Galectin-1 Overexpression Activates the FAK/PI3K/AKT/mTOR Pathway and Is Correlated with Upper Urinary Urothelial Carcinoma Progression and Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Patients and Tumor Samples
2.3. IHC Analysis
2.4. Cell Lines and Culture
2.5. Immunoblotting
2.6. GAL1 Knockdown Cells with shRNA
2.7. Stable Knockdown of GAL1 by Using Lentiviruses
2.8. Transwell Migration and Invasion Assay
2.9. RNA Isolation and Quantitive PCR
2.10. Gene Expression Microarray Assay
2.11. Bioinformatics Analysis
2.12. Statistical Analysis
3. Results
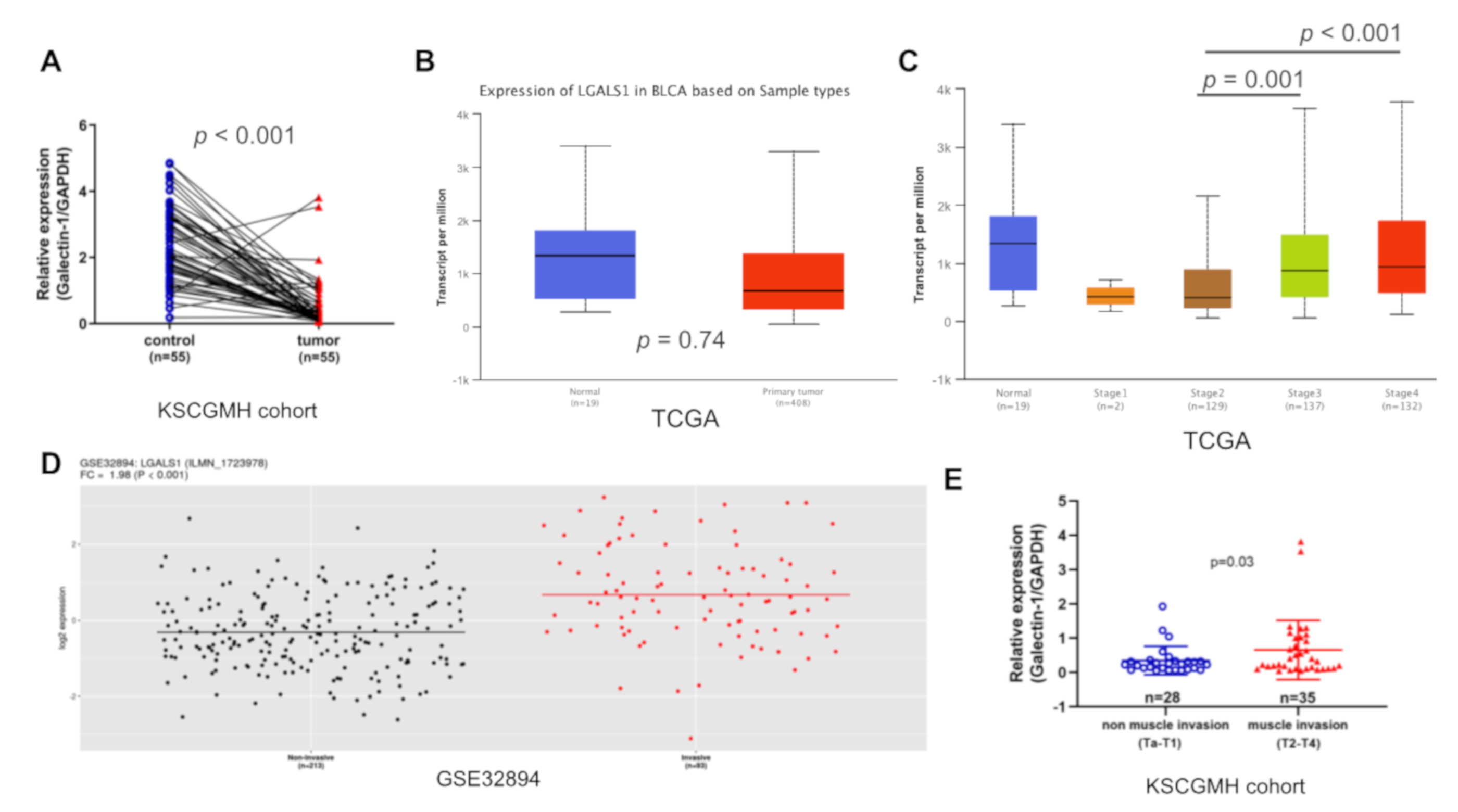
3.1. LGALS1 mRNA Expression Increased Significantly in Advanced UC
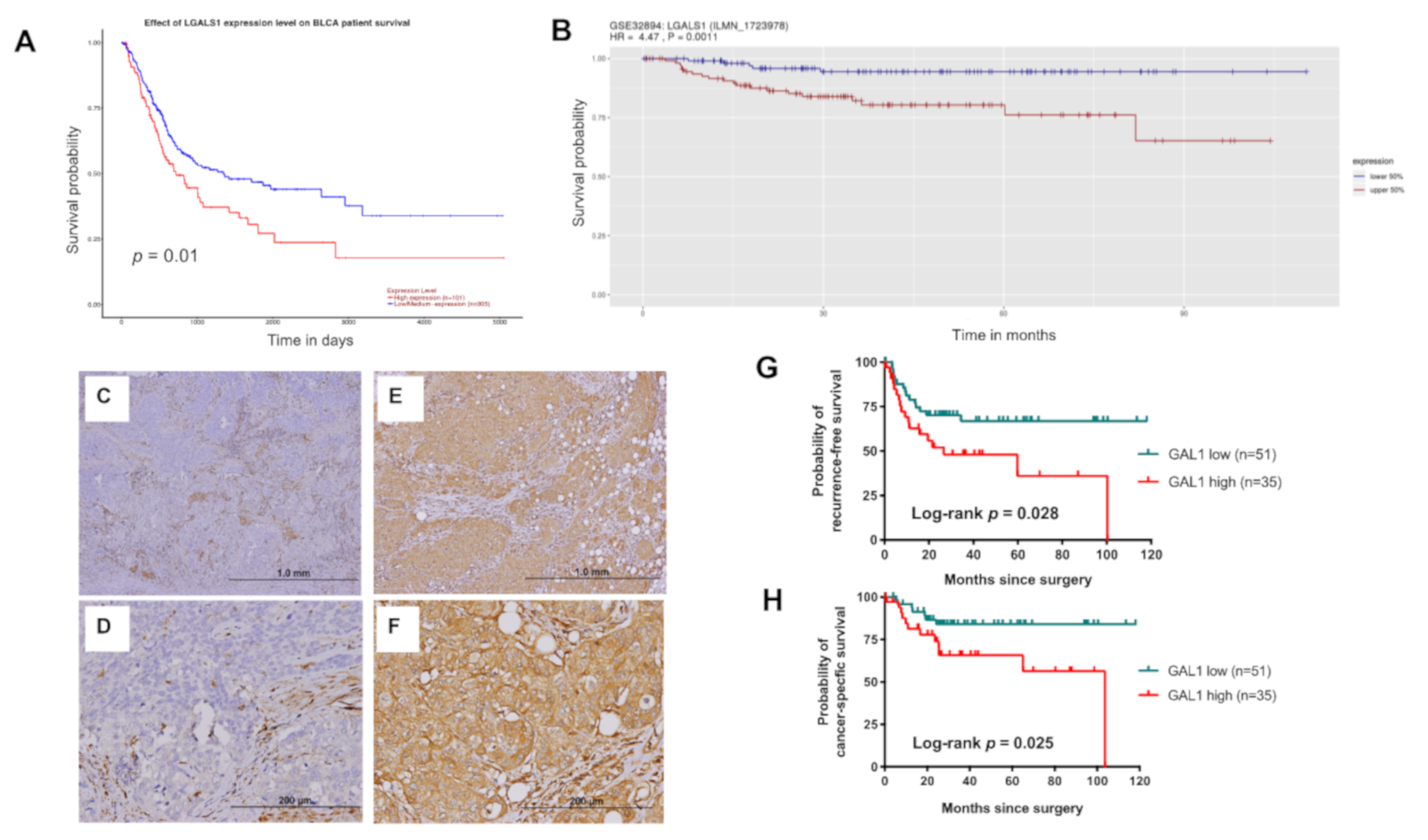
3.2. High Expression of GAL1 is Associated with Poor Disease Recurrence and CSS
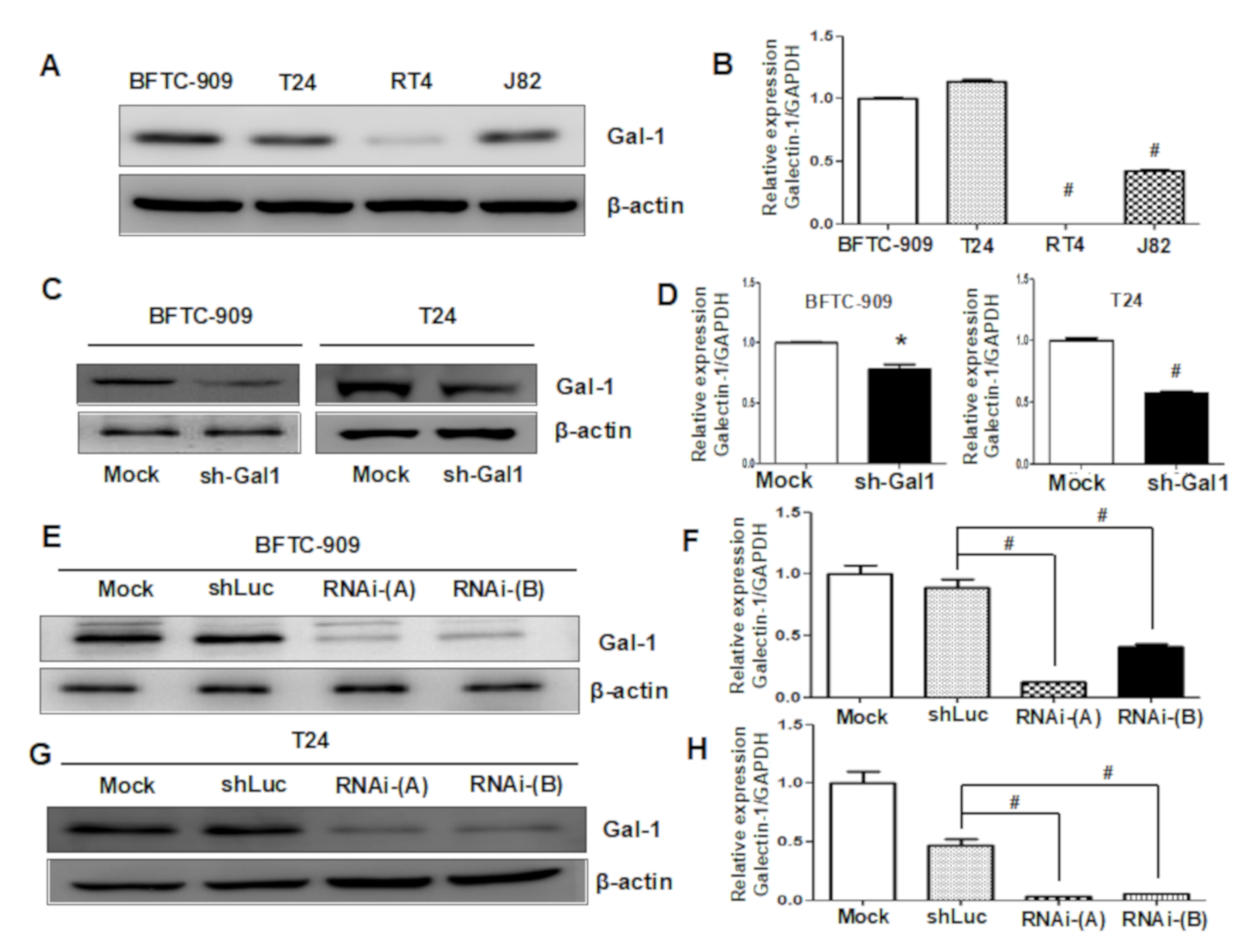
3.3. Downregulation of GAL1 in UC Cell Lines
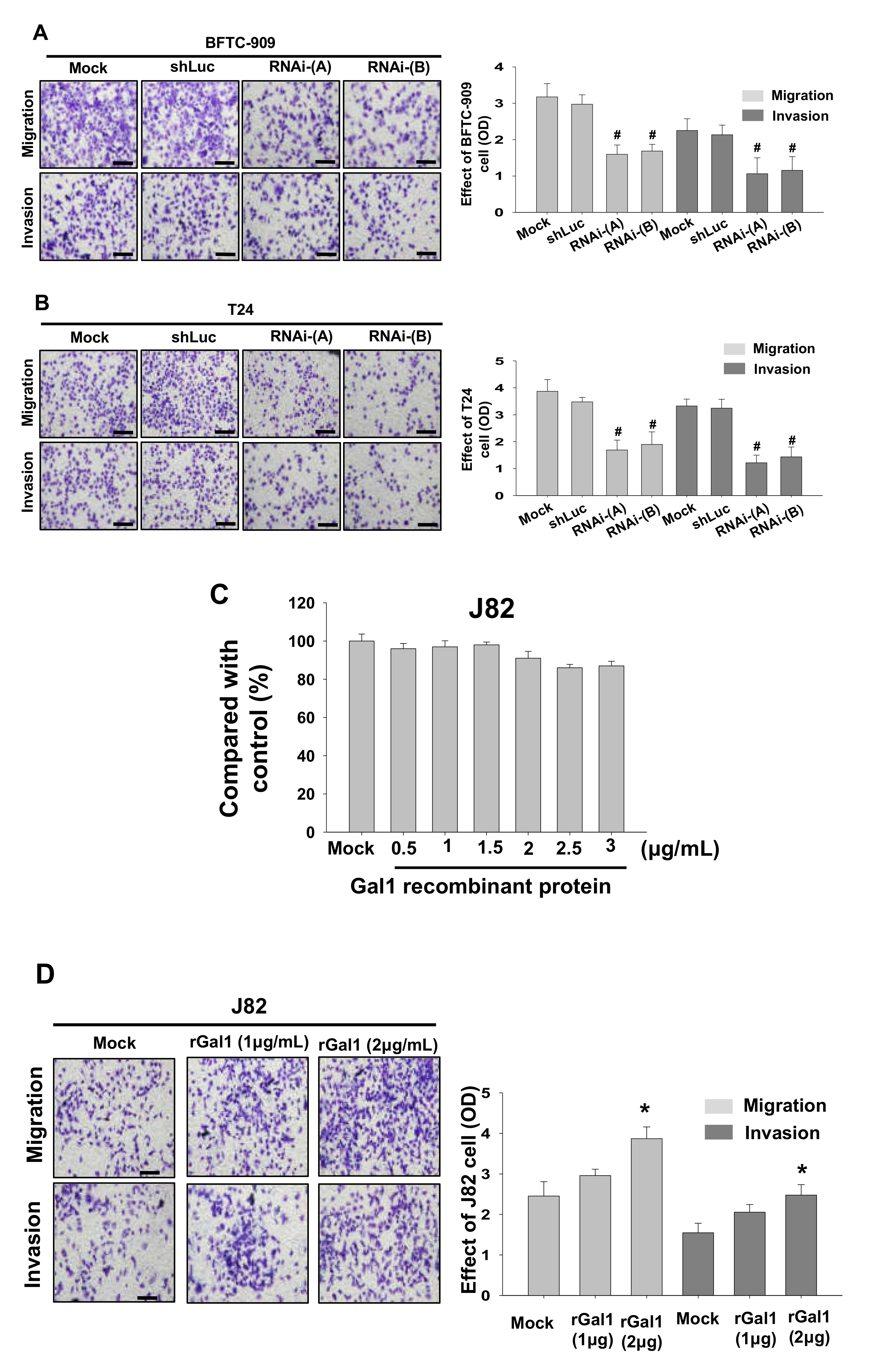
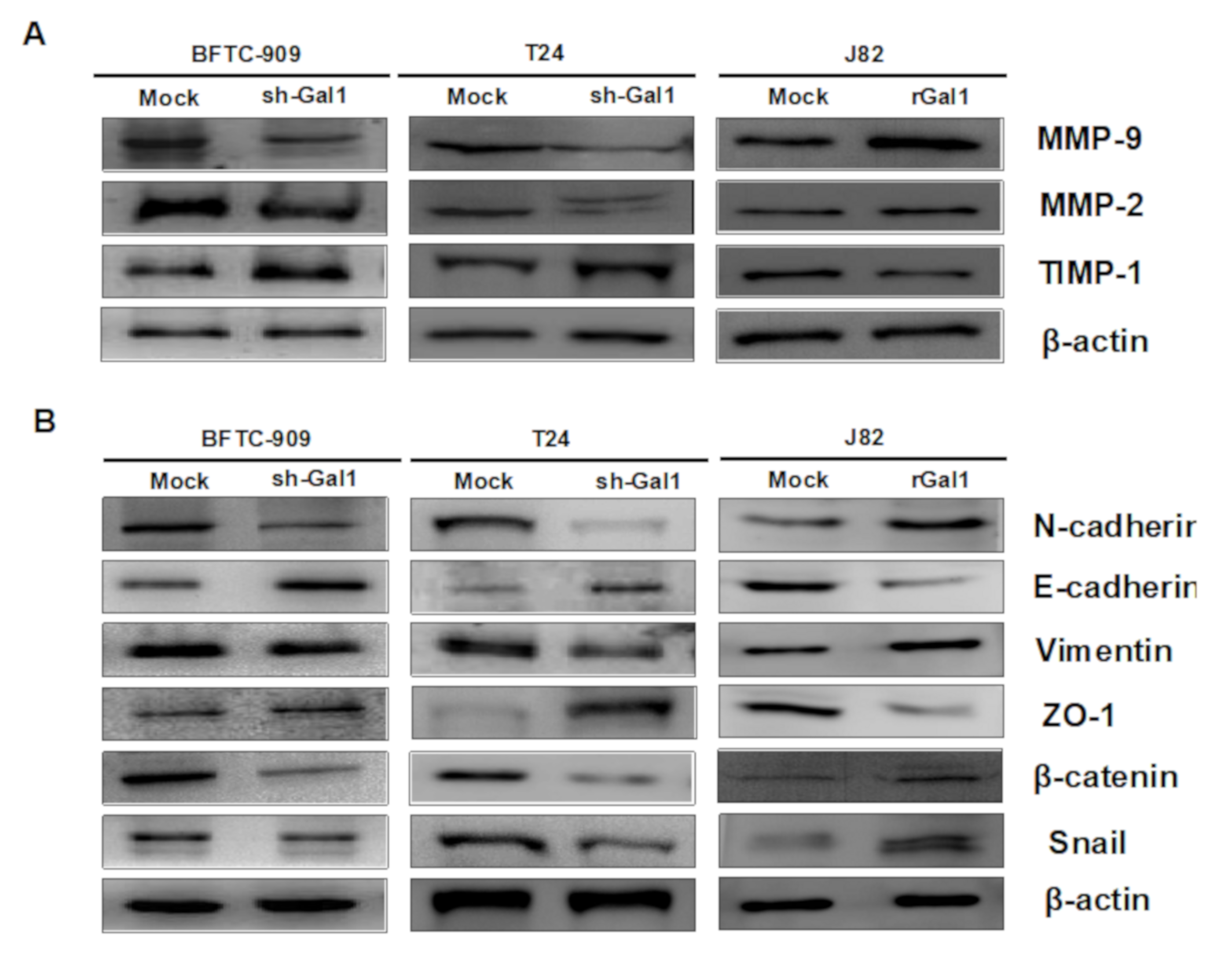
3.4. GAL1 Expression Increased Tumor Invasiveness and Migration
3.5. GAL1-Mediated Epithelial–Mesenchymal Transition in UC
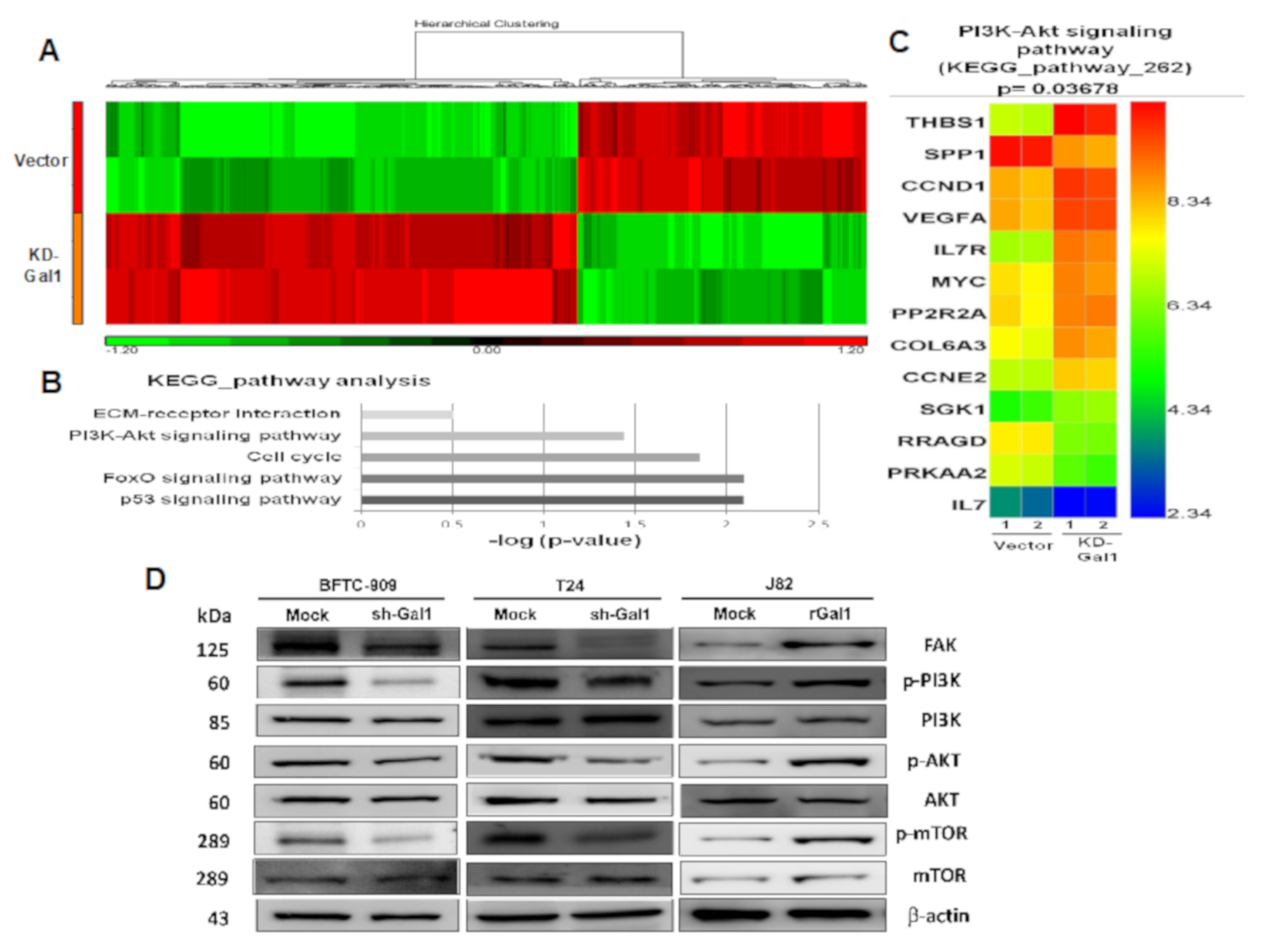
3.6. Gene Expression Variations Among Samples
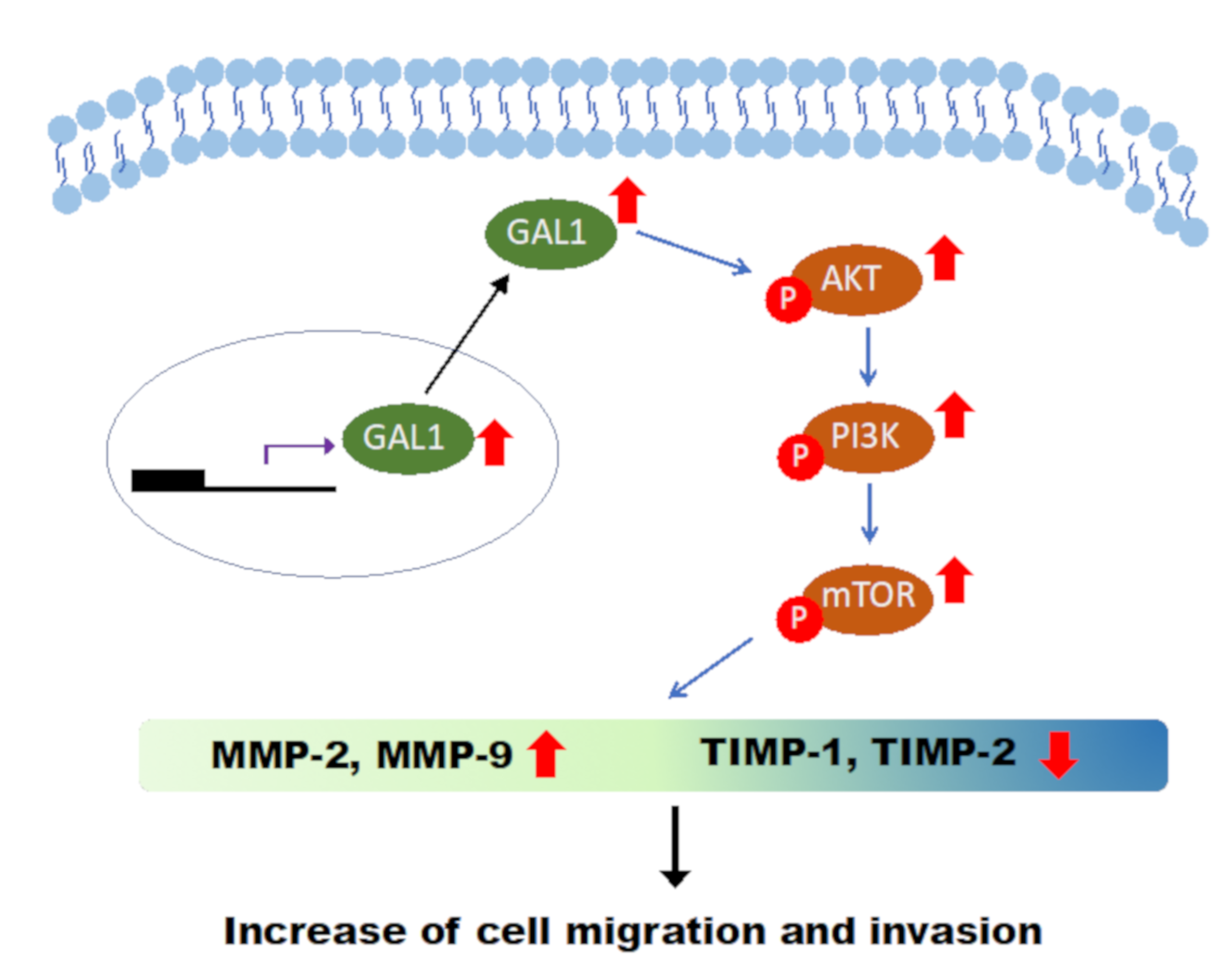
3.7. Downregulation of GAL1 Suppressed FAK and Phosphorylated PI3K-AKT-mTOR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CIS: | carcinoma in situ; |
| CSS: | cancer-specific survival; |
| ECM: | extracellular matrix; |
| EMT: | epithelial-mesenchymal transition; |
| FAK: | focal adhesion kinase; |
| GAL1: | galectin-1; |
| HR: | hazard ratio; |
| IHC: | immunohistochemical; |
| IQR: | interquartile range; |
| LVI: | lymphovascular invasion; |
