P0-Related Protein Accelerates Human Mesenchymal Stromal Cell Migration by Modulating VLA-5 Interactions with Fibronectin
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cells
2.2. Cell Lines and Stable Transfectants
2.3. Cell Staining and Analysis
2.3.1. Cell Counts and Viability
2.3.2. Antibodies and Flow Cytometric Analysis of Cell Surface Antigens
2.4. Hunam Bone Marrow Mesenchymal Stromal Cells (hBM MSC) Lineage Differentiation
2.5. Quantitative Real Time PCR (qRT-PCR)
2.6. siRNA Knockdown
2.7. Functional Studies
2.7.1. Coating Surfaces with Extracellular Matrix Proteins
2.7.2. In Vitro hBM MSC Adhesion Assay
2.7.3. In Vitro hBM MSC Spreading Assay
2.7.4. In Vitro hBM MSC and NIH3T3 Transfectant Migration Assays-Wound Healing Assay
2.8. Immunoprecipitation and Immunoblotting
2.8.1. Cell Lysis and Immunoprecipitation
2.8.2. Gel Electrophoresis and Western Blotting
2.9. Confocal Microscopy
2.9.1. Co-Localization Studies for Integrins and PZR Isoforms
2.9.2. Staining for Phosphorylated-Focal Adhesion Kinase (PFAK) and Vinculin
3. Statistics
4. Results
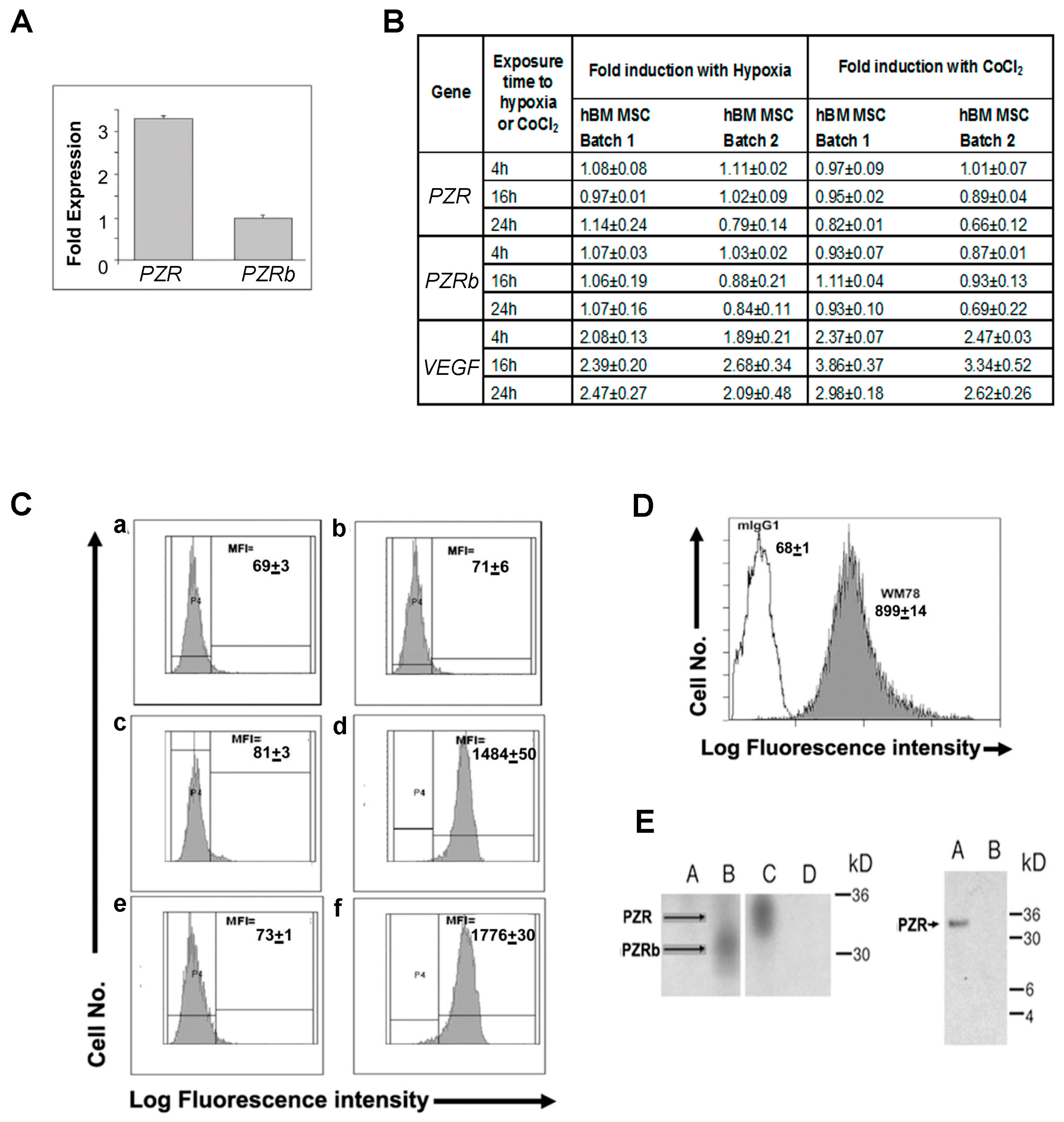
4.1. P0-Related Protein (PZR) is the Predominant Isoform on hBM MSCs
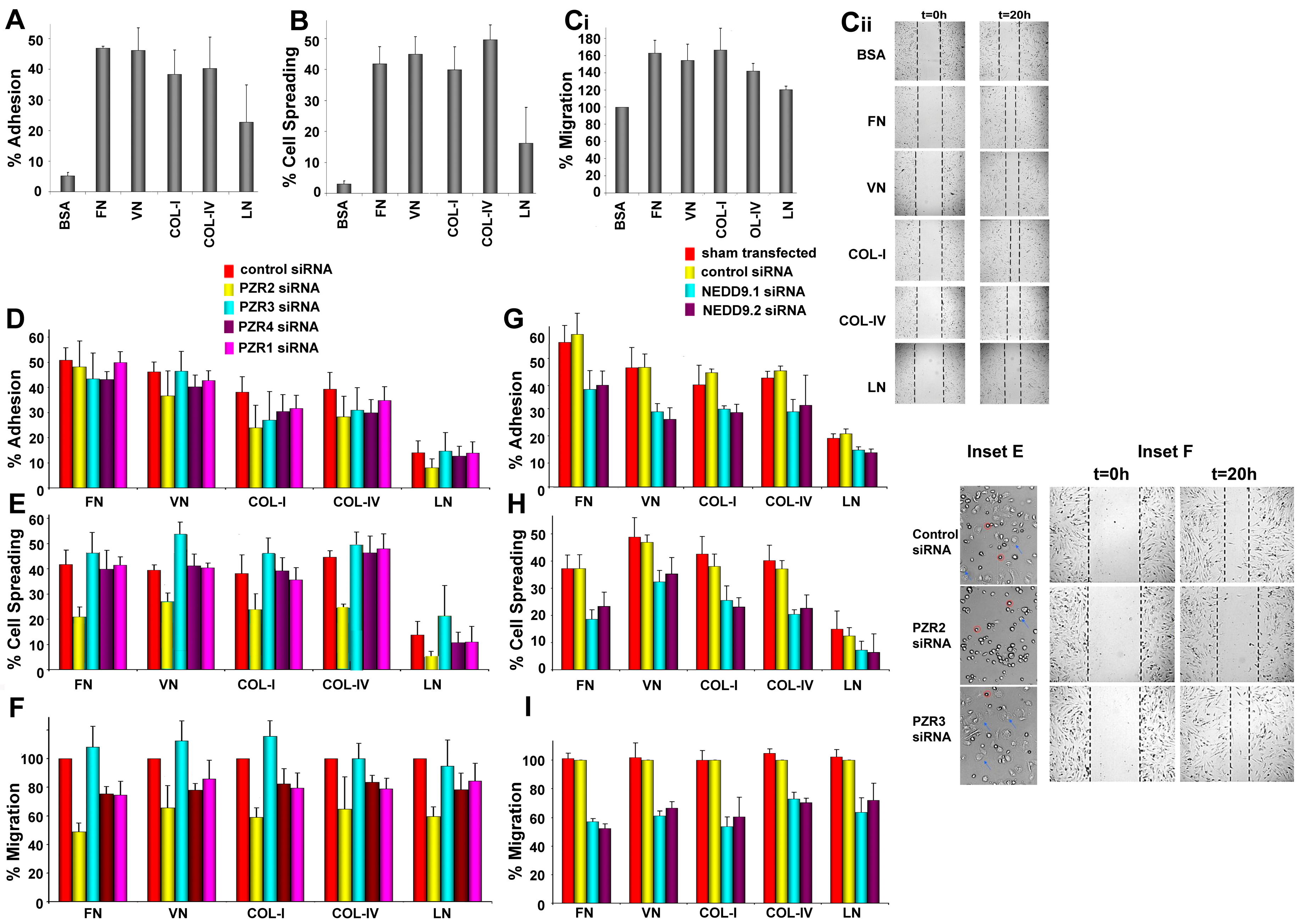
4.2. Differential Interaction of hBM MSCs with ECM Substrates
4.3. Knockdown of Human PZR Principally Reduces hBM MSC Migration on ECM Substrates
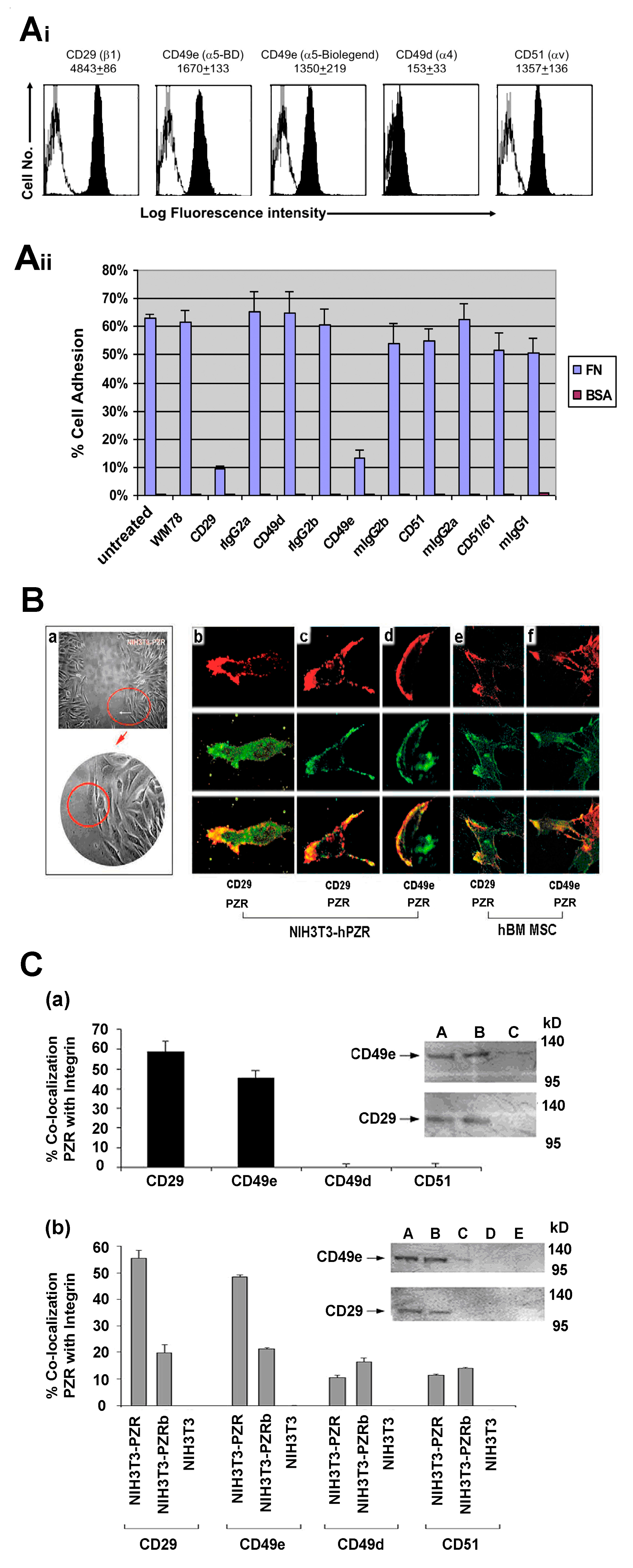
4.4. NIH3T3 Stable Transfectants Expressing Human PZR Show Enhanced Migration on Fibronectin
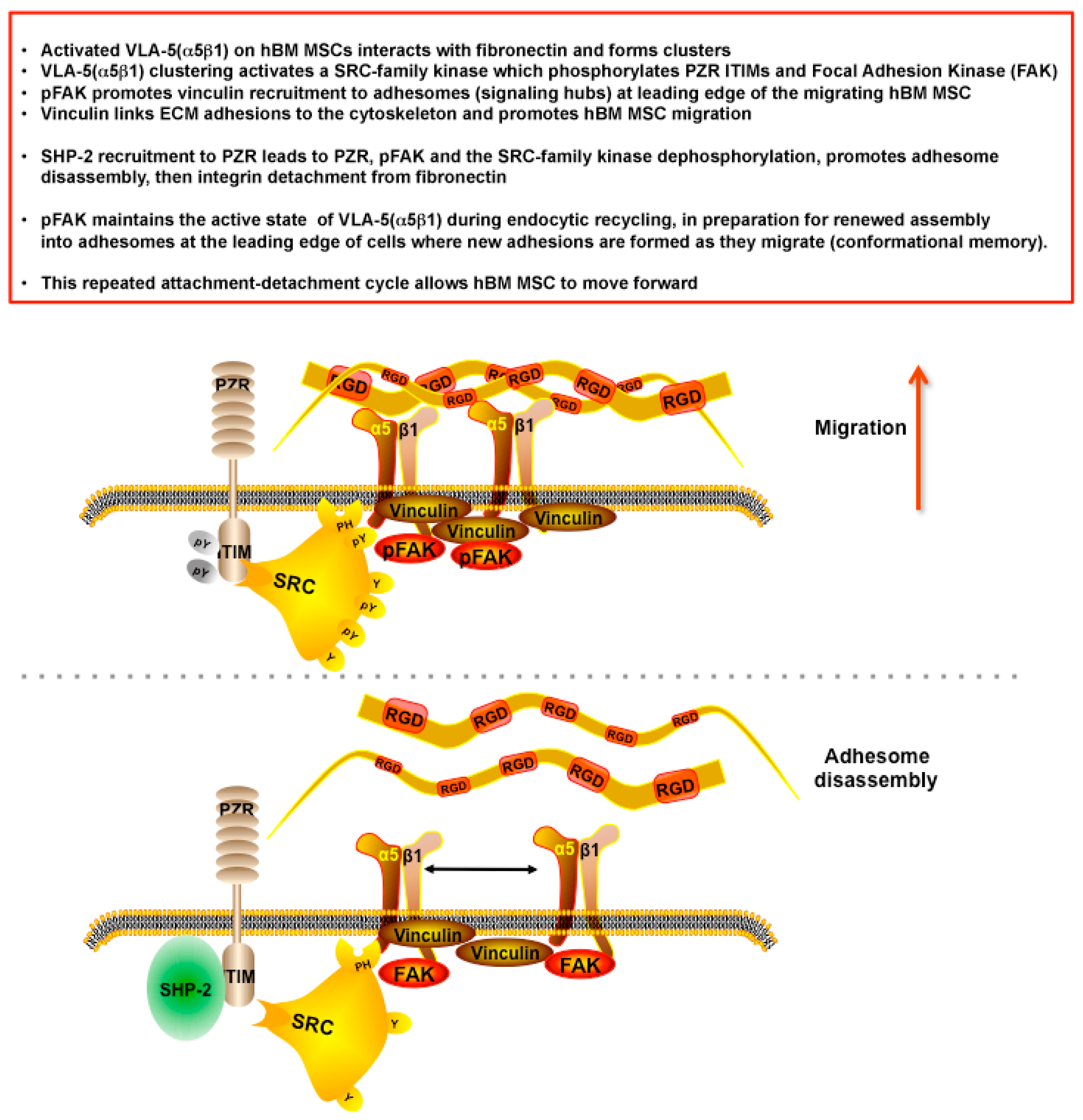
hPZR Clusters with the Fibronectin Receptor VLA-5 during Migration on Fibronectin and Promotes Focal Adhesion Kinase Phosphorylation
5. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tajan, M.; Paccoud, R.; Branka, S.; Edouard, T.; Yart, A. The RASopathy family: Consequences of germline activation of the RAS/MAPK pathway. Endocr. Rev. 2018, 39, 676–700. [Google Scholar] [CrossRef]
- Tajan, M.; de Rocca Serra, A.; Valet, P.; Edouard, T.; Yart, A. SHP2 sails from physiology to pathology. Eur. J. Med. Genet. 2015, 58, 509–525. [Google Scholar] [CrossRef]
- Barford, D.; Neel, B.G. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 1998, 6, 249–254. [Google Scholar] [CrossRef]
- Hof, P.; Pluskey, S.; Dhe-Paganon, S.; Eck, M.J.; Shoelson, S.E. Crystal structure of the tyrosine phosphatase SHP-2. Cell 1998, 92, 441–450. [Google Scholar] [CrossRef]
- Qiu, W.; Wang, X.; Romanov, V.; Hutchinson, A.; Lin, A.; Ruzanov, M.; Battaile, K.P.; Pai, E.F.; Neel, B.G.; Chirgadze, N.Y. Structural insights into Noonan/LEOPARD syndrome-related mutants of protein-tyrosine phosphatase SHP2 (PTPN11). BMC Struct. Biol. 2014, 14, 10–20. [Google Scholar] [CrossRef]
- Yu, D.H.; Qu, C.K.; Henegariu, O.; Lu, X.; Feng, G.S. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 1998, 273, 21125–21131. [Google Scholar] [CrossRef]
- Mañes, S.; Mira, E.; Gómez-Mouton, C.; Zhao, Z.J.; Lacalle, R.A.; Martínez, A.C. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell Biol. 1999, 19, 3125–3135. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Yang, W.; Kontaridis, M.I.; Bivona, T.G.; Wen, G.; Araki, T.; Luo, J.; Thompson, J.A.; Schraven, B.L.; Philips, M.R.; et al. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell. 2004, 13, 341–355. [Google Scholar] [CrossRef]
