1. Introduction
The neuronal ceroid lipofuscinoses (NCLs) constitute a family of fatal pediatric neurodegenerative disorders that primarily affect the central nervous system (CNS) [
1]. NCLs are atypical lysosomal storage disorders that manifest accumulation of lipopigments in the lysosomes of neurons and other cell types [
2]. CLN3 disease arises due to mutations in the CLN3 gene and is the most common variant of the NCL group [
3]. This neurological disease manifests at four to eight years of age with progressive visual deterioration, seizures, blindness, motor and cognitive decline, mental retardation, epilepsy and early death during the second or third decade of life [
4]. Massive cortical neuronal cell loss due to neuronal apoptosis within the cortex [
5], and neuronal loss in hippocampus and microglial activation in this region are documented [
6]. Eighty five per cent of patients with CLN3 disease harbor a 1.02 kb deletion eliminating exons 7/8 and creating a truncated CLN3 protein [
7,
8].
CLN3 protein influences major cellular functions, including apoptosis and cell growth 9. Apoptosis is the mechanism of neuronal and photoreceptor cell loss in human brain from patients with CLN3 disease 3 [
9]. Ceramide, a pro-apoptotic lipid second messenger, mediates anti-proliferative events of apoptosis, growth inhibition, cell differentiation, and senescence [
10].
Ceramide levels are increased in CLN3-deficient cells and in brain of CLN3 patients [
11]. Studies confirm that CLN3 protein expression modulates brain ceramide levels. Levels of lipids ceramide, SM, GalCer, GluCer, and globoside are elevated in human CLN3-deficient fibroblasts. Ceramide levels normalized following restoration of CLN3 function, but not following caspase inhibition by zVAD, a pan-inhibitor of caspases [
8,
11,
12]. Overexpressing CLN3 protein results in a drop in ceramide levels [
13]. Increased ceramide levels and neuronal cell loss are evident in brain sections from post-mortem CLN3 disease patients and in brains and sera of
Cln3Δex7/8 mice [
14]. Treatment regimens for CLN3 disease are largely supportive, not curative, and do not target the underlying causes of the disease.
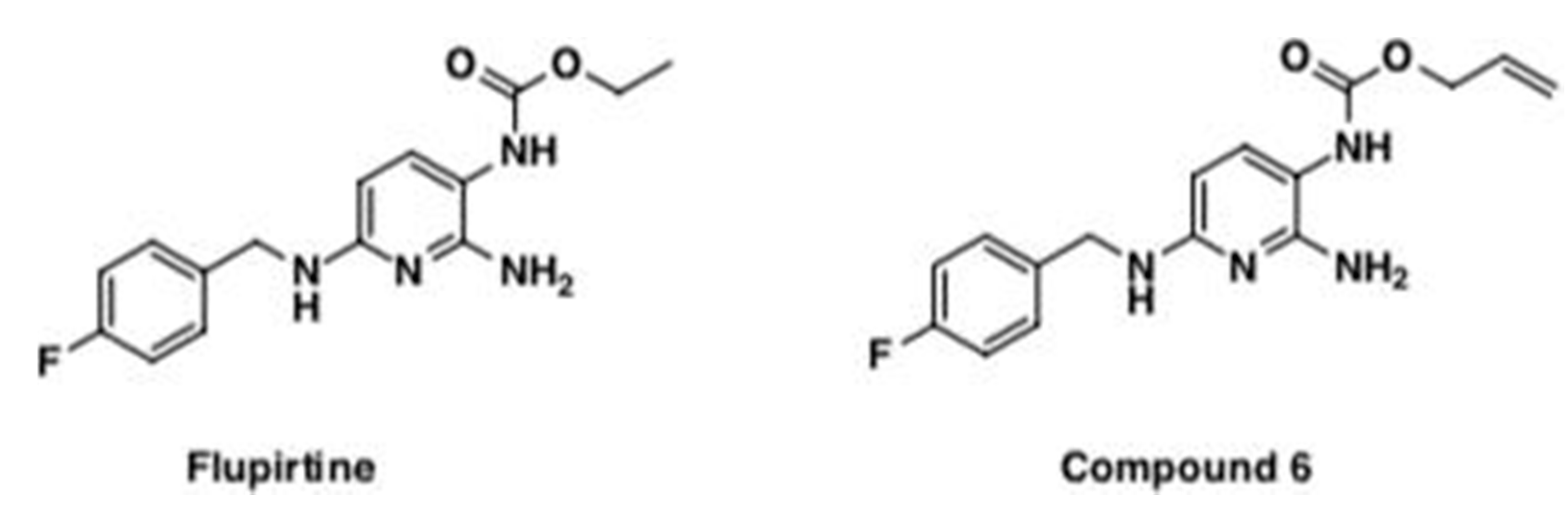
Flupirtine is a centrally acting non-opioid drug previously widely used in clinics as an analgesic [
15,
16]. Flupirtine maleate is the salt of this drug, henceforth, referred to as just flupirtine. It is neuroprotective, has muscle relaxant and anticonvulsant properties [
17] and suppresses neuronal hyper-excitability [
18]. Flupirtine protects photoreceptor and neuronal cells from apoptosis induced by various insults [
13]. There is evidence suggesting that flupirtine reduces brain injury, induces remodeling of brain tissue, and diminishes cognitive impairment in in vivo animal models of ischemic stroke [
15]. Flupirtine protects lymphoblasts, differentiated human post-mitotic hNT neurons and PC12 neuronal precursor cells from apoptosis induced by etoposide [
13,
19]. A newly synthesized allyl carbamate derivative of flupirtine (compound 6) has shown potential neuroprotective effects in vitro, as one of nine flupirtine aromatic carbamate derivative [
19,
20,
21]. Compound 6 imparted a 150% increase in Bcl-2/Bax ratio in vitro which is protective [
19]. Flupirtine and its allyl carbamate derivative (compound 6) increased cell viability in human CLN3 patient-derived lymphoblasts and in neuronal precursor PC12 cells transfected with siRNA directed against CLN3, exhibiting significant anti-apoptotic and neuroprotective effects [
19].
This study tests, in vivo, oral supplementation of flupirtine for a period of 14 weeks in Cln3Δex7/8 knock-in mice. Outcomes of efficacy include improved behavioral measures, altered gene expression profiles, decreased glial immunoreactivity, and increased neuronal cell numbers in specific brain regions. Supplementation of compound 6 assessed effectiveness in several parameters, as a first step in also developing it as potential treatment for CLN3 disease.
2. Materials and Methods
2.1. Animals
This mouse work was conducted in accordance with an approved American University of Beirut (AUB) Institutional Animal Care and Use Committee (IACUC) protocol (IACUC approval #18-08-496). Animal testing was carried out at the AUB Animal Care Facility where animals were housed. C57BL/6J (JAX stock number: 000664) and homozygous Cln3Δex7/8 (JAX stock number: 029471) mice were obtained from the Jackson laboratory, kept in a 12-h light/dark cycle (lights onset at 6:00 am) and supplied with access to food and water ad libitum. Room temperature was maintained between 18–26 °C, and relative humidity between 30–70%. Mice were housed in groups of 3–4/cage. All efforts to minimize number of animals and animal suffering were applied. Mice were monitored for weekly weights, basic behavior and general health throughout the study, and were bred in-house.
