Short-Term Rapamycin Preconditioning Diminishes Therapeutic Efficacy of Human Adipose-Derived Stem Cells in a Murine Model of Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
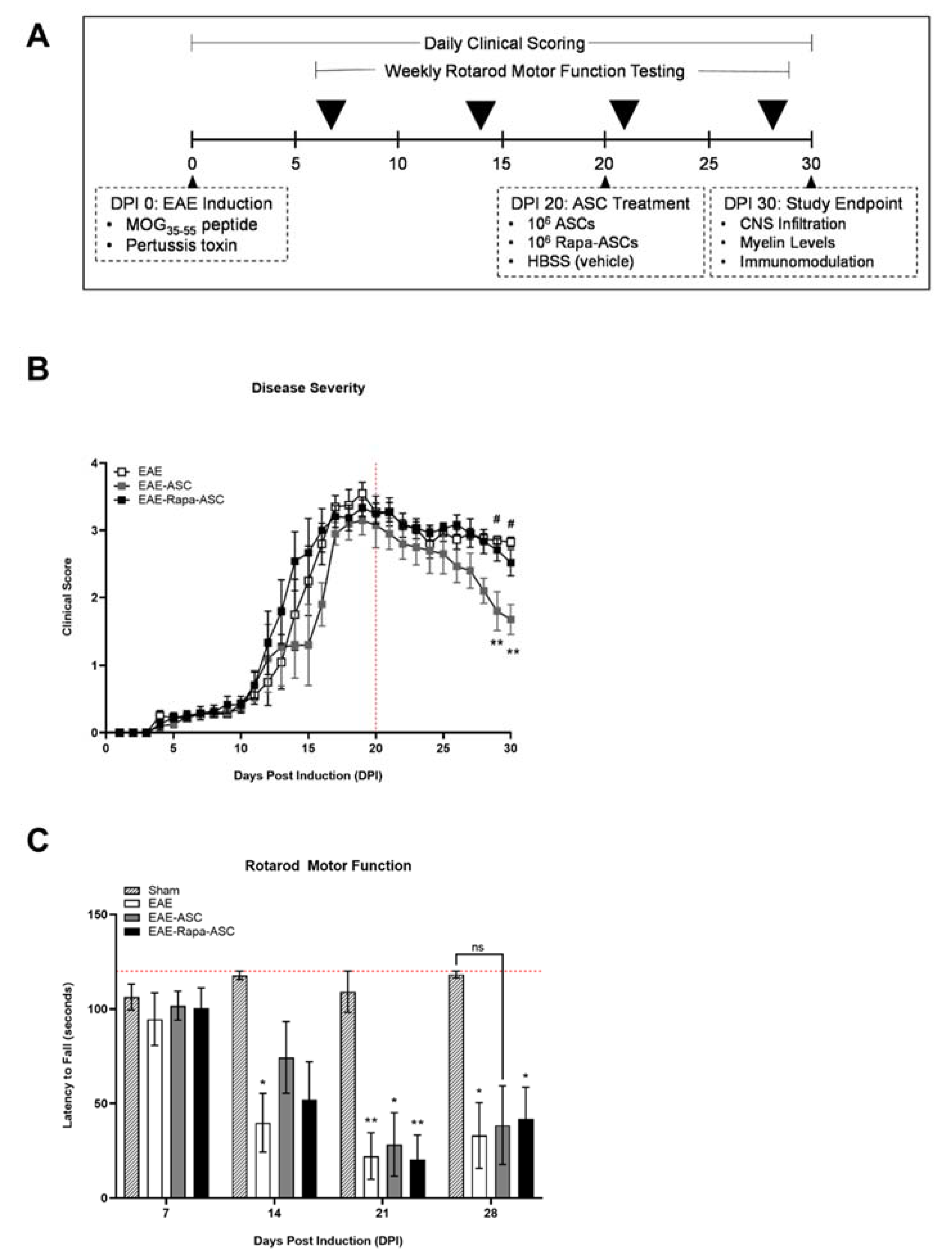
2.1. Induction of EAE with Myelin Oligodendrocyte Glycoprotein (MOG)35–55 Peptide
2.2. Rotarod Analysis
2.3. Cells and Cell Culture
2.4. Preparation and Injection of Cells
2.5. Tissue Harvest and Processing
2.6. Flow Cytometric Staining and Analysis
2.7. RNA Isolation and Quantitative Reverse-Transcription PCR (qRT-PCR)
2.8. Histological Analysis of Spinal Cords
2.9. Statistical Analysis
3. Results
3.1. ASCs, but Not Rapa-ASCs, Modestly Improved Rotarod Performance in EAE Mice
3.2. Reduced Myelin Content of the CNS in ASC-, but Not Rapa-ASC-Treated EAE Mice
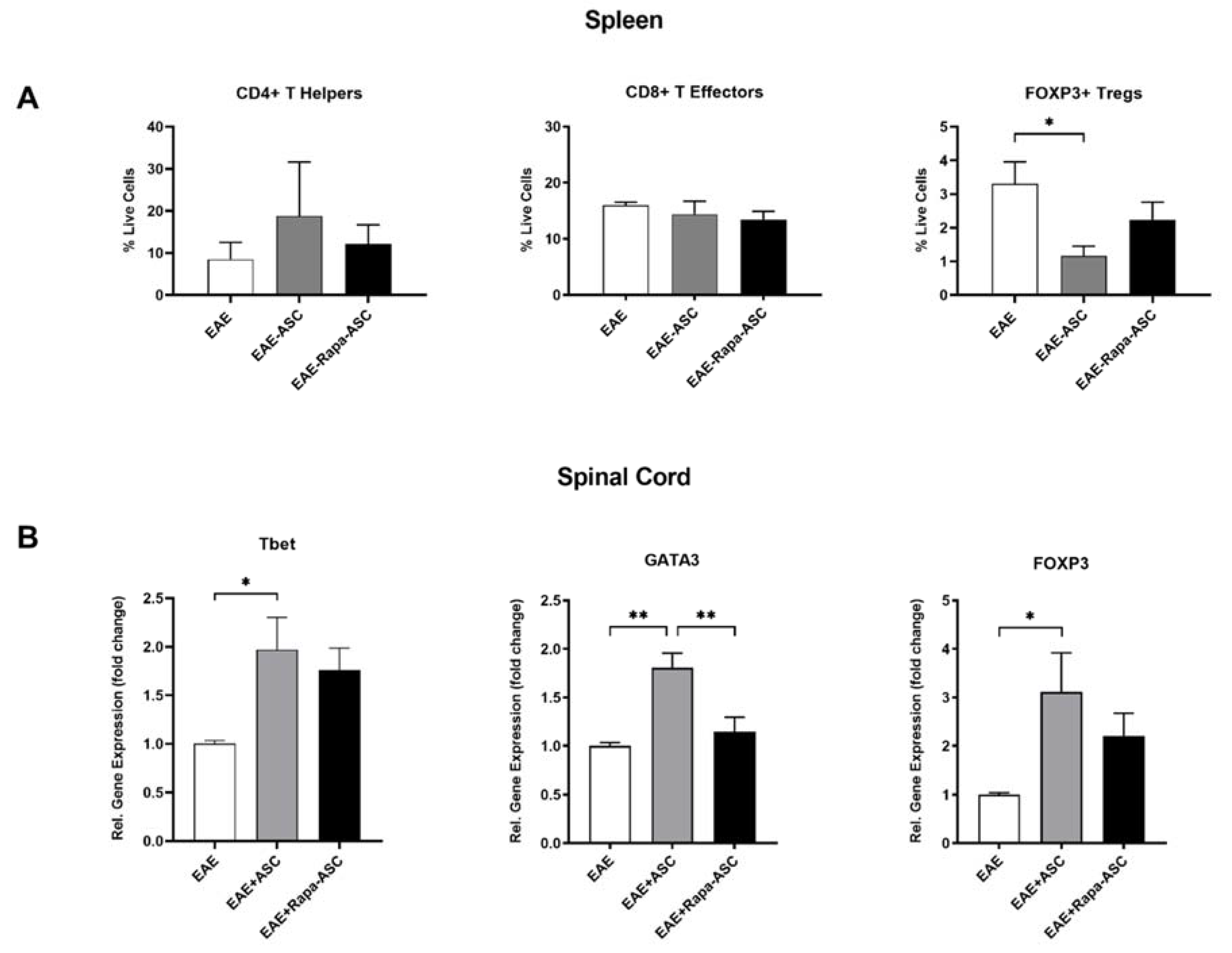
3.3. ASC, but Not Rapa-ASC, Treatment Resulted in Elevated T Cell Marker in the CNS of EAE Mice
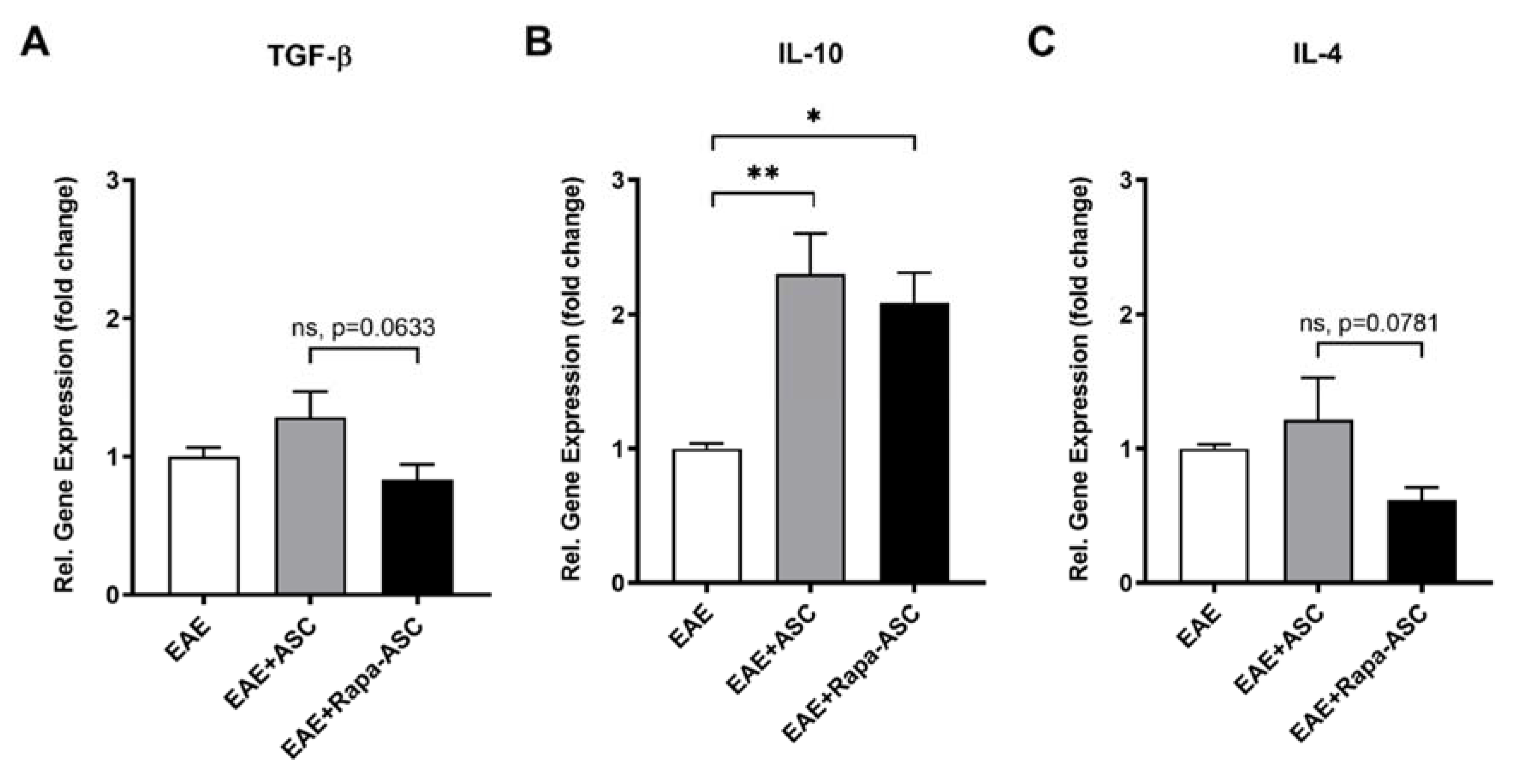
3.4. IL-10 Gene Expression is Significantly Increased with ASC- and Rapa-ASC-Treated EAE Mice
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H.G. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of multiple sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Mckay, K.A.; Kwan, V.; Duggan, T.; Tremlett, H. Risk Factors Associated with the Onset of Relapsing-Remitting and Primary Progressive Multiple Sclerosis: A Systematic Review. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Zhao, Y.; Devonshire, V. Natural history of secondary-progressive multiple sclerosis. Mult. Scler. 2008, 14, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Bross, M.; Hackett, M.; Bernitsas, E. Approved and Emerging Disease Modifying Therapies on Neurodegeneration in Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 4312. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Sabol, R.A.; Bowles, A.C.; Côté, A.; Wise, R.; Pashos, N.; Bunnell, B.A. Therapeutic Potential of Adipose Stem Cells; Springer: New York, NY, USA, 2018; pp. 1–11. [Google Scholar]
- Zheng, G.; Qiu, G.; Ge, M.; He, J.; Huang, L.; Chen, P.; Wang, W.; Xu, Q.; Hu, Y.; Shu, Q.; et al. Human adipose-derived mesenchymal stem cells alleviate obliterative bronchiolitis in a murine model via IDO. Respir. Res. 2017, 18, 119. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Semon, J.A.; Maness, C.; Zhang, X.; Sharkey, S.A.; Beuttler, M.M.; Shah, F.S.; Pandey, A.C.; Gimble, J.M.; Zhang, S.; Scruggs, B.A.; et al. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res. Ther. 2014, 5, 2. [Google Scholar] [CrossRef]
