Ischemia-Like Stress Conditions Stimulate Trophic Activities of Adipose-Derived Stromal/Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
2.2. Ischemic Culture Conditions
2.3. Live/Dead Staining
2.4. Quantification of DNA
2.5. MTT Assay
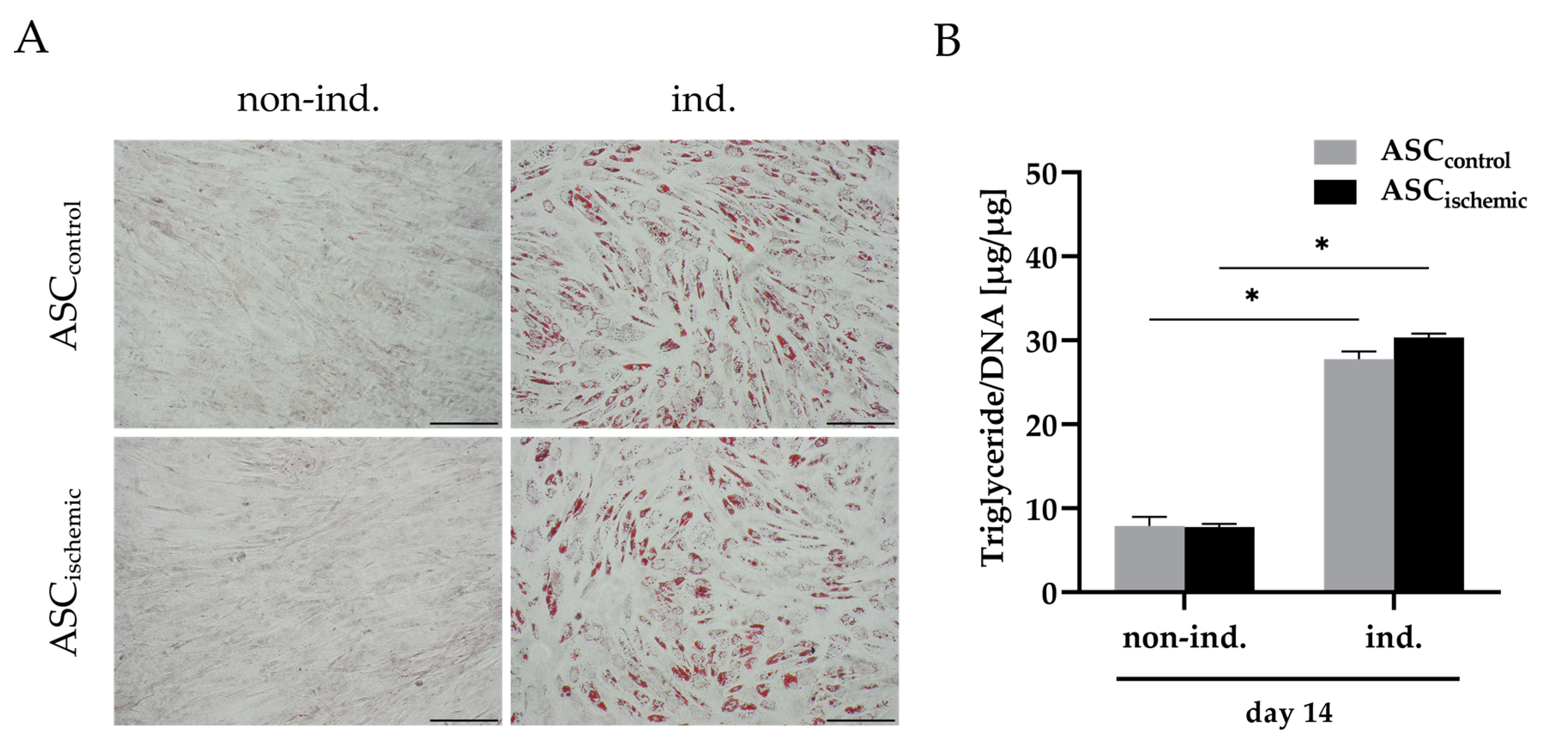
2.6. Adipogenic Differentiation of Ischemia-Treated ASCs
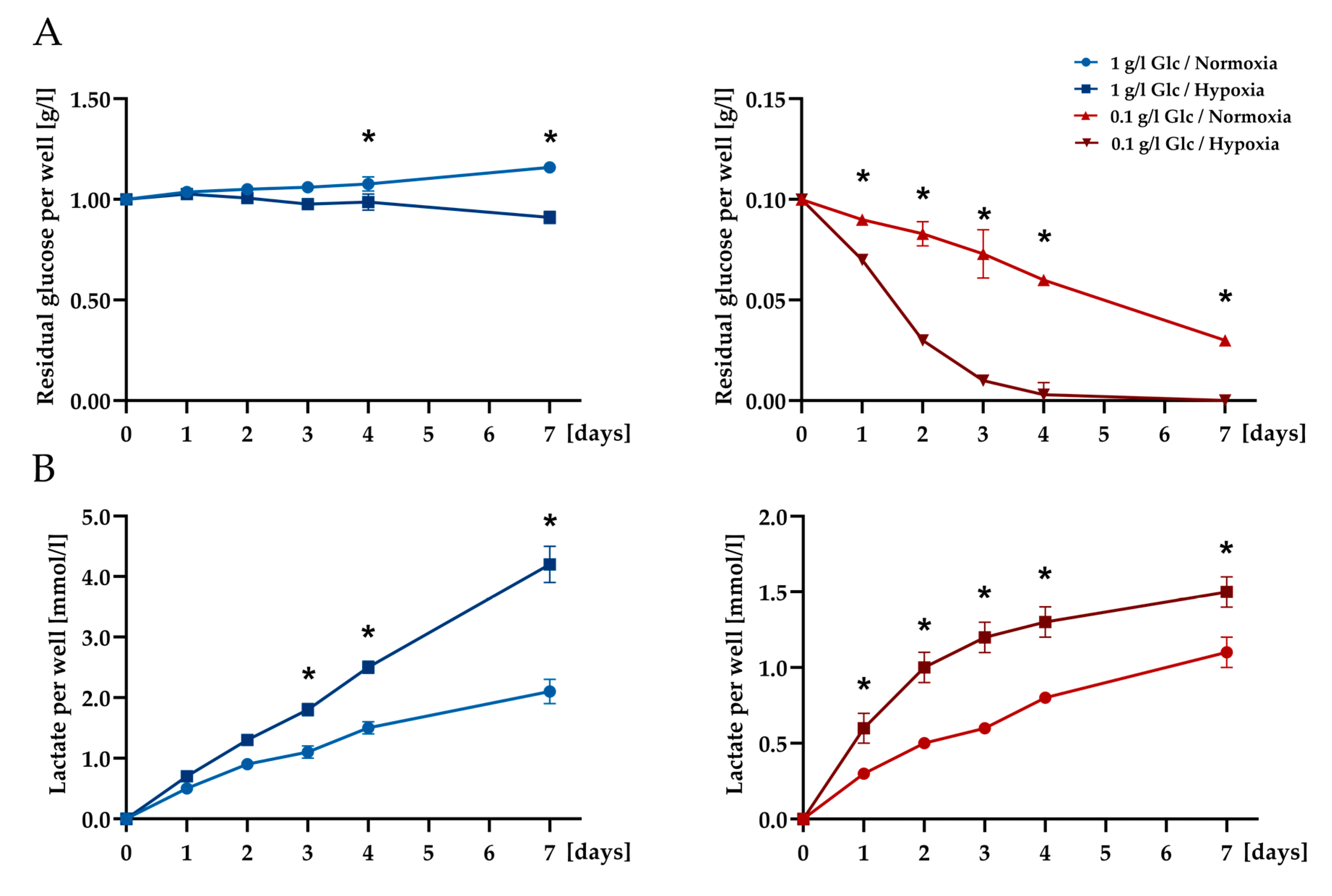
2.7. Glucose and Lactate Determination
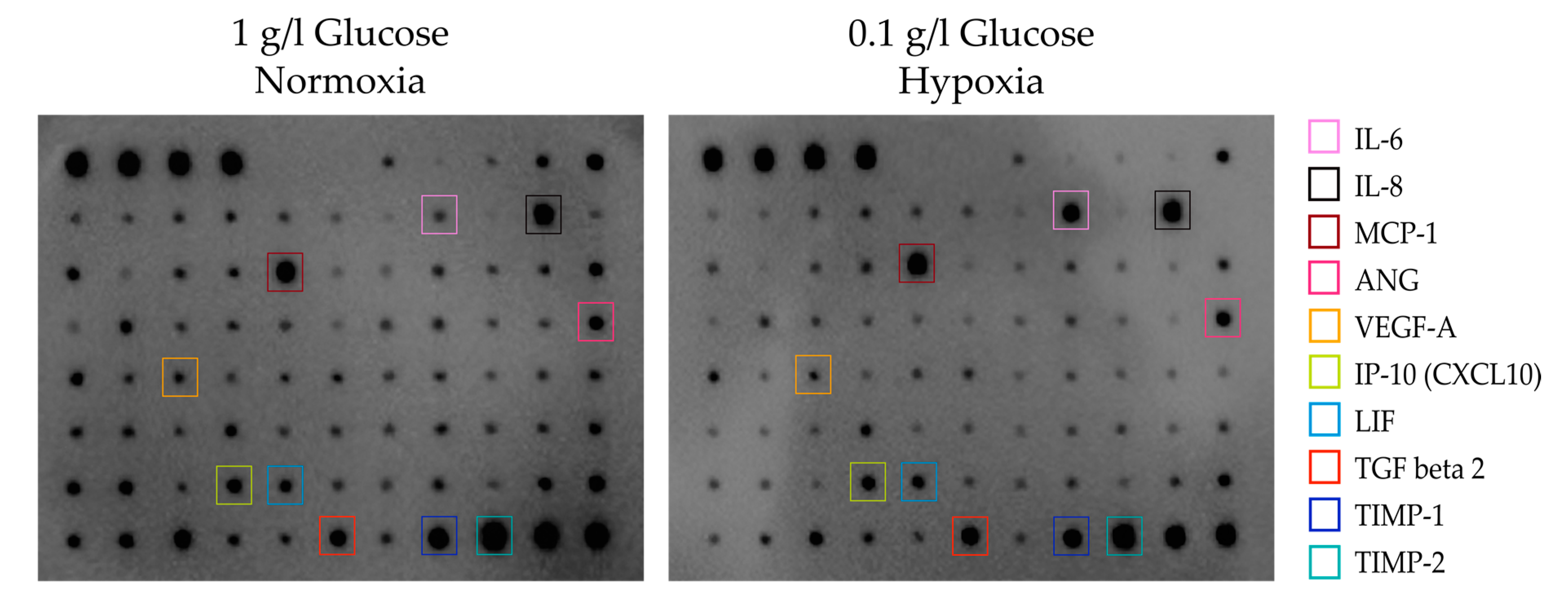
2.8. Assays of Cytokines
2.9. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.10. Preparation of Conditioned Medium
2.11. Tube Formation Assay
2.12. Proliferation and Metabolic Activity of Fibroblasts
2.13. Fibroblast Migration Assay
2.14. Statistical Analysis
3. Results
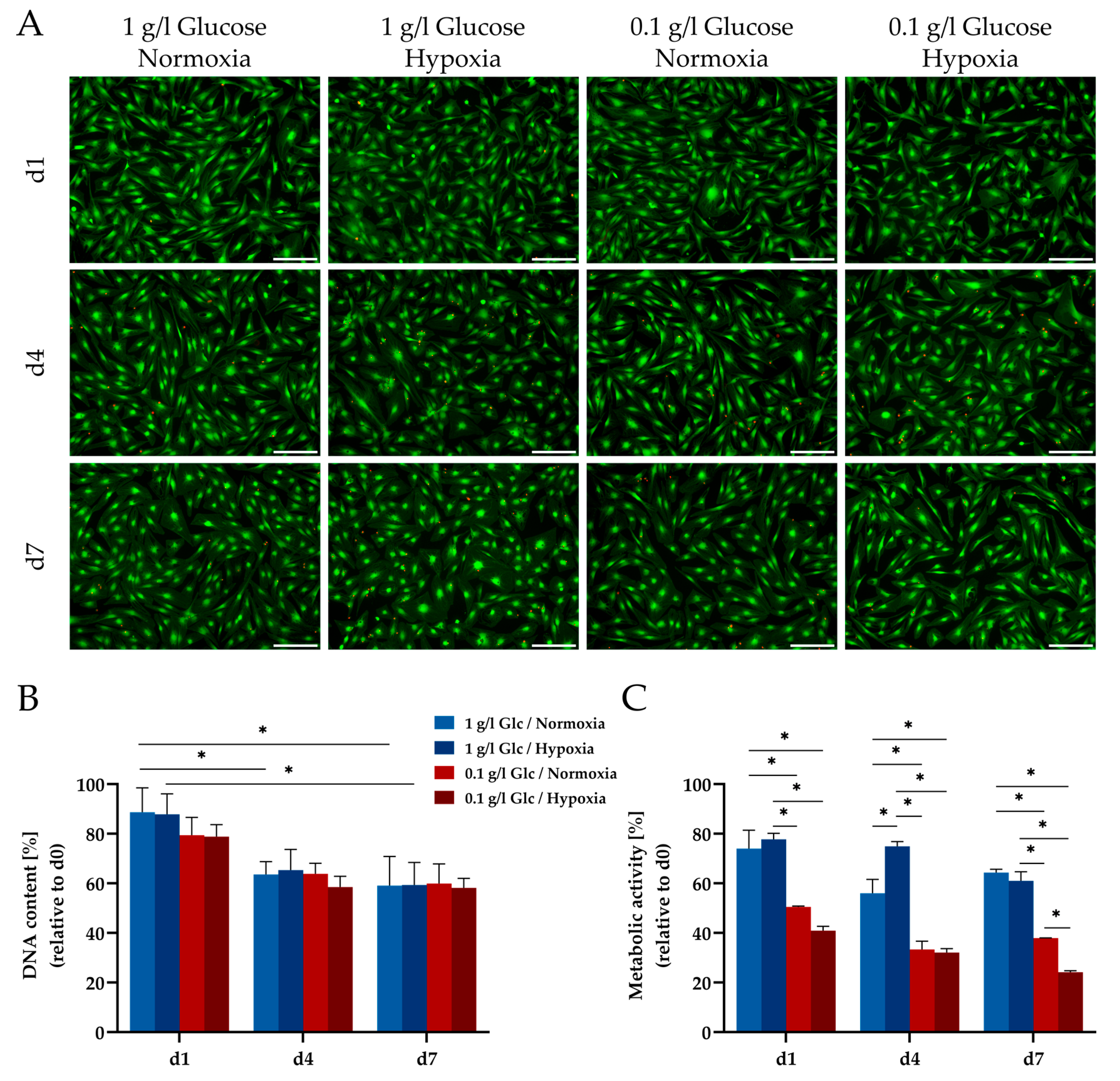
3.1. Characterization of ASCs under Glucose/Oxygen Deprivation
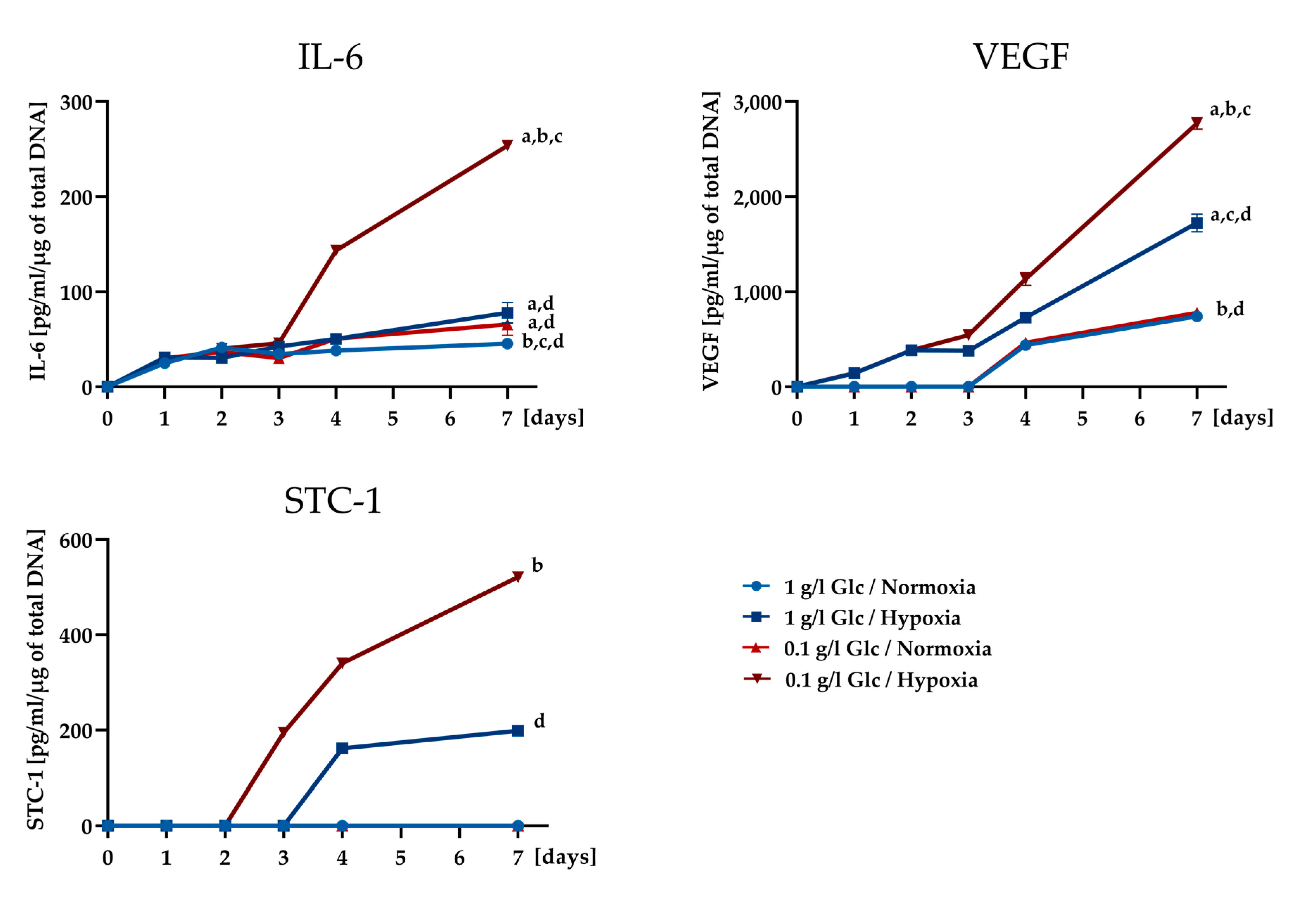
3.2. Secretory Potential of ASCs under Glucose/Oxygen Deprivation
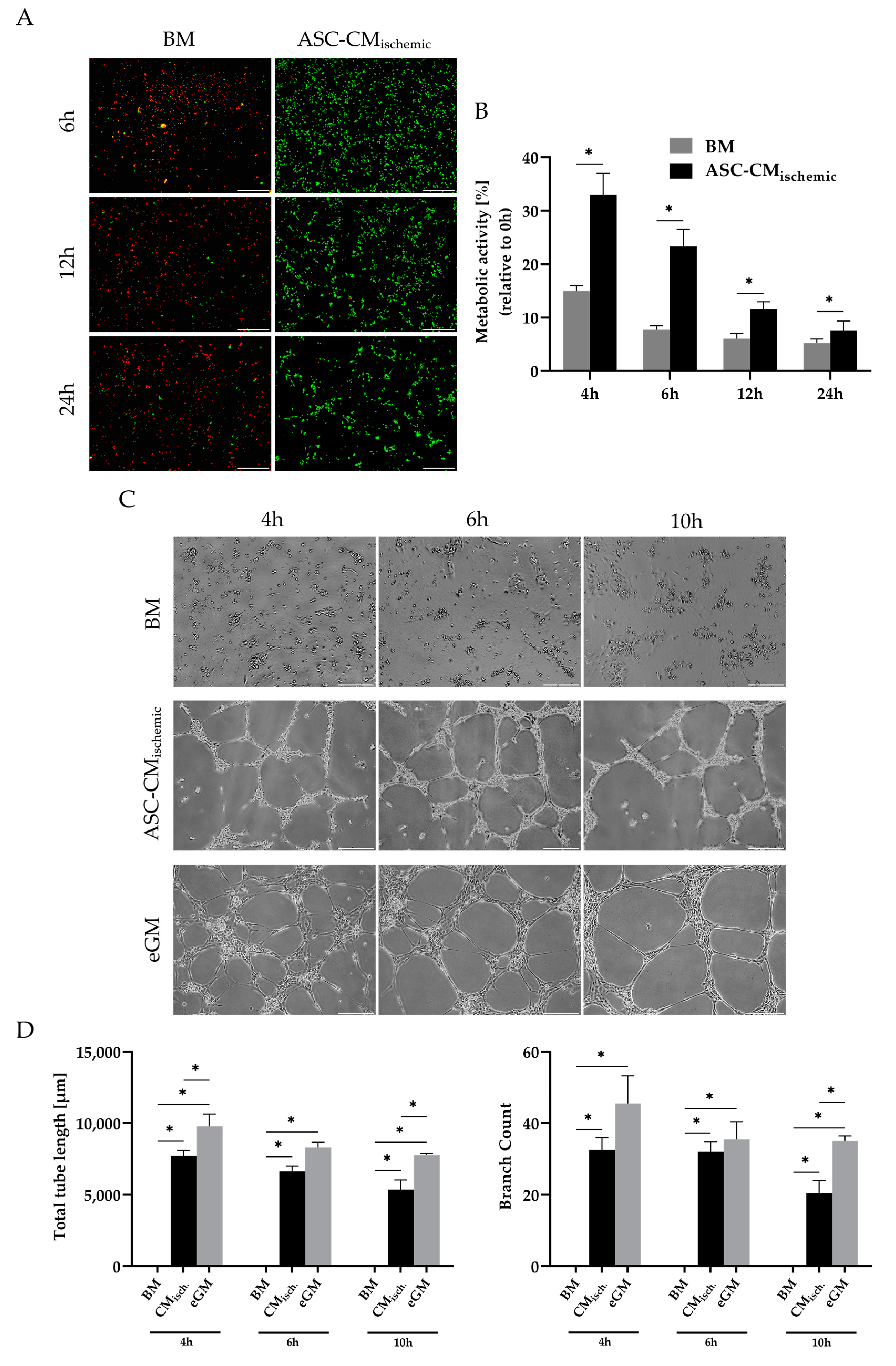
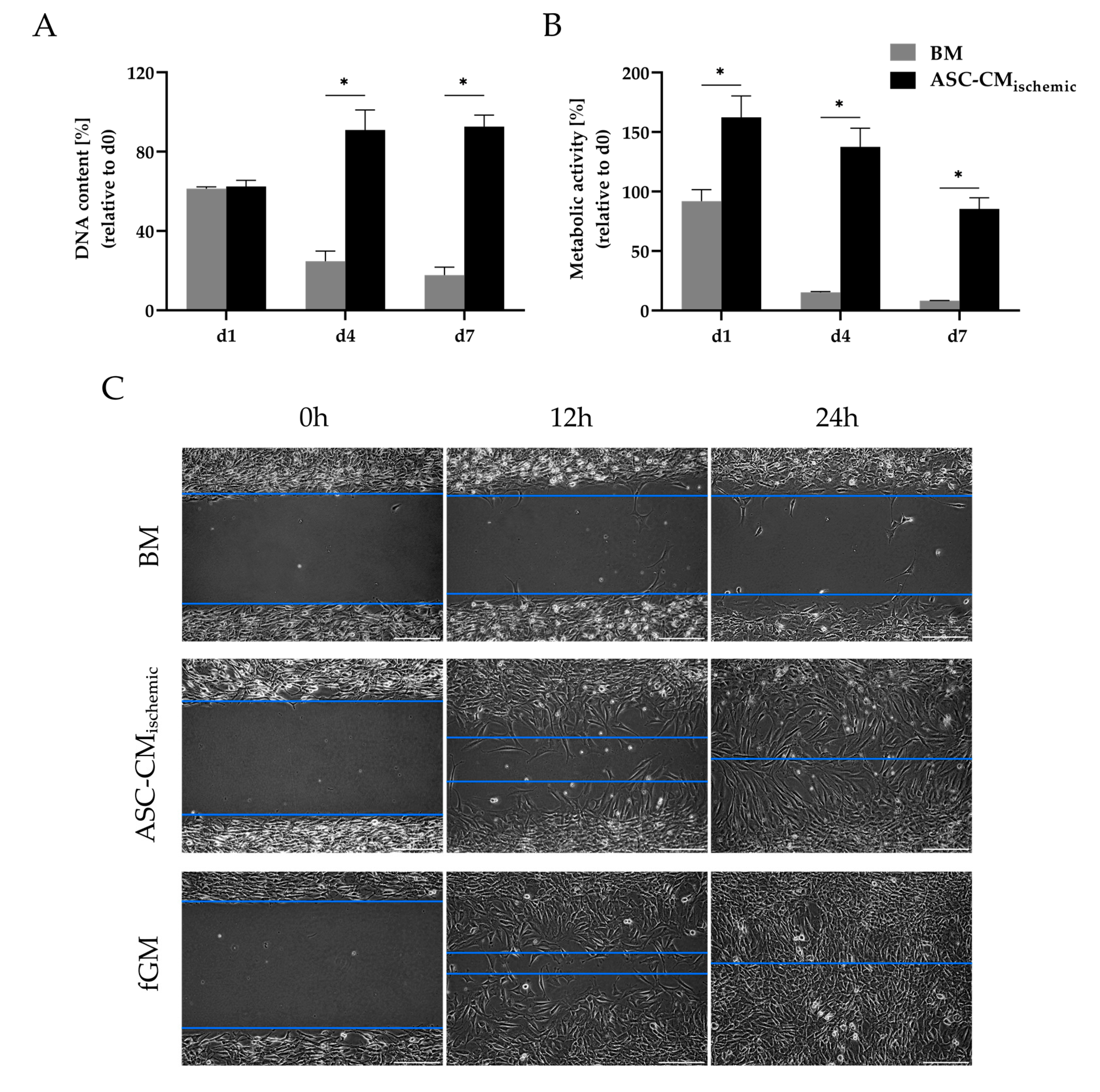
3.3. Regenerative Effects of Conditioned Medium from Glucose/Oxygen-Deprived ASCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Gimble, J.M.; Lee, K.; Marra, K.G.; Rubin, J.P.; Yoo, J.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Adipose tissue engineering for soft tissue regeneration. Tissue Eng. Part B Rev. 2010, 16, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Han, J.; Hwang, S.J.; Sung, J.H. An update on niche composition, signaling and functional regulation of the adipose-derived stem cells. Expert Opin. Biol. Ther. 2014, 14, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Bauer-Kreisel, P.; Goepferich, A.; Blunk, T. Cell-delivery therapeutics for adipose tissue regeneration. Adv. Drug Deliv. Rev. 2010, 62, 798–813. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast. Surg. 2008, 32, 48–55. [Google Scholar] [CrossRef]
