Potential Role of Circulating Endoglin in Hypertension via the Upregulated Expression of BMP4
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
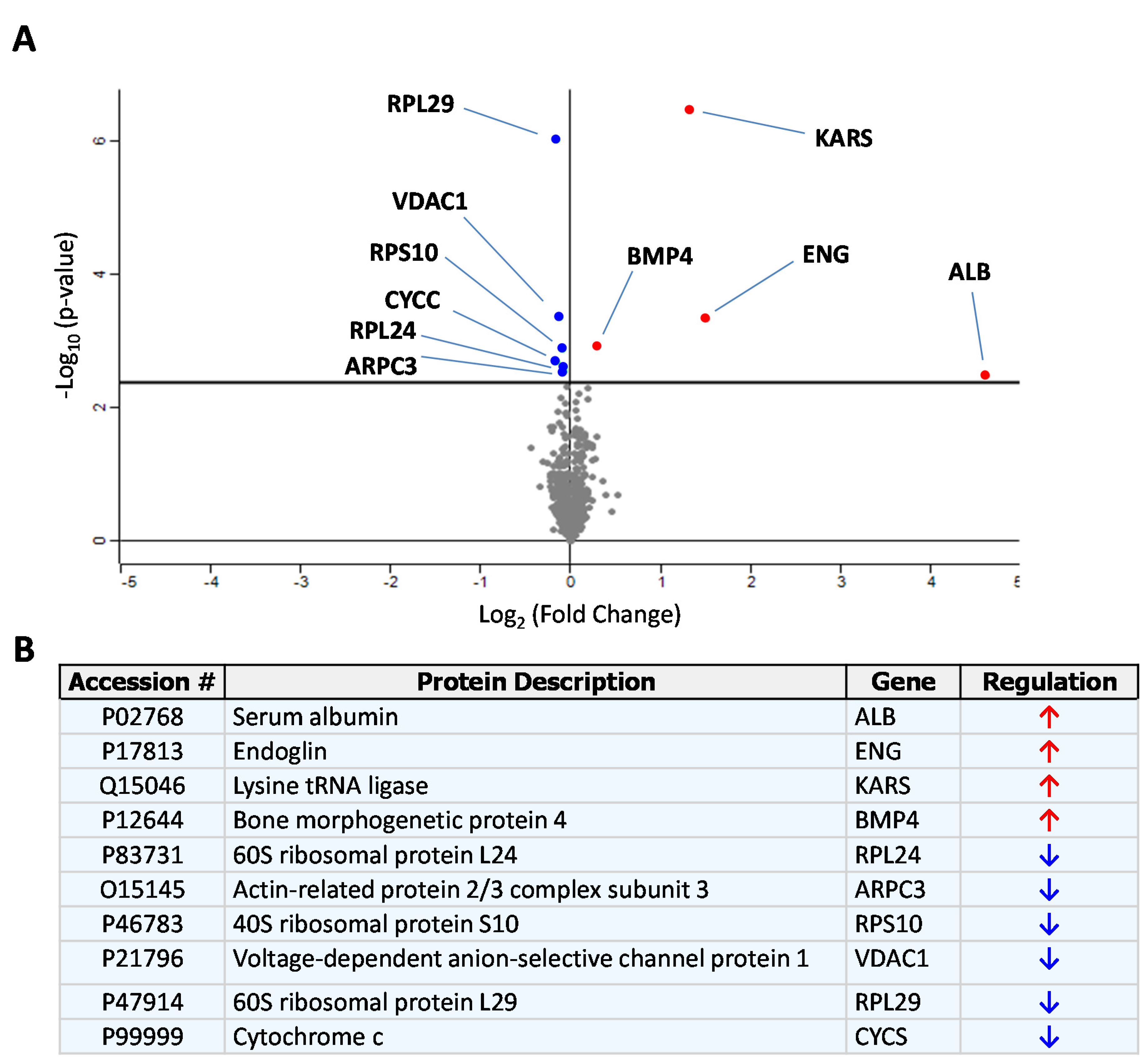
2.2. Protein Quantification of the Endothelial Secretome Using Isobaric Labeling (iTraq)
2.3. RNA Expression Analysis by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.4. ELISA of BMP4, sEng, and sFlts1
2.5. Plasmids, Cell Transfections and Reporter Assays
2.6. Human Blood Samples
2.7. Mice
2.8. Mouse Blood and Tissue Collection
2.9. In Vivo Experiments with Osmotic Pumps
2.10. Mouse Model of Preeclampsia
2.11. Blood Pressure Measurements in Mice
2.12. Immunohistochemistry
2.13. Statistical Analysis
3. Results
3.1. Identification of sEng-Induced Downstream Mediators in Human Endothelial Cells
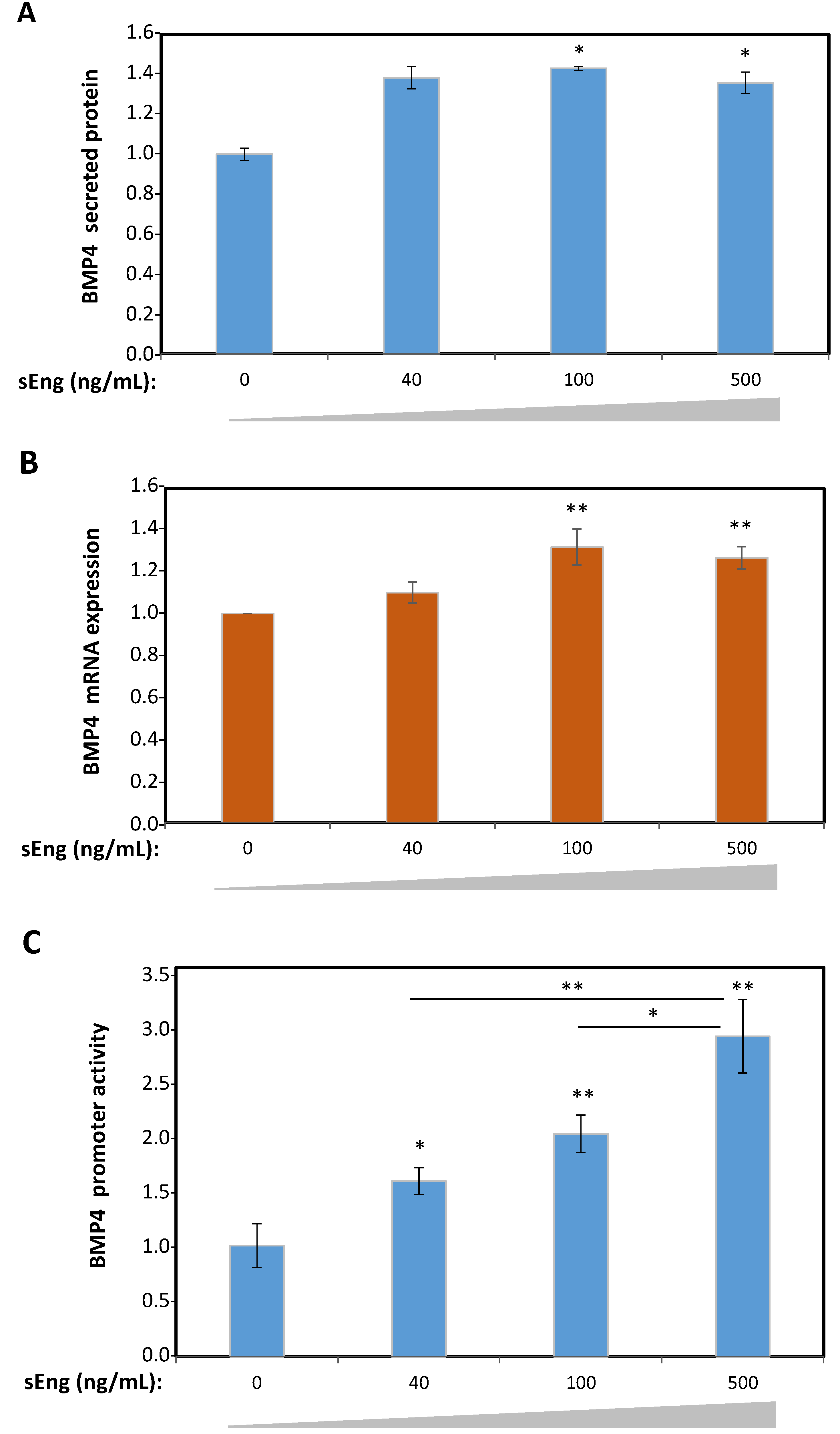
3.2. Expression of BMP4 is Induced by sEng in Human Endothelial Cells In Vitro
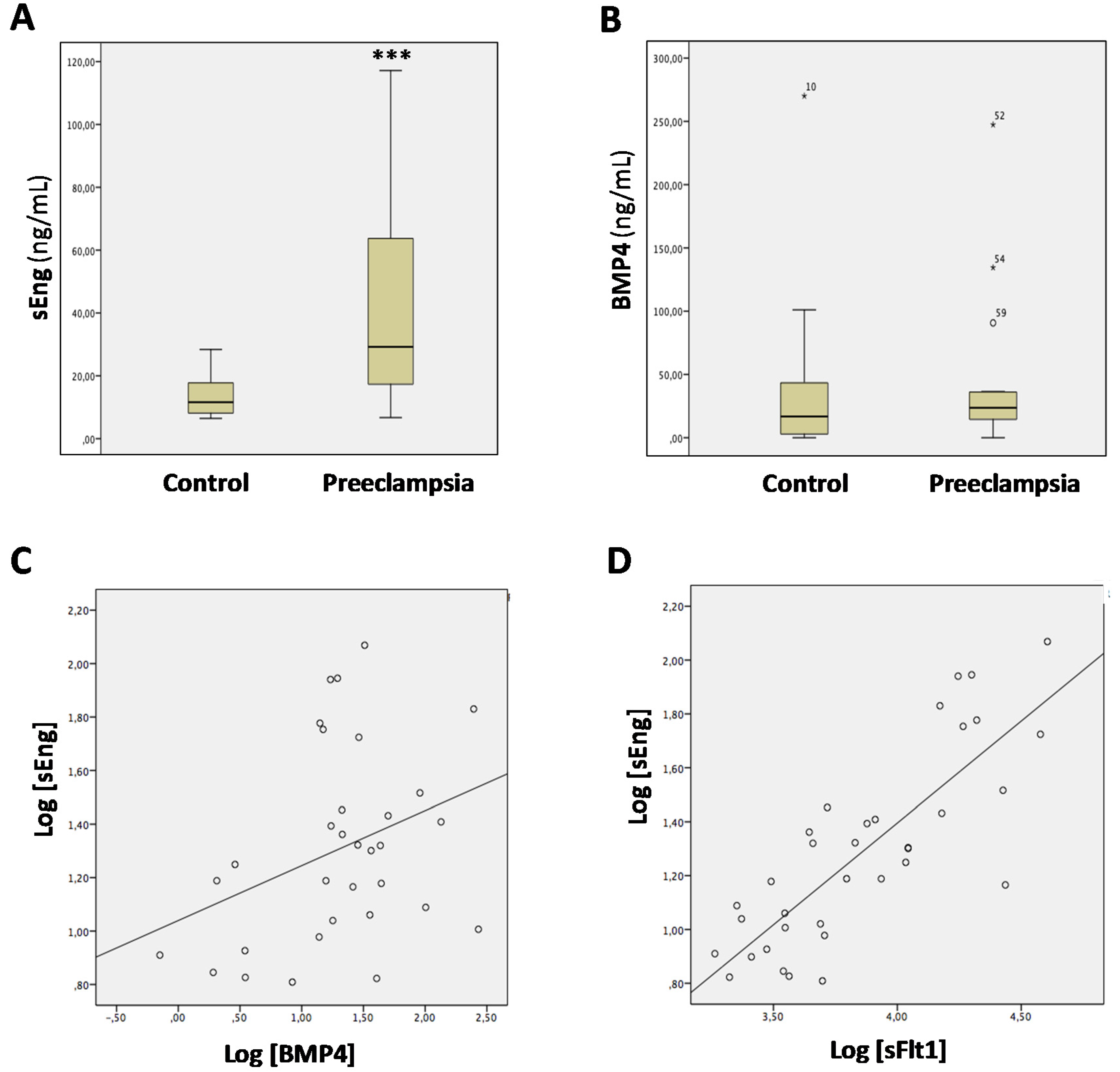
3.3. Levels of sEng and BMP4 Correlate with Each Other in Sera from Pregnant Women
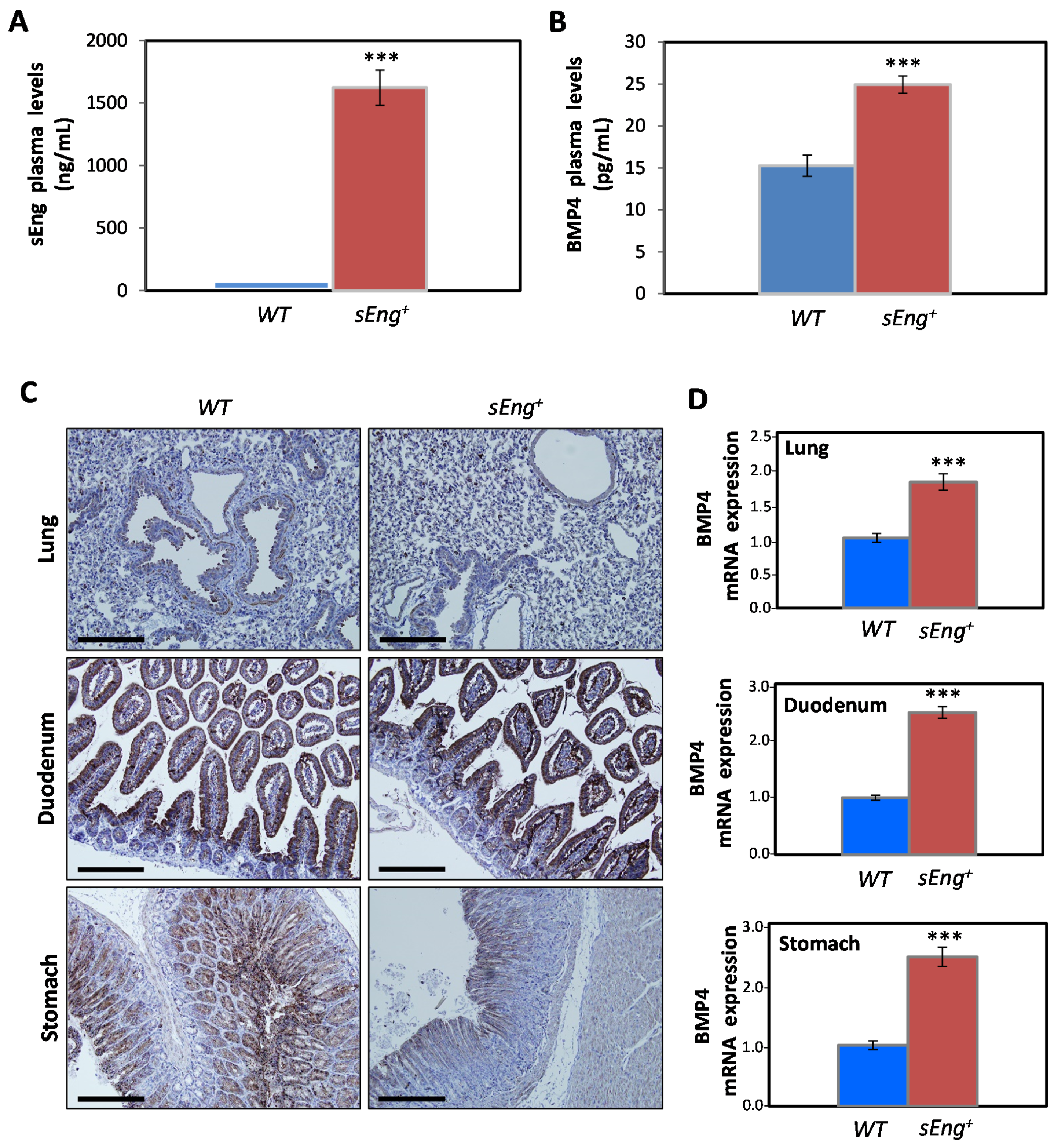
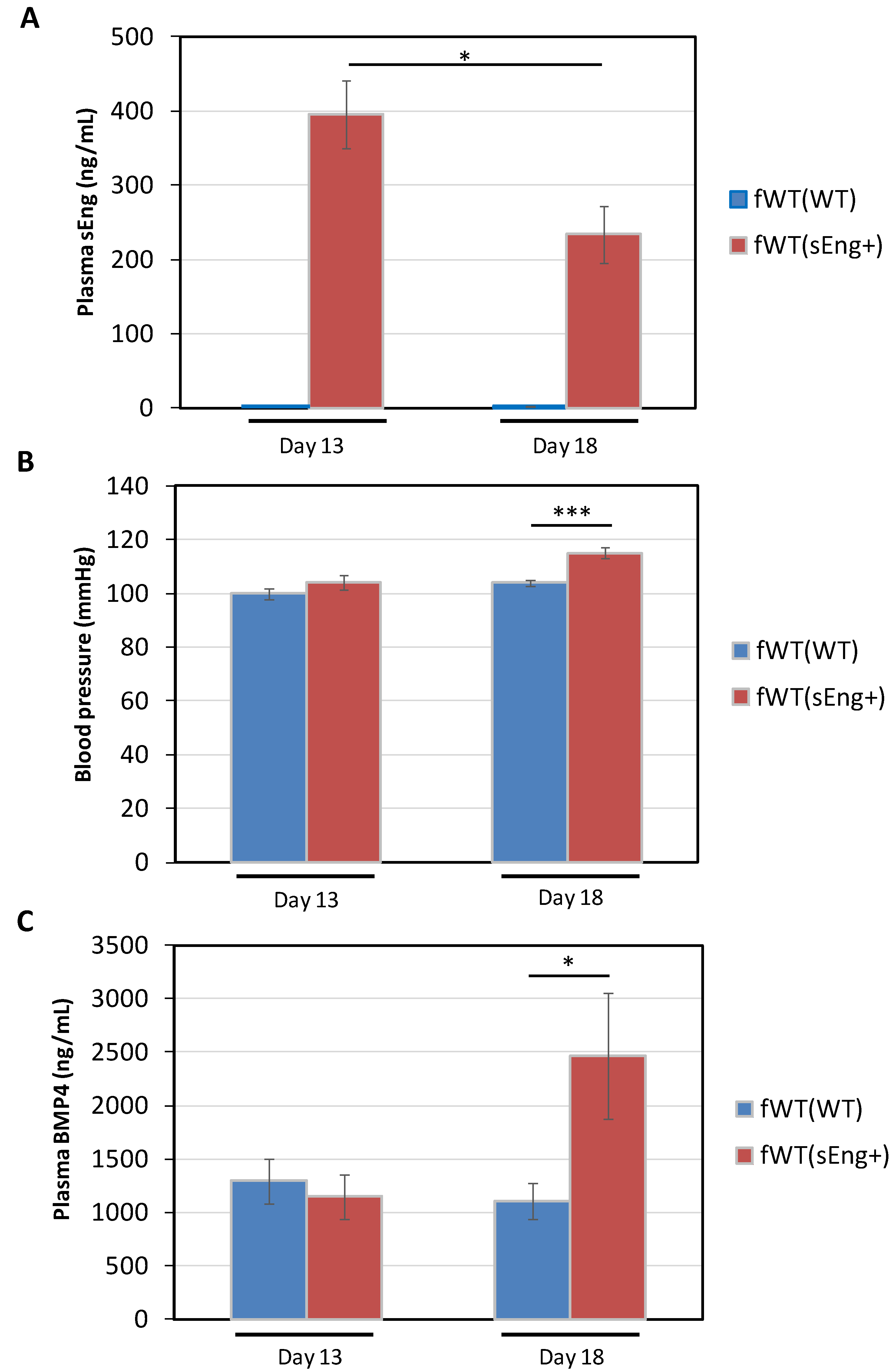
3.4. Expression of BMP4 is Enhanced by sEng in a Mouse Model
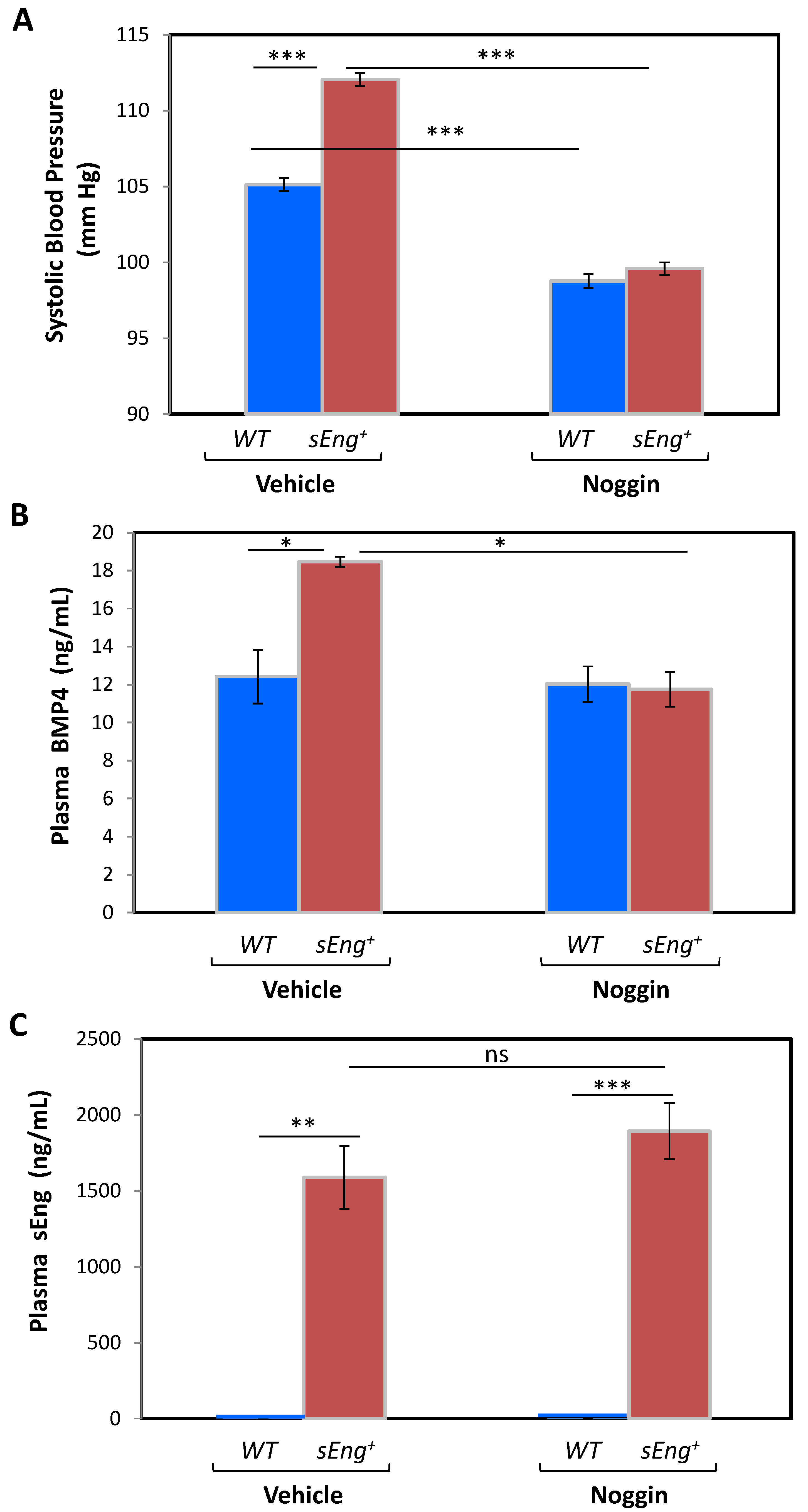
3.5. BMP4 Is a Mediator of the sEng-Dependent Hypertensive Effect in Mice
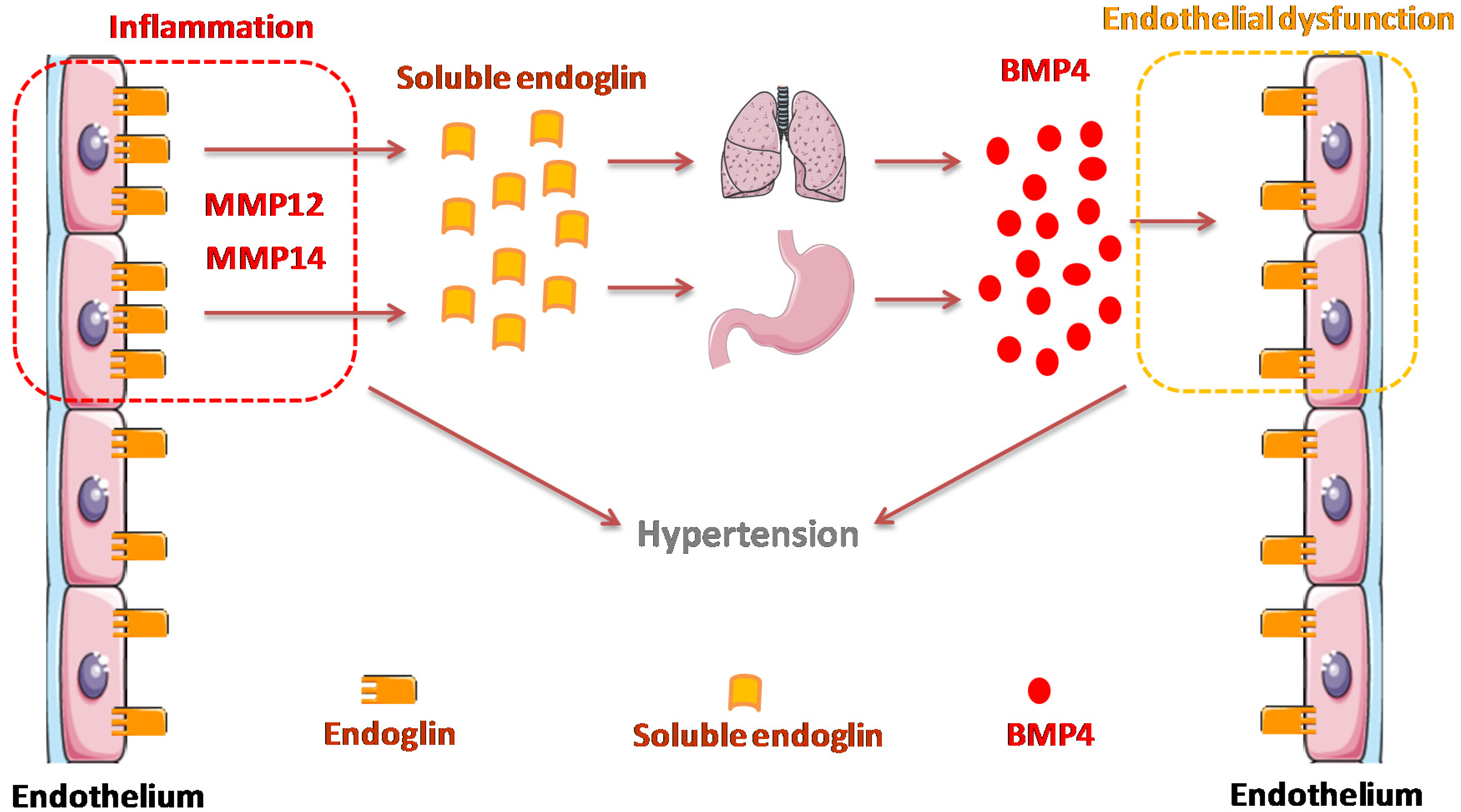
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ten Dijke, P.; Arthur, H.M. Extracellular control of TGFbeta signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 857–869. [Google Scholar] [CrossRef]
- Santibañez, J.F.; Quintanilla, M.; Bernabeu, C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin. Sci. (Lond) 2011, 121, 233–251. [Google Scholar] [CrossRef]
- Cunha, S.I.; Magnusson, P.U.; Dejana, E.; Lampugnani, M.G. Deregulated TGF-β/BMP Signaling in Vascular Malformations. Circ. Res. 2017, 121, 981–999. [Google Scholar] [CrossRef]
- Gatica, S.; Cabello-Verrugio, C.; Simon, F. Transforming Growth Factor-Beta Family: Advances in Vascular Function and Signaling. Curr. Protein Pept. Sci. 2018, 19, 1164–1171. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- López-Novoa, J.M.; Bernabeu, C. The physiological role of endoglin in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H959–H974. [Google Scholar] [CrossRef]
- Núñez-Gómez, E.; Pericacho, M.; Ollauri-Ibáñez, C.; Bernabéu, C.; López-Novoa, J.M. The role of endoglin in post-ischemic revascularization. Angiogenesis 2017, 20, 1–24. [Google Scholar] [CrossRef]
- Ruiz-Llorente, L.; Gallardo-Vara, E.; Rossi, E.; Smadja, D.M.; Botella, L.M.; Bernabeu, C. Endoglin and alk1 as therapeutic targets for hereditary hemorrhagic telangiectasia. Expert Opin. Ther. Targets 2017, 21, 933–947. [Google Scholar] [CrossRef]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrell, J.; et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994, 8, 345–351. [Google Scholar] [CrossRef]
