Bondy, C.A. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. FASEB J. 2001, 15, 907–915. [Google Scholar] [CrossRef][Green Version]
- Hewitt, S.C.; Li, Y.; Li, L.; Korach, K.S. Estrogen-mediated regulation of IGF1 transcription and uterine growth involves direct binding of estrogen receptor α to estrogen-responsive elements. J. Biol. Chem. 2010, 285, 2676–2685. [Google Scholar] [CrossRef]
- Henderson, N.A.; Cooke, G.M.; Robaire, B. Region-specific expression of androgen and growth factor pathway genes in the rat epididymis and the effects of dual 5α-reductase inhibition. J. Endocrinol. 2006, 190, 779–791. [Google Scholar] [CrossRef]
- Koike, H.; Ito, K.; Takezawa, Y.; Oyama, T.; Yamanaka, H.; Suzuki, K. Insulin-like growth factor binding protein-6 inhibits prostate cancer cell proliferation: Implication for anticancer effect of diethylstilbestrol in hormone refractory prostate cancer. Br. J. Cancer 2005, 92, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Mulholland, D.J.; Ettinger, S.; Fazli, L.; Nelson, C.C.; Gleave, M.E. Differential regulation of IGFBP-3 by the androgen receptor in the lineage-related androgen-dependent LNCaP and androgen-independent C4-2 prostate cancer models. Prostate 2006, 66, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, J.; Malloy, P.J.; Feldman, D. The role of insulin-like growth factor binding protein-3 in the growth inhibitory actions of androgens in LNCaP human prostate cancer cells. Int. J. Cancer 2008, 122, 558–566. [Google Scholar] [CrossRef]
- Grimberg, A.; Cohen, P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell. Physiol. 2000, 183, 1–9. [Google Scholar] [CrossRef]
- Pollak, M. Insulin-like growth factor physiology and cancer risk. Eur. J. Cancer 2000, 36, 1224–1228. [Google Scholar] [CrossRef]
- Duan, C.; Xu, Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen. Comp. Endocrinol. 2005, 142, 44–52. [Google Scholar] [CrossRef]
- Schmid, C.H.; Rutishauser, J.; Schläpfer, I.; Froesch, E.R.; Zapf, J. Intact but not truncated insulin-like growth factor binding protein-3 (IGFBP-3) blocks IGF I-induced stimulation of osteoblasts: Control of IGF signalling to bone cells by IGFBP-3-specific proteolysis? Biochem. Biophys. Res. Commun. 1991, 179, 579–585. [Google Scholar] [CrossRef]
- Valentinis, B.; Bhala, A.; DeAngelis, T.; Baserga, R.; Cohen, P. The human insulin-like growth factor (IGF) binding protein-3 inhibits the growth of fibroblasts with a targeted disruption of the Igf-1 receptor gene. Mol. Endocrinol. 1995, 9, 361–367. [Google Scholar]
- Umayahara, Y.; Kawamori, R.; Watada, H.; Imano, E.; Iwama, N.; Morishima, T.; Yamasaki, Y.; Kajimoto, Y.; Kamada, T. Estrogen regulation of the insulin-like growth factor 1 gene transcription involves an AP-1 enhancer. J. Biol. Chem. 1994, 269, 16433–16442. [Google Scholar]
- Kerr, J.; Beck, S.; Handa, R. Androgens selectively modulate C-Fos messenger RNA induction in the rat hippocampus following novelty. Neuroscience 1996, 74, 757–766. [Google Scholar] [CrossRef]
- Pelzer, A.E.; Bektic, J.; Haag, P.; Berger, A.P.; Pycha, A.; Schäfer, G.; Rogatsch, H.; Horninger, W.; Bartsch, G.; Klocker, H. The expression of transcription factor activating transcription factor 3 in the human prostate and its regulation by androgen in prostate cancer. J. Urol. 2006, 175, 1517–1522. [Google Scholar] [CrossRef]
- Angel, P.; Karin, M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta Rev. Cancer 1991, 1072, 129–157. [Google Scholar] [CrossRef]