- Vyas, K.S.; Vasconez, H.C.; Morrison, S.; Mogni, B.; Linton, S.; Hockensmith, L.; Kabir, T.; Zielins, E.; Najor, A.; Bakri, K.; et al. Fat Graft Enrichment Strategies: A Systematic Review. Plast. Reconstr. Surg. 2020, 145, 827–841. [Google Scholar] [CrossRef]
- Kølle, S.F.T.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.L.M.; et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Salgado, A.J.; Reis, R.L.; Sousa, N.; Gimble, J.M. Adipose Tissue Derived Stem Cells Secretome: Soluble Factors and Their Roles in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef]
- Li, X.; Ma, T.; Sun, J.; Shen, M.; Xue, X.; Chen, Y.; Zhang, Z. Harnessing the secretome of adipose-derived stem cells in the treatment of ischemic heart diseases. Stem Cell Res. Ther. 2019, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Mooney, D.J.; Vandenburgh, H. Cell Delivery Mechanisms for Tissue Repair. Cell Stem Cell 2008, 2, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y.Y.G. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE 2008, 3, 1886. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Praveen Kumar, L.; Sangeetha, K.; Ranjita, M.; Vijayalakshmi, S.; Rajagopal, K.; Rama Shanker, V. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Meiliana, A.; Dewi, N.M.; Wijaya, A. Mesenchymal stem cell secretome: Cell-free therapeutic strategy in regenerative medicine. Indones. Biomed. J. 2019, 11, 113–124. [Google Scholar] [CrossRef]
- Fu, Y.; Karbaat, L.; Wu, L.; Leijten, J.; Both, S.K.; Karperien, M. Trophic Effects of Mesenchymal Stem Cells in Tissue Regeneration. Tissue Eng. Part B Rev. 2017, 23, 515–528. [Google Scholar] [CrossRef]
- Weiser, B.; Prantl, L.; Schubert, T.E.O.; Zellner, J.; Fischbach-Teschl, C.; Spruss, T.; Seitz, A.K.; Tessmar, J.; Goepferich, A.; Blunk, T. In Vivo Development and Long-Term Survival of Engineered Adipose Tissue Depend on In Vitro Precultivation Strategy. Tissue Eng. Part A 2008, 14, 275–284. [Google Scholar] [CrossRef]