- Shovlin, C.L. Hereditary haemorrhagic telangiectasia: Pathophysiology, diagnosis and treatment. Blood Rev. 2010, 24, 203–219. [Google Scholar] [CrossRef]
- Tual-Chalot, S.; Oh, S.P.; Arthur, H.M. Mouse models of hereditary hemorrhagic telangiectasia: Recent advances and future challenges. Front. Genet. 2015, 6, 25. [Google Scholar] [CrossRef]
- Gougos, A.; Letarte, M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J. Biol. Chem. 1990, 265, 8361–8364. [Google Scholar] [PubMed]
- Bernabeu, C.; Conley, B.A.; Vary, C.P. Novel biochemical pathways of endoglin in vascular cell physiology. J. Cell. Biochem. 2007, 102, 1375–1388. [Google Scholar] [CrossRef]
- Hawinkels, L.J.; Kuiper, P.; Wiercinska, E.; Verspaget, H.W.; Liu, Z.; Pardali, E.; Sier, C.F.; ten Dijke, P. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010, 70, 4141–4150. [Google Scholar] [CrossRef]
- Valbuena-Diez, A.C.; Blanco, F.J.; Oujo, B.; Langa, C.; Gonzalez-Nuñez, M.; Llano, E.; Pendas, A.M.; Díaz, M.; Castrillo, A.; Lopez-Novoa, J.M.; et al. Oxysterol-induced soluble endoglin release and its involvement in hypertension. Circulation 2012, 126, 2612–2624. [Google Scholar] [CrossRef]
- Gallardo-Vara, E.; Blanco, F.J.; Roqué, M.; Friedman, S.L.; Suzuki, T.; Botella, L.M.; Bernabeu, C. Transcription factor KLF6 upregulates expression of metalloprotease MMP14 and subsequent release of soluble endoglin during vascular injury. Angiogenesis 2016, 19, 155–171. [Google Scholar] [CrossRef]
- Ermini, L.; Ausman, J.; Melland-Smith, M.; Yeganeh, B.; Rolfo, A.; Litvack, M.L.; Todros, T.; Letarte, M.; Post, M.; Caniggia, I. A Single Sphingomyelin Species Promotes Exosomal Release of Endoglin into the Maternal Circulation in Preeclampsia. Sci. Rep. 2017, 7, 12172. [Google Scholar] [CrossRef]
- Chang, X.; Yao, J.; He, Q.; Liu, M.; Duan, T.; Wang, K. Exosomes From Women With Preeclampsia Induced Vascular Dysfunction by Delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to Endothelial Cells. Hypertension 2018, 72, 1381–1390. [Google Scholar] [CrossRef]
- Aristorena, M.; Gallardo-Vara, E.; Vicen, M.; de Las Casas-Engel, M.; Ojeda-Fernandez, L.; Nieto, C.; Blanco, F.J.; Valbuena-Diez, A.C.; Botella, L.M.; Nachtigal, P.; et al. MMP-12, secreted by pro-inflammatory macrophages, targets endoglin in human macrophages and endothelial cells. Int. J. Mol. Sci. 2019, 20, 3107. [Google Scholar] [CrossRef]
- Llorca, O.; Trujillo, A.; Blanco, F.J.; Bernabeu, C. Structural model of human endoglin, a transmembrane receptor responsible for hereditary hemorrhagic telangiectasia. J. Mol. Biol. 2007, 365, 694–705. [Google Scholar] [CrossRef]
