Social Stress Increases Vulnerability to High-Fat Diet-Induced Insulin Resistance by Enhancing Neutrophil Elastase Activity in Adipose Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Assessment of Body Weight and Food Intake
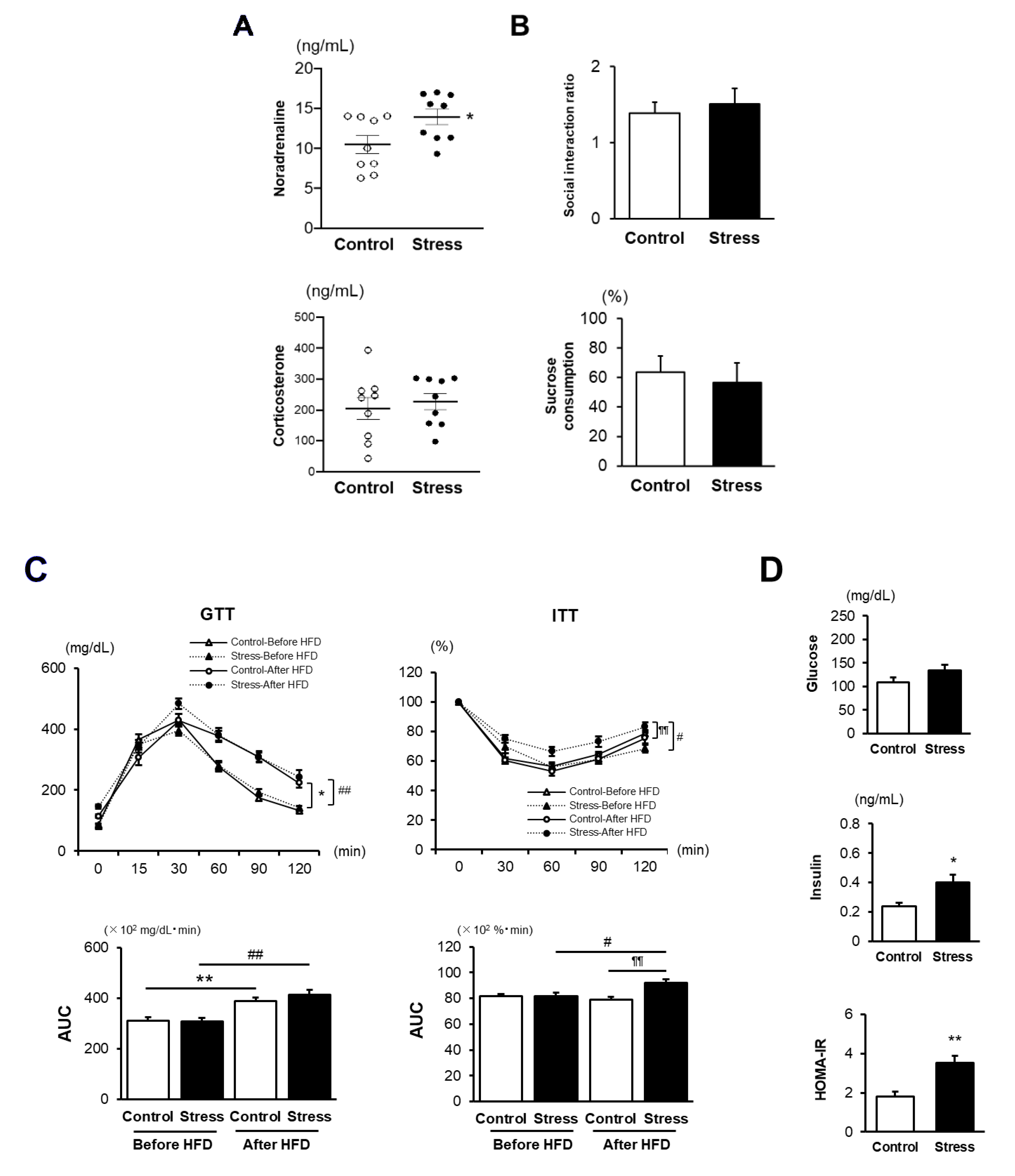
2.3. Behavior Analysis
2.4. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
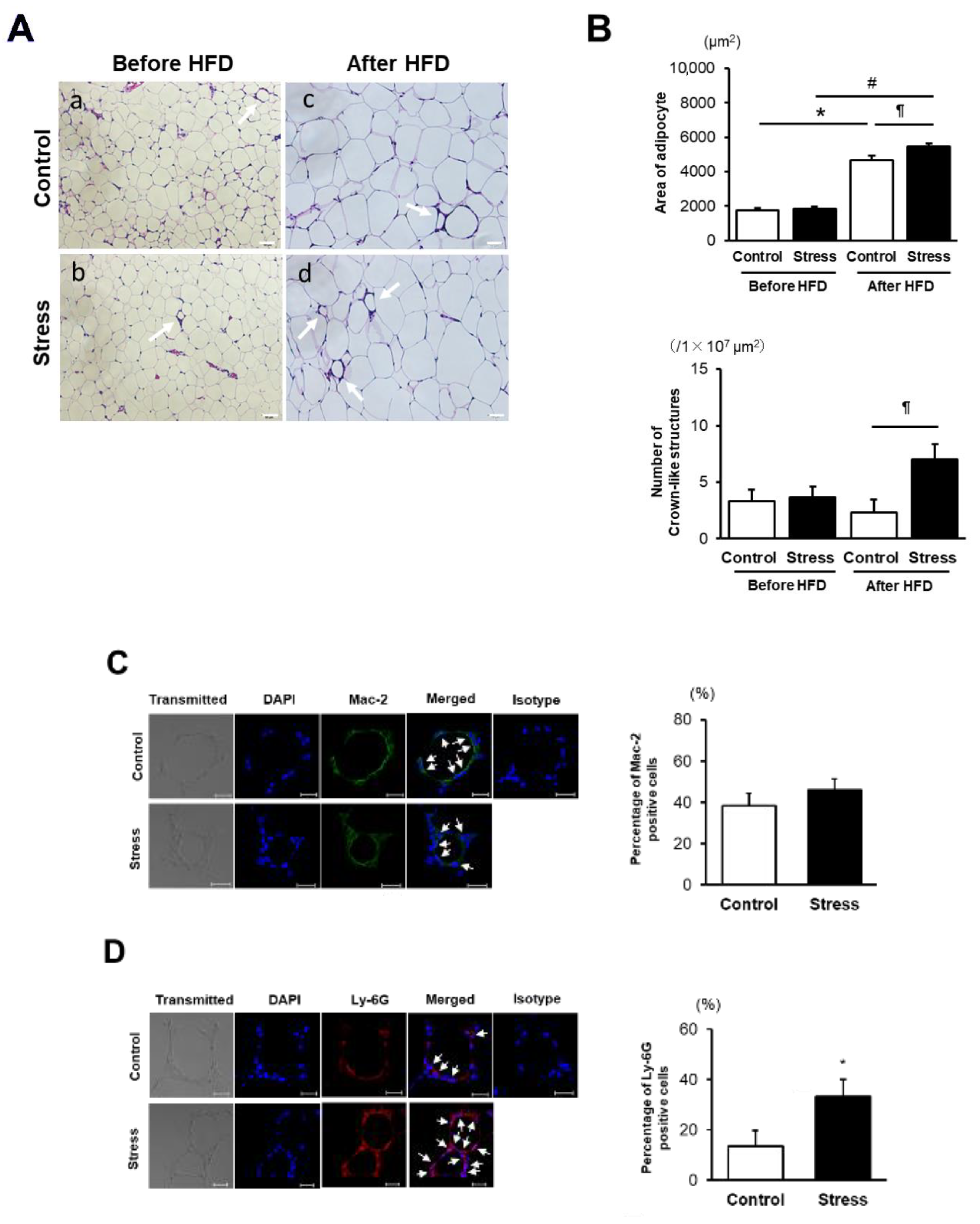
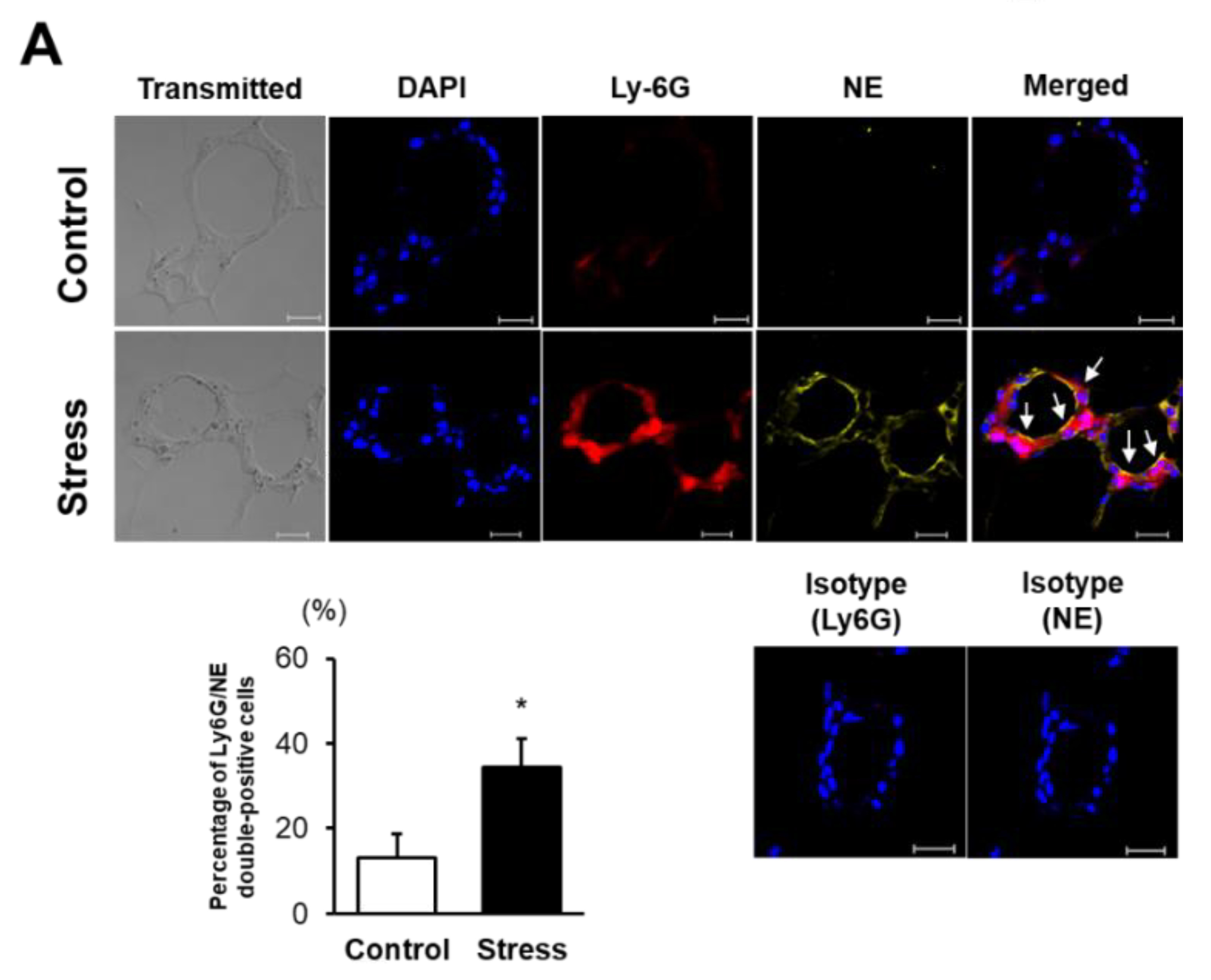
2.6. Immunohistochemistry
2.7. Blood Leukocyte Counts
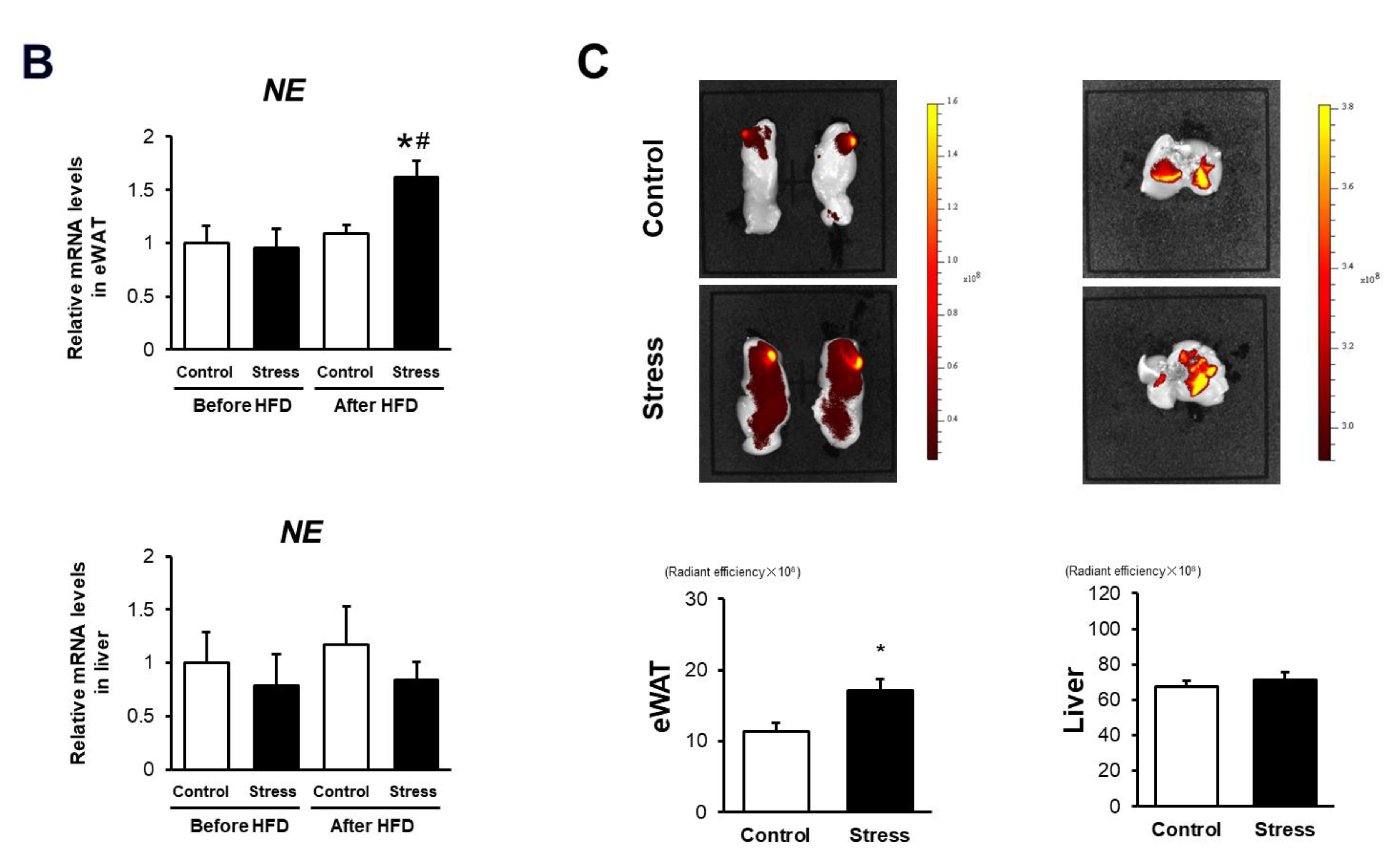
2.8. Real-Time Polymerase Chain Reaction (RT-PCR)
2.9. Flow Cytometry and Cell Sorting (FACS)
2.10. Ex Vivo Neutrophil Elastase (NE) Activity
2.11. In Vitro Activation of Bone Marrow (BM) Neutrophils Upon Stimulation with Formyl Peptide Receptor (FPR) 1 Agonist
2.12. Statistical Analysis
3. Results
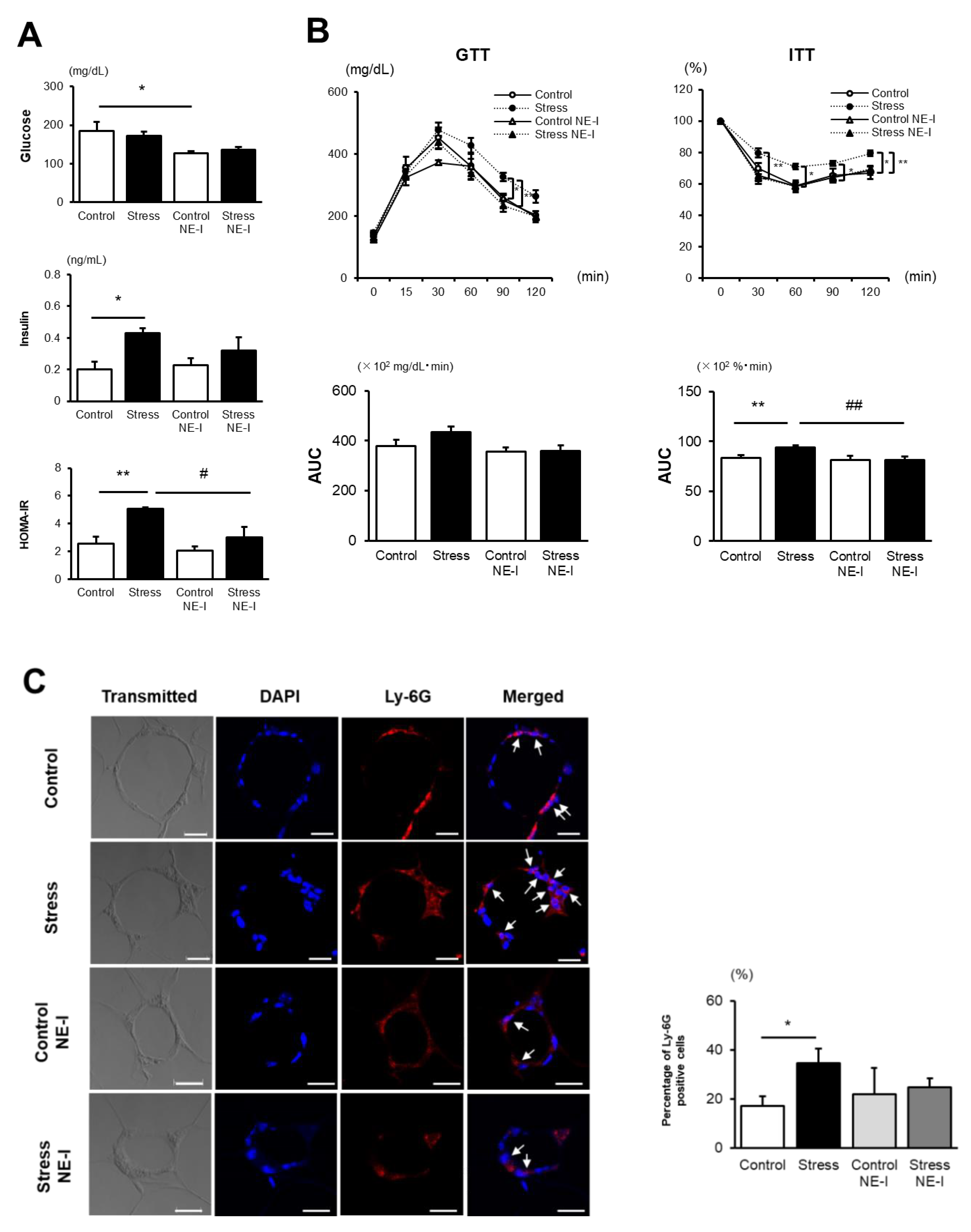
3.1. SS Increases the Vulnerability to the Development of HFD-Induced IR
3.2. SS Augments Neutrophil Accumulation in eWAT
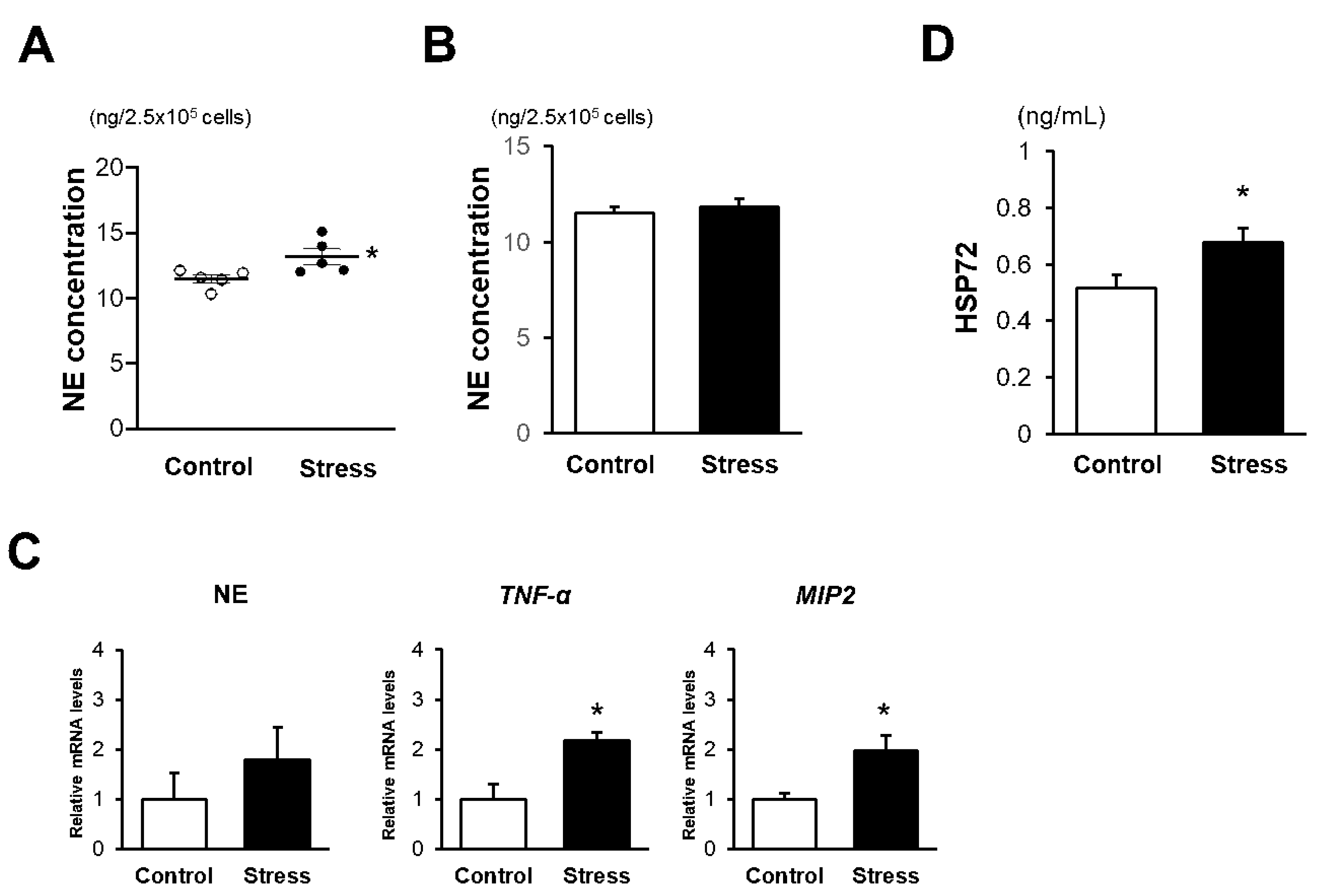
3.3. NE Activation Is Augmented in eWAT of Stressed Mice
3.4. NE Inhibitor Treatment Completely Abolishes the Accelerated IR Development in Stressed Mice
