Transcriptome Analysis and Identification of Insecticide Tolerance-Related Genes after Exposure to Insecticide in Sitobion avenae
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Chemicals
2.2. Bioassay
2.3. Sample Preparation
2.4. RNA Isolation, cDNA Library Construction, and Illumina Sequencing
2.5. Sequencing Data Analysis
2.6. Analysis of Differential Expressed Unigenes (DEUs), GO Annotation, and Pathway Enrichment
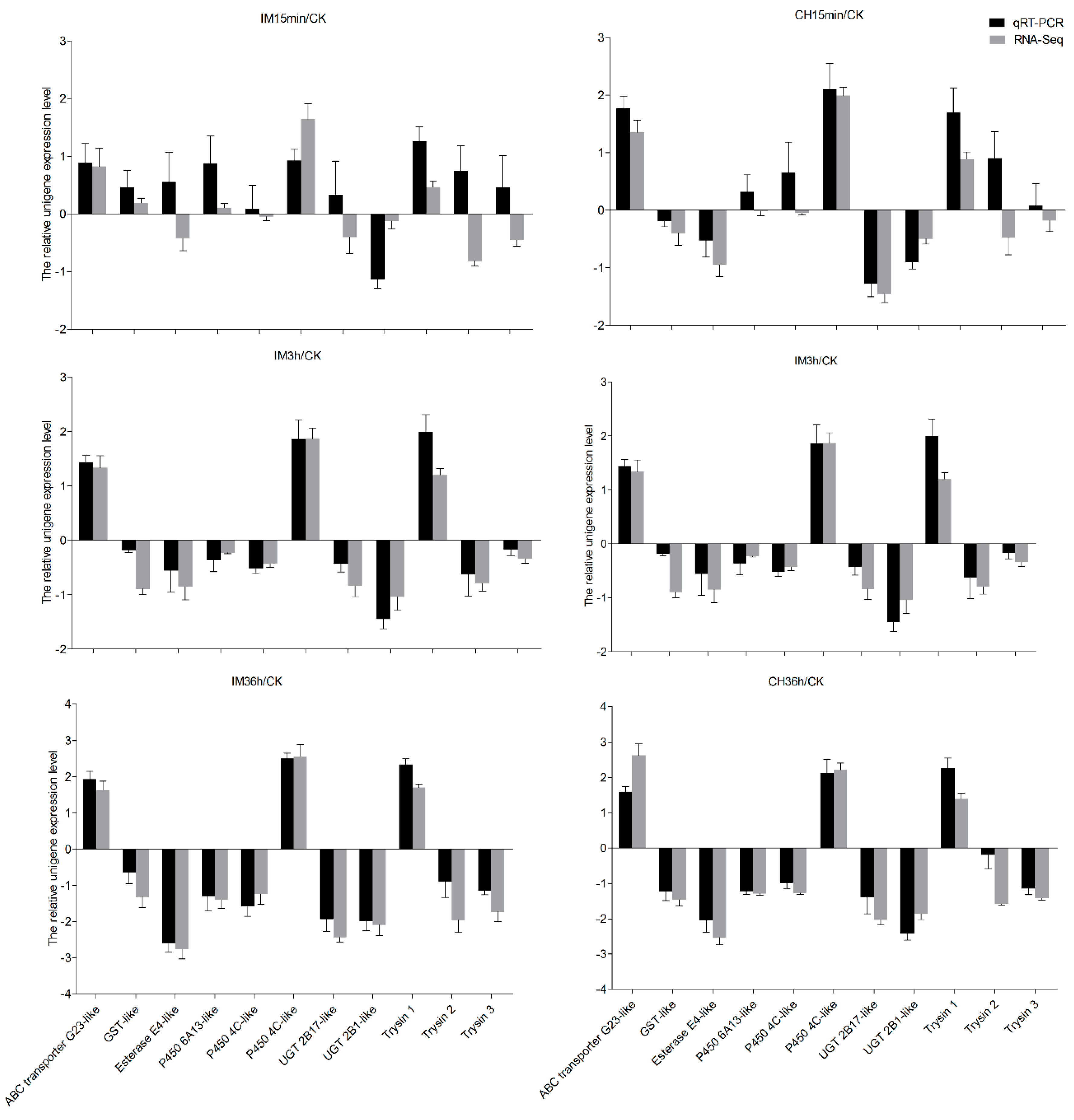
2.7. Sequence Confirmation and qRT-PCR Validation
3. Results
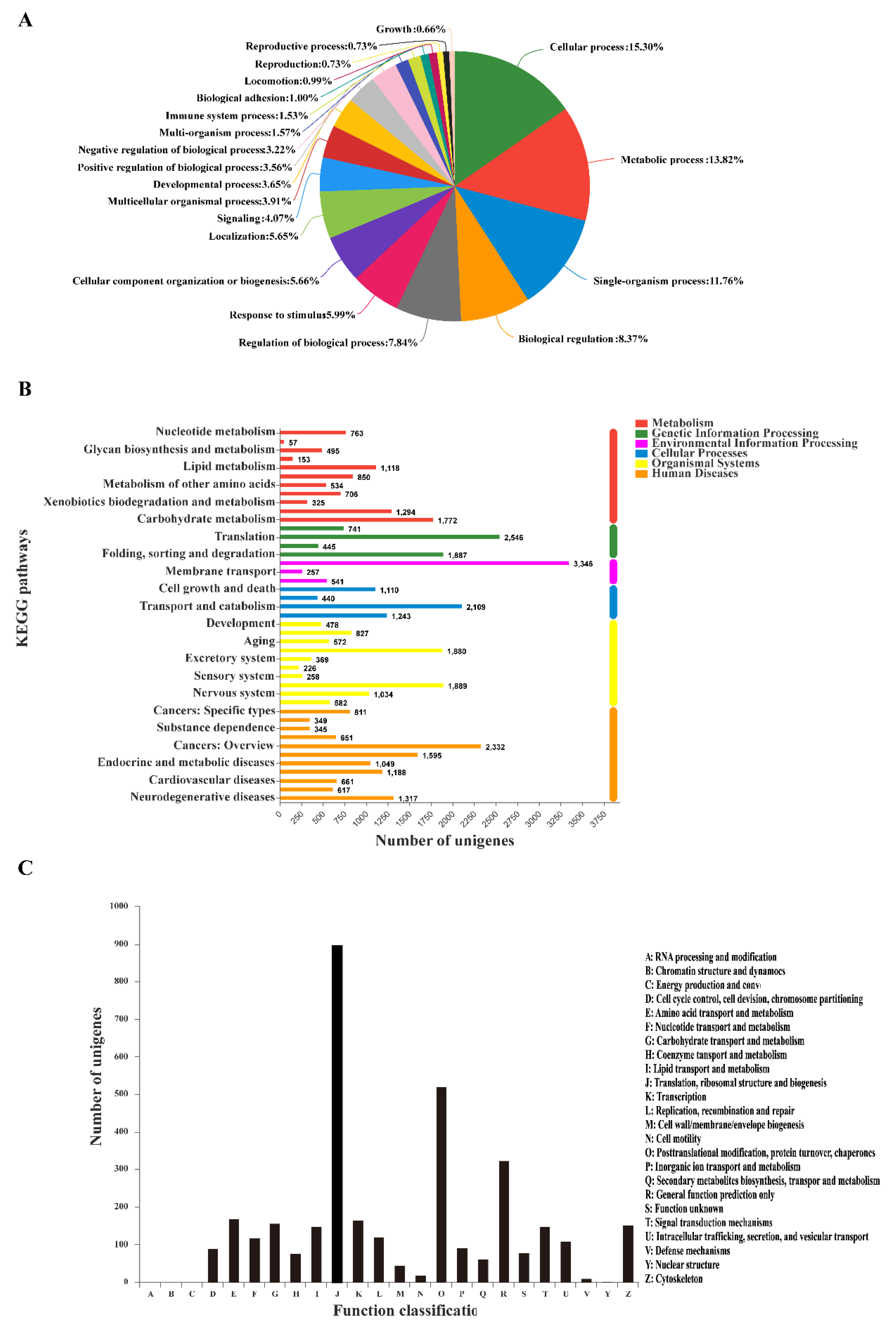
3.1. Bioassay, RNA Sequencing, Assembly, and Annotation
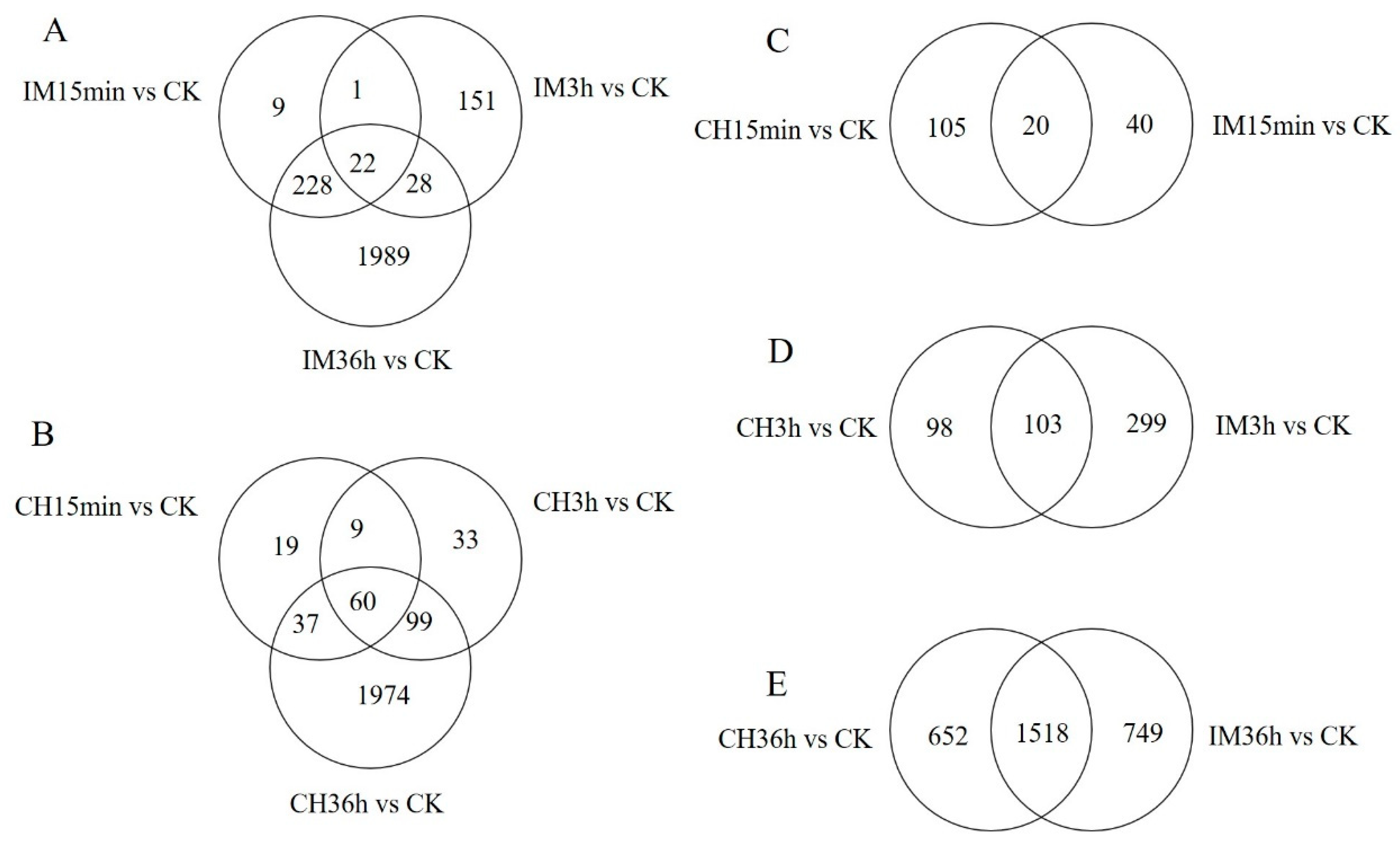
3.2. Unigene Expression Analysis for Insecticide Treatments
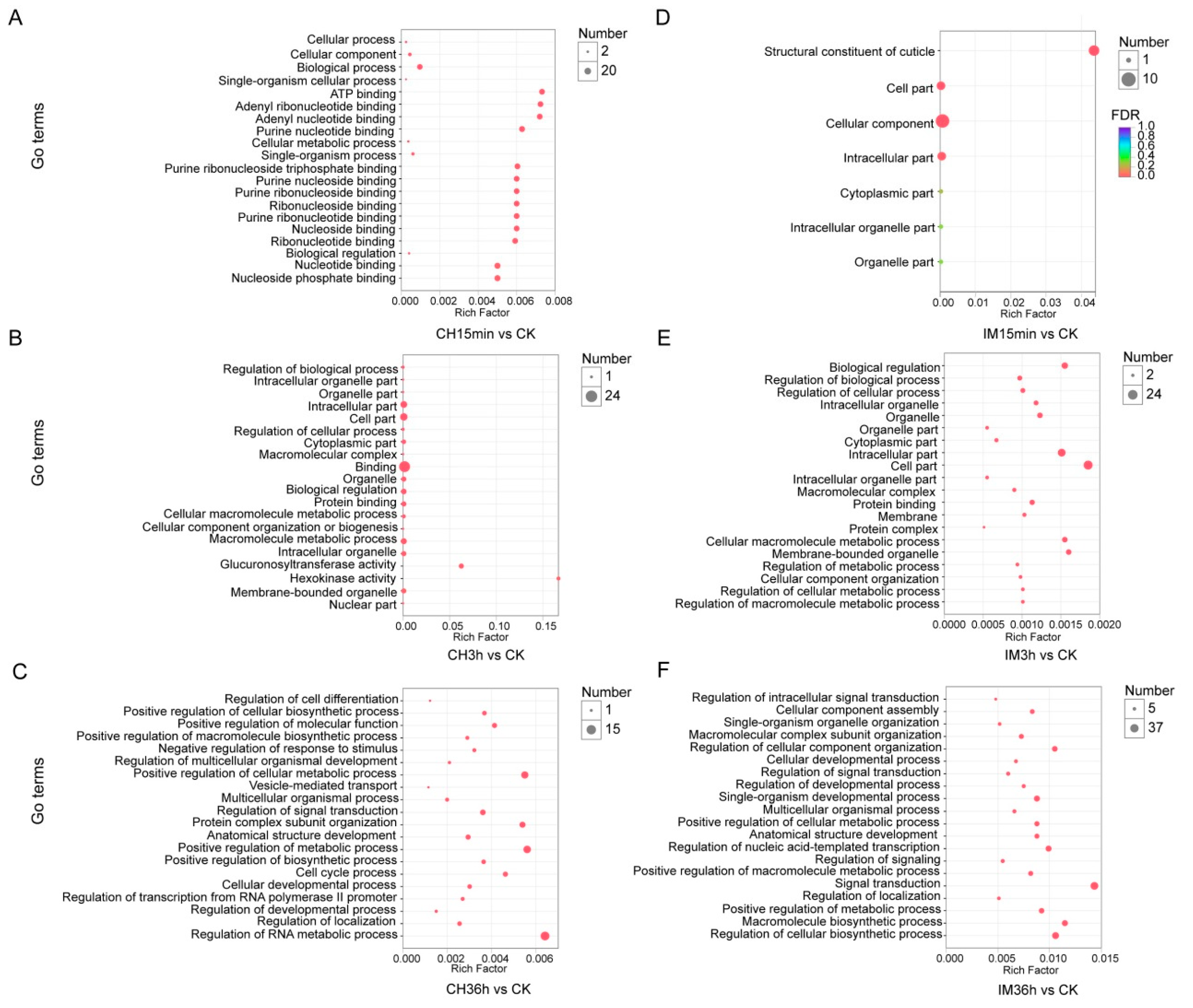
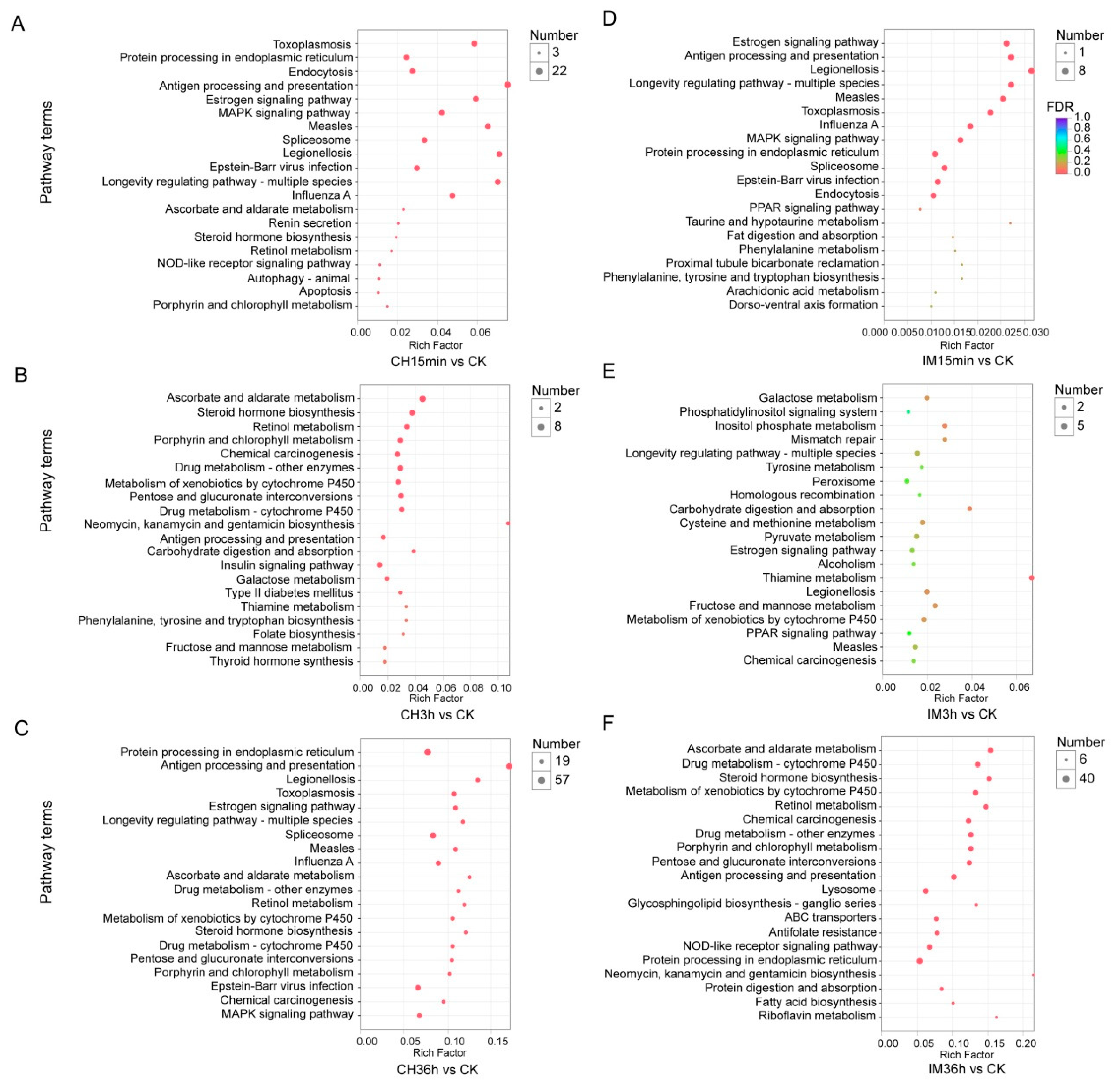
3.3. GO Classification and KEGG Pathway Identification of DEUs
3.4. Insecticide Tolerance Related Unigenes Analysis and Quantitative Real-Time PCR (qRT-PCR) Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cao, Y.; Yin, J.; Li, K.; Zhang, K.; Li, X. Discussion on the causes of resurgence and management strategy of wheat aphid. Plant Prot. 2006, 32, 72–75. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S. Wheat Aphid and Its Comprehensive Management; Agricultural Science Technology Press: Beijing, China, 1990; pp. 316–339. [Google Scholar]
- Li, Q.; Wu, Y.; Li, S.; Liu, A.; Liu, Y. Effect of wheat aphid damage on the quality of high-quality bread wheat. Plant Prot. 2003, 29, 43–44. [Google Scholar] [CrossRef]
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq methods for transcriptome analysis. Wires RNA 2017, 8, e1364. [Google Scholar] [CrossRef] [PubMed]