- Saito, T.; Bokhove, M.; Croci, R.; Zamora-Caballero, S.; Han, L.; Letarte, M.; de Sanctis, D.; Jovine, L. Structural Basis of the Human Endoglin-BMP9 Interaction: Insights into BMP Signaling and HHT1. Cell Rep. 2017, 19, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, R.; Werner, E.D.; Matthews, R.G.; Presman, E.; Mulivor, A.W.; Solban, N.; Sako, D.; Pearsall, R.S.; Underwood, K.W.; Seehra, J.; et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J. Biol. Chem. 2011, 286, 30034–30046. [Google Scholar] [CrossRef] [PubMed]
- Alt, A.; Miguel-Romero, L.; Donderis, J.; Aristorena, M.; Blanco, F.J.; Round, A.; Rubio, V.; Bernabeu, C.; Marina, A. Structural and functional insights into endoglin ligand recognition and binding. PLoS ONE 2012, 7, e29948. [Google Scholar] [CrossRef] [PubMed]
- Lawera, A.; Tong, Z.; Thorikay, M.; Redgrave, R.E.; Cai, J.; van Dinther, M.; Morrell, N.W.; Afink, G.B.; Charnock-Jones, D.S.; Arthur, H.M.; et al. Role of soluble endoglin in BMP9 signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 17800–17808. [Google Scholar] [CrossRef] [PubMed]
- Dyer, L.A.; Pi, X.; Patterson, C. The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol. Metab. 2014, 25, 472–480. [Google Scholar] [CrossRef]
- Cheifetz, S.; Bellón, T.; Calés, C.; Vera, S.; Bernabeu, C.; Massagué, J.; Letarte, M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem. 1992, 267, 19027–19030. [Google Scholar] [PubMed]
- Bellón, T.; Corbí, A.; Lastres, P.; Calés, C.; Cebrián, M.; Vera, S.; Cheifetz, S.; Massague, J.; Letarte, M.; Bernabéu, C. Identification and expression of two forms of the human transforming growth factor-beta-binding protein endoglin with distinct cytoplasmic regions. Eur. J. Immunol. 1993, 23, 2340–2345. [Google Scholar] [CrossRef]
- Lastres, P.; Letamendía, A.; Zhang, H.; Rius, C.; Almendro, N.; Raab, U.; López, L.A.; Langa, C.; Fabra, A.; Letarte, M.; et al. Endoglin modulates cellular responses to TGF-beta 1. J. Cell Biol. 1996, 133, 1109–1121. [Google Scholar] [CrossRef]
- Mahmoud, M.; Upton, P.D.; Arthur, H.M. Angiogenesis regulation by TGFβ signalling: Clues from an inherited vascular disease. Biochem. Soc. Trans. 2011, 39, 1659–1666. [Google Scholar] [CrossRef]
- Guerrero-Esteo, M.; Sanchez-Elsner, T.; Letamendia, A.; Bernabeu, C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II. J. Biol. Chem. 2002, 277, 29197–29209. [Google Scholar] [CrossRef]