3.5. In Vitro NE Release Is Augmented in BM Neutrophils of Stressed Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nabi, H.; Kivimäki, M.; Batty, G.D.; Shipley, M.J.; Britton, A.; Brunner, E.J.; Vahtera, J.; Lemogne, C.; Elbaz, A.; Singh-Manoux, A. Increased risk of coronary heart disease among individuals reporting adverse impact of stress on their health: The Whitehall II prospective cohort study. Eur. Heart J. 2013, 34, 2697–2705. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Nyberg, S.T.; Batty, G.D.; Fransson, E.I.; Heikkilä, K.; Alfredsson, L.; Bjorner, J.B.; Borritz, M.; Burr, H.; Casini, A.; et al. Consortium. Job strain as a risk factor for coronary heart disease: A collaborative meta-analysis of individual participant data. Lancet 2012, 380, 1491–1497. [Google Scholar] [CrossRef]
- Chandola, T.; Brunner, E.; Marmot, M. Chronic stress at work and the metabolic syndrome: Prospective study. BMJ 2006, 332, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Sun, N.; Zhan, L.; Lu, X.; Chen, T.; Mao, X. Association between work-related stress and risk for type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. PLoS ONE 2016, 11, e0159978. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Chawla, A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013, 339, 172–177. [Google Scholar] [CrossRef]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef]