- Lapinski, P.E.; Meyer, M.F.; Feng, G.S.; Kamiya, N.; King, P.D. Deletion of SHP-2 in mesenchymal stem cells causes growth retardation, limb and chest deformity, and calvarial defects in mice. Dis. Model Mech. 2013, 6, 1448–1458. [Google Scholar] [CrossRef]
- Ran, H.; Tsutsumi, R.; Araki, T.; Neel, B.G. Sticking It to cancer with molecular glue for SHP2. Cancer Cell. 2016, 30, 194–196. [Google Scholar] [CrossRef]
- Zannettino, A.C.; Roubelakis, M.; Welldon, K.J.; Jackson, D.E.; Simmons, P.J.; Bendall, L.J.; Henniker, A.; Harrison, K.L.; Niutta, S.; Bradstock, K.F.; et al. Novel mesenchymal and haematopoietic cell isoforms of the SHP-2 docking receptor, PZR: Identification, molecular cloning and effects on cell migration. Biochem. J. 2003, 370, 537–549. [Google Scholar] [CrossRef]
- Roubelakis, M.G.; Martin-Rendon, E.; Tsaknakis, G.; Stavropoulos, A.; Watt, S.M. The murine ortholog of the SHP-2 binding molecule, PZR accelerates cell migration on fibronectin and is expressed in early embryo formation. J. Cell. Biochem. 2007, 102, 955–969. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Zhao, R. Purification and cloning of PZR, a binding protein and putative physiological substrate of tyrosine phosphatase SHP-2. J. Biol. Chem. 1998, 273, 29367–29372. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, Z.J. Dissecting the interaction of SHP-2 with PZR, an immunoglobulin family protein containing immunoreceptor tyrosine-based inhibitory motifs. J. Biol. Chem. 2000, 275, 5453–5459. [Google Scholar] [CrossRef]
- Zhao, R.; Guerrah, A.; Tang, H.; Zhao, Z.J. Cell surface glycoprotein PZR is a major mediator of concanavalin A-induced cell signaling. J. Biol. Chem. 2002, 277, 7882–7888. [Google Scholar] [CrossRef]