- Blanco, F.J.; Santibanez, J.F.; Guerrero-Esteo, M.; Langa, C.; Vary, C.P.; Bernabeu, C. Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. J. Cell. Physiol. 2005, 204, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Bokhove, M.; Jovine, L. Structure of Zona Pellucida Module Proteins. Curr. Top. Dev. Biol. 2018, 130, 413–442. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Sanz-Rodriguez, F.; Eleno, N.; Düwell, A.; Blanco, F.J.; Langa, C.; Botella, L.M.; Cabañas, C.; Lopez-Novoa, J.M.; Bernabeu, C. Endothelial endoglin is involved in inflammation: Role in leukocyte adhesion and transmigration. Blood 2013, 121, 403–415. [Google Scholar] [CrossRef]
- Rossi, E.; Smadja, D.M.; Boscolo, E.; Langa, C.; Arevalo, M.A.; Pericacho, M.; Gamella-Pozuelo, L.; Kauskot, A.; Botella, L.M.; Gaussem, P.; et al. Endoglin regulates mural cell adhesion in the circulatory system. Cell. Mol. Life Sci. 2016, 73, 1715–1739. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Pericacho, M.; Bachelot-Loza, C.; Pidard, D.; Gaussem, P.; Poirault-Chassac, S.; Blanco, F.J.; Langa, C.; González-Manchón, C.; Lopez-Novoa, J.M.; et al. Human endoglin as a potential new partner involved in platelet-endothelium interactions. Cell. Mol. Life Sci. 2018, 75, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Bernabeu, C.; Smadja, D.M. Endoglin as an Adhesion Molecule in Mature and Progenitor Endothelial Cells: A Function Beyond TGF-β. Front. Med. (Lausanne). 2019, 6, 10. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef]
- Gregory, A.L.; Xu, G.; Sotov, V.; Letarte, M. Review: The enigmatic role of endoglin in the placenta. Placenta 2014, 35, S93–S99. [Google Scholar] [CrossRef]
- Li, C.; Guo, B.; Ding, S.; Rius, C.; Langa, C.; Kumar, P.; Bernabeu, C.; Kumar, S. TNF alpha down-regulates CD105 expression in vascular endothelial cells: A comparative study with TGF beta 1. Anticancer Res. 2003, 23, 1189–1196. [Google Scholar] [PubMed]
- Sunderland, N.S.; Thomson, S.E.; Heernan, S.J.; Lim, S.; Thompson, J.; Ogle, R.; McKenzie, P.; Kirwan, P.J.; Makris, A.; Hennessy, A. Tumor necrosis factor induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 2011, 56, 192–199. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.; Karumanchi, S.A. Preeclampsia. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Tomimatsu, T.; Mimura, K.; Matsuzaki, S.; Endo, M.; Kumasawa, K.; Kimura, T. Preeclampsia: Maternal Systemic Vascular Disorder Caused by Generalized Endothelial Dysfunction Due to Placental Antiangiogenic Factors. Int. J. Mol. Sci. 2019, 20, 4246. [Google Scholar] [CrossRef] [PubMed]
- Vitverova, B.; Blazickova, K.; Najmanova, I.; Vicen, M.; Hyšpler, R.; Dolezelova, E.; Nemeckova, I.; Tebbens, J.D.; Bernabeu, C.; Pericacho, M.; et al. Soluble endoglin and hypercholesterolemia aggravate endothelial and vessel wall dysfunction in mouse aorta. Atherosclerosis 2018, 271, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Vara, E.; Tual-Chalot, S.; Botella, L.M.; Arthur, H.M.; Bernabeu, C. Soluble endoglin regulates expression of angiogenesis-related proteins and induction of arteriovenous malformations in a mouse model of hereditary hemorrhagic telangiectasia. Dis. Model Mech. 2018, 11, dmm034397. [Google Scholar] [CrossRef]