- Laakso, M.; Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 2014, 10, 293–302. [Google Scholar] [CrossRef]
- Uchida, Y.; Takeshita, K.; Yamamoto, K.; Kikuchi, R.; Nakayama, T.; Nomura, M.; Cheng, X.W.; Egashira, K.; Matsushita, T.; Nakamura, H.; et al. Stress augments insulin resistance and prothrombotic state: Role of visceral adipose-derived monocyte chemoattractant protein-1. Diabetes 2012, 61, 1552–1561. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Fang, C.; Zhang, H.; Yang, J.; Xuan, C.; Wang, F.; Lu, H.; Cao, S.; Wang, Y.; et al. Chronic noise-exposure exacerbates insulin resistance and promotes the manifestations of the type 2 diabetes in a high-fat diet mouse model. PLoS ONE 2018, 13, e0195411. [Google Scholar] [CrossRef]
- Pasquali, R. The hypothalamic-pituitary-adrenal axis and sex hormones in chronic stress and obesity: Pathophysiological and clinical aspects. Ann. N. Y. Acad. Sci. 2012, 1264, 20–35. [Google Scholar] [CrossRef]
- Hering, D.; Lachowska, K.; Schlaich, M. Role of the sympathetic nervous system in stress-mediated cardiovascular disease. Curr. Hypertens. Rep. 2015, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Battista, M.; Kao, W.M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.D.; Sloan, E.K.; Bailey, M.T.; Arevalo, J.M.; Miller, G.E.; Chen, E.; Kobor, M.S.; Reader, B.F.; Sheridan, J.F.; Cole, S.W. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA 2013, 110, 16574–16579. [Google Scholar] [CrossRef]
- Heidt, T.; Sager, H.B.; Courties, G.; Dutta, P.; Iwamoto, Y.; Zaltsman, A.; von Zur Muhlen, C.; Bode, C.; Fricchione, G.L.; Denninger, J.; et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014, 20, 754–758. [Google Scholar] [CrossRef]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef]
- Mansuy-Aubert, V.; Zhou, Q.L.; Xie, X.; Gong, Z.; Huang, J.Y.; Khan, A.R.; Aubert, G.; Candelaria, K.; Thomas, S.; Shin, D.J.; et al. Imbalance between neutrophil elastase and its inhibitor α1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013, 17, 534–548. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E., 3rd; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef]
- Vincent, M.Y.; Jacobson, L. Glucocorticoid receptor deletion from the dorsal raphé nucleus of mice reduces dysphoria-like behavior and impairs hypothalamic-pituitary-adrenocortical axis feedback inhibition. Eur. J. Neurosci. 2014, 39, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Libby, P.; Aikawa, E.; Alcaide, P.; Luscinskas, F.W.; Weissleder, R.; Pittet, M.J. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Investig. 2007, 117, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.W.; Morris, D.L.; Lumeng, C.N. Flow cytometry analyses of adipose tissue macrophages. Methods Enzymol. 2014, 537, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Taggart, C.; Coakley, R.J.; Greally, P.; Canny, G.; O’Neill, S.J.; McElvaney, N.G. Increased elastase release by CF neutrophils is mediated by tumor necrosis factor-alpha and interleukin-8. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L33–L41. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Johnson, J.D.; Campisi, J.; Sharkey, C.M.; Kennedy, S.L.; Nickerson, M.; Fleshner, M. Adrenergic receptors mediate stress-induced elevations in extracellular Hsp72. J. Appl. Physiol. 2005, 99, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.S.; Chase, M.A.; Senft, A.P.; Poynter, S.E.; Wong, H.R.; Page, K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respir. Res. 2009, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, M.; Frank, M.; Maier, S.F. Danger signals and inflammasomes: Stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology 2017, 42, 36–45. [Google Scholar] [CrossRef]
- Fleshner, M.; Crane, C.R. Exosomes, DAMPs and miRNA: Features of stress physiology and immune homeostasis. Trends Immunol. 2017, 38, 768–776. [Google Scholar] [CrossRef]