| MMP: | matrix metalloproteinase; |
| mTOR: | mammalian target of rapamycin; |
| PI3K: | phosphoinositide 3-kinase; |
| q-PCR: | quantitative polymerase chain reaction; |
| RFS: | recurrence-free survival; |
| TCGA: | The Cancer Genome Atlas; |
| TIMP: | tissue inhibitors of metalloproteinases; |
| UC: | urothelial carcinoma; |
| UCB: | urothelial carcinoma of the bladder; |
| UTUC: | upper tract urothelial carcinoma. |
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics. Cancer J. Clin. 2013, 63, 6311–6330. [Google Scholar]
- Munoz, J.J.; Ellison, L.M. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J. Urol. 2000, 164, 1523–1525. [Google Scholar] [CrossRef]
- Raman, J.D.; Messer, J.; Sielatycki, J.A.; Hollenbeak, C.S. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA 1973–2005. BJU Int. 2011, 107, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Chang, T.H.; Wu, W.J.; Ke, H.L.; Huang, S.P.; Tsai, P.C.; Chang, S.J.; Shen, J.T.; Chou, Y.H.; Huang, C.H. Significant predictive factors for prognosis of primary upper urinary tract cancer after radical nephroureterectomy in Taiwanese patients. Eur. Urol. 2008, 54, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.H.; Huang, C.H. Unusual clinical presentation of upper urothelial carcinoma in Taiwan. Cancer 1999, 85, 1342–1344. [Google Scholar] [CrossRef]
- Cukuranovic, R.; Ignjatovic, I.; Visnjic, M.; Velickovic, L.J.; Petrovic, B.; Potic, M.; Stefanovic, V. Characteristics of upper urothelial carcinoma in an area of Balkan endemic nephropathy in south Serbia. A fifty-year retrospective study. Tumori 2010, 96, 674–679. [Google Scholar] [CrossRef]