- Vicen, M.; Vitverova, B.; Havelek, R.; Blazickova, K.; Machacek, M.; Rathouska, J.; Najmanová, I.; Dolezelova, E.; Prasnicka, A.; Sternak, M.; et al. Regulation and role of endoglin in cholesterol-induced endothelial and vascular dysfunction in vivo and in vitro. FASEB J. 2019, 33, 6099–6114. [Google Scholar] [CrossRef]
- Casado-Vela, J.; Martínez-Esteso, M.J.; Rodriguez, E.; Borrás, E.; Elortza, F.; Bru-Martínez, R. iTRAQ-based quantitative analysis of protein mixtures with large fold change and dynamic range. Proteomics 2010, 10, 343–347. [Google Scholar] [CrossRef]
- García-Palmero, I.; Torres, S.; Bartolomé, R.A.; Peláez-García, A.; Larriba, M.J.; Lopez-Lucendo, M.; Peña, C.; Escudero-Paniagua, B.; Muñoz, A.; Casal, J.I. Twist1-induced activation of human fibroblasts promotes matrix stiffness by upregulating palladin and collagen α1(VI). Oncogene 2016, 35, 5224–5236. [Google Scholar] [CrossRef]
- Gallardo-Vara, E. Soluble Endoglin: Generation Mechanism and Function in Endothelial Cells and its Effect in Vascular Remodeling. Ph.D. Thesis, Complutense University, Madrid, Spain, 2017. [Google Scholar]
- Pérez-Roque, L. El Papel de Endoglina Soluble en la Fisiopatología de la Preeclampsia. Ph.D. Thesis, University of Salamanca, Salamanca, Spain, 2017. [Google Scholar]
- Fink, G.D. Does Tail-Cuff Plethysmography Provide a Reliable Estimate of Central Blood Pressure in Mice? J. Am. Heart Assoc. 2017, 6, e006554. [Google Scholar] [CrossRef]
- Wu, J.; Yu, Z.; Su, D. BMP4 protects rat pulmonary arterial smooth muscle cells from apoptosis by PI3K/AKT/Smad1/5/8 signaling. Int. J. Mol. Sci. 2014, 15, 13738–13754. [Google Scholar] [CrossRef]
- Tang, Y.; Qian, S.W.; Wu, M.Y.; Wang, J.; Lu, P.; Li, X.; Huang, H.Y.; Guo, L.; Sun, X.; Xu, C.J.; et al. BMP4 mediates the interplay between adipogenesis and angiogenesis during expansion of subcutaneous white adipose tissue. J. Mol. Cell Biol. 2016, 8, 302–312. [Google Scholar] [CrossRef]
- Lochab, A.K.; Extavour, C.G. Bone Morphogenetic Protein (BMP) signaling in animal reproductive system development and function. Dev. Biol. 2017, 427, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Leaños-Miranda, A.; Navarro-Romero, C.S.; Sillas-Pardo, L.J.; Ramírez-Valenzuela, K.L.; Isordia-Salas, I.; Jiménez-Trejo, L.M. Soluble Endoglin As a Marker for Preeclampsia, Its Severity, and the Occurrence of Adverse Outcomes. Hypertension 2019, 74, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Schmella, M.J.; Assibey-Mensah, V.; Parks, W.T.; Roberts, J.M.; Jeyabalan, A.; Hubel, C.A.; Catov, J.M. Plasma concentrations of soluble endoglin in the maternal circulation are associated with maternal vascular malperfusion lesions in the placenta of women with preeclampsia. Placenta 2019, 78, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, I.; Karumanchi, S.A. Preeclampsia and the Anti-Angiogenic State. Pregnancy Hypertens. 