- Eminaga, S.; Bennett, A.M. Noonan syndrome-associated SHP-2/Ptpn11 mutants enhance SIRPα and PZR tyrosyl phosphorylation and promote adhesion-mediated ERK activation. J. Biol. Chem. 2008, 283, 15328–15338. [Google Scholar] [CrossRef]
- Paardekooper Overman, J.; Yi, J.S.; Bonetti, M.; Soulsby, M.; Preisinger, C.; Stokes, M.P.; Hui, L.; Silva, J.C.; Overvoorde, J.; Giansanti, P.; et al. PZR coordinates Shp2 Noonan and LEOPARD syndrome signaling in zebrafish and mice. Mol. Cell. Biol. 2014, 34, 2874–2889. [Google Scholar] [CrossRef]
- Yi, J.S.; Huang, Y.; Kwaczala, A.T.; Kuo, I.Y.; Ehrlich, B.E.; Campbell, S.G.; Giordano, F.J.; Bennett, A.M. Low-dose dasatinib rescues cardiac function in Noonan syndrome. JCI Insight. 2016, 1, e90220. [Google Scholar] [CrossRef]
- Gripp, K.W.; Schill, L.; Schoyer, L.; Stronach, B.; Bennett, A.M.; Blaser, S.; Brown, A.; Burdine, R.; Burkitt-Wright, E.; Castel, P.; et al. The sixth international RASopathies symposium: Precision medicine-From promise to practice. Am. J. Med. Genet. A. 2019, 182, 597–606. [Google Scholar] [CrossRef]
- Kusano, K.; Thomas, T.N.; Fujiwara, K. Phosphorylation and localization of protein-zero related (PZR) in cultured endothelial cells. Endothelium. 2008, 15, 127–136. [Google Scholar] [CrossRef]
- Roubelakis, M.G. The role of SHP-2 and its partners in regulating stem/progenitor cell fate. Ph.D. Thesis, University of Oxford, Oxford, UK, 2004. [Google Scholar]
- Tsaknakis, G. Molecular mechanisms of stem cell migration, homing and engraftment. Ph.D. Thesis, University of Oxford, Oxford, UK, 2008. [Google Scholar]
- Roubelakis, M.G.; Tsaknakis, G.; Pappa, K.I.; Anagnou, N.P.; Watt, S.M. Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization. PLoS ONE 2013, 8, e54747. [Google Scholar] [CrossRef] [PubMed]