2.2. Flupirtine and Compound 6 Treatment
Flupirtine and compound 6 were dissolved in vehicle (0.5% di-methyl sulphoxide (DMSO) in 10% phosphate-buffered saline (PBS)), at a dose of 30 mg/kg body weight for a period of 14 weeks starting at 4 weeks of age. Vehicle treatment consisted of 0.5% DMSO in 10% PBS. Mice were treated ‘per os’ by drinking water with a consistent supply in a volume of ≈8 mL/day/mouse. Both drugs were synthesized by Dr. Paul Trippier at the department of pharmaceutical sciences in the School of Pharmacy at Texas Tech University Health Sciences Center (
Figure 1). Mice were divided into five groups, consisting of 16 mice each (eight males and females) and consisted of C57BL/6J vehicle-treated WT mice, C57BL/6J compound 6-treated WT mice, vehicle-treated
Cln3Δex7/8 mice, flupirtine-treated
Cln3Δex7/8 mice and compound 6-treated
Cln3Δex7/8 mice. Genotype was confirmed by PCR of DNA mouse blood.
2.3. Behavioral Studies
Mice were held in their cages in the behavioral room for testing, with lights dimmed for 60 min prior to onset of tests, for habituation. All behavioral assays were performed during the light cycle. Each test was performed at n, the same time of the day and within the same hour, when possible, to minimize variability between cohorts. Comparison between groups was carried out on males and females separately.
Open field: A mouse was placed on the periphery of a transparent Plexiglas cubic box (dimensions: 50 cm width, 50 cm length and 30 cm in height, with the center 16.4 cm2) so that locomotion would be apparent to the operator and exploratory behavior videotaped. Specific parameters were recorded by a top-mounted video-recorder using EthoVision software (Noldus Information Technology, Wageningen, The Netherlands) for each animal including total distance traveled, average speed, mobility duration, rearing and walling frequencies. Each mouse was allowed to move in the arena freely for 5 min. The box was wiped with 50% ethanol between each mouse to avoid olfactory cuing.
Pole climbing: Mice were habituated to the task in five trials/day for 1 day. On the next test day, five trial measures/mouse were performed by placing the mouse head upward on top of a rough- surfaced pole (1 cm in diameter and 60 cm in height) wrapped with tape to prevent slipping. The time for the mouse to completely turn its head down (tturn), time to reach the middle of the pole (t1/2), time it takes to descend and settle on the floor (ttotal), and time the mouse spent freezing during descent (tstop) were recorded. Each mouse had a maximum time of two minutes to climb down to avoid exhaustion.
Morris water maze (MWZ): The spatial learning abilities of mice were assessed in a MWM task. The apparatus consisted of a circular pool 100 cm diameter, filled up to 50 cm with water made opaque by addition of a small amount of non-toxic white paint and maintained at 21–22 °C. The pool is virtually subdivided into four quadrants using the software. A circular escape platform was placed in a fixed south-west (SW) location hidden 0.5 cm below the surface of the water, and five fixed-position geometric visual cues were kept in the brightly lit room throughout the period of testing. A digital camera was positioned above the center of the tank and linked to a tracking system (ANY-Maze behavioral tracking software, version 6.3, USA) in order to record escape latencies, path distance (m), percentage of thigmotaxis path and swim speed for each trial, together with time spent swimming in each of the four quadrants of the maze.
Mice (eight males and females from each treatment group) were given four consecutive days of acquisition training sessions that consisted of four trials per day. Throughout the course of this acquisition period the position of the hidden platform remained fixed (SW) and the entry point was varied from trial to trial, but the sequence remained fixed for all mice within that day. We used four different entry points (north, south, east, and west). The sequence of starting points was modified from one day to the other. Mice were given 60 s to find the platform, and if the mouse failed to locate the platform within this period, it was guided onto it. All mice were allowed to rest on the platform for a 30-s interval after each trial. At the end of the training block, mice were put in a drying cage and allowed to dry prior to being returned to their experimental cages. The last day of the test, the ‘probe trial’ was performed with the platform removed from the maze and the rodent released from the North entry point of the pool to find the previous location of the hidden platform.
2.4. Corticosterone Immunoassay Kit
Blood was collected from the inferior vena cava, left to clot, centrifuged and spun for 15 min at 10,000 rpm at 4 °C. Serum corticosterone levels were determined using DetectX® Corticosterone Enzyme Immunoassay Kit (Cat. No K014-H5, Arbor Assays, Ann Arbor, MI, USA), according to manufacturer’s instructions. Five μL of standards or mouse serum samples were assayed in duplicate and run altogether on one single plate simultaneously. The absorbance was measured using TriStar2S microplate reader (Berthold Technologies, Bad Wildbad, Germany) at 450 nm.
2.5. RNA Extraction from Brain Tissue
Mice were deeply anesthetized with a mixture of xylazine and ketamine (10 mg/kg and 100 mg/kg, respectively) and brains were rapidly dissected and “snap” frozen in liquid nitrogen to preserve RNA integrity, and stored at −80 °C. A total of 30 mg ground fresh brain tissue (from four males and females in each treatment group) were homogenized using a motorized rotor-stator homogenizer and RNA extracted using RNeasyPlus Mini Kits (Cat No. 74134, Qiagen, Germantown, MD, USA) according to manufacturer’s instructions. For assessing RNA quality, A260/A280 and A260/A230 ratios for RNA are analyzed with the ExperionTM Automated Electrophoresis System (BioRad, Hercules, CA, USA). RNA concentrations are determined by absorption at 260 nm wavelength with a ND-1000 spectrometer (Nanodrop Technologies LLC, Wilmington, DE, USA).
2.6. Quantitative Real-Time PCR (qRT-PCR)
Total RNA extracted from fresh brain tissues was reverse transcribed using RevertAid Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA, USA) with 2 µg of input RNA and random primers (Thermo Fisher Scientific, USA). qRT-PCR reactions were performed in 384-well plates using specific primers (Tm = 60 °C) (TIB MOLBIOL, Berlin, Germany) (
Table 1) and iTaq SYBR Green Supermix (BioRad, Hercules, CA, USA) as a fluorescent detection dye, in CFX384TM Real-Time PCR (BioRad, USA), in a final volume of 10 µL. To characterize generated amplicons and to control contamination by unspecific by-products, melt curve analysis was applied. Results were normalized to β-actin or Gapdh mRNA level. All reactions were performed in duplicate, and results were calculated using the ∆∆Ct method.
