Transplantation of Endothelial Progenitor Cells in Obese Diabetic Rats Following Myocardial Infarction: Role of Thymosin Beta-4
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Peripheral Blood Mononuclear Cell Isolation and Culture
2.3. EPC Isolation and Culture
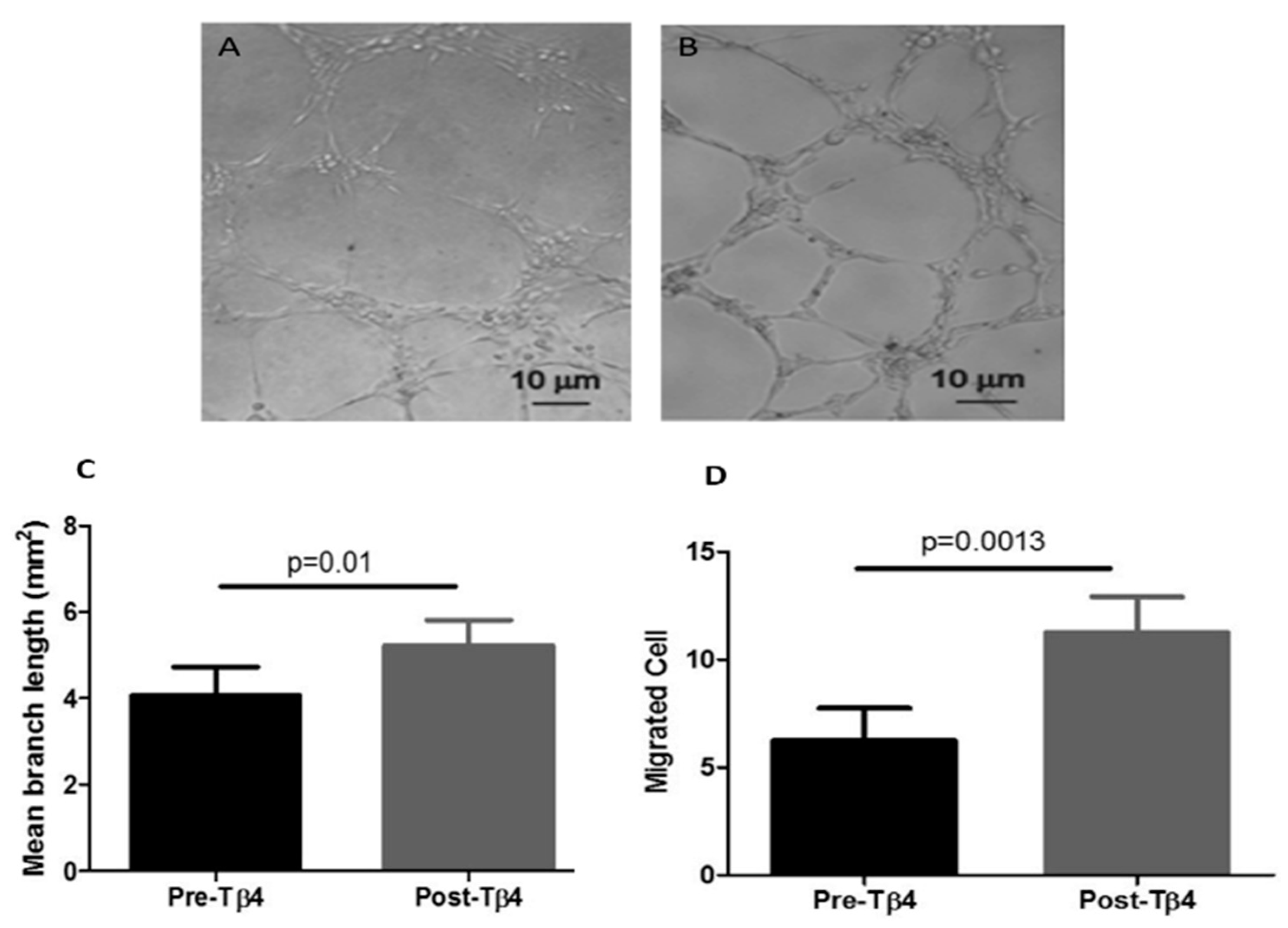
2.4. Migration and Tubule Formation Assays
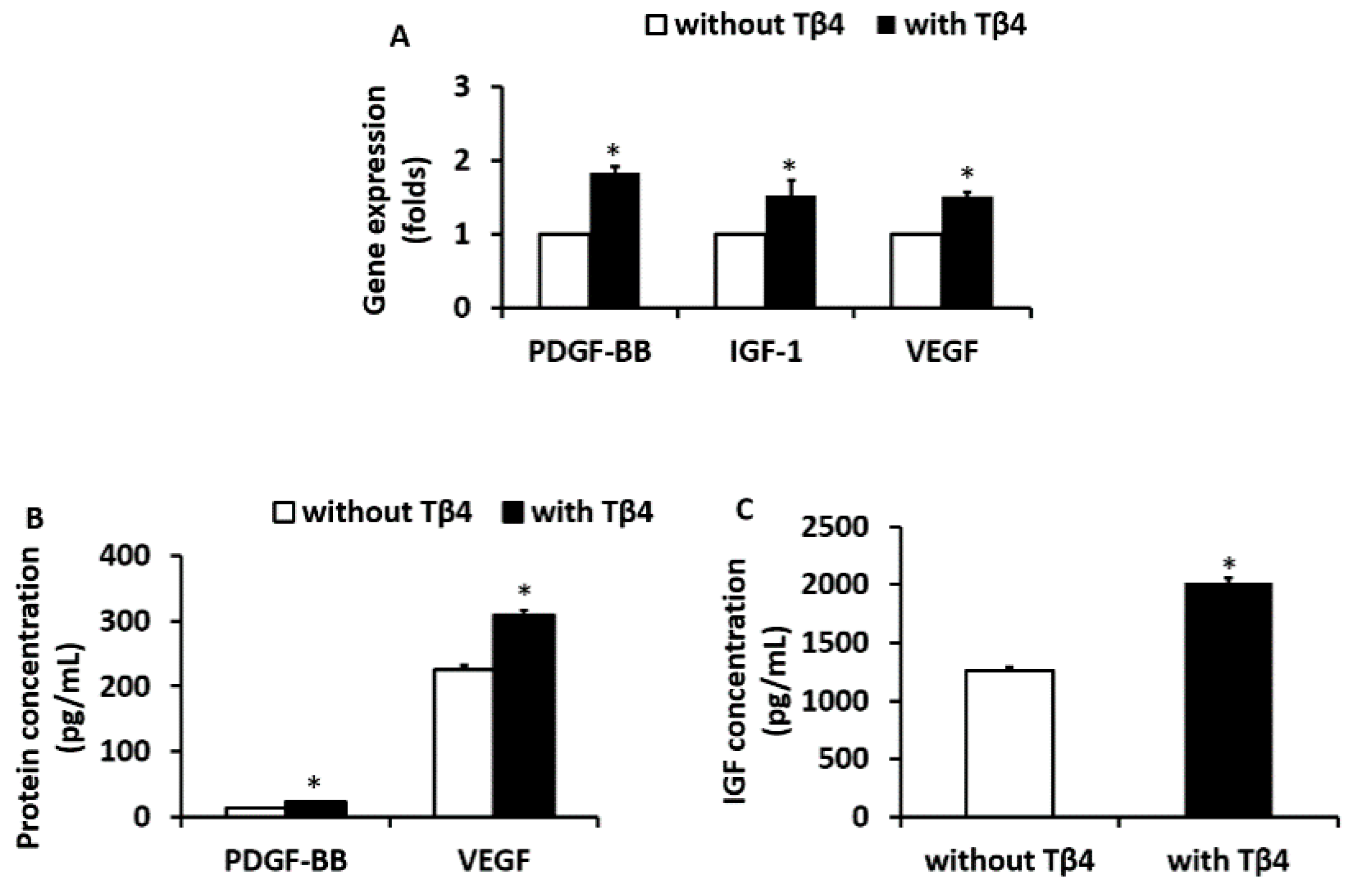
2.5. Quantification of Paracrine Factors Released by EPCs
2.6. Rat Heart Model of Myocardial Infarction and EPC Transplantation
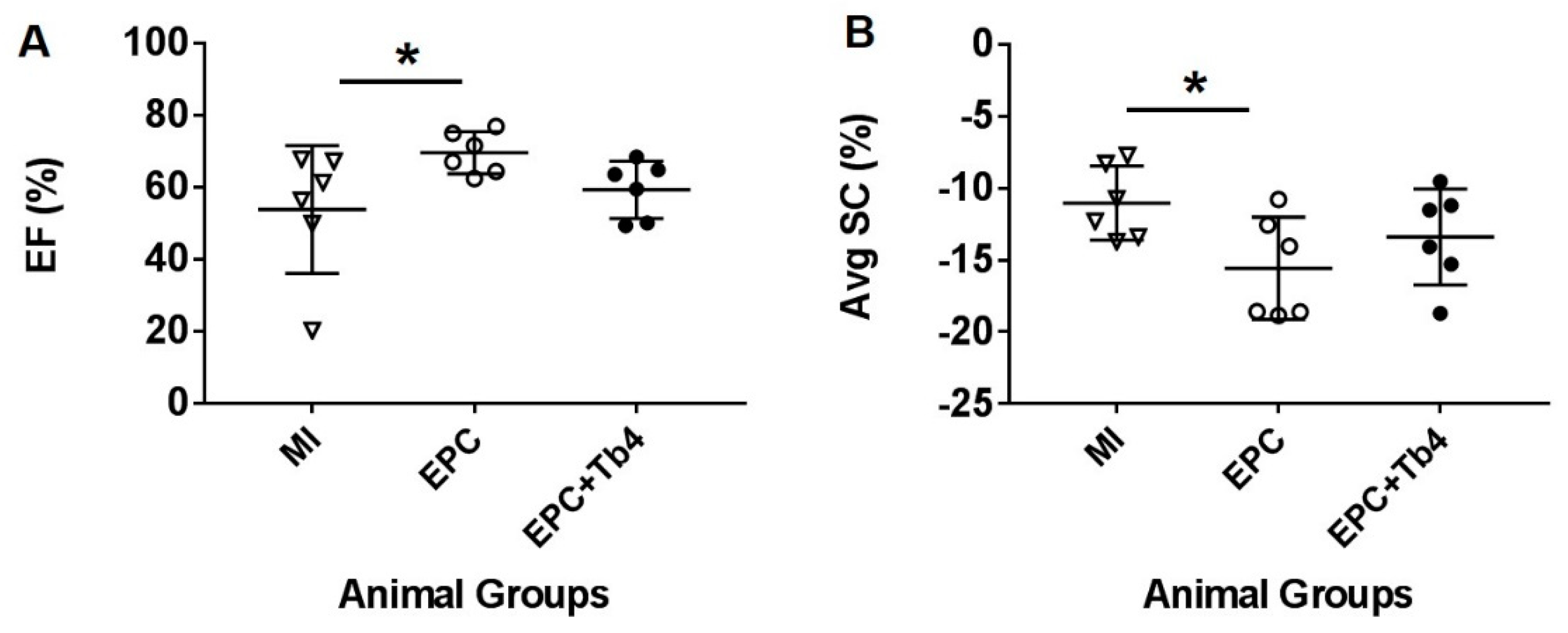
2.7. Echocardiographic Protocol
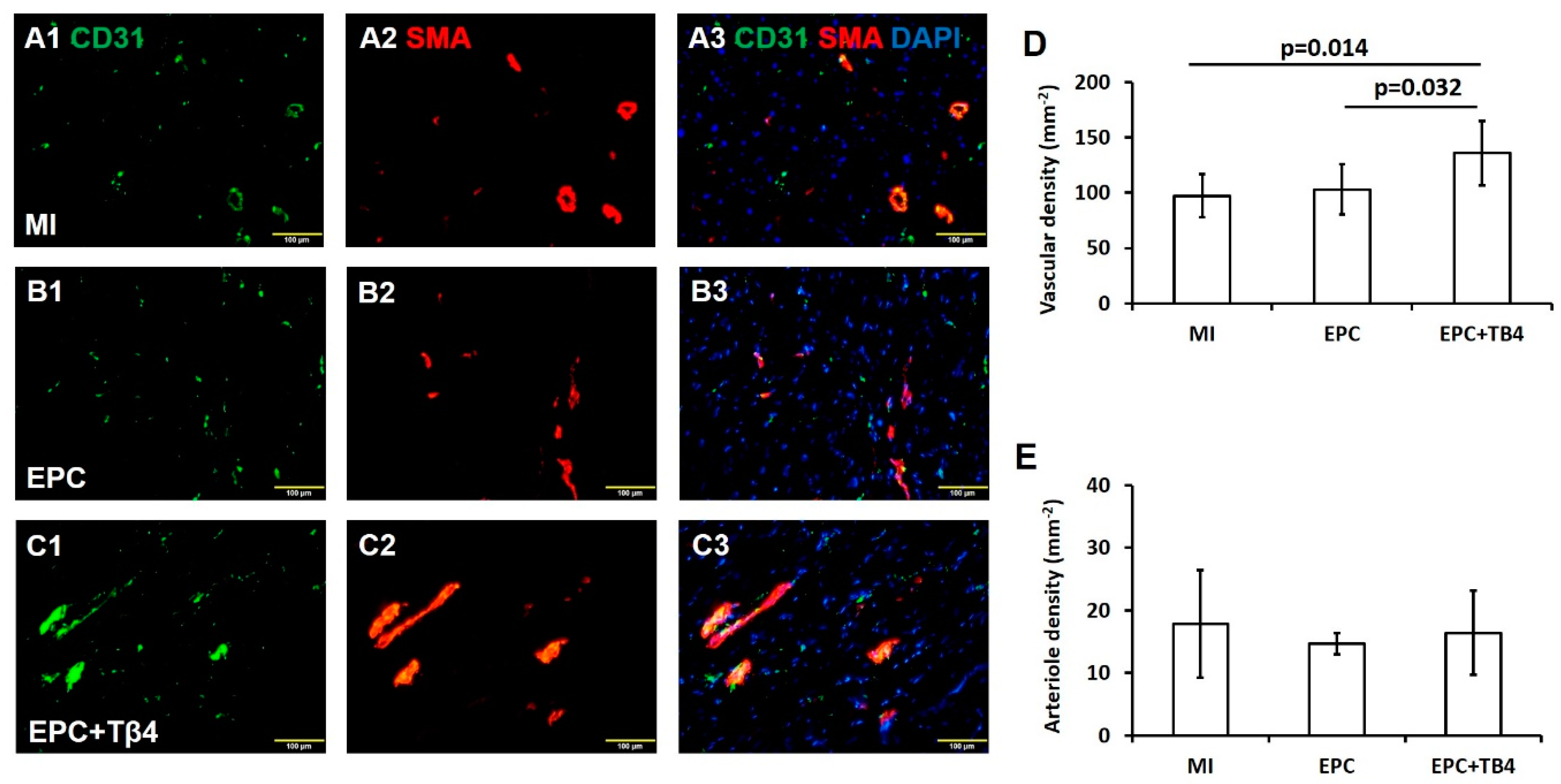