- Poon, S.L.; Pang, S.T.; McPherson, J.R.; Yu, W.; Huang, K.K.; Guan, P.; Weng, W.H.; Siew, E.Y.; Liu, Y.; Heng, H.L.; et al. Genome-wide mutational signatures of aristolochic acid and its application as a screening tool. Sci. Transl. Med. 2013, 5, 197ra101. [Google Scholar] [CrossRef]
- Roupret, M.; Yates, D.R.; Comperat, E.; Cussenot, O. Upper urinary tract urothelial cell carcinomas and other urological malignancies involved in the hereditary nonpolyposis colorectal cancer (lynch syndrome) tumor spectrum. Eur. Urol. 2008, 54, 1226–1236. [Google Scholar] [CrossRef]
- Roupret, M.; Babjuk, M.; Comperat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Bohle, A.; Van Rhijn, B.W.; Kaasinen, E.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. Eur. Urol. 2015, 68, 868–879. [Google Scholar] [CrossRef]
- Patel, N.; Arya, M.; Muneer, A.; Powles, T.; Sullivan, M.; Hines, J.; Kelly, J. Molecular aspects of upper tract urothelial carcinoma. Urol. Oncol. 2014, 32, e11–e20. [Google Scholar] [CrossRef]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef]
- Adams, L.; Scott, G.K.; Weinberg, C.S. Biphasic modulation of cell growth by recombinant human galectin-1. Biochim. Biophys. Acta. 1996, 1312, 137–144. [Google Scholar] [CrossRef]
- Wells, V.; Mallucci, L. Identification of an autocrine negative growth factor: Mouse beta-galactoside-binding protein is a cytostatic factor and cell growth regulator. Cell 1991, 64, 91–97. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Postel, R.; Brandwijk, R.J.; Dings, R.P.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Barrow, H.; Rhodes, J.M.; Yu, L.G. The role of galectins in colorectal cancer progression. Int. J. Cancer 2011, 129, 1–8. [Google Scholar] [CrossRef]