2.7. Immunohistochemistry
For morphological and immunohistochemical sectioning, 4 mice/treatment group were deeply anesthetized with a mixture of xylazine and ketamine (10 mg/kg and 100 mg/kg, respectively) and fixed by cardiac puncture with 30 mL of 4% paraformaldehyde (PFA) in PBS. Brains were carefully isolated and fixed with 4% PFA solution (pH of 7.4) for 2 h at 4 °C, then cryoprotected in a solution of 20% sucrose overnight. The following day, brains were processed and frozen using embedding medium, Optimal Cutting Temperature (OCT) compound, for later tangential sectioning on glass slides. Brains were cut in coronal sections (20 μm) using a cryostat and sections stored at −20 °C for further analysis. Brain coronal cryosections were treated with PBS for 5 min twice, then incubated with PBST (0.1% Triton X-100 in PBS) for 10 min twice. Sections were incubated with blocking solution (PBST 0.1%-FBS 10%) for 1 h at room temperature (RT). They were then incubated with each of the primary antibodies: anti-GFAP (1:500, Abcam, Cambridge, UK, catalogue #ab7260) and anti-NeuN antibody (1/300, Abcam, Cambridge, UK, catalogue #ab104225) in antibody solution (PBST 0.1%-FBS 1%) overnight at 4 °C. After washing in PBST, slides were treated with Sudan black for 40 min, then washed with PBS three times. Brain cryosections were then incubated with biotinylated secondary antibody diluted in antibody solution at RT for 1 h. Samples were counterstained with 1:10,000 Hoechst (Sigma, St. Louis, MO, USA) and then mounted in Fluoromount (Sigma, St. Louis, MO, USA).
Signal quantification was assessed using Leica microscope software imaging. For microscopic imaging, three sections/mouse were selected. Primary motor cortex was viewed at 40× magnification with three to four photos/section for motor cortex layers (I–VI). Hippocampus images for GFAP were obtained at 40× magnification. Intensity quantification was depicted by the ratio of integrated density over total area of image using Image J software, version 1.52a. NeuN positive cells were quantified manually using Image J software. Number of NeuN positive cells divided by total area of image.
2.8. Statistical Analysis
Basic statistical analysis was conducted using GraphPad Prism 6 statistical package (GraphPad Software version 6.04, San Diego, CA, USA). Data was expressed as mean ± standard error of the mean (SEM). For two group comparisons, Student’s t-test was used with quantitative continuous variables. Comparisons between different groups were statistically tested with either one-way analysis of variants (ANOVA) or two-factorial ANOVA followed by Tukey’s post-hoc test for multiple group comparisons. All tests are two sided and a p-value < 0.05 is considered as statistically significant.
4. Discussion
This study addresses novel small molecule treatment strategies for CLN3 disease in a Cln3Δex7/8 knock-in mouse model. Hopefully, this will translate into future knowledge to improve the lives of CLN3 patients.
Flupirtine is well known for its significant powerful anti-oxidative, anti-apoptotic, and neuroprotective effects in vitro and in vivo. The effectiveness of the chosen daily dose of flupirtine (30 mg/kg per os) has been demonstrated in testing for anti-nociceptive, anticonvulsant, and anti-apoptotic activity in rodents [
23,
24,
25,
26,
27]. This study is the first to evaluate the use of flupirtine as potential treatment for CLN3 disease in an animal model, i.e., in vivo. Previous studies proved that flupirtine reaches brain regions, including hippocampus and cortex, and that it rapidly crosses the blood brain barrier and enters other tissues. The liver is the primary organ responsible for its metabolism [
15].
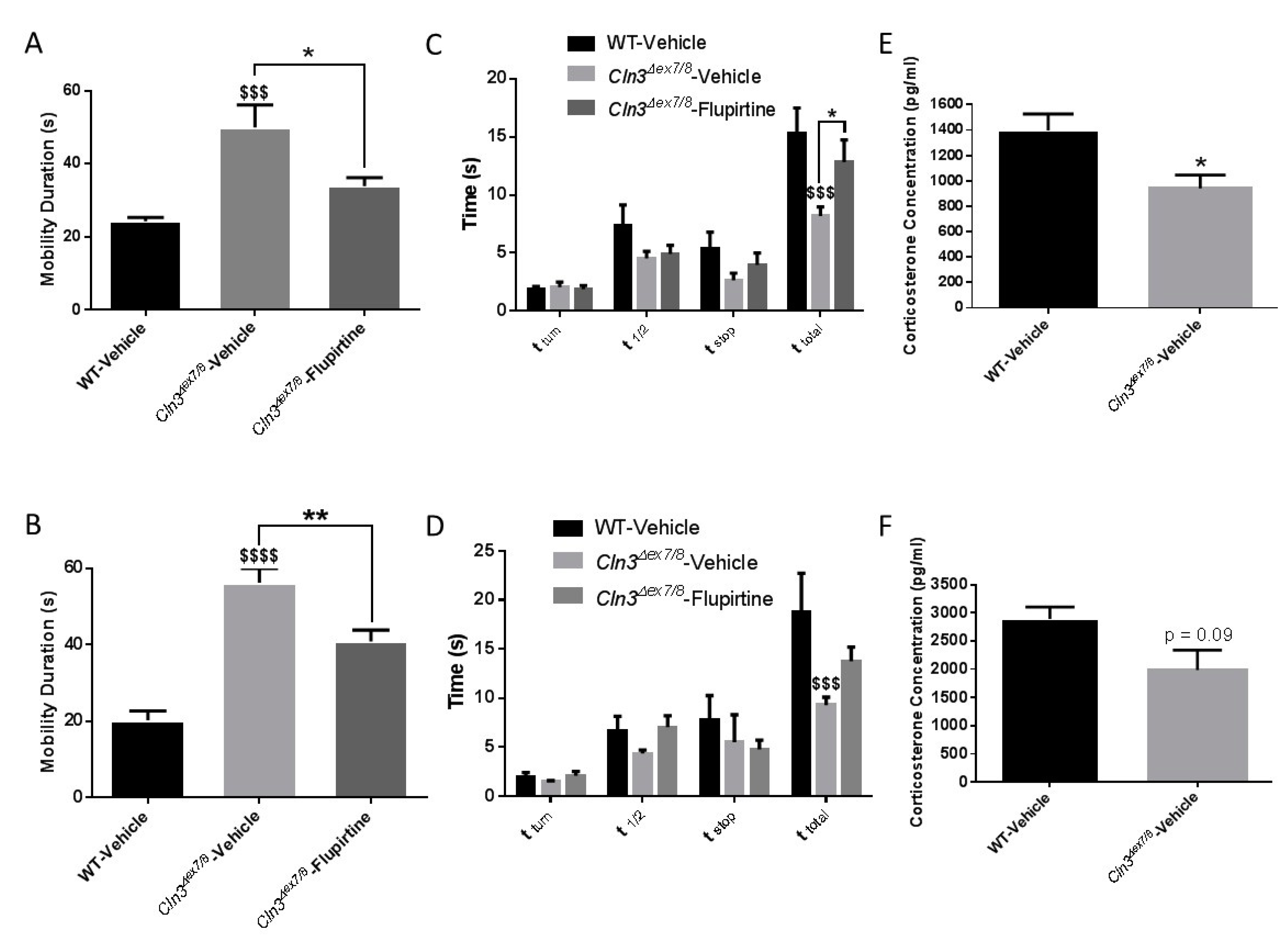
Behavioral tests assessed different aspects of
Cln3Δex7/8 mouse motor strength, coordination, balance, as well as learning and spatial memory functions before and after flupirtine treatment. Male and female vehicle-treated
Cln3Δex7/8 mice exhibit significantly increased mobility with respect to WT controls in the open field test. The hyperactive phenotype prominent in vehicle-treated
Cln3Δex7/8 mice is described for the first time here in mice at of 18 weeks of age. This was not previously documented in
Cln3Δex7/8 mice at 40 weeks of age [
28]. This suggests that, at a young age,
Cln3Δex7/8 mice express a distinct behavioral phenotype prior to onset of more severe CLN3 disease symptoms, including motor decline.