- Seifert, A.W.; Harfe, B.D.; Cohn, M.J. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev. Biol. 2008, 318, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.W.; Bouldin, C.M.; Choi, K.S.; Harfe, B.D.; Cohn, M.J. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development 2009, 136, 3949–3957. [Google Scholar] [CrossRef]
- Hyuga, T.; Suzuki, K.; Acebedo, A.R.; Hashimoto, D.; Kajimoto, M.; Miyagawa, S.; Enmi, J.-I.; Yoshioka, Y.; Yamada, G. Regulatory roles of epithelial-mesenchymal interaction (EMI) during early and androgen dependent external genitalia development. Differentiation 2019, 110, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Dravis, C.; Yokoyama, N.; Chumley, M.J.; Cowan, C.A.; Silvany, R.E.; Shay, J.; Baker, L.A.; Henkemeyer, M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev. Biol. 2004, 271, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Gredler, M.L.; Seifert, A.W.; Cohn, M.J. Tissue-specific roles of Fgfr2 in development of the external genitalia. Development 2015, 142, 2203–2212. [Google Scholar] [CrossRef]
- Harada, M.; Omori, A.; Nakahara, C.; Nakagata, N.; Akita, K.; Yamada, G. Tissue-specific roles of FGF signaling in external genitalia development. Dev. Dyn. 2015, 244, 759–773. [Google Scholar] [CrossRef]
- Bhushan, A.; Itoh, N.; Kato, S.; Thiery, J.P.; Czernichow, P.; Bellusci, S.; Scharfmann, R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 2001, 128, 5109–5117. [Google Scholar]
- Hart, A.; Papadopoulou, S.; Edlund, H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev. Dyn. 2003, 228, 185–193. [Google Scholar] [CrossRef]
- Steinberg, Z.; Myers, C.; Heim, V.M.; Lathrop, C.A.; Rebustini, I.T.; Stewart, J.S.; Larsen, M.; Hoffman, M.P. Fgfr2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development 2005, 132, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Weiler, S.; Rohrbach, V.; Pulvirenti, T.; Adams, R.; Ziemiecki, A.; Andres, A.C. Mammary epithelial-specific knockout of the ephrin-B2 gene leads to precocious epithelial cell death at lactation. Dev. Growth Differ. 2009, 51, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Bendall, A.J.; Hu, G.; Levi, G.; Abate-Shen, C. Dlx5 regulates chondrocyte differentiation at multiple stages. Int. J. Dev. Biol. 2003, 47, 335–344. [Google Scholar] [PubMed]
- Yu, H.; Jiang, H.; Xu, D.; Jin, J.; Zhao, Z.; Ma, Y.; Liang, J. Transcription factor MafB promotes hepatocellular carcinoma cell proliferation through up-regulation of cyclin D1. Cell. Physiol. Biochem. 2016, 39, 700–708. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. Ap-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef]
- Egea, J.; Klein, R. Bidirectional Eph–ephrin signaling during axon guidance. Trends Cell Biol. 2007, 17, 230–238. [Google Scholar] [CrossRef]
- Suzuki, K.; Haraguchi, R.; Ogata, T.; Barbieri, O.; Alegria, O.; Vieux-Rochas, M.; Nakagata, N.; Ito, M.; Mills, A.A.; Kurita, T. Abnormal urethra formation in mouse models of split-hand/split-foot malformation type 1 and type 4. Eur. J. Hum. Genet. 2008, 16, 36–44. [Google Scholar] [CrossRef]
- Chen, Y.; Kuroki, Y.; Shaw, G.; Pask, A.J.; Yu, H.; Toyoda, A.; Fujiyama, A.; Renfree, M.B. Androgen and oestrogen affect the expression of long non-coding rnas during phallus development in a marsupial. Non-Coding RNA 2019, 5, 3. [Google Scholar] [CrossRef]
