HSP90 Interacts with the Fibronectin N-terminal Domains and Increases Matrix Formation
Abstract
1. Introduction
2. Materials and Methods
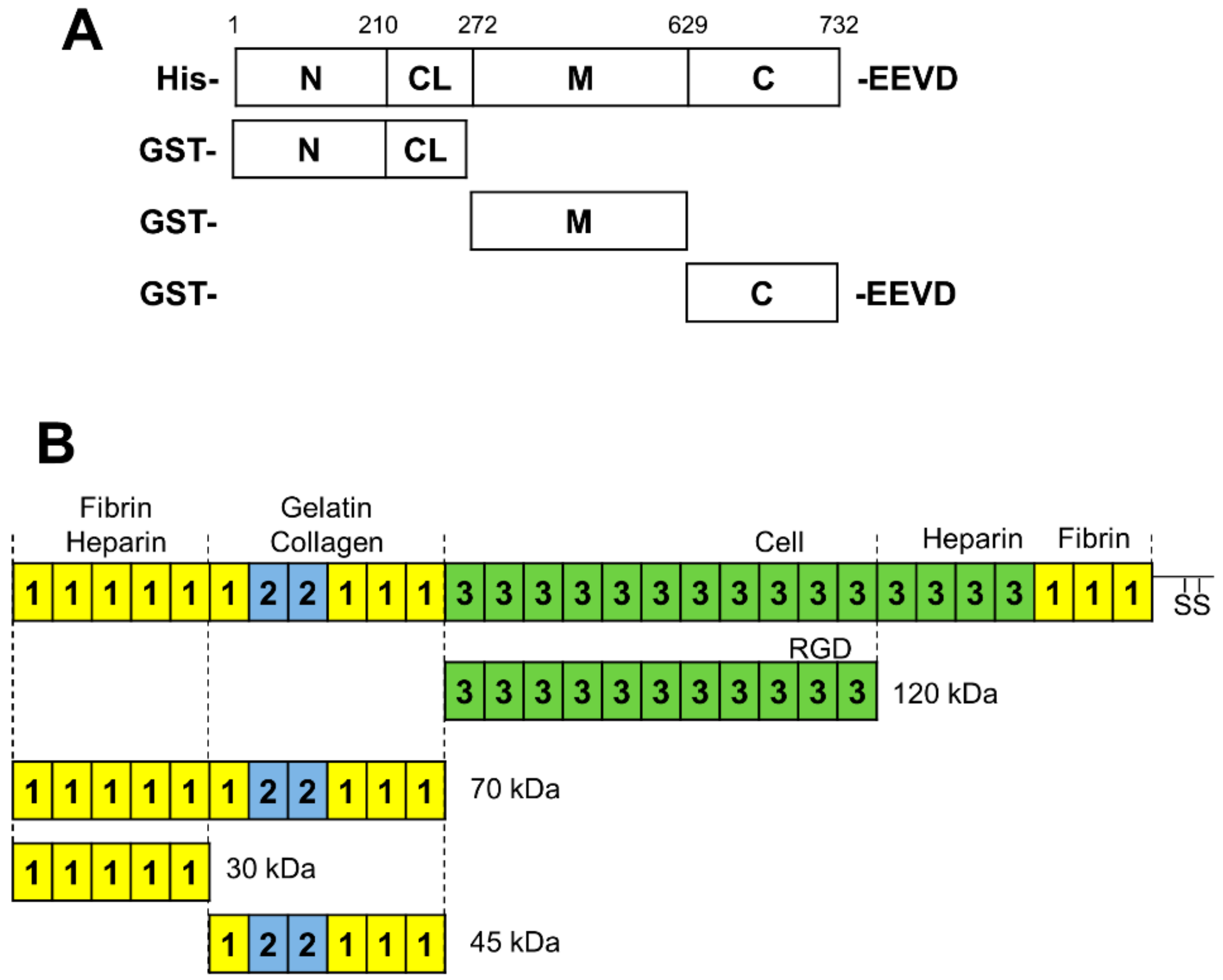
2.1. Proteins, Antibodies, and Plasmids
2.2. Plasmids
2.3. Cell Line Maintenance and Transfection
2.4. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot Analysis