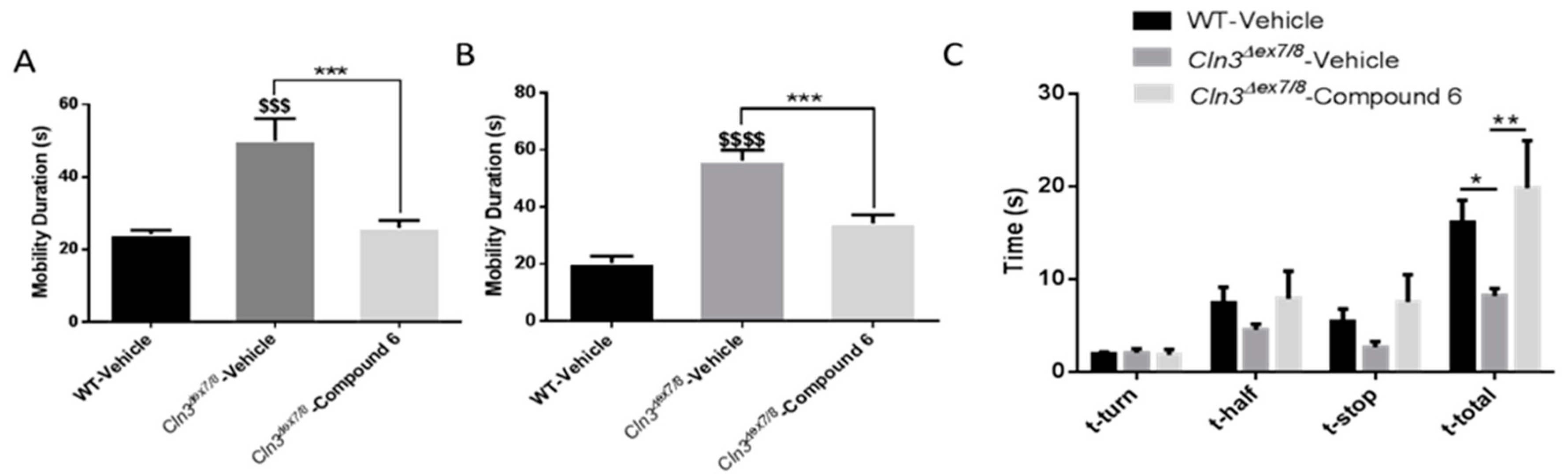
Cln3Δex7/8 mice also showed increased random and chaotic exploratory activity compared to WT controls in a novel environment. This indicates inattentiveness and diminished executive function. Treatment with flupirtine significantly attenuated this abnormal mobility in male and female
Cln3Δex7/8 mice. This phenotype may be consistent with the notion that diminished executive function of vehicle-treated
Cln3Δex7/8 mice is driving their inattentive hyperactivity. The vertical pole test evaluates spatial and motor orientation and balance of mice [
29]. Reduced latency of vehicle-treated
Cln3Δex7/8 mice to descend the pole is explained by their quick and less controlled behavior compared to WT animals, consistent with open field behavioral assessments of excessive mobility. The aforementioned behavioral test reveals prominent hyperactivity and attentional deficits in vehicle-treated
Cln3Δex7/8 mice, while treatment with flupirtine lessened this impulsive phenotype. These results are in line with other mouse models of neurodevelopmental psychiatric disorder of attention-deficit hyperactivity disorder (ADHD). Mice recapitulating this disease are characterized by impulsivity, inattentiveness, and hyperactivity [
30]. Stress has a direct profound effect on rodent behavior and physical activity [
31]. Chronic stress leads to increased corticosterone levels [
31], as observed in WT male and female mice with respect to
Cln3Δex7/8 mice. Several studies elucidate that animals who do not experience stress show higher exploration, locomotion, and physical activity in an open field test, as a reaction to an unknown environment [
32,
33]. Therefore, the markedly decreased corticosterone levels in serum of
Cln3Δex7/8 mice with respect to WT animals explains their hyperactivity resulting from reduced stress levels in male and female
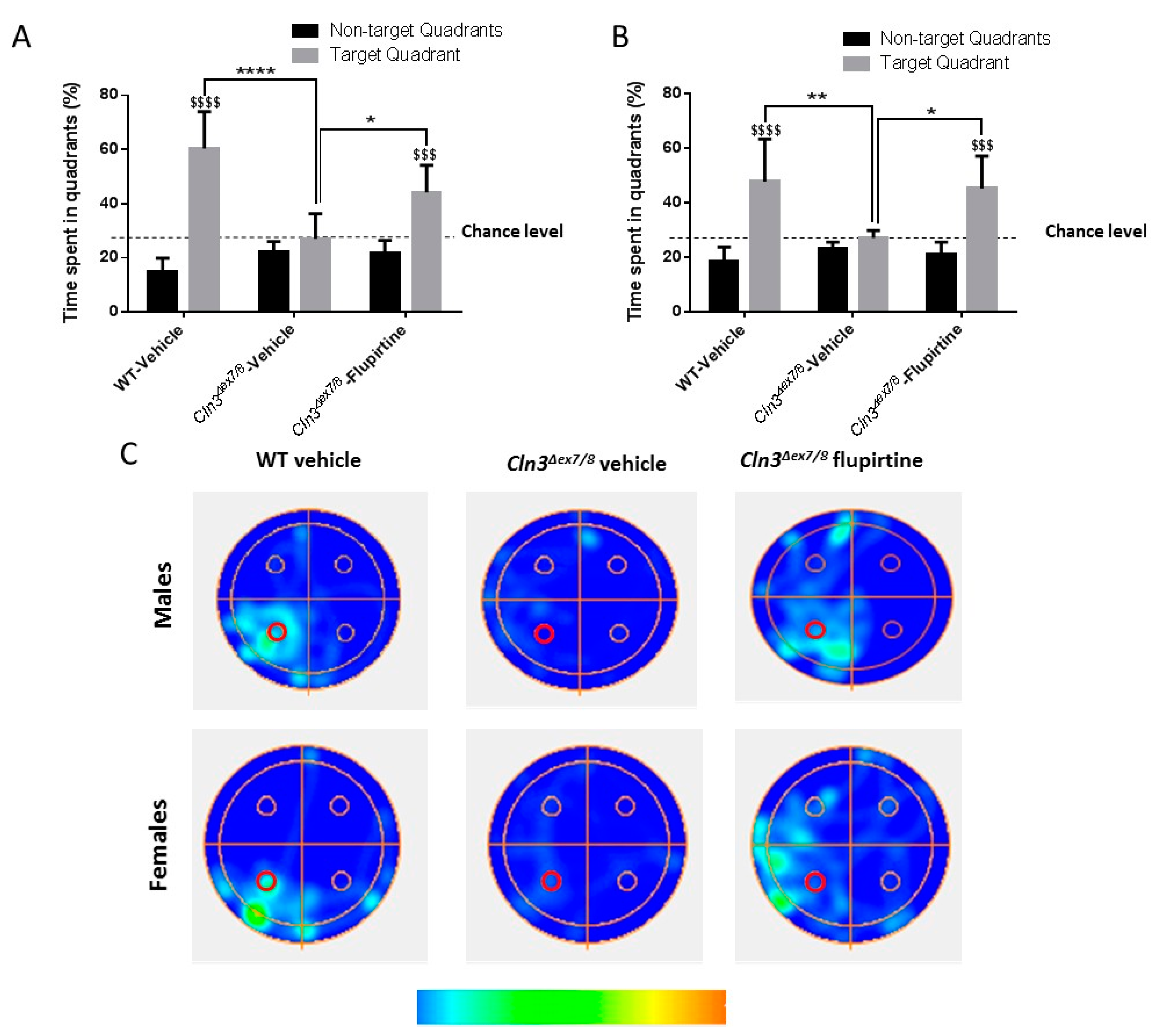
Cln3Δex7/8 mice. Learning and cognitive ability of mice was tested at 16 weeks of age using the Morris water maze (MWM) test. Analysis of the swim paths of mice during the probe trial showed that flupirtine-treated male and female
Cln3Δex7/8 mice adopted strategies and maintained spatial preference in the target quadrant, contrary to vehicle-treated
Cln3Δex7/8 mice that show coverage of the whole maze. Flupirtine significantly enhanced spatial learning, navigation and memory retention in
Cln3Δex7/8 male and female mice.