- Dalotto-Moreno, T.; Croci, D.O.; Cerliani, J.P.; Martinez-Allo, V.C.; Dergan-Dylon, S.; Mendez-Huergo, S.P.; Stupirski, J.C.; Mazal, D.; Osinaga, E.; Toscano, M.A.; et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013, 73, 1107–1117. [Google Scholar] [CrossRef]
- Szoke, T.; Kayser, K.; Baumhakel, J.D.; Trojan, I.; Furak, J.; Tiszlavicz, L.; Horvath, A.; Szluha, K.; Gabius, H.J.; Andre, S. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncol. 2005, 69, 167–174. [Google Scholar] [CrossRef]
- Huang, E.Y.; Chanchien, C.C.; Lin, H.; Wang, C.C.; Wang, C.J.; Huang, C.C. Galectin-1 is an independent prognostic factor for local recurrence and survival after definitive radiation therapy for patients with squamous cell carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 975–982. [Google Scholar] [CrossRef]
- Ouyang, J.; Plutschow, A.; Pogge von Strandmann, E.; Reiners, K.S.; Ponader, S.; Rabinovich, G.A.; Neuberg, D.; Engert, A.; Shipp, M.A. Galectin-1 serum levels reflect tumor burden and adverse clinical features in classical Hodgkin lymphoma. Blood 2013, 121, 3431–3433. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, S.J.; Zhang, Y.; Zhang, G.Q.; Zha, T.Z.; Feng, Y.Z.; Zhang, K. Clinicopathological and prognostic significance of galectin-1 and vascular endothelial growth factor expression in gastric cancer. World J. Gastroenterol. 2013, 19, 2073–2079. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Do, I.G.; Jeon, H.K.; Cho, Y.J.; Park, Y.A.; Choi, J.J.; Sung, C.O.; Lee, Y.Y.; Choi, C.H.; Kim, T.J.; et al. Galectin 1 expression is associated with tumor invasion and metastasis in stage IB to IIA cervical cancer. Hum. Pathol. 2013, 44, 62–68. [Google Scholar] [CrossRef]