- Bowles, A.C.; Strong, A.L.; Wise, R.M.; Thomas, R.C.; Gerstein, B.Y.; Dutreil, M.F.; Hunter, R.S.; Gimble, J.M.; Bunnell, B.A. Adipose Stromal Vascular Fraction-Mediated Improvements at Late-Stage Disease in a Murine Model of Multiple Sclerosis. Stem Cells 2017, 35, 532–544. [Google Scholar] [CrossRef]
- Bowles, A.C.; Wise, R.M.; Gerstein, B.Y.; Thomas, R.C.; Ogelman, R.; Febbo, I.; Bunnell, B.A. Immunomodulatory Effects of Adipose Stromal Vascular Fraction Cells Promote Alternative Activation Macrophages to Repair Tissue Damage. Stem Cells 2017, 35, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bowles, A.C.; Semon, J.A.; Scruggs, B.A.; Zhang, S.; Strong, A.L.; Gimble, J.M.; Bunnell, B.A. Transplantation of Autologous Adipose Stem Cells Lacks Therapeutic Efficacy in the Experimental Autoimmune Encephalomyelitis Model. PLoS ONE 2014, 9, e85007. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose Tissue-Derived Multipotent Stromal Cells Have a Higher Immunomodulatory Capacity Than Their Bone Marrow-Derived Counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Anderson, P.; Gonzalez-Rey, E.; O’valle, F.; Martin, F.; Oliver, F.J.; Delgado, M. Allogeneic Adipose-Derived Mesenchymal Stromal Cells Ameliorate Experimental Autoimmune Encephalomyelitis by Regulating Self-Reactive T Cell Responses and Dendritic Cell Function. Stem Cells Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Payne, N.L.; Sun, G.; Mcdonald, C.; Layton, D.; Moussa, L.; Emerson-Webber, A.; Veron, N.; Siatskas, C.; Herszfeld, D.; Price, J.; et al. Distinct immunomodulatory and migratory mechanisms underpin the therapeutic potential of human mesenchymal stem cells in autoimmune demyelination. Cell Transplant. 2013, 22, 1409–1425. [Google Scholar] [CrossRef]
- Constantin, G.; Marconi, S.; Rossi, B.; Angiari, S.; Calderan, L.; Anghileri, E.; Gini, B.; Bach, S.D.; Martinello, M.; Bifari, F.; et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 2009, 27, 2624–2635. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Sabbah, N.A.; Saber, T.; Abdel Hamid, R.A. Adipose-derived mesenchymal stem cells modulate the immune response in chronic experimental autoimmune encephalomyelitis model. IUBMB Life 2016, 68, 106–115. [Google Scholar] [CrossRef]
- Strong, A.L.; Bowles, A.C.; Wise, R.M.; Morand, J.P.; Dutreil, M.F.; Gimble, J.M.; Bunnell, B.A. Human Adipose Stromal/Stem Cells from Obese Donors Show Reduced Efficacy in Halting Disease Progression in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Stem Cells 2016, 34, 614–626. [Google Scholar] [CrossRef]