Apoptosis is a naturally-occurring mechanism of cell death and helps maintain tissue homeostasis [
34]. Neuronal cell loss is evident in brain sections from post-mortem CLN3 disease patients [
3]. Numerous apoptotic cells are present within cortical brain sections from CLN3 disease patients [
3]. CLN3 patient-derived lymphoblasts have decreased growth rate compared to normal lymphoblasts, validating that apoptosis is one of the mechanisms implicated in CLN3 disease pathogenesis [
35]. Other studies demonstrate damage and apoptosis of neuronal and glial cells in hippocampus and cortex of CLN3 patients in addition to marked loss of cortical neurons due to apoptotic cell death [
6]. CLN3 defects also perturb calcium signaling, leading to a profound defects in neuronal survival [
36].
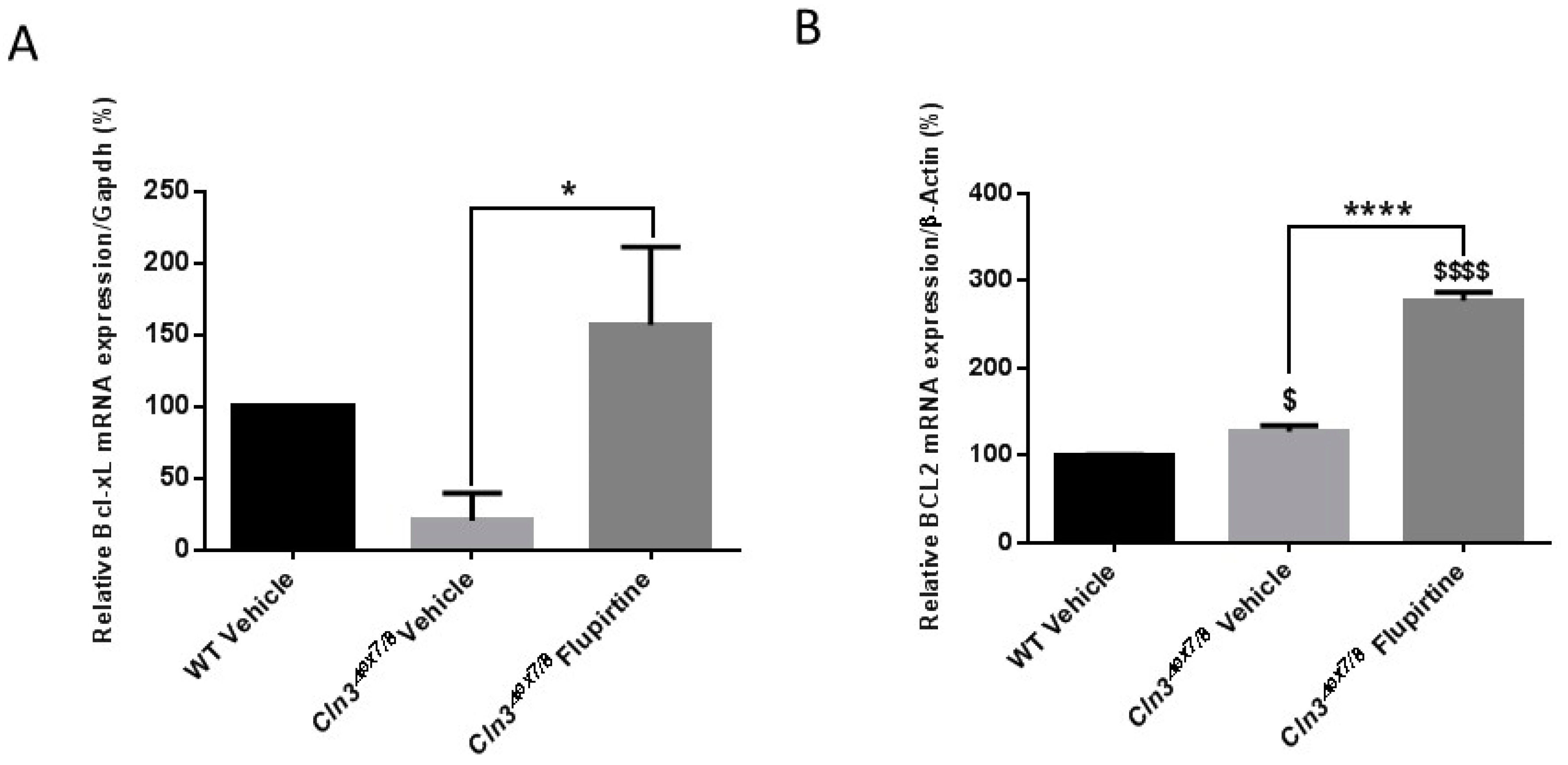
Most neuronal death in CLN2 and CLN3 brains takes place via apoptosis, and the surviving neurons upregulate Bcl-2 [
5]. Treatment with flupirtine significantly upregulated expression of anti-apoptotic BCL-2 in CLN3-deficient cells in vitro [
20]. This is the case
in vivo in this current study as flupirtine-treated
Cln3Δex7/8 female mice show a remarkable increase in
Bcl-2 expression. In males, another anti-apoptotic protein,
Bcl-xl, was upregulated following treatment with flupirtine. Different proteins were impacted in male versus female mice, yet the end result was upregulation of anti-apoptotic pathways and hence, reduction of cell death in brains of
Cln3Δex7/8 mice given flupirtine. This variation among sexes is not a new observation in this disease. We documented this in another study using exogenous galactosylceramide as potential treatment for CLN3 disease [
28].