- David, J.P.; Coissac, E.; Melodelima, C.; Poupardin, R.; Riaz, M.A.; Chandor-Proust, A. Transcriptome response to pollutants and insecticides in the dengue vector Aedes aegypti using next-generation sequencing technology. BMC Genom. 2010, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, L.; Ali, F.; Xie, M.; Zhang, G.; Su, W. Using next-generation sequencing to detect differential expression genes in Bradysia odoriphaga after exposure to insecticides. Int. J. Mol. Sci. 2017, 18, 2445. [Google Scholar] [CrossRef]
- Lei, Y.; Zhu, X.; Xie, W.; Wu, Q.; Wang, S.; Guo, Z.; Xu, B.; Li, X.; Zhou, X.; Zhang, Y. Midgut transcriptome response to a Cry toxin in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Gene 2014, 533, 180–187. [Google Scholar] [CrossRef]
- Xu, L.; Duan, X.; Lv, Y.; Zhang, X.; Liang, R. Silencing of an aphid carboxylesterase gene by use of plant-mediated RNAi impairs Sitobionavenae tolerance of Phoxim insecticides. Transgenic Res. 2014, 23, 389–396. [Google Scholar] [CrossRef]
- Xiao, D.; Lu, Y.; Shang, Q.; Song, D.; Gao, X. Gene silencing of two acetylcholinesterases reveals their cholinergic and non-cholinergic functions in Rhopalosiphum padi and Sitobionavenae. Pest Manag. Sci. 2015, 71, 523–530. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, T.; Gao, X. Establishment of baseline susceptibility data to various insecticides for aphids Rhopalosiphum padi (Linnaeus) and Sitobionavenae (Fabricius) (Homoptera: Aphididae) by the method of residual film in glass tube. Acta Entomol. Sin. 2009, 52, 52–58. [Google Scholar] [CrossRef]
- SPSS Inc. Statistical Package for Social Sciences; SPSS Inc.: Chicago, IL, USA, 2007. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Huang, X.; Liu, D.; Zhang, R.; Shi, X. Transcriptional responses in defense-related genes of Sitobion avenae (Hemiptera: Aphididae) feeding on wheat and barley. J. Econ. Entom. 2019, 112, 382–395. [Google Scholar] [CrossRef]