- Scruggs, B.A.; Semon, J.A.; Zhang, X.; Zhang, S.; Bowles, A.C.; Pandey, A.C.; Imhof, K.M.P.; Kalueff, A.V.; Gimble, J.M.; Bunnell, B.A. Age of the Donor Reduces the Ability of Human Adipose-Derived Stem Cells to Alleviate Symptoms in the Experimental Autoimmune Encephalomyelitis Mo. Stem Cells Transl. Med. 2013, 2, 797–807. [Google Scholar] [CrossRef]
- Seo, Y.; Shin, T.H.; Kim, H.S. Current strategies to enhance adipose stem cell function: An update. Int. J. Mol. Sci. 2019, 20, 3827. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Moon, S.-J.; Park, M.-J.; Kim, B.-M.; Kim, E.-K.; Lee, S.-H.; Lee, E.-J.; Chung, B.-H.; Yang, C.-W.; Cho, M.-L. Optimization of adipose tissue-derived mesenchymal stem cells by rapamycin in a murine model of acute graft-versus-host disease. Stem Cell Res. Ther. 2015, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Huang, Z.; Sun, X.; Zhu, X.; Zhou, L.; Li, M.; Cheng, B.; Liu, X.; He, C. Microglia Polarization with M1/M2 Phenotype Changes in rd1 Mouse Model of Retinal Degeneration. Front. Neuroanat. 2017, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.-T.; Chen, J.-B.; Zhao, Y.-L.; Yang, S.-P.; He, L. Resveratrol attenuates senescence of adipose-derived mesenchymal stem cells and restores their paracrine effects on promoting insulin secretion of INS-1 cells through Pim-1. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1203–1213. [Google Scholar]
- Gharibi, B.; Farzadi, S.; Ghuman, M.; Hughes, F.J. Inhibition of Akt/mTOR Attenuates Age-Related Changes in Mesenchymal Stem Cells. Stem Cells 2014, 32, 2256–2266. [Google Scholar] [CrossRef]
- Antonioli, E.; Torres, N.; Ferretti, M.; de Azevedo Piccinato, C.; Sertie, A.L. Individual response to mTOR inhibition in delaying replicative senescence of mesenchymal stromal cells. PLoS ONE 2019, 14, e0204784. [Google Scholar] [CrossRef]
- Li, C.; Ye, L.; Yang, L.; Yu, X.; He, Y.; Chen, Z.; Li, L.; Zhang, D. Rapamycin Promotes the Survival and Adipogenesis of Ischemia-Challenged Adipose Derived Stem Cells by Improving Autophagy. Cell. Physiol. Biochem. 2018, 44, 1762–1774. [Google Scholar] [CrossRef]