- Chung, J.; Nguyen, A.K.; Henstridge, D.C.; Holmes, A.G.; Chan, M.H.; Mesa, J.L.; Lancaster, G.I.; Southgate, R.J.; Bruce, C.R.; Duffy, S.J.; et al. HSP72 protects against obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 1739–1744. [Google Scholar] [CrossRef]
- Henstridge, D.C.; Bruce, C.R.; Drew, B.G.; Tory, K.; Kolonics, A.; Estevez, E.; Chung, J.; Watson, N.; Gardner, T.; Lee-Young, R.S.; et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 2014, 63, 1881–1894. [Google Scholar] [CrossRef]
- Rodrigues-Krause, J.; Krause, M.; O’Hagan, C.; De Vito, G.; Boreham, C.; Murphy, C.; Newsholme, P.; Colleran, G. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: Does fat matter? Cell Stress Chaperones 2012, 17, 293–302. [Google Scholar] [CrossRef]
- Krause, M.; Keane, K.; Rodrigues-Krause, J.; Crognale, D.; Egan, B.; De Vito, G.; Murphy, C.; Newsholme, P. Elevated levels of extracellular heat-shock protein 72 (eHSP72) are positively correlated with insulin resistance in vivo and cause pancreatic β-cell dysfunction and death in vitro. Clin. Sci. 2014, 126, 739–752. [Google Scholar] [CrossRef]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Brake, D.K.; Smith, E.O.; Mersmann, H.; Smith, C.W.; Robker, R.L. ICAM-1 expression in adipose tissue: Effects of diet-induced obesity in mice. Am. J. Physiol. Cell. Physiol. 