- Bustin, S.A.; Beaulieu, J.F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef]
- Bass, C.; Zimmer, C.T.; Riveron, J.M.; Wilding, C.S.; Wondji, C.S.; Kaussmann, M.; Field, L.M.; Williamson, M.S.; Nauen, R. Gene amplification and microsatellite polymorphism underlie a recent insect host shift. Proc. Natl. Acad. Sci. USA 2013, 110, 19460–19465. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Kawashima, M.; Banba, S. Differential metabolism of neonicotinoids by Myzus persicae CYP6CY3 stably expressed in Drosophila S2 cells. J. Pestic. Sci. 2019, 44, 177–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.; Han, Y.; Wang, L.; Liu, Z.; Guo, H.; Fang, J. Transcript-level analysis of detoxification gene mutation-mediated chlorpyrifos resistance in Laodelphax striatellus (Hemiptera: Delphacidae). J. Econ.Entomol. 2019, 112, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Puinean, A.M.; Foster, S.P.; Oliphant, L.; Denholm, I.; Field, L.M.; Millar, N.S.; Williamson, M.S.; Bass, C. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010, 6, e1000999. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, H.; Pan, Y.; Gao, X.; Xi, J.; Zhang, J.; Shang, Q. Expression profile changes of cytochrome P450 genes between thiamethoxam susceptible and resistant strains of Aphis gossypii Glover. Pestic. Biochem. Physiol. 2018, 149, 1–7. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Zhang, B.; Liang, P.; Wang, B.; Gao, X. Overexpression of multiple cytochrome P450 genes associated with sulfoxaflor resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 157, 204–210. [Google Scholar] [CrossRef]
- Scott, J.G. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef]
- Chen, C.; Shan, T.; Liu, Y.; Wang, C.; Shi, X.; Gao, X. Identification and functional analysis of a cytochrome P450 gene involved in imidacloprid resistance in Bradysia odoriphaga Yang et Zhang. Pestic. Biochem. Phys. 2019, 153, 129–135. [Google Scholar] [CrossRef]