- Javorkova, E.; Vackova, J.; Hajkova, M.; Hermankova, B.; Zajicova, A.; Holan, V.; Krulova, M. The effect of clinically relevant doses of immunosuppressive drugs on human mesenchymal stem cells. Biomed. Pharmacother. 2018, 97, 402–411. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Y.; Hu, Y.; Shan, W.; Liu, S.; Xu, Y.; Zhang, H.; Cai, S.; Yu, X.; Cai, Z.; et al. mTOR inhibition improves the immunomodulatory properties of human bone marrow mesenchymal stem cells by inducing COX-2 and PGE2. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Gao, L.; Cen, S.; Wang, P.; Xie, Z.; Liu, Z.; Deng, W.; Su, H.; Wu, X.; Wang, S.; Li, J.; et al. Autophagy Improves the Immunosuppression of CD4+ T Cells by Mesenchymal Stem Cells Through Transforming Growth Factor-β1. Stem Cells Transl. Med. 2016, 5, 1496–1505. [Google Scholar] [CrossRef]
- Brock, T.G.; Mcnish, R.W.; Peters-Golden, M. Arachidonic Acid Is Preferentially Metabolized by Cyclooxygenase-2 to Prostacyclin and Prostaglandin E2. J. Biol. Chem. 1999, 274, 11660–11666. [Google Scholar] [CrossRef] [PubMed]
- Gazdic, M.; Volarevic, V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal Stem Cells: A Friend or Foe in Immune-Mediated Diseases. Stem Cell Rev. Rep. 2015, 11, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, M.; Orlowski, W.; Fortak-Michalska, M.; Jurewicz, A.; Selmaj, K. Immunoregulatory function of bone marrow mesenchymal stem cells in EAE depends on their differentiation state and secretion of PGE2. J. Neuroimmunol. 2011, 233, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, S.; Weigert, A.; Männich, J.; Eberle, M.; Birod, K.; Häussler, A.; Ferreiros, N.; Schreiber, Y.; Kunkel, H.; Grez, M.; et al. PGE2/EP4 signaling in peripheral immune cells promotes development of experimental autoimmune encephalomyelitis. Biochem. Pharmacol. 2014, 87, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Matsushita, T.; Kita, Y.; Uematsu, S.; Akira, S.; Kira, J.I.; Ishii, S.; Shimizu, T. Targeted lipidomics reveals mPGES-1-PGE2 as a therapeutic target for multiple sclerosis. Proc. Natl. Acad. Sci. USA 2009, 106, 21807–21812. [Google Scholar] [CrossRef]
- Esaki, Y.; Li, Y.; Sakata, D.; Yao, C.; Segi-Nishida, E.; Matsuoka, T.; Fukuda, K.; Narumiya, S. Dual roles of PGE2-EP4 signaling in mouse experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2010, 107, 12233–12238. [Google Scholar] [CrossRef]
- Dutra, R.C.; Moreira, E.L.G.; Alberti, T.B.; Marcon, R.; Prediger, R.D.; Calixto, J.B. Spatial reference memory deficits precede motor dysfunction in an experimental autoimmune encephalomyelitis model: The role of kallikrein-kinin system. Brain. Behav. Immun. 2013, 33, 90–101. [Google Scholar] [CrossRef]