2011, 1, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Afink, G.B.; Dijke, P.T. Soluble fms-like tyrosine kinase 1 and soluble endoglin are elevated circulating anti-angiogenic factors in pre-eclampsia. Pregnancy Hypertens. 2012, 2, 358–367. [Google Scholar] [CrossRef]
- Szpera-Gozdziewicz, A.; Breborowicz, G.H. Endothelial dysfunction in the pathogenesis of pre-eclampsia. Front. Biosci. (Landmark Ed) 2014, 19, 734–746. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Miriyala, S.; Gongora Nieto, M.C.; Mingone, C.; Smith, D.; Dikalov, S.; Harrison, D.G.; Jo, H. Bone morphogenic protein-4 induces hypertension in mice: Role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation 2006, 113, 2818–2825. [Google Scholar] [CrossRef]
- Wong, W.T.; Tian, X.Y.; Chen, Y.; Leung, F.P.; Liu, L.; Lee, H.K.; Ng, C.F.; Xu, A.; Yao, X.; Vanhoutte, P.M.; et al. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: Implications on hypertension. Circ. Res. 2010, 107, 984–991. [Google Scholar] [CrossRef]
- Hirsinger, E.; Duprez, D.; Jouve, C.; Malapert, P.; Cooke, J.; Pourquié, O. Noggin acts downstream of Wnt and Sonic Hedgehog to antagonize BMP4 in avian somite patterning. Development 1997, 124, 4605–4614. [Google Scholar] [PubMed]
- Krause, C.; Guzman, A.; Knaus, P. Noggin. Int. J. Biochem. Cell Biol. 2011, 43, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Brazil, D.P.; Church, R.H.; Surae, S.; Godson, C.; Martin, F. BMP signalling: Agony and antagony in the family. Trends Cell Biol. 2015, 25, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-L, A.; Garrido-Martin, E.M.; Sanz-Rodriguez, F.; Pericacho, M.; Rodriguez-Barbero, A.; Eleno, N.; Lopez-Novoa, J.M.; Düwell, A.; Vega, M.A.; Bernabeu, C.; et al. Gene expression fingerprinting for human hereditary hemorrhagic telangiectasia. Hum. Mol. Genet. 2007, 16, 1515–1533. [Google Scholar] [CrossRef]
- Thomas, B.; Eyries, M.; Montagne, K.; Martin, S.; Agrapart, M.; Simerman-François, R.; Letarte, M.; Soubrier, F. Altered endothelial gene expression associated with hereditary haemorrhagic telangiectasia. Eur. J. Clin. Investig. 2007, 37, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Ojeda-Fernandez, L.; Aristorena, M.; Gallardo-Vara, E.; Benguria, A.; Dopazo, A.; Langa, C.; Botella, L.M.; Bernabeu, C. Genome-wide transcriptional and functional analysis of endoglin isoforms in the human promonocytic cell line U937. J. Cell. Physiol. 2015, 230, 947–958. [Google Scholar] [CrossRef]