CLN3 is directly implicated in apoptotic cell death signaling cascades by activating caspase-dependent and caspase-independent pathways [
8]. Ceramide is a major sphingolipid second messenger implicated in several cell processes and impacts divergent pathways [
37]. Sphingolipids are major bioactive lipids involved in homeostasis, growth, proliferation and cell death [
38]. Ceramide mediates anti-proliferative events, such as apoptosis, growth inhibition, cell differentiation, and senescence [
10]. This biomolecule possesses complex biophysical properties and acts as a central hub. Regulation of its levels affects catabolism and break-down of various sphingolipid species [
10]. Ceramide is generated through several complex interrelated pathways either via the de novo pathway, sphingomyelin, or cerebroside catabolism. Ceramide is synthesized de novo in the endoplasmic reticulum or through breakdown of sphingomyelin in Golgi, plasma membrane, or mitochondrial membrane [
39]. Defects in ceramide signaling pathways often result in augmenting programmed cell death in multiple cell types, including neurons [
40]. Previous studies show that ceramide levels are increased in CLN3-deficient cells and brain of CLN3 patients [
11]. Published reports demonstrate that 17 week-old
Cln3Δex7/8 mice express higher levels of ceramide in brain compared to age-matched WT mice [
14]. Sptlc3 catalyzes the initial steps in formation of ceramide via the de novo pathway by condensing serine and palmitoyl Co-A to generate 3-ketoshphinganine (3-KDS) [
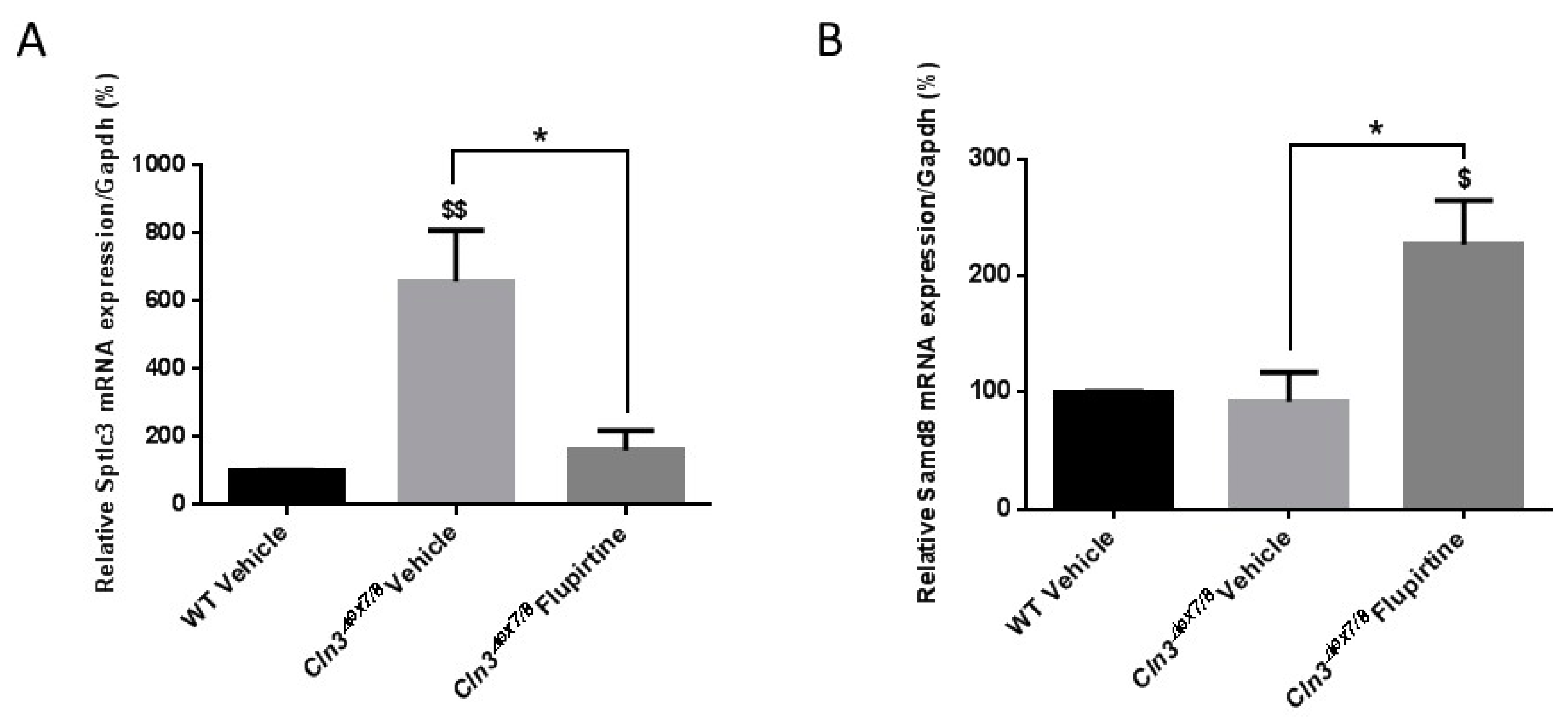
10]. We documented decreased
Sptlc3 levels in flupirtine-treated
Cln3Δex7/8 mice versus vehicle-treated
Cln3Δex7/8 male mice. Downregulation of
Sptlc3 leads to diminution of ceramide generation via the de novo ceramide pathway. As for the other enzymes of the de novo pathway, the expression of
Sptlc2 and
Degs1 also decreased with flupirtine treatment, but did not reach significance (data not shown). This supports our conclusion that flupirtine treatment in male mice impacts the de novo synthesis pathway. In females, however, a different pathway in ceramide signaling is at play.
Samd8 is an ER transferase that converts phosphatidylethanolamine (PE) and ceramide to ceramide phosphoethanolamine (CPE).
Samd8 levels are increased in flupirtine-treated
Cln3Δex7/8 female mice.
Samd8 operates as a ceramide sensor to control ceramide homeostasis in the ER rather than a converter of ceramides. This implicates that the ceramide salvage pathway is modulated in flupirtine-treated
Cln3Δex7/8 female mice with the end result of diminished ceramide levels. Empirical evidence from our study confirms reduced synthesis (decreased
Sptlc3 expression) in flupirtine-treated male mice, but increased degradation of ceramide (increased
Samd8 expression) in female flupirtine-treated mice. Physiologic gender differences affects drug activity and charactersitics, including pharmacokinetics [
41]. Differences in body size result in larger distribution volumes, faster total clearance, and less tissue absorption of some medications in men compared to women [
42]. This may explain higher brain absorption in female compared to male mice. Moreover, sex hormones, in females, have a direct effect on drug absorption, distribution, metabolism, elimination and adverse effects [
43].This study implies that sex-specific drug dosing regimens may be warranted for treatment of neurological diseases that affect the blood brain barrier, including CLN3 disease in mouse and man.