2.8. Immunohistochemistry Assessments
2.9. Statistical Analysis
3. Results
3.1. Tβ4 Treatment Increased Diabetic EPC Migration and Tubule Formation
3.2. Tβ4 Treatment Increased the Expression of Angiogenic Growth Factors from EPCs Isolated from ZDF Rats
3.3. EPC but not EPC + Tβ4 Treatment Improved Cardiac Function
3.4. Tβ4 Treatment EPCs Increased Angiogenesis Post-Myocardial Infarction (MI)
3.5. Tβ4 Treated EPCs Increases the Recruitment of Endogenous Cardiac Progenitor Cells (CPCs) Post-MI
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethics Approval
Abbreviations
| CPCs | Cardiac progenitor cells |
| DM | Diabetes Mellitus |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| eNOS | Endothelial nitric oxide synthase |
| FBS | Fetal bovine serum |
| FITC | Fluorescein isothiocyanate |
| hFGF-B | Human fibroblast growth factor-B |
| IGF-1 | Insulin growth factor-1 |
| IHD | Ischemic heart disease |
| mRNA | Messenger ribonucleic acid |
| MI | myocardial infarction |
| MSCs | Mesenchymal stem cells |
| PBS | Phosphate buffered saline |
| PCR | Polymerase chain reaction |
| PDGF-BB | Platelet-derived growth factor-BB |
| rhEGF | Recombinant human epidermal growth factor |
| RNA | Ribonucleic acid |
| SMA | Smooth muscle actin |
| TRITC | Tetramethylrhodamine |
| VEGF | Vascular endothelial growth factor |
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Desouza, C.V.; Hamel, F.G.; Bidasee, K.; O’Connell, K. Role of Inflammation and Insulin Resistance in Endothelial Progenitor Cell Dysfunction. Diabetes 2011, 60, 1286–1294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devaraj, S.; Jialal, I. Dysfunctional endothelial progenitor cells in metabolic syndrome. Exp. Diabetes Res. 2012, 2012, 585018. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organisation: Obesity and Overweight Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 December 2018).