- Lu, F.; Li, J.; Gao, J.; Ogawa, R.; Ou, C.; Yang, B.; Fu, B. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast. Reconstr. Surg. 2009, 124, 1437–1446. [Google Scholar] [CrossRef]
- Moya, A.; Paquet, J.; Deschepper, M.; Larochette, N.; Oudina, K.; Denoeud, C.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Human Mesenchymal Stem Cell Failure to Adapt to Glucose Shortage and Rapidly Use Intracellular Energy Reserves Through Glycolysis Explains Poor Cell Survival After Implantation. Stem Cells 2018, 36, 363–376. [Google Scholar] [CrossRef]
- Deschepper, M.; Oudina, K.; David, B.; Myrtil, V.; Collet, C.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J. Cell. Mol. Med. 2011, 15, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Mylotte, L.A.; Duffy, A.M.; Murphy, M.; O’Brien, T.; Samali, A.; Barry, F.; Szegezdi, E. Metabolic Flexibility Permits Mesenchymal Stem Cell Survival in an Ischemic Environment. Stem Cells 2008, 26, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Mischen, B.T.; Follmar, K.E.; Moyer, K.E.; Buehrer, B.; Olbrich, K.C.; Levin, L.S.; Klitzman, B.; Erdmann, D. Metabolic and functional characterization of human adipose-derived stem cells in tissue engineering. Plast. Reconstr. Surg. 2008, 122, 725–738. [Google Scholar] [CrossRef]
- Faghih, H.; Javeri, A.; Taha, M.F. Impact of early subcultures on stemness, migration and angiogenic potential of adipose tissue-derived stem cells and their resistance to in vitro ischemic condition. Cytotechnology 2017, 69, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Block, G.J.; Ohkouchi, S.; Fung, F.; Frenkel, J.; Gregory, C.; Pochampally, R.; Dimattia, G.; Sullivan, D.E.; Prockop, D.J. Multipotent Stromal Cells (MSCs) are Activated to Reduce Apoptosis in Part by Upregulation and Secretion of Stanniocalcin-1 (STC-1) HHS Public Access. Stem Cells 2009, 27, 670–681. [Google Scholar] [CrossRef]