2006, 291, C1232–C1239. [Google Scholar] [CrossRef]
- Walsh, D.E.; Greene, C.M.; Carroll, T.P.; Taggart, C.C.; Gallagher, P.M.; O’Neill, S.J.; McElvaney, N.G. Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J. Biol. Chem. 2001, 276, 35494–35499. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Wernstedt, A.I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef]
- Laurencikiene, J.; van Harmelen, V.; Arvidsson, N.E.; Dicker, A.; Blomqvist, L.; Näslund, E.; Langin, D.; Arner, P.; Rydén, M. NF-kappaB is important for TNF-alpha-induced lipolysis in human adipocytes. J. Lipid Res. 2007, 48, 1069–1077. [Google Scholar] [CrossRef]
- Ranjit, S.; Boutet, E.; Gandhi, P.; Prot, M.; Tamori, Y.; Chawla, A.; Greenberg, A.S.; Puri, V.; Czech, M.P. Regulation of fat specific protein 27 by isoproterenol and TNF-α to control lipolysis in murine adipocytes. J. Lipid Res. 2011, 52, 221–236. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motoyama, S.; Yamada, H.; Yamamoto, K.; Wakana, N.; Terada, K.; Kikai, M.; Wada, N.; Saburi, M.; Sugimoto, T.; Kubota, H.; et al. Social Stress Increases Vulnerability to High-Fat Diet-Induced Insulin Resistance by Enhancing Neutrophil Elastase Activity in Adipose Tissue. Cells 2020, 9, 996. https://doi.org/10.3390/cells9040996