- Dutra, R.C.; Leite, D.F.P.; Bento, A.F.; Manjavachi, M.N.; Patrício, E.S.; Figueiredo, C.P.; Pesquero, J.B.; Calixto, J.B. The Role of Kinin Receptors in Preventing Neuroinflammation and Its Clinical Severity during Experimental Autoimmune Encephalomyelitis in Mice. PLoS ONE 2011, 6, e27875. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Diaz, Z.T.; Singer, H.J.; Mert, K.B.; Bunnell, B.A. Increase in Leptin and PPAR-γ Gene Expression in Lipedema Adipocytes Differentiated in vitro from Adipose-Derived Stem Cells. Cells 2020, 9, 430. [Google Scholar] [CrossRef]
- Sabol, R.A.; Villela, V.A.; Denys, A.; Freeman, B.T.; Hartono, A.B.; Wise, R.M.; Harrison, M.A.A.; Sandler, M.B.; Hossain, F.; Miele, L.; et al. Obesity-altered adipose stem cells promote radiation resistance of estrogen receptor positive breast cancer through paracrine signaling. Int. J. Mol. Sci. 2020, 21, 2722. [Google Scholar] [CrossRef]
- Strong, A.L.; Hunter, R.S.; Jones, R.B.; Bowles, A.C.; Dutreil, M.F.; Gaupp, D.; Hayes, D.J.; Gimble, J.M.; Levi, B.; McNulty, M.A.; et al. Obesity inhibits the osteogenic differentiation of human adipose-derived stem cells. J. Transl. Med. 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Jafarinia, M.; Alsahebfosoul, F.; Salehi, H.; Eskandari, N.; Azimzadeh, M.; Mahmoodi, M.; Asgary, S.; Ganjalikhani Hakemi, M. Therapeutic effects of extracellular vesicles from human adipose-derived mesenchymal stem cells on chronic experimental autoimmune encephalomyelitis. J. Cell. Physiol. 2020, 235, 8779–8790. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Chen, Z.; Huang, Y.; Yang, D.; Su, Z.; Weng, Y.; Li, X.; Zhang, X. Therapeutic effects of human adipose tissue-derived stem cell (hADSC) transplantation on experimental autoimmune encephalomyelitis (EAE) mice. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kanhere, A.; Hertweck, A.; Bhatia, U.; Gökmen, M.R.; Perucha, E.; Jackson, I.; Lord, G.M.; Jenner, R.G. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat. Commun. 2012, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. J. Immunol. 2017, 198, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Immunity Review Transforming Growth Factor-b Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Serejo, T.R.T.; Silva-Carvalho, A.É.; Braga, L.D.d.C.F.; Neves, F.d.A.R.; Pereira, R.W.; Carvalho, J.L.d.; Saldanha-Araujo, F. Assessment of the Immunosuppressive Potential of INF-γ Licensed Adipose Mesenchymal Stem Cells, Their Secretome and Extracellular Vesicles. Cells 2019, 8, 22. [Google Scholar] [CrossRef]