- Steinberger, J.; Daniels, S.R. Obesity, Insulin Resistance, Diabetes, and Cardiovascular Risk in Children. Circulation 2003, 107, 1448–1453. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Mechanisms of Disease: Endothelial dysfunction in insulin resistance and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 46–56. [Google Scholar] [CrossRef]
- Sosne, G.; Qiu, P.; Goldstein, A.L.; Wheater, M. Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB J. 2010, 24, 2144–2151. [Google Scholar] [CrossRef]
- Hinkel, R.; Klett, K.; Bähr, A.; Kupatt, C. Thymosin β4-mediated protective effects in the heart. Expert Opin. Biol. Ther. 2018, 18, 121–129. [Google Scholar] [CrossRef]
- Bock-Marquette, I.; Saxena, A.; White, M.D.; DiMaio, J.M.; Srivastava, D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 2004, 432, 466–472. [Google Scholar] [CrossRef]
- Hinkel, R.; El-Aouni, C.; Olson, T.; Horstkotte, J.; Mayer, S.; Muller, S.; Willhauck, M.; Spitzweg, C.; Gildehaus, F.J.; Munzing, W.; et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation 2008, 117, 2232–2240. [Google Scholar] [CrossRef]
- Philp, D.; Huff, T.; Gho, Y.S.; Hannappel, E.; Kleinman, H.K. The actin binding site on thymosin β4 promotes angiogenesis. FASEB J. 2003, 17, 2103–2105. [Google Scholar] [CrossRef]
- Smart, N.; Risebro, C.A.; Melville, A.A.; Moses, K.; Schwartz, R.J.; Chien, K.R.; Riley, P.R. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007, 445, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Haider, H.; Esa, W.B.; Law, P.K.; Zhang, W.; Su, L.; Zhang, Y.; Sim, E.K. Nonviral vector-based gene transfection of primary human skeletal myoblasts. Exp. Biol. Med. (Maywood) 2007, 232, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Haider, H.; Tan, R.; Toh, W.; Law, P.K.; Tan, W.; Su, L.; Zhang, W.; Ge, R.; Zhang, Y.; et al. Transplantation of nanoparticle transfected skeletal myoblasts overexpressing vascular endothelial growth factor-165 for cardiac repair. Circulation 2007, 116, I113–I120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ye, L.; Zhang, P.; Duval, S.; Su, L.; Xiong, Q.; Zhang, J. Thymosin beta4 increases the potency of transplanted mesenchymal stem cells for myocardial repair. Circulation 2013, 128, S32–S41. [Google Scholar] [CrossRef] [PubMed]
- Skeehan, T.M.; Schuler, H.G.; Riley, J.L. Comparison of the alteration of cardiac function by sevoflurane, isoflurane, and halothane in the isolated working rat heart. J. Cardiothorac. Vasc. Anesth. 1995, 9, 706–712. [Google Scholar] [CrossRef]
- Djohan, A.H.; Sia, C.H.; Lee, P.S.; Poh, K.K. Endothelial Progenitor Cells in Heart Failure: An Authentic Expectation for Potential Future Use and a Lack of Universal Definition. J. Cardiovasc. Transl. Res. 2018, 11, 393–402. [Google Scholar] [CrossRef]
- Marks, E.D.; Kumar, A. Thymosin beta4: Roles in Development, Repair, and Engineering of the Cardiovascular System. Vitam. Horm. 2016, 102, 227–249. [Google Scholar]