- Merryweather-Clarke, A.T.; Cook, D.; Lara, B.J.; Hua, P.; Repapi, E.; Ashley, N.; Lim, S.Y.; Watt, S.M. Does osteogenic potential of clonal human bone marrow mesenchymal stem/stromal cells correlate with their vascular supportive ability? Stem Cell Res. Ther. 2018, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rendon, E.; Hale, S.J.; Ryan, D.; Baban, D.; Forde, S.P.; Roubelakis, M.; Sweeney, D.; Moukayed, M.; Harris, A.L.; Davies, K.; et al. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells. 2007, 25, 1003–1012. [Google Scholar] [CrossRef]
- Wielockx, B.; Grinenko, T.; Mirtschink, P.; Chavakis, T. Hypoxia pathway proteins in normal and malignant hematopoiesis. Cells. 2019, 8, E155. [Google Scholar] [CrossRef]
- Klamer, S.; Voermans, C. The role of novel and known extracellular matrix and adhesion molecules in the homeostatic and regenerative bone marrow microenvironment. Cell Adh. Migr. 2014, 8, 563–577. [Google Scholar] [CrossRef]
- Hynes, R.O. Stretching the boundaries of extracellular matrix research. Nat. Rev. Mol. Cell Biol. 2014, 15, 761–763. [Google Scholar] [CrossRef]
- Klein, G. The extracellular matrix of the hematopoietic microenvironment. Experientia 1995, 51, 914–926. [Google Scholar] [CrossRef]
- Humphries, J.D.; Chastney, M.R.; Askari, J.A.; Humphries, M.J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 2019, 56, 14–21. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Waterman, C.M. Localization-based super-resolution imaging of cellular structures. Methods Mol. Biol. 2013, 1046, 59–84. [Google Scholar]