- Yeung, B.H.Y.; Law, A.Y.S.; Wong, C.K.C. Evolution and roles of stanniocalcin. Mol. Cell. Endocrinol. 2012, 349, 272–280. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Z.; Pu, F. Role of stanniocalcin-1 in breast cancer (Review). Oncol. Lett. 2019, 18, 3946–3953. [Google Scholar] [CrossRef]
- He, X.; Zhong, X.; Ni, Y.; Liu, M.; Liu, S.; Lan, X. Effect of ASCs on the graft survival rates of fat particles in rabbits. J. Plast. Surg. Hand Surg. 2013, 47, 3–7. [Google Scholar] [CrossRef]
- Matsumoto, D.; Sato, K.; Gonda, K.; Takaki, Y.; Shigeura, T.; Sato, T.; Aiba-Kojima, E.; Iizuka, F.; Inoue, K.; Suga, H.; et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006, 12, 3375–3382. [Google Scholar] [CrossRef]
- Zhao, L.; Johnson, T.; Liu, D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res. Ther. 2017, 8, 125. [Google Scholar] [CrossRef]
- Copland, I.B.; Lord-Dufour, S.; Cuerquis, J.; Coutu, D.L.; Annabi, B.; Wang, E.; Galipeau, J. Improved Autograft Survival of Mesenchymal Stromal Cells by Plasminogen Activator Inhibitor 1 Inhibition. Stem Cells 2009, 27, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.P.; Gimble, J.M.; Kheterpal, I.; Rowan, B.G. Impact of low oxygen on the secretome of human adipose-derived stromal/stem cell primary cultures. Biochimie 2013, 95, 2286–2296. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Pirkmajer, S.; Chibalin, A.V. Serum starvation: Caveat emptor. Am. J. Physiol. Cell Physiol. 2011, 301, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Nuschke, A.; Rodrigues, M.; Wells, A.W.; Sylakowski, K.; Wells, A. Mesenchymal stem cells/multipotent stromal cells (MSCs) are glycolytic and thus glucose is a limiting factor of in vitro models of MSC starvation. Stem Cell Res. Ther. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Moya, A.; Larochette, N.; Paquet, J.; Deschepper, M.; Bensidhoum, M.; Izzo, V.; Kroemer, G.; Petite, H.; Logeart-Avramoglou, D. Quiescence Preconditioned Human Multipotent Stromal Cells Adopt a Metabolic Profile Favorable for Enhanced Survival under Ischemia. Stem Cells 2017, 35, 181–196. [Google Scholar] [CrossRef]
- Li, C.; Ye, L.; Yang, L.; Yu, X.; He, Y.; Chen, Z.; Li, L.; Zhang, D. Rapamycin Promotes the Survival and Adipogenesis of Ischemia-Challenged Adipose Derived Stem Cells by Improving Autophagy. Cell. Physiol. Biochem. 2018, 44, 1762–1774. [Google Scholar] [CrossRef]
- Pan, J.S.C.; Huang, L.; Belousova, T.; Lu, L.; Yang, Y.; Reddel, R.; Chang, A.; Ju, H.; DiMattia, G.; Tong, Q.; et al. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J. Am. Soc. Nephrol. 2015, 26, 364–378. [Google Scholar] [CrossRef]
- Higuera, G.A.; Fernandes, H.; Spitters, T.W.G.M.; van de Peppel, J.; Aufferman, N.; Truckenmueller, R.; Escalante, M.; Stoop, R.; van Leeuwen, J.P.; de Boer, J.; et al. Spatiotemporal proliferation of human stromal cells adjusts to nutrient availability and leads to stanniocalcin-1 expression in vitro and in vivo. Biomaterials 2015, 61, 190–202. [Google Scholar] [CrossRef]
- Bironaite, D.; Westberg, J.A.; Andersson, L.C.; Venalis, A. A variety of mild stresses upregulate stanniocalcin-1 (STC-1) and induce mitohormesis in neural crest-derived cells. J. Neurol. Sci. 2013, 329, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Westberg, J.A.; Serlachius, M.; Lankila, P.; Penkowa, M.; Hidalgo, J.; Andersson, L.C. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke 2007, 38, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Paquet, J.; Deschepper, M.; Moya, A.; Logeart-Avramoglou, D.; Boisson-Vidal, C.; Petite, H. Oxygen Tension Regulates Human Mesenchymal Stem Cell Paracrine Functions. Stem Cells Transl. Med. 2015, 4, 809–821. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Kritis, A.; Andreadis, D.; Papachristou, E.; Leyhausen, G.; Koidis, P.; Geurtsen, W.; Tsiftsoglou, A. Angiogenic Potential and Secretome of Human Apical Papilla Mesenchymal Stem Cells in Various Stress Microenvironments. Stem Cells Dev. 2015, 24, 2496–2512. [Google Scholar] [CrossRef] [PubMed]
- Stein, I.; Neeman, M.; Shweiki, D.; Itin, A.; Keshet, E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol. Cell. Biol. 1995, 15, 5363–5368. [Google Scholar] [CrossRef]
- Shweiki, D.; Neeman, M.; Itin, A.; Keshet, E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: Implications for tumor angiogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 768–772. [Google Scholar] [CrossRef]
- Deschepper, M.; Manassero, M.; Oudina, K.; Paquet, J.; Monfoulet, L.E.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Proangiogenic and prosurvival functions of glucose in human mesenchymal stem cells upon transplantation. Stem Cells 2013, 31, 526–535. [Google Scholar] [CrossRef]
- Choi, S.J.; Shin, I.J.; Je, K.H.; Min, E.K.; Kim, E.J.; Kim, H.S.; Choe, S.; Kim, D.E.; Lee, D.K. Hypoxia Antagonizes Glucose Deprivation on Interleukin 6 Expression in an Akt Dependent, but HIF-1/2α Independent Manner. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef][Green Version]
- Fougeray, S.; Bouvier, N.; Beaune, P.; Legendre, C.; Anglicheau, D.; Thervet, E.; Pallet, N. Metabolic stress promotes renal tubular inflammation by triggering the unfolded protein response. Cell Death Dis. 2011, 2, e143. [Google Scholar] [CrossRef]
- He, L.F.; Wang, T.T.; Gao, Q.Y.; Zhao, G.F.; Huang, Y.H.; Yu, L.K.; Hou, Y.Y. Stanniocalcin-1 promotes tumor angiogenesis through up-regulation of VEGF in gastric cancer cells. J. Biomed. Sci. 2011, 18, 39. [Google Scholar] [CrossRef]