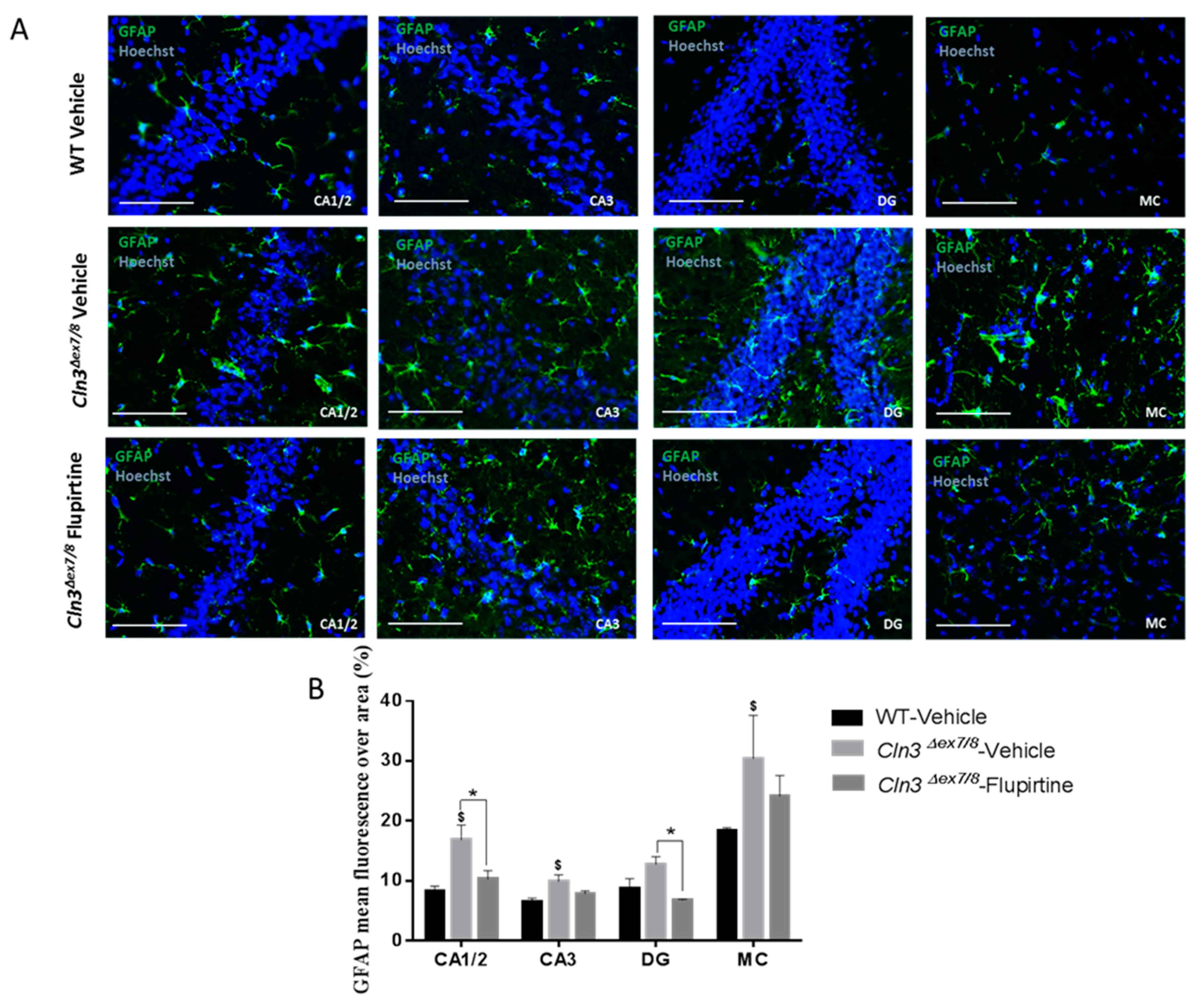
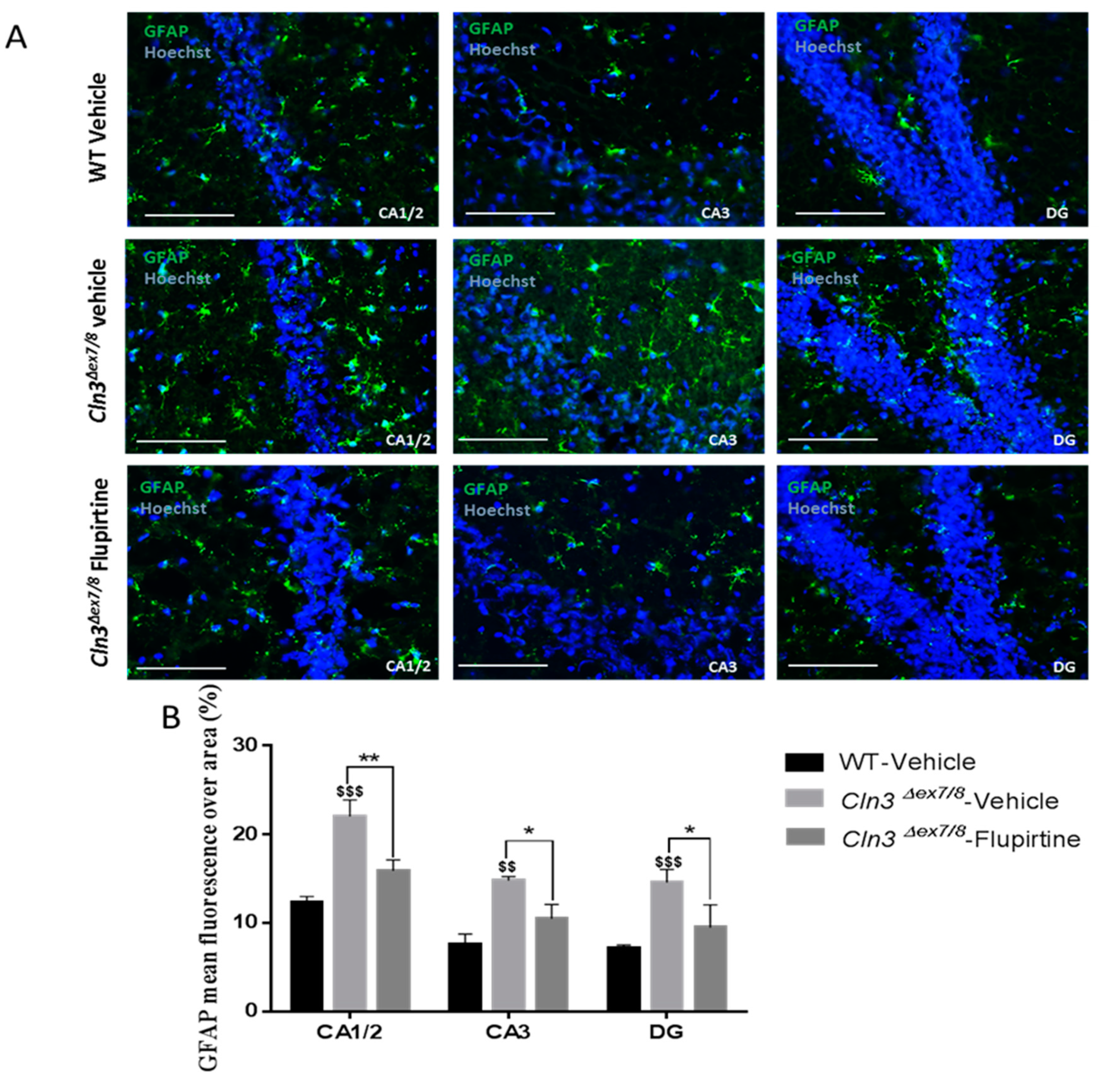
Activation of the glial cell population contributes to imbalance in CNS function and impacts cognitive function [
44]. Hyperactive astrocytes are observed in neurodegenerative disorders and following brain injury documented by GFAP as biomarker. In males, microscopic inspection of vehicle-treated
Cln3Δex7/8 mouse brain documents widespread intracellular GFAP staining in hippocampal regions (CA1, CA2, CA3, and the dentate gyrus) and in the MC relative to age-matched, vehicle-treated WT animals. Mice treated with flupirtine had significantly lower levels of GFAP staining in these regions. This data provides evidence that flupirtine attenuates astrogliosis at the level of the hippocampus and MC in
Cln3Δex7/8 male mice. In females, flupirtine was able to attenuate GFAP immunostaining in CA1/2, CA3 and DG regions of the hippocampus. The motor cortex did not show any difference in vehicle-treated
Cln3Δex7/8 female mice compared to WT. This may explain better performance in
Cln3Δex7/8 female mice on the rotarod compared to males as it tests motor skills (data not shown).