- Schulkens, I.A.; Heusschen, R.; Van den Boogaart, V.; van Suylen, R.J.; Dingemans, A.M.; Griffioen, A.W.; Thijssen, V.L. Galectin expression profiling identifies galectin-1 and Galectin-9Delta5 as prognostic factors in stage I/II non-small cell lung cancer. PLoS ONE 2014, 9, e107988. [Google Scholar] [CrossRef]
- Punt, S.; Thijssen, V.L.; Vrolijk, J.; de Kroon, C.D.; Gorter, A.; Jordanova, E.S. Galectin-1, -3 and -9 Expression and Clinical Significance in Squamous Cervical Cancer. PLoS ONE 2015, 10, e0129119. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.F.; Li, C.F.; Chien, L.H.; Shen, K.H.; Huang, H.Y.; Su, C.C.; Liao, A.C. Galectin-1 dysregulation independently predicts disease specific survival in bladder urothelial carcinoma. J. Urol. 2015, 193, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.H.; Li, C.F.; Chien, L.H.; Huang, C.H.; Su, C.C.; Liao, A.C.; Wu, T.F. Role of galectin-1 in urinary bladder urothelial carcinoma cell invasion through the JNK pathway. Cancer Sci. 2016, 107, 1390–1398. [Google Scholar] [CrossRef]
- Chung, L.Y.; Tang, S.J.; Sun, G.H.; Chou, T.Y.; Yeh, T.S.; Yu, S.L.; Sun, K.H. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin. Cancer Res. 2012, 18, 4037–4047. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Rodriguez, I.P.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Dumas, J.; Gargano, M.A.; Dancik, G.M.; Shiny, G.E.O. A web-based application for analyzing gene expression omnibus datasets. Bioinformatics 2016, 32, 3679–3681. [Google Scholar] [CrossRef]