- Fontanilla, C.V.; Gu, H.; Liu, Q.; Zhu, T.Z.; Zhou, C.; Johnstone, B.H.; March, K.L.; Pascuzzi, R.M.; Farlow, M.R.; Du, Y. Adipose-derived Stem Cell Conditioned Media Extends Survival time of a mouse model of Amyotrophic Lateral Sclerosis. Sci. Rep. 2015, 5, 16953. [Google Scholar] [CrossRef]
- Patrikoski, M.; Mannerström, B.; Miettinen, S. Perspectives for Clinical Translation of Adipose Stromal/Stem Cells. Stem Cells Int. 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Wang, G.; Yu, G.; Wang, D.; Guo, S.; Shan, F. Comparison of the purity and vitality of natural killer cells with different isolation kits. Exp. Ther. Med. 2017, 13, 1875–1883. [Google Scholar] [CrossRef]
- Bowles, A.C.; Wise, R.M.; Gerstein, B.Y.; Thomas, R.C.; Ogelman, R.; Manayan, R.C.; Bunnell, B.A. Adipose stromal vascular fraction attenuates TH1 cell-mediated pathology in a model of multiple sclerosis. J. Neuroinflamm. 2018, 15, 77. [Google Scholar] [CrossRef]
- Targoni, O.S.; Baus, J.; Hofstetter, H.H.; Hesse, M.D.; Karulin, A.Y.; Boehm, B.O.; Forsthuber, T.G.; Lehmann, P.V. Frequencies of Neuroantigen-Specific T Cells in the Central Nervous System Versus the Immune Periphery During the Course of Experimental Allergic Encephalomyelitis. J. Immunol. 2001, 166, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Kuerten, S.; Lehmann, P.V. The immune pathogenesis of experimental autoimmune encephalomyelitis: Lessons learned for multiple sclerosis? J. Interf. Cytokine Res. 2011, 31, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.-L.; Simon, A.K.; Prescott, M.; Menendez, J.A.; Liu, F.; Wang, F.; Wang, C.; Wolvetang, E.; Vazquez-Martin, A.; Zhang, J. Autophagy in stem cells. Autophagy 2013, 9, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Fehervari, Z.; Yamaguchi, T.; Sakaguchi, S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA 2008, 105, 10113–10118. [Google Scholar] [CrossRef]
- Koutrolos, M.; Berer, K.; Kawakami, N.; Wekerle, H.; Krishnamoorthy, G. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol. Commun. 2014, 2, 163. [Google Scholar] [CrossRef]
- Zhang, X.; Koldzic, D.N.; Izikson, L.; Reddy, J.; Nazareno, R.F.; Sakaguchi, S.; Kuchroo, V.K.; Weiner, H.L. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25 + CD4 + regulatory T cells. Int. Immunol. 2004, 16, 249–256. [Google Scholar] [CrossRef]
- Saravia, J.; Chapman, N.M.; Chi, H. Helper T cell differentiation. Cell. Mol. Immunol. 2019, 16, 634–643. [Google Scholar] [CrossRef]
- Restorick, S.M.; Durant, L.; Kalra, S.; Hassan-Smith, G.; Rathbone, E.; Douglas, M.R.; Curnow, S.J. CCR6+ Th cells in the cerebrospinal fluid of persons with multiple sclerosis are dominated by pathogenic non-classic Th1 cells and GM-CSF-only-secreting Th cells. Brain. Behav. Immun. 2017, 64, 71–79. [Google Scholar] [CrossRef]