- Karunker, I.; Morou, E.; Nikou, D.; Nauen, R.; Sertchook, R.; Stevenson, B.J. Structural model and functional characterization of the Bemisia tabaci CYP6CM1VQ, a cytochrome P450 associated with high levels of imidacloprid resistance. Insect Biochem. Mol. Biol. 2009, 39, 697–706. [Google Scholar] [CrossRef]
- Dermauw, W.; Leeuwen, T.V. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Molecul. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef]
- You, M.; Yue, Z.; He, W.; Yang, X.; Yang, G.; Xie, M.; Zhan, D.; Baxter, S.W.; Vasseur, L.; Gurr, G.M.; et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Gaddelapati, S.C.; Kalsi, M.; Roy, A.; Palli, S.R. Cap ‘n’ collar C regulates genes responsible for imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Insect Biochem. Molecul. Biol. 2018, 99, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Pu, J.; Chen, F.; Wang, J.; Han, Z. Multiple ATP-binding cassette transporters are involved in insecticide resistance in the small brown planthopper, Laodelphax striatellus. Insect Molecul. Biol. 2017, 26, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Mastrantonio, V.; Ferrari, M.; Negri, A.; Sturmo, T.; Favia, G.; Porretta, D.; Epis, S.; Urbanelli, S. Insecticide exposure triggers a modulated expression of ABC transporter genes in larvae of Anopheles gambiae s.s. Insects 2019, 10, 66. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Fang, F.; Wang, W.; Zhang, D.; Lv, Y.; Zhou, D.; Ma, L.; Zhu, C. The cuticle proteins: A putative role for deltamethrin resistance in Culex pipienspallens. Parasitol. Res. 2015, 114, 4421–4429. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Q.; Sun, X.; Zhang, C.; Xu, N.; Xu, Y.; Shen, B. Culex pipienspallens cuticular protein CPLCG5 participates in pyrethroid resistance by forming a rigid matrix. Parasites Vectors 2018, 11, 6. [Google Scholar] [CrossRef]