- Tinari, N.; Kuwabara, I.; Huflejt, M.E.; Shen, P.F.; Iacobelli, S.; Liu, F.T. Glycoprotein 90K/MAC-2BP interacts with galectin-1 and mediates galectin-1-induced cell aggregation. Int. J. Cancer 2001, 91, 167–172. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, P.; Shi, B.; Zhou, M.; Jiang, H.; Zhang, H.; Pan, X.; Gao, H.; Sun, H.; Li, Z. Galectin-1 overexpression promotes progression and chemoresistance to cisplatin in epithelial ovarian cancer. Cell Death Dis. 2014, 5, e991. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.Y.; Yen, S.L.; Huang, C.C.; Huang, E.Y. Galectin-1 is a poor prognostic factor in patients with glioblastoma multiforme after radiotherapy. BMC Cancer 2018, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Shen, K.H.; Chien, L.H.; Huang, C.H.; Wu, T.F.; He, H.L. Proteomic Identification of the Galectin-1-Involved Molecular Pathways in Urinary Bladder Urothelial Carcinoma. Int. J. Mol. Sci. 2018, 19, E1242. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Haklai, R.; Elad-Sfadia, G.; Ballan, E.; Kloogm, Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001, 20, 7486–7493. [Google Scholar] [CrossRef]
- Vyakarnam, A.; Dagher, S.F.; Wang, J.L.; Patterson, R.J. Evidence for a role for galectin-1 in pre-mRNA splicing. Mol. Cell Biol. 1997, 17, 4730–4737. [Google Scholar] [CrossRef]
- Zhang, P.F.; Li, K.S.; Shen, Y.H.; Gao, P.T.; Dong, Z.R.; Cai, J.B.; Zhang, C.; Huang, X.Y.; Tian, M.X.; Hu, Z.Q.; et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis. 2016, 7, e2201. [Google Scholar] [CrossRef]
| GAL1-Low | GAL1-High | p-Value | |
|---|---|---|---|
| (N = 51, %) | (N = 35, %) | ||
| Age (years) | 70.5 ± 10.8 | 68.0 ± 9.8 | 0.27 |
| Gender | |||
| Female | 31 (60.8) | 18 (51.4) | 0.39 |
| Male | 20 (39.2) | 17 (48.6) | |
| Smoking | |||