- Califano, D.; Sweeney, K.J.; Le, H.; VanValkenburgh, J.; Yager, E.; O’Connor, W.; Kennedy, J.S.; Jones, D.M.; Avram, D. Diverting T helper cell trafficking through increased plasticity attenuatesautoimmune encephalomyelitis. J. Clin. Investig. 2014, 124, 174–187. [Google Scholar] [CrossRef]
- Ria, F.; Penna, G.; Adorini, L. Th1 cells induce and Th2 inhibit antigen-dependent IL-12 secretion by dendritic cells. Eur. J. Immunol. 1998, 28, 2003–2016. [Google Scholar] [CrossRef]
- Lafaille, J.J.; Van De Keere, F.; Hsu, A.L.; Baron, J.L.; Haas, W.; Raine, C.S.; Tonegawa, S. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 1997, 186, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, B.W.; Lai, M.; Hodgkinson, S.; Barkhof, F.; Miller, D.H.; Moseley, I.F.; Thompson, A.J.; Rudge, P.; McDougall, A.; McLeod, J.G.; et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: Results of a randomized, double-blind, placebo-controlled, MR- monitored phase II trial. Neurology 1997, 49, 351–357. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 1998, 8, 275–283. [Google Scholar] [CrossRef]
- Paludan, S.R. Interleukin-4 and interferon-γ: The quintessence of a mutual antagonistic relationship. Scand. J. Immunol. 1998, 48, 459–468. [Google Scholar] [CrossRef]
- Prajeeth, C.K.; Löhr, K.; Floess, S.; Zimmermann, J.; Ulrich, R.; Gudi, V.; Beineke, A.; Baumgärtner, W.; Müller, M.; Huehn, J.; et al. Effector molecules released by Th1 but not Th17 cells drive an M1 response in microglia. Brain. Behav. Immun. 2014, 37, 248–259. [Google Scholar] [CrossRef]
- Katakura, T.; Miyazaki, M.; Kobayashi, M.; Herndon, D.N.; Suzuki, F. CCL17 and IL-10 as Effectors That Enable Alternatively Activated Macrophages to Inhibit the Generation of Classically Activated Macrophages. J. Immunol. 2004, 172, 1407–1413. [Google Scholar] [CrossRef]
- Town, T.; Nikolic, V.; Tan, J. The microglial “activation” continuum: From innate to adaptive responses. J. Neuroinflamm. 2005, 2, 24. [Google Scholar] [CrossRef]
- Bogie, J.F.J.; Stinissen, P.; Hendriks, J.J.A. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. 2014, 128, 191–213. [Google Scholar] [CrossRef]
- Luo, C.; Jian, C.; Liao, Y.; Huang, Q.; Wu, Y.; Liu, X.; Zou, D.; Wu, Y. The role of microglia in multiple sclerosis. Neuropsychiatr. Dis. Treat. 2017, 13, 1661–1667. [Google Scholar] [CrossRef]