- Wang, W.; Lv, Y.; Fang, F.; Hong, S.; Guo, Q.; Hu, S.; Zhou, D. Identification of proteins associated with pyrethroid resistance by iTRAQ-based quantitative proteomic analysis in Culex pipienspallens. Parasites Vectors 2015, 8, 95. [Google Scholar] [CrossRef]
- Hsu, J.; Lin, Y.; Chang, C.; Hua, K.; Chen, M.; Huang, L. Discovery of organophosphate resistance-related genes associated with well-known resistance mechanisms of Plutella xylostella (L.) (Lepidoptera: Plutellidae) by RNA-Seq. J. Econ. Entomol. 2016, 109, 1378–1386. [Google Scholar] [CrossRef]
- Wu, H.; Tian, H.; Wu, G.; Langdon, G.; Kurtis, J.; Shen, B.; Zhu, C. Culex pipienspallens: Identification of genes differentially expressed in deltamethrin-resistant and-susceptible strains. Pestic. Biochem. Phys. 2004, 79, 75–83. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.; Jin, D. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 2018, 8, 8773. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Peng, T.; Gao, X.; Zhang, L.; Yang, C.; Xi, J.; Shang, Q. Transcriptomic comparison of thiamethoxam-resistance adaptation in resistant and susceptible strains of Aphis gossypii Glover. Comp. Biochem. Phys. D 2015, 13, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, C.; Gao, X.; Peng, T.; Bi, R.; Xi, J.; Shang, Q. Spirotetramat resistance adaption analysis of Aphis gossypii Glover by transcriptomic survey. Pestic. Biochem. Phys. 2015, 124, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bozzolan, F.; Siaussat, D.; Maria, A.; Durand, N.; Pottier, M.A.; Chertemps, T.; Maïbèche-Coisne, M. Antennal uridine diphosphate (UDP)-glycosyltransferases in a pest insect: Diversity and putative function in odorant