| Yes | 8 (15.7) | 4 (11.4) | 0.75 |
| No | 43 (84.3) | 31 (88.6) | |
| Primary site | |||
| Renal pelvis | 26 (51.0) | 26 (74.3) | 0.09 |
| Ureter | 19 (37.3) | 7 (20.1) | |
| Both | 6 (11.8) | 2 (5.7) | |
| Grade | |||
| Low | 0 (0) | 1 (2.9) | 0.41 |
| High | 51 (100) | 34 (97.1) | |
| Histopathologic variant | |||
| Presence | 20 (39.2) | 14 (40) | 0.94 |
| Absence | 31 (60.8) | 21 (60) | |
| CIS | |||
| Presence | 17 (33.3) | 13 (37.1) | 0.72 |
| Absence | 34 (66.7) | 22 (62.9) | |
| LVI | |||
| Presence | 20 (39.2) | 14 (40) | 0.94 |
| Absence | 31 (60.8) | 21 (60) | |
| Tumor necrosis | |||
| Presence | 18 (35.3) | 16 (45.7) | 0.33 |
| Absence | 33 (64.7) | 19 (54.3) | |
| Multicentricity | |||
| Yes | 12 (23.5) | 5 (14.3) | 0.29 |
| No | 39 (76.5) | 30 (85.7) | |
| Papillary feature | |||
| Presence | 31 (60.8) | 15 (42.9) | 0.10 |
| Absence | 20 (39.2) | 20 (57.1) |
| Characteristics | RFS | CSS | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| p-Value | HR (95% CI) | p-Value | p-Value | HR (95% CI) | p-Value | |
| Age | ||||||
| ≥65 vs. <65 | 0.99 | 0.67 | ||||
| Gender | ||||||
| Male vs. female | 0.17 | 0.41 | ||||
| Smoking history | ||||||
| Yes vs. no | 0.16 | 0.08 | ||||
| Primary site | ||||||
| Kidney vs. ureter | 0.24 | 2.03 (0.96–4.28) | 0.06 | 0.23 | ||
| Histological variant | ||||||
| Presence vs. absence | 0.74 | 0.26 | 3.65 (1.20–11.12) | 0.023 | ||
| LVI | ||||||
| Presence vs. absence | 0.043 | 2.41 (1.15–5.05) | 0.02 | 0.18 | 3.56 (1.06–11.88) | 0.039 |
| Tumor necrosis | ||||||
| Presence vs. absence | 0.25 | 0.35 | ||||
| CIS | ||||||
| Presence vs. absence | 0.97 | 0.96 | ||||
| Papillary feature | ||||||