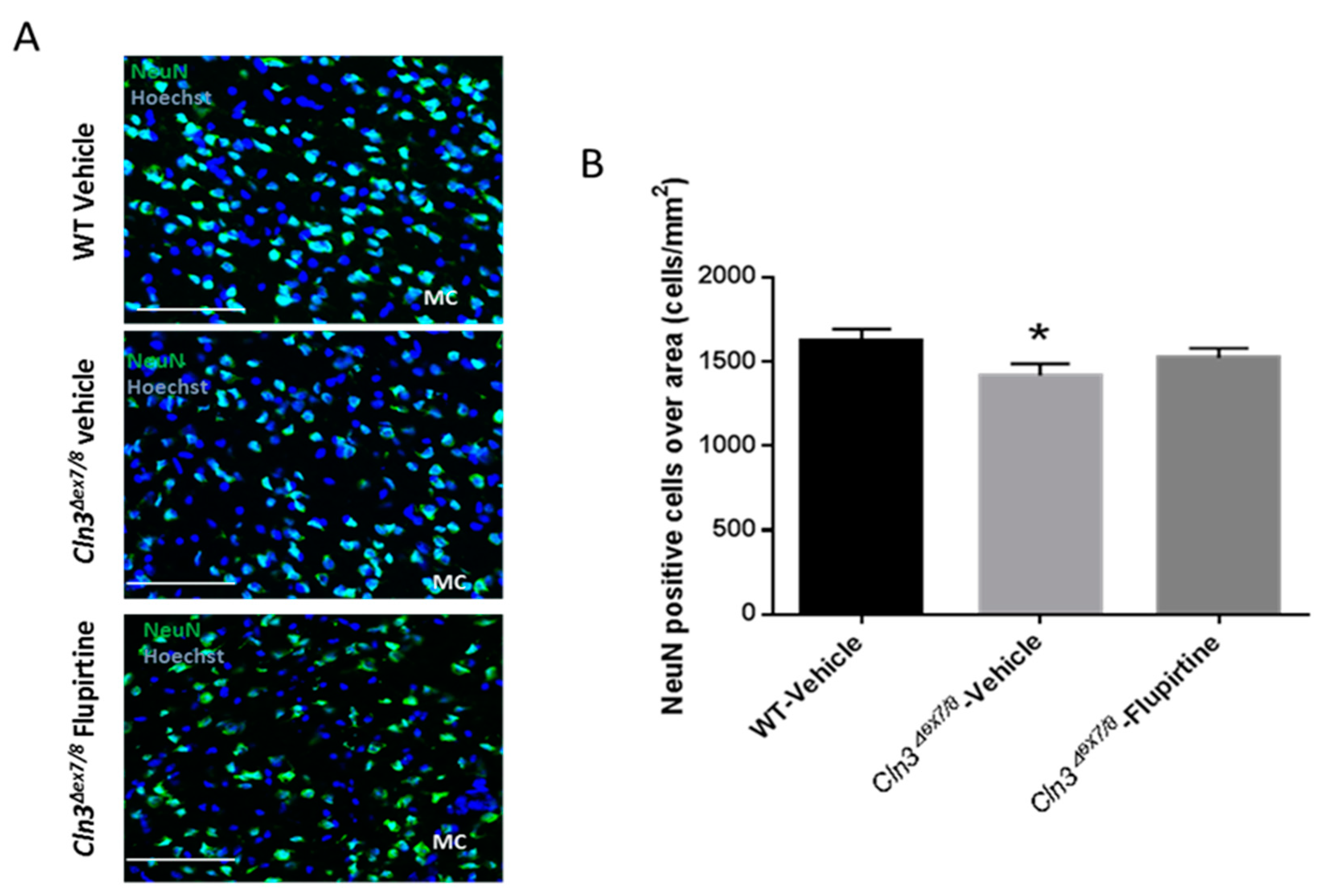
The neuronal nuclear protein (NeuN) is a marker not detected in glial cells or other cells in the brain [
28]. NeuN assesses neuronal health and loss of this protein is indicative of damage. Neuronal cell loss in CLN3 patients is at the root of CLN3 disease pathogenesis [
45]. NeuN-positive cell were significantly ablated in vehicle-treated
Cln3Δex7/8 mouse. Although it did not reach significance, flupirtine resulted in an increase in the neuronal population in motor cortex (MC) of male mice. This suggests that flupirtine conferred neuroprotection and reduced cell death in brains of
Cln3Δex7/8 mice. In females, there was no difference in neuronal counts in motor cortex of vehicle-treated
Cln3Δex7/8 female mice compared to WT. In a previous study, affected female mice 44 weeks of age did show a diminution in NeuN positive cells [
28], suggesting that unlike males, neuronal loss in females starts at a later age.
The novel, flupirtine-like allyl carbamate derivative, compound 6, developed to possess physicochemical properties desirable for CNS therapeutics had an improved Multiparameter optimization (MPO) score. The latter predicts blood–brain barrier (BBB) penetration, an essential parameter for neuroprotective compounds, and was ≥ 4 more effective [
19]. The newly synthesized allyl carbamate derivative of flupirtine, compound 6, showed potential for improved neuroprotection, after screening in vitro nine flupirtine derivatives [
20]. Here, we report early promising behavioral in vivo results for compound 6 for treatment of
Cln3Δex7/8 mice. Although pharmacokinetics and toxicological safety remain to be established for compound 6, the promising behavioral data obtained in this study are worth reporting. Treatment with compound 6 significantly attenuated the high mobility documented by open field and pole climbing in
Cln3Δex7/8 mice, suggesting more work is necessary to determine optimal dosing for this compound.