and xenobiotics clearance. Insect Mol. Bio. 2014, 23, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Lazard, D.; Zupko, K.; Poria, Y.; Nef, P.; Lazarovits, J.; Horn, S.; Khen, M.; Lancet, D. Odorant signal termination by olfactory UDP-glucuronosyltransferase. Nature 1991, 349, 790–793. [Google Scholar] [CrossRef]
- Bull, D.L.; Whitten, C.J. Factors influencing organophosphorus insecticide resistance in tobacco budworms. J. Agric. Food Chem. 1972, 20, 561–564. [Google Scholar] [CrossRef]

| Insecticide | Regression Equation | LC50 (mg/L) | 95% Confidence Interval | LC10 (mg/L) | 95% Confidence Interval | X2 | p-Value |
|---|---|---|---|---|---|---|---|
| chlorpyrifos | y = −0.89+1.39x | 3.93 | 2.76–5.13 | 0.47 | 0.17–0.88 | 3.90 | 0.27 |
| imidacloprid | y = −3.23+1.23x | 429.90 | 172.80–784.54 | 38.90 | 1.43–115.15 | 10.16 | 0.038 |
| Unigene ID | Fold Change | Annotation |
|---|---|---|
| DN58420_c0_g1 | 4.96 | anthranilate synthase component 1 |
| DN68934_c1_g1 | 4.61 | no result |
| DN56676_c0_g1 | 4.60 | ribosomal large subunit pseudouridine synthase C |
| DN69579_c4_g1 | 4.53 | WD repeat-containing protein 6 isoform X2 |
| DN63141_c10_g2 | 3.95 | uncharacterized family 31 glucosidase KIAA1161 |
| DN64362_c2_g2 | 3.95 | uncharacterized protein LOC111041562 |
| DN59858_c0_g2 | 3.77 | putative signal recognition particle protein |
| DN58147_c0_g1 | 3.64 | regucalcin like isoform X1 |
| DN69340_c3_g2 | 3.57 | no result |
| DN62276_c7_g3 | 3.52 | uncharacterized protein LOC111032214 |
| DN62849_c0_g1 | −4.39 | uncharacterized protein LOC100169299 |
| DN63539_c2_g2 | −3.99 | uncharacterized protein LOC111038926 |
| DN59661_c1_g1 | −3.98 | gamma glutamyl transpeptidase 1 isoform X2 |
| DN66753_c0_g1 | −3.96 | gamma glutamyl transpeptidase 1 isoform X2 |
| DN61744_c1_g4 | −3.78 | uncharacterized protein LOC100574123 |