| Presence vs. absence | 0.039 | 0.62 | ||||
| Multicentricity | ||||||
| Yes vs. no | 0.74 | 0.82 | ||||
| Galectin-1 | ||||||
| High vs. low | 0.028 | 2.43 (1.17–5.05) | 0.018 | 0.025 | 4.04 (1.25–13.03) | 0.019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-L.; Luo, H.-L.; Huang, C.-C.; Liu, T.-T.; Huang, E.-Y.; Sung, M.-T.; Lin, J.-J.; Chiang, P.-H.; Chen, Y.-T.; Kang, C.-H.; et al. Galectin-1 Overexpression Activates the FAK/PI3K/AKT/mTOR Pathway and Is Correlated with Upper Urinary Urothelial Carcinoma Progression and Survival. Cells 2020, 9, 806. https://doi.org/10.3390/cells9040806
Su Y-L, Luo H-L, Huang C-C, Liu T-T, Huang E-Y, Sung M-T, Lin J-J, Chiang P-H, Chen Y-T, Kang C-H, et al. Galectin-1 Overexpression Activates the FAK/PI3K/AKT/mTOR Pathway and Is Correlated with Upper Urinary Urothelial Carcinoma Progression and Survival. Cells. 2020; 9(4):806. https://doi.org/10.3390/cells9040806
Chicago/Turabian StyleSu, Yu-Li, Hao-Lun Luo, Chun-Chieh Huang, Ting-Ting Liu, Eng-Yen Huang, Ming-Tse Sung, Jen-Jie Lin, Po-Hui Chiang, Yen-Ta Chen, Chih-Hsiung Kang, and et al. 2020. "Galectin-1 Overexpression Activates the FAK/PI3K/AKT/mTOR Pathway and Is Correlated with Upper Urinary Urothelial Carcinoma Progression and Survival" Cells 9, no. 4: 806. https://doi.org/10.3390/cells9040806
APA StyleSu, Y.-L., Luo, H.-L., Huang, C.-C., Liu, T.-T., Huang, E.-Y., Sung, M.-T., Lin, J.-J., Chiang, P.-H., Chen, Y.-T., Kang, C.-H., & Cheng, Y.-T. (2020). Galectin-1 Overexpression Activates the FAK/PI3K/AKT/mTOR Pathway and Is Correlated with Upper Urinary Urothelial Carcinoma Progression and Survival. Cells, 9(4), 806. https://doi.org/10.3390/cells9040806