- Law, A.Y.S.; Wong, C.K.C. Stanniocalcin-1 and -2 promote angiogenic sprouting in HUVECs via VEGF/VEGFR2 and angiopoietin signaling pathways. Mol. Cell. Endocrinol. 2013, 374, 73–81. [Google Scholar] [CrossRef]
- Suga, H.; Glotzbach, J.P.; Sorkin, M.; Longaker, M.T.; Gurtner, G.C. Paracrine mechanism of angiogenesis in adipose-derived stem cell transplantation. Ann. Plast. Surg. 2014, 72, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999, 56, 794–814. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Karaman, S.; Leppänen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145. [Google Scholar] [CrossRef]
- Pu, C.M.; Liu, C.W.; Liang, C.J.; Yen, Y.H.; Chen, S.H.; Jiang-Shieh, Y.F.; Chien, C.L.; Chen, Y.C.; Chen, Y.L. Adipose-Derived Stem Cells Protect Skin Flaps against Ischemia/Reperfusion Injury via IL-6 Expression. J. Investig. Dermatol. 2017, 137, 1353–1362. [Google Scholar] [CrossRef]
- Fan, Y.; Ye, J.; Shen, F.; Zhu, Y.; Yeghiazarians, Y.; Zhu, W.; Chen, Y.; Lawton, M.T.; Young, W.L.; Yang, G.Y. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J. Cereb. Blood Flow Metab. 2008, 28, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, L.; Liu, J.; Gong, N.; Chen, L. The effects of cytokines in adipose stem cell-conditioned medium on the migration and proliferation of skin fibroblasts in vitro. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jin, S.Y.; Song, J.S.; Seo, K.K.; Cho, K.H. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann. Dermatol. 2012, 24, 136–143. [Google Scholar] [CrossRef]
- Luckett, L.R.; Gallucci, R.M. Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. Br. J. Dermatol. 2007, 156, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-Q.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; Kemény, L.; Peter, R.U.; Beetz, A.; Ried, C.; Arenberger, P.; Ruzicka, T. Interleukin-8 receptor-mediated chemotaxis of normal human epidermal cells. FEBS Lett. 1992, 305, 241–243. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Reintjes, M.G.; Low, Q.E.H.; Levi, B.; Gamelli, R.L. Modulation of macrophage recruitment into wounds by monocyte chemoattractant protein-1. Wound Repair Regen. 2001, 9, 28–33. [Google Scholar] [CrossRef]
- Mazur, S.; Zołocińska, A.; Siennicka, K.; Janik-Kosacka, K.; Chrapusta, A.; Pojda, Z. Safety of adipose-derived cell (stromal vascular fraction—SVF) augmentation for surgical breast reconstruction in cancer patients. Adv. Clin. Exp. Med. 2018, 27, 1085–1090. [Google Scholar] [CrossRef]
- Waked, K.; Colle, J.; Doornaert, M.; Cocquyt, V.; Blondeel, P. Systematic review: The oncological safety of adipose fat transfer after breast cancer surgery. Breast 2017, 31, 128–136. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachmann, J.; Ehlert, E.; Becker, M.; Otto, C.; Radeloff, K.; Blunk, T.; Bauer-Kreisel, P. Ischemia-Like Stress Conditions Stimulate Trophic Activities of Adipose-Derived Stromal/Stem Cells. Cells 2020, 9, 1935. https://doi.org/10.3390/cells9091935
Bachmann J, Ehlert E, Becker M, Otto C, Radeloff K, Blunk T, Bauer-Kreisel P. Ischemia-Like Stress Conditions Stimulate Trophic Activities of Adipose-Derived Stromal/Stem Cells. Cells. 2020; 9(9):1935. https://doi.org/10.3390/cells9091935
Chicago/Turabian StyleBachmann, Julia, Elias Ehlert, Matthias Becker, Christoph Otto, Katrin Radeloff, Torsten Blunk, and Petra Bauer-Kreisel. 2020. "Ischemia-Like Stress Conditions Stimulate Trophic Activities of Adipose-Derived Stromal/Stem Cells" Cells 9, no. 9: 1935. https://doi.org/10.3390/cells9091935
APA StyleBachmann, J., Ehlert, E., Becker, M., Otto, C., Radeloff, K., Blunk, T., & Bauer-Kreisel, P. (2020). Ischemia-Like Stress Conditions Stimulate Trophic Activities of Adipose-Derived Stromal/Stem Cells. Cells, 9(9), 1935. https://doi.org/10.3390/cells9091935