- Bachir, A.I.; Zareno, J.; Moissoglu, K.; Plow, E.F.; Gratton, E.; Horwitz, A.R. Integrin-associated complexes form hierarchically with variable stoichiometry in nascent adhesions. Curr. Biol. 2014, 24, 1845–1853. [Google Scholar] [CrossRef]
- Winograd-Katz, S.E.; Fassler, R.; Geiger, B.; Legate, K.R. The integrin adhesome: From genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 273–288. [Google Scholar] [CrossRef]
- Horton, E.R.; Byron, A.; Askari, J.A.; Ng, D.H.J.; Millon-Fremillon, A.; Robertson, J.; Koper, E.J.; Paul, N.R.; Warwood, S.; Knight, D.; et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 2015, 17, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Horton, E.R.; Humphries, J.D.; James, J.; Jones, M.C.; Askari, J.A.; Humphries, M.J. The integrin adhesome network at a glance. J. Cell. Sci. 2016, 129, 4159–4163. [Google Scholar] [CrossRef] [PubMed]
- Ajeian, J.N.; Horton, E.R.; Astudillo, P.; Byron, A.; Askari, J.A.; Millon-Frémillon, A.; Knight, D.; Kimber, S.J.; Humphries, M.J.; Humphries, J.D. Proteomic analysis of integrin-associated complexes from mesenchymal stem cells. Proteomics Clin. Appl. 2016, 10, 51–57. [Google Scholar] [CrossRef]
- Zhong, J.; Baquiran, J.B.; Bonakdar, N.; Lees, J.; Ching, Y.W.; Pugacheva, E.; Fabry, B.; O’Neill, G. NEDD9 stabilizes focal adhesions, increases binding to the extra-cellular matrix and differentially effects 2D versus 3D cell migration. PLoS ONE 2012, 7, e35058. [Google Scholar] [CrossRef]
- Kleinschmidt, E.G.; Schlaepfer, D.D. Focal adhesion kinase signaling in unexpected places. Curr. Opin. Cell Biol. 2017, 45, 24–30. [Google Scholar] [CrossRef]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase, in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef]