- Yang, H.; Cheng, X.; Yao, Q.; Li, J.; Ju, G. The promotive effects of thymosin beta4 on neuronal survival and neurite outgrowth by upregulating L1 expression. Neurochem. Res. 2008, 33, 2269–2280. [Google Scholar] [CrossRef]
- Philp, D.; Nguyen, M.; Scheremeta, B.; St-Surin, S.; Villa, A.M.; Orgel, A.; Kleinman, H.K.; Elkin, M. Thymosin β4 increases hair growth by activation of hair follicle stem cells. FASEB J. 2004, 18, 385–387. [Google Scholar] [CrossRef]
- Chiu, L.L.Y.; Radisic, M. Controlled release of thymosin β4 using collagen–chitosan composite hydrogels promotes epicardial cell migration and angiogenesis. J. Control. Release 2011, 155, 376–385. [Google Scholar] [CrossRef]
- Tokura, Y.; Nakayama, Y.; Fukada, S.; Nara, N.; Yamamoto, H.; Matsuda, R.; Hara, T. Muscle injury-induced thymosin beta4 acts as a chemoattractant for myoblasts. J. Biochem. 2011, 149, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Li-Ping, S.; Wei-Feng, P.; Peter, K.L. Role of Thymosin β4 on Skeletal Myoblast Migration, Proliferation, and Survival. Recent Pat. Regen. Med. 2012, 2, 146–155. [Google Scholar] [CrossRef]
- Philp, D.; Scheremeta, B.; Sibliss, K.; Zhou, M.; Fine, E.L.; Nguyen, M.; Wahl, L.; Hoffman, M.P.; Kleinman, H.K. Thymosin beta4 promotes matrix metalloproteinase expression during wound repair. J. Cell. Physiol. 2006, 208, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Sosne, G.; Christopherson, P.L.; Barrett, R.P.; Fridman, R. Thymosin-beta4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injury. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2388–2395. [Google Scholar] [CrossRef]
- Qiu, P.; Wheater, M.K.; Qiu, Y.; Sosne, G. Thymosin beta4 inhibits TNF-alpha-induced NF-kappaB activation, IL-8 expression, and the sensitizing effects by its partners PINCH-1 and ILK. FASEB J. 2011, 25, 1815–1826. [Google Scholar] [CrossRef]
- Sopko, N.; Qin, Y.; Finan, A.; Dadabayev, A.; Chigurupati, S.; Qin, J.; Penn, M.S.; Gupta, S. Significance of thymosin beta4 and implication of PINCH-1-ILK-alpha-parvin (PIP) complex in human dilated cardiomyopathy. PLoS ONE 2011, 6, e20184. [Google Scholar] [CrossRef]
- Crea, F.; Libby, P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation 2017, 136, 1155–1166. [Google Scholar] [CrossRef]
- Grant, D.S.; Rose, W.; Yaen, C.; Goldstein, A.; Martinez, J.; Kleinman, H. Thymosin B4 enhances endothelial cell differentiation and angiogenesis. Angiogenesis 1999, 3, 125–135. [Google Scholar] [CrossRef]
- Lv, S.; Cheng, G.; Zhou, Y.; Xu, G. Thymosin beta4 induces angiogenesis through Notch signaling in endothelial cells. Mol. Cell. Biochem. 2013, 381, 283–290. [Google Scholar] [CrossRef]
- Qiu, F.Y.; Song, X.; Zheng, H.; Zhao, Y.B. Thymosin B4 induces endothelial progenitor cell migration via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2009, 53, 209–214. [Google Scholar] [CrossRef]
- Fang, S.; Wei, J.; Pentinmikko, N.; Leinonen, H.; Salven, P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012, 10, e1001407. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Sartore, S.; Agostini, C.; Avogaro, A. Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care 2007, 30, 1305–1313. [Google Scholar] [CrossRef] [PubMed]