| DN62043_c5_g2 | −3.73 | calphotin like |
| DN61971_c3_g5 | −3.58 | calphotin like |
| DN60187_c0_g1 | −3.50 | RNA binding protein 14 |
| DN62043_c4_g1 | −3.44 | MAGE like protein 2 |
| DN67869_c1_g3 | −3.43 | phytoene desaturase |
| Unigene ID | Fold Change | Annotation |
|---|---|---|
| DN58420_c0_g1 | 4.41 | trpE |
| DN56676_c0_g1 | 4.11 | ribosomal large subunit pseudouridine synthase C like |
| DN63141_c10_g2 | 4.07 | uncharacterized family 31 glucosidase KIAA1161 like |
| DN69579_c4_g1 | 3.59 | WD repeat-containing protein 6 isoform X2 |
| DN58900_c5_g1 | 3.53 | no result |
| DN68934_c1_g1 | 3.47 | no result |
| DN58147_c0_g1 | 3.26 | regucalcin like isoform X1 |
| DN59687_c3_g4 | 3.17 | uncharacterized protein LOC108376199 |
| DN69016_c3_g2 | 3.05 | no result |
| DN59858_c0_g | 2.97 | putative signal recognition particle protein |
| DN61971_c3_g5 | −4.04 | calphotin like |
| DN63539_c2_g2 | −3.98 | uncharacterized protein LOC111038926 |
| DN62043_c5_g2 | −3.94 | calphotin like |
| DN66074_c0_g1 | −3.80 | MAGE-like protein 2 |
| DN62043_c4_g1 | −3.71 | MAGE-like protein 2 |
| DN61744_c1_g4 | −3.49 | uncharacterized protein LOC100574123 |
| DN60266_c0_g1 | −3.43 | probable NADP-dependent mannitol dehydrogenase |
| DN66753_c0_g1 | −3.35 | UGT 2C1 like |
| DN61958_c0_g1 | −3.33 | protein-glutamate O-methyltransferase like |
| DN59661_c1_g1 | −3.30 | gamma-glutamyl transpeptidase 1 isoform X2 |
| Gene Type | Unigenes Number in De Novo Database | IM 15 min vs. CK | IM 3 h vs. CK | IM 36 h vs. CK | CH 15 min vs. CK | CH 3 h vs. CK | CH 36 h vs. CK | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | Up | Down | Up | Down | Up | Down | ||
| Glutathione s-transferase | 90 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Carboxylesterase | 81 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 7 |