- Gallardo-Vara, E.; Ruiz-Llorente, L.; Casado-Vela, J.; Ruiz-Rodríguez, M.J.; López-Andrés, N.; Pattnaik, A.K.; Quintanilla, M.; Bernabeu, C. Endoglin Protein Interactome Profiling Identifies TRIM21 and Galectin-3 as New Binding Partners. Cells 2019, 8, 1082. [Google Scholar] [CrossRef]
- Powe, C.; Levine, R.; Karumanchi, S. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Haramis, A.P.; Begthel, H.; van den Born, M.; van Es, J.; Jonkheer, S.; Offerhaus, G.J.; Clevers, H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 2004, 303, 1684–1686. [Google Scholar] [CrossRef]
- Ishizuya-Oka, A.; Hasebe, T. Sonic hedgehog and bone morphogenetic protein-4 signaling pathway involved in epithelial cell renewal along the radial axis of the intestine. Digestion 2008, 77 (Suppl. 1), 42–47. [Google Scholar] [CrossRef]
- Frank, D.B.; Abtahi, A.; Yamaguchi, D.J.; Manning, S.; Shyr, Y.; Pozzi, A.; Baldwin, H.S.; Johnson, J.E.; de Caestecker, M.P. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ. Res. 2005, 97, 496–504. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Li, Y.; Southwood, M.; Ye, L.; Long, L.; Al-Lamki, R.S.; Morrell, N.W. Id proteins are critical downstream effectors of BMP signaling in human pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L312–L321. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Kovacs, L.; Dong, S.; Wu, G.; Su, Y. BMP4 inhibits PDGF-induced proliferation and collagen synthesis via PKA-mediated inhibition of calpain-2 in pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L638–L648. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.L.; Yang, J.; Strulovici-Barel, Y.; Salit, J.; Rostami, M.; Mezey, J.G.; O’Beirne, S.L.; Kaner, R.J.; Crystal, R.G. Exaggerated BMP4 signalling alters human airway basal progenitor cell differentiation to cigarette smoking-related phenotypes. Eur. Respir. J. 2019, 53, 1702553. [Google Scholar] [CrossRef]
- Buda, V.; Andor, M.; Baibata, D.E.; Cozlac, R.; Radu, G.; Coricovac, D.; Danciu, C.; Ledeti, I.; Cheveresan, A.; Nica, C.; et al. Decreased sEng plasma levels in hypertensive patients with endothelial dysfunction under chronic treatment with Perindopril. Drug Des. Devel. Ther. 2019, 13, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Tian, X.Y.; Wong, W.T.; Chen, Y.; Wang, L.; Luo, J.; Cheang, W.S.; Lau, C.W.; Kwan, K.M.; et al. Inhibition of bone morphogenic protein 4 restores endothelial function in db/db diabetic mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Varejckova, M.; Gallardo-Vara, E.; Vicen, M.; Vitverova, B.; Fikrova, P.; Dolezelova, E.; Rathouska, J.; Prasnicka, A.; Blazickova, K.; Micuda, S.; et al. Soluble endoglin modulates the pro-inflammatory mediators NF-κB and IL-6 in cultured human endothelial cells. Life Sci. 2017, 175, 52–60. [Google Scholar] [CrossRef]