Motoyama S, Yamada H, Yamamoto K, Wakana N, Terada K, Kikai M, Wada N, Saburi M, Sugimoto T, Kubota H, et al. Social Stress Increases Vulnerability to High-Fat Diet-Induced Insulin Resistance by Enhancing Neutrophil Elastase Activity in Adipose Tissue. Cells. 2020; 9(4):996. https://doi.org/10.3390/cells9040996
Chicago/Turabian StyleMotoyama, Shinichiro, Hiroyuki Yamada, Keita Yamamoto, Noriyuki Wakana, Kensuke Terada, Masakazu Kikai, Naotoshi Wada, Makoto Saburi, Takeshi Sugimoto, Hiroshi Kubota, and et al. 2020. "Social Stress Increases Vulnerability to High-Fat Diet-Induced Insulin Resistance by Enhancing Neutrophil Elastase Activity in Adipose Tissue" Cells 9, no. 4: 996. https://doi.org/10.3390/cells9040996
APA StyleMotoyama, S., Yamada, H., Yamamoto, K., Wakana, N., Terada, K., Kikai, M., Wada, N., Saburi, M., Sugimoto, T., Kubota, H., Miyawaki, D., Kami, D., Ogata, T., Ibi, M., Yabe-Nishimura, C., & Matoba, S. (2020). Social Stress Increases Vulnerability to High-Fat Diet-Induced Insulin Resistance by Enhancing Neutrophil Elastase Activity in Adipose Tissue. Cells, 9(4), 996. https://doi.org/10.3390/cells9040996