| Cytochrome P450 | 198 | 1 | 1 | 7 | 0 | 5 | 6 | 1 | 1 | 1 | 1 | 5 | 6 |
| NADH dehydrogenase | 135 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| Trypsin | 218 | 0 | 0 | 2 | 3 | 15 | 17 | 1 | 0 | 1 | 0 | 8 | 12 |
| Superoxide dismutase | 26 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| ABC transporter | 381 | 0 | 0 | 0 | 1 | 8 | 21 | 0 | 0 | 0 | 1 | 8 | 17 |
| Cuticle protein | 120 | 0 | 5 | 0 | 0 | 4 | 46 | 0 | 5 | 0 | 4 | 3 | 40 |
| UGT | 112 | 0 | 0 | 0 | 3 | 1 | 23 | 0 | 3 | 0 | 6 | 0 | 18 |
| Acetylcholine receptor | 12 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chloride channel | 11 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, N.; Zhong, Y.; Lin, L.; Xie, M.; Zhang, G.; Su, W.; Li, C.; Chen, H. Transcriptome Analysis and Identification of Insecticide Tolerance-Related Genes after Exposure to Insecticide in Sitobion avenae. Genes 2019, 10, 951. https://doi.org/10.3390/genes10120951
Wei N, Zhong Y, Lin L, Xie M, Zhang G, Su W, Li C, Chen H. Transcriptome Analysis and Identification of Insecticide Tolerance-Related Genes after Exposure to Insecticide in Sitobion avenae. Genes. 2019; 10(12):951. https://doi.org/10.3390/genes10120951
Chicago/Turabian StyleWei, Ning, Yongzhi Zhong, Lulu Lin, Minghui Xie, Guangling Zhang, Weihua Su, Chuanren Li, and Haoliang Chen. 2019. "Transcriptome Analysis and Identification of Insecticide Tolerance-Related Genes after Exposure to Insecticide in Sitobion avenae" Genes 10, no. 12: 951. https://doi.org/10.3390/genes10120951
APA StyleWei, N., Zhong, Y., Lin, L., Xie, M., Zhang, G., Su, W., Li, C., & Chen, H. (2019). Transcriptome Analysis and Identification of Insecticide Tolerance-Related Genes after Exposure to Insecticide in Sitobion avenae. Genes, 10(12), 951. https://doi.org/10.3390/genes10120951