| MI (n = 6) | EPC (n = 6) | EPC + TB4 (n = 6) | |
|---|---|---|---|
| Body weight (g) | 403 ± 34.5 | 423.83 ± 15.2 | 418.3 ± 4 |
| LVIDd (mm) | 8.7 ± 0.83 | 8.95 ± 1.4 | 7.9 6 ± 0.4 |
| LVIDs (mm) | 5.87 ± 1.43 | 4.95 ± 1.13 | 5.04 ± 0.3 |
| LVEF (%) | 53.9 ± 17.8 | 69.6 ± 5.9# | 59.34 ± 8 |
| Mitral E (cm/s) | 109.3 ± 14 | 105.7 ± 7.3 | 115.83 ± 58 |
| HR (bpm) | 206.3 ± 31.5 | 226.7 ± 21.1 | 234.67 ± 40.3 |
| Septal E’ (mm) | 62.7 ± 25.7 | 49 ± 10.5 | 64.83 ± 45 |
| Septal S’ (mm) | 45.2 ± 21.5 | 45.3 ± 11.6 | 40 ± 16.89 |
| LAT E’ (mm) | 49.5 ± 10.5 | 43.2 ± 11.1 | 50.67 ± 35.3 |
| LAT S’ (mm) | 36.7 ± 11.1 | 38 ± 10.5 | 33.67 ± 17.4 |
| Avg E’ (mm) | 56.1 ± 19.9 | 46.1 ± 10.7 | 57.8 ± 39.3 |
| Avg S’ (mm) | 40.9 ± 16.9 | 41.7 ± 11.2 | 36.8 ± 16.7 |
| Avg SC (%) | −11 ± 2.6 | −15.6 ± 3.6# | −13. 4 ± 3. 3 |
| Avg SR (%) | 17.1 ± 8.6 | 27.7 ± 16.1 | 22.6 ± 10.4 |
| Avg SrC S (1/s) | −3.49 ± 0.87 | −3.64 ± 0.35 | −3.64 ± 0.3 |
| Avg SrC E (1/s) | 3.82 ± 1.69 | 4.71 ± 0.82 | 4.95 ± 0. 0.86 |
| Avg SrC A (1/s) | 3.82 ± 1.64 | 3.59 ± 0.62 | 3.37 ± 0.7 |
| Avg SrR S (1/s) | 4.81 ± 1.26 | 4.5 ± 0.92 | 5.14 ± 1.08 |
| Avg SrR E (1/s) | −4.08 ± 3.75 | −6.67 ± 2.66 | −7.21 ± 1. 36 |
| Avg SrR A (1/s) | −5.26 ± 1.58 | −4.19 ± 2.27 | −4.18 ± 0.69 |
| Avg Rot S (°) | −2.7 ± 2 | −0.09 ± 2.65 | 0.1 ± 1.5 |
| Avg RotR S (°/s) | −101.8 ± 52.6 | −96.7 ± 78.7 | −68 ± 18.43 |
| Avg RotR E (°/s) | 111.6 ± 52.3 | 112.6 ± 47.5 | 118.76 ± 23.08 |
| Avg RotR A (°/s) | 119.1 ± 50.2 | 112.8 ± 42.6 | 90.22 ± 28.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poh, K.K.; Lee, P.S.S.; Djohan, A.H.; Galupo, M.J.; Songco, G.G.; Yeo, T.C.; Tan, H.C.; Richards, A.M.; Ye, L. Transplantation of Endothelial Progenitor Cells in Obese Diabetic Rats Following Myocardial Infarction: Role of Thymosin Beta-4. Cells 2020, 9, 949. https://doi.org/10.3390/cells9040949
Poh KK, Lee PSS, Djohan AH, Galupo MJ, Songco GG, Yeo TC, Tan HC, Richards AM, Ye L. Transplantation of Endothelial Progenitor Cells in Obese Diabetic Rats Following Myocardial Infarction: Role of Thymosin Beta-4. Cells. 2020; 9(4):949. https://doi.org/10.3390/cells9040949
Chicago/Turabian StylePoh, Kian Keong, Poay Sian Sabrina Lee, Andie Hartanto Djohan, Mary Joyce Galupo, Geronica Gorospe Songco, Tiong Cheng Yeo, Huay Cheem Tan, Arthur Mark Richards, and Lei Ye. 2020. "Transplantation of Endothelial Progenitor Cells in Obese Diabetic Rats Following Myocardial Infarction: Role of Thymosin Beta-4" Cells 9, no. 4: 949. https://doi.org/10.3390/cells9040949
APA StylePoh, K. K., Lee, P. S. S., Djohan, A. H., Galupo, M. J., Songco, G. G., Yeo, T. C., Tan, H. C., Richards, A. M., & Ye, L. (2020). Transplantation of Endothelial Progenitor Cells in Obese Diabetic Rats Following Myocardial Infarction: Role of Thymosin Beta-4. Cells, 9(4), 949. https://doi.org/10.3390/cells9040949