- Giannone, G.; Sheetz, M.P. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006, 16, 213–223. [Google Scholar] [CrossRef]
- Ginsberg, M.H.; Partridge, A.; Shattil, S.J. Integrin regulation. Curr. Opin. Cell Biol. 2005, 17, 509–516. [Google Scholar] [CrossRef]
- Ziegler, W.H.; Liddington, R.C.; Critchley, D.R. The structure and regulation of vinculin. Trends Cell Biol. 2006, 16, 453–460. [Google Scholar] [CrossRef]
- Nader, G.P.; Ezratty, E.J.; Gundersen, G.G. FAK, talin and PIPKIgamma regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat. Cell Biol. 2016, 18, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Vitillo, L.; Kimber, S.J. Integrin and FAK regulation of human pluripotent stem cells. Curr. Stem Cell Rep. 2017, 3, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T.S. Nuclear FAK: A new mode of gene regulation from cellular adhesions. Mol. Cells. 2013, 36, 1–6. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on Cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Marin, T.M.; Clemente, C.F.; Santos, A.M.; Picardi, P.K.; Pascoal, V.D.; Lopes-Cendes, I.; Saad, M.J.; Franchini, K.G. Shp2 negatively regulates growth in cardiomyocytes by controlling focal adhesion kinase/Src and mTOR pathways. Circ. Res. 2008, 103, 813–824. [Google Scholar] [CrossRef]
- Walker, N.G.; Mistry, A.R.; Smith, L.E.; Eves, P.C.; Tsaknakis, G.; Forster, S.; Watt, S.M.; Macneil, S. A chemically defined carrier for the delivery of human mesenchymal stem/stromal cells to skin wounds. Tissue Eng. Part. C Methods. 2012, 18, 143–155. [Google Scholar] [CrossRef]