- Honsawek, S.; Tanavalee, A.; Yuktanandana, P. Elevated circulating and synovial fluid endoglin are associated with primary knee osteoarthritis severity. Arch. Med. Res. 2009, 40, 590–594. [Google Scholar] [CrossRef]
- Blázquez-Medela, A.M.; García-Ortiz, L.; Gómez-Marcos, M.A.; Recio-Rodríguez, J.I.; Sánchez-Rodríguez, A.; López-Novoa, J.M.; Martínez-Salgado, C. Increased plasma soluble endoglin levels as an indicator of cardiovascular alterations in hypertensive and diabetic patients. BMC Med. 2010, 8, 86. [Google Scholar] [CrossRef]
- Pohl, D.; Andrýs, C.; Borská, L.; Fiala, Z.; Hamaková, K.; Ettler, K.; Krejsek, J. Serum level of a soluble form of endoglin (CD105) is decreased after Goeckerman’s therapy of psoriasis. Acta Med. (Hradec Kral) 2011, 54, 59–62. [Google Scholar] [CrossRef][Green Version]
- Ligi, D.; Croce, L.; Mosti, G.; Raffetto, J.D.; Mannello, F. Chronic Venous Insufficiency: Transforming Growth Factor-β Isoforms and Soluble Endoglin Concentration in Different States of Wound Healing. Int. J. Mol. Sci. 2017, 18, 2206. [Google Scholar] [CrossRef]
- Li, Q.; Lin, F.; Ke, D.; Cheng, Q.; Gui, Y.; Zhou, Y.; Wu, Y.; Wang, Y.; Zhu, P. Combination of Endoglin and ASCVD Risk Assessment Improves Carotid Subclinical Atherosclerosis Recognition. J. Atheroscler. Thromb. 2020, 27, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo-Vara, E.; Gamella-Pozuelo, L.; Perez-Roque, L.; Bartha, J.L.; Garcia-Palmero, I.; Casal, J.I.; López-Novoa, J.M.; Pericacho, M.; Bernabeu, C. Potential Role of Circulating Endoglin in Hypertension via the Upregulated Expression of BMP4. Cells 2020, 9, 988. https://doi.org/10.3390/cells9040988
Gallardo-Vara E, Gamella-Pozuelo L, Perez-Roque L, Bartha JL, Garcia-Palmero I, Casal JI, López-Novoa JM, Pericacho M, Bernabeu C. Potential Role of Circulating Endoglin in Hypertension via the Upregulated Expression of BMP4. Cells. 2020; 9(4):988. https://doi.org/10.3390/cells9040988
Chicago/Turabian StyleGallardo-Vara, Eunate, Luis Gamella-Pozuelo, Lucía Perez-Roque, José L. Bartha, Irene Garcia-Palmero, J. Ignacio Casal, José M. López-Novoa, Miguel Pericacho, and Carmelo Bernabeu. 2020. "Potential Role of Circulating Endoglin in Hypertension via the Upregulated Expression of BMP4" Cells 9, no. 4: 988. https://doi.org/10.3390/cells9040988
APA StyleGallardo-Vara, E., Gamella-Pozuelo, L., Perez-Roque, L., Bartha, J. L., Garcia-Palmero, I., Casal, J. I., López-Novoa, J. M., Pericacho, M., & Bernabeu, C. (2020). Potential Role of Circulating Endoglin in Hypertension via the Upregulated Expression of BMP4. Cells, 9(4), 988. https://doi.org/10.3390/cells9040988