- Vogel, D.Y.S.; Vereyken, E.J.F.; Glim, J.E.; Heijnen, P.D.A.M.; Moeton, M.; van der Valk, P.; Amor, S.; Teunissen, C.E.; van Horssen, J.; Dijkstra, C.D. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflamm. 2013, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Wang, J.; Yang, B.; Weng, Q.; He, Q. Targeting Microglia and Macrophages: A Potential Treatment Strategy for Multiple Sclerosis. Front. Pharmacol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.E.; Boyd, A.; Zhao, J.W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; Van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Beta-actin | GTGGGCCGCCCTAGGCACCA | TTAGCACGCACTGTAGTTTCTC |
| TGF-β | CGTCAGACATTCGGGAAGCA | TGCCGTACAACTCCAGTGAC |
| IL-10 | GCTCTTGCACTACCAAAGCC | CTGCTGATCCTCATGCCAGT |
| IL-4 | GGTCTCAACCCCCAGCTAGT | GCCGATGATCTCTCTCAAGTGAT |
| Tbet | CACTAAGCAAGGACGGCGAA | TAATGGCTTGTGGGCTCCAG |
| GATA3 | TGTCTGCGAACACTGAGCTG | CGATCACCTGAGTAGCAAGGA |
| FOXP3 | CCCATCCCCAGGAGTCTTG | ACCATGACTAGGGGCACTGTA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wise, R.M.; Harrison, M.A.A.; Sullivan, B.N.; Al-Ghadban, S.; Aleman, S.J.; Vinluan, A.T.; Monaco, E.R.; Donato, U.M.; Pursell, I.A.; Bunnell, B.A. Short-Term Rapamycin Preconditioning Diminishes Therapeutic Efficacy of Human Adipose-Derived Stem Cells in a Murine Model of Multiple Sclerosis. Cells 2020, 9, 2218. https://doi.org/10.3390/cells9102218
Wise RM, Harrison MAA, Sullivan BN, Al-Ghadban S, Aleman SJ, Vinluan AT, Monaco ER, Donato UM, Pursell IA, Bunnell BA. Short-Term Rapamycin Preconditioning Diminishes Therapeutic Efficacy of Human Adipose-Derived Stem Cells in a Murine Model of Multiple Sclerosis. Cells. 2020; 9(10):2218. https://doi.org/10.3390/cells9102218
Chicago/Turabian StyleWise, Rachel M., Mark A. A. Harrison, Brianne N. Sullivan, Sara Al-Ghadban, Sarah J. Aleman, Amber T. Vinluan, Emily R. Monaco, Umberto M. Donato, India A. Pursell, and Bruce A. Bunnell. 2020. "Short-Term Rapamycin Preconditioning Diminishes Therapeutic Efficacy of Human Adipose-Derived Stem Cells in a Murine Model of Multiple Sclerosis" Cells 9, no. 10: 2218. https://doi.org/10.3390/cells9102218
APA StyleWise, R. M., Harrison, M. A. A., Sullivan, B. N., Al-Ghadban, S., Aleman, S. J., Vinluan, A. T., Monaco, E. R., Donato, U. M., Pursell, I. A., & Bunnell, B. A. (2020). Short-Term Rapamycin Preconditioning Diminishes Therapeutic Efficacy of Human Adipose-Derived Stem Cells in a Murine Model of Multiple Sclerosis. Cells, 9(10), 2218. https://doi.org/10.3390/cells9102218