- Jiang, D.; Qi, Y.; Walker, N.G.; Sindrilaru, A.; Hainzl, A.; Wlaschek, M.; MacNeil, S.; Scharffetter-Kochanek, K. The effect of adipose tissue derived MSCs delivered by a chemically defined carrier on full-thickness cutaneous wound healing. Biomaterials. 2013, 34, 2501–2515. [Google Scholar] [CrossRef]
- Nakao, M.; Kim, K.; Nagase, K.; Grainger, D.W.; Kanazawa, H.; Okano, T. Phenotypic traits of mesenchymal stem cell sheets fabricated by temperature-responsive cell culture plate: Structural characteristics of MSC sheets. Stem Cell Res. Ther. 2019, 10, 353–366. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roubelakis, M.G.; Tsaknakis, G.; Lyu, F.-J.; Trohatou, O.; Zannettino, A.C.W.; Watt, S.M. P0-Related Protein Accelerates Human Mesenchymal Stromal Cell Migration by Modulating VLA-5 Interactions with Fibronectin. Cells 2020, 9, 1100. https://doi.org/10.3390/cells9051100
Roubelakis MG, Tsaknakis G, Lyu F-J, Trohatou O, Zannettino ACW, Watt SM. P0-Related Protein Accelerates Human Mesenchymal Stromal Cell Migration by Modulating VLA-5 Interactions with Fibronectin. Cells. 2020; 9(5):1100. https://doi.org/10.3390/cells9051100
Chicago/Turabian StyleRoubelakis, Maria G., Grigorios Tsaknakis, Feng-Juan Lyu, Ourania Trohatou, Andrew C. W. Zannettino, and Suzanne M. Watt. 2020. "P0-Related Protein Accelerates Human Mesenchymal Stromal Cell Migration by Modulating VLA-5 Interactions with Fibronectin" Cells 9, no. 5: 1100. https://doi.org/10.3390/cells9051100
APA StyleRoubelakis, M. G., Tsaknakis, G., Lyu, F.-J., Trohatou, O., Zannettino, A. C. W., & Watt, S. M. (2020). P0-Related Protein Accelerates Human Mesenchymal Stromal Cell Migration by Modulating VLA-5 Interactions with Fibronectin. Cells, 9(5), 1100. https://doi.org/10.3390/cells9051100