- Lindsey, R.C.; Rundle, C.H.; Mohan, S. Role of IGF-I and EFN-EPH signaling in skeletal metabolism. J. Mol. Endocrinol. 2018, 61, T87–T102. [Google Scholar] [CrossRef]
- Lovrecic, L.; Rudolf, G.; Veble, A.; Peterlin, B. A new case of rare proximal 3q13 interstitial deletion. Open Med. 2011, 6, 625–630. [Google Scholar] [CrossRef]
- Walsh, D.M.; Shalev, S.A.; Simpson, M.A.; Morgan, N.V.; Gelman-Kohan, Z.; Chemke, J.; Trembath, R.C.; Maher, E.R. Acrocallosal syndrome: Identification of a novel KIF7 mutation and evidence for oligogenic inheritance. Eur. J. Med. Genet. 2013, 56, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-P.; Lin, C.-J.; Chang, T.-Y.; Chern, S.-R.; Wu, P.-S.; Chen, Y.-T.; Su, J.-W.; Lee, C.-C.; Chen, L.-F.; Wang, W. Prenatal diagnosis of ring chromosome 2 with lissencephaly and 2p25.3 and 2q37.3 microdeletions detected using array comparative genomic hybridization. Gene 2013, 519, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Armfield, B.A.; Cohn, M.J. Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proc. Natl. Acad. Sci. USA 2015, 112, E7194–E7203. [Google Scholar] [CrossRef] [PubMed]
- Govers, L.C.; Phillips, T.R.; Mattiske, D.M.; Rashoo, N.; Black, J.R.; Sinclair, A.; Baskin, L.S.; Risbridger, G.P.; Pask, A.J. A critical role for estrogen signaling in penis development. FASEB J. 2019, 33, fj-201802586RR. [Google Scholar] [CrossRef]
- Carmon, K.S.; Gong, X.; Lin, Q.; Thomas, A.; Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 11452–11457. [Google Scholar] [CrossRef]
- Carmon, K.S.; Gong, X.; Yi, J.; Thomas, A.; Liu, Q. RSPO–LGR4 functions via IQGAP1 to potentiate Wnt signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E1221–E1229. [Google Scholar] [CrossRef]
- Chen, P.-H.; Chen, X.; Lin, Z.; Fang, D.; He, X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 2013, 27, 1345–1350. [Google Scholar] [CrossRef]
- Kim, K.-A.; Zhao, J.; Andarmani, S.; Kakitani, M.; Oshima, T.; Binnerts, M.E.; Abo, A.; Tomizuka, K.; Funk, W.D. R-Spondin proteins: A novel link to β-catenin activation. Cell Cycle 2006, 5, 23–26. [Google Scholar] [CrossRef]
- Wieczorek, D.; Köster, B.; Gillessen-Kaesbach, G. Absence of thumbs, A/hypoplasia of radius, hypoplasia of ulnae, retarded bone age, short stature, microcephaly, hypoplastic genitalia, and mental retardation. Am. J. Med. Genet. Part A 2002, 108, 209–213. [Google Scholar] [CrossRef]
- Fischer, S.; Kohlhase, J.; Böhm, D.; Schweiger, B.; Hoffmann, D.; Heitmann, M.; Horsthemke, B.; Wieczorek, D. Biallelic loss of function of the promyelocytic leukaemia zinc finger (PLZF) gene causes severe skeletal defects and genital hypoplasia. J. Med. Genet. 2008, 45, 731–737. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Renfree, M.B. Hormonal and Molecular Regulation of Phallus Differentiation in a Marsupial Tammar Wallaby. Genes 2020, 11, 106. https://doi.org/10.3390/genes11010106
Chen Y, Renfree MB. Hormonal and Molecular Regulation of Phallus Differentiation in a Marsupial Tammar Wallaby. Genes. 2020; 11(1):106. https://doi.org/10.3390/genes11010106
Chicago/Turabian StyleChen, Yu, and Marilyn B. Renfree. 2020. "Hormonal and Molecular Regulation of Phallus Differentiation in a Marsupial Tammar Wallaby" Genes 11, no. 1: 106. https://doi.org/10.3390/genes11010106
APA StyleChen, Y., & Renfree, M. B. (2020). Hormonal and Molecular Regulation of Phallus Differentiation in a Marsupial Tammar Wallaby. Genes, 11(1), 106. https://doi.org/10.3390/genes11010106