2.5. Solid Phase Protein–Protein Interaction Assay
2.6. Gelatin and Heparin Agarose Pull-Down Assay
2.7. Immunoprecipitation and Nickel Affinity Pull-Down Assay
2.8. Protein Thermal Shift Unfolding Assay
2.9. Confocal Microscopy for Colocalization and Biomolecular Fluorescence Complementation (BiFC) Assay
2.10. Confocal Microscopy Analysis of FN Matrix
2.11. Cell Migration Assays
2.12. Statistical Analysis and Reproducibility
3. Results
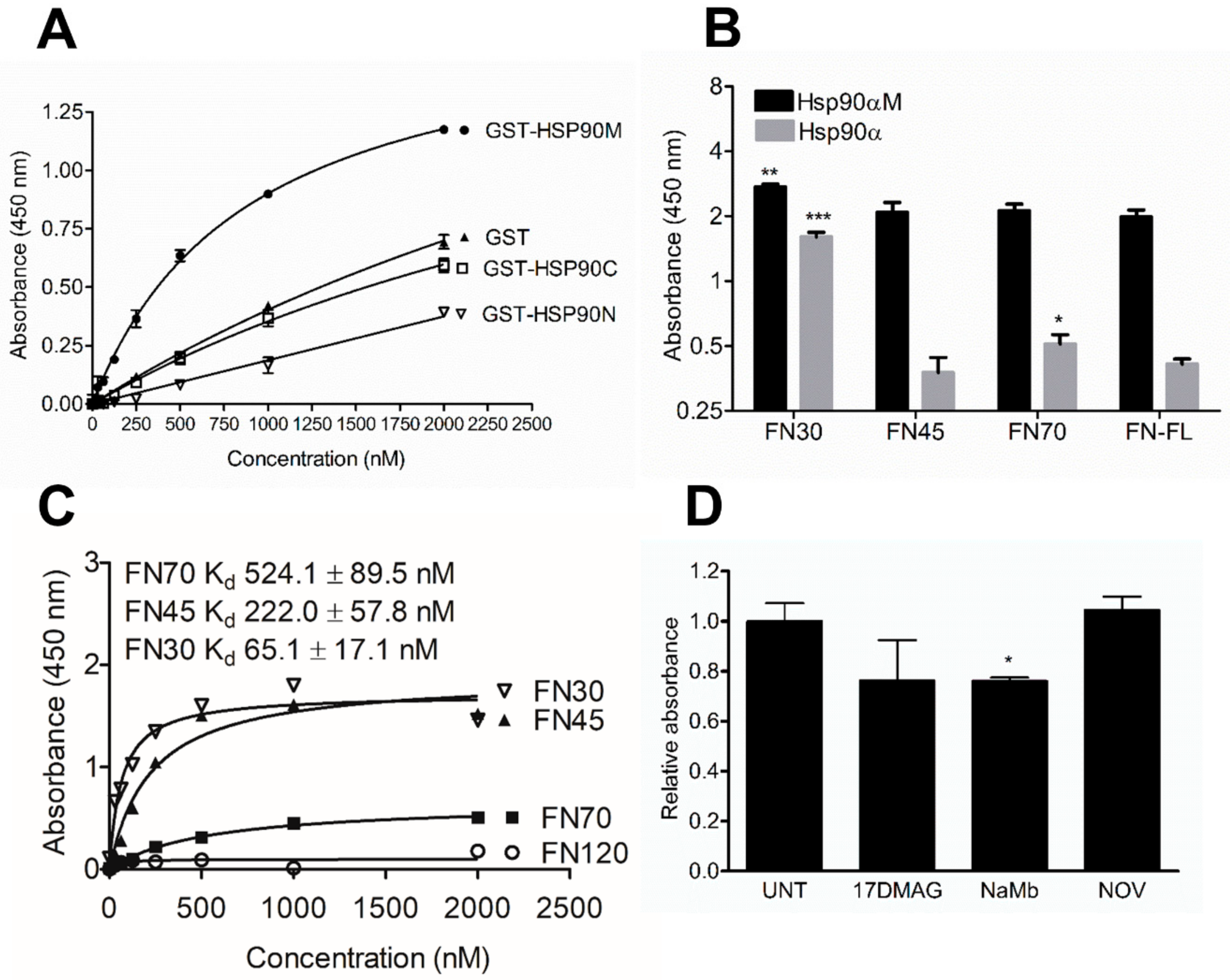
3.1. Identification of the Interaction Domains in HSP90 and FN
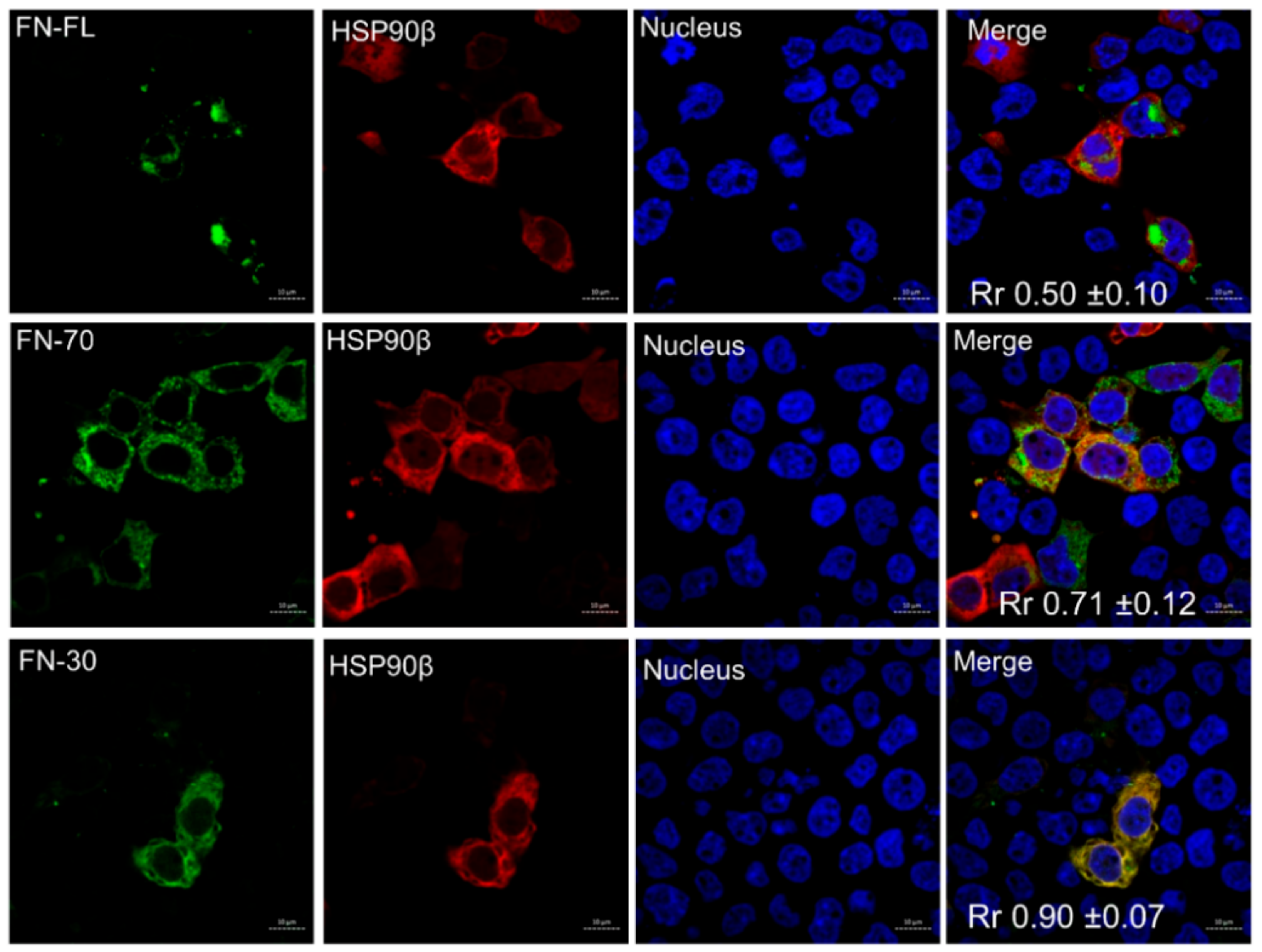
3.2. Colocalization of Fibronectin and HSP90α in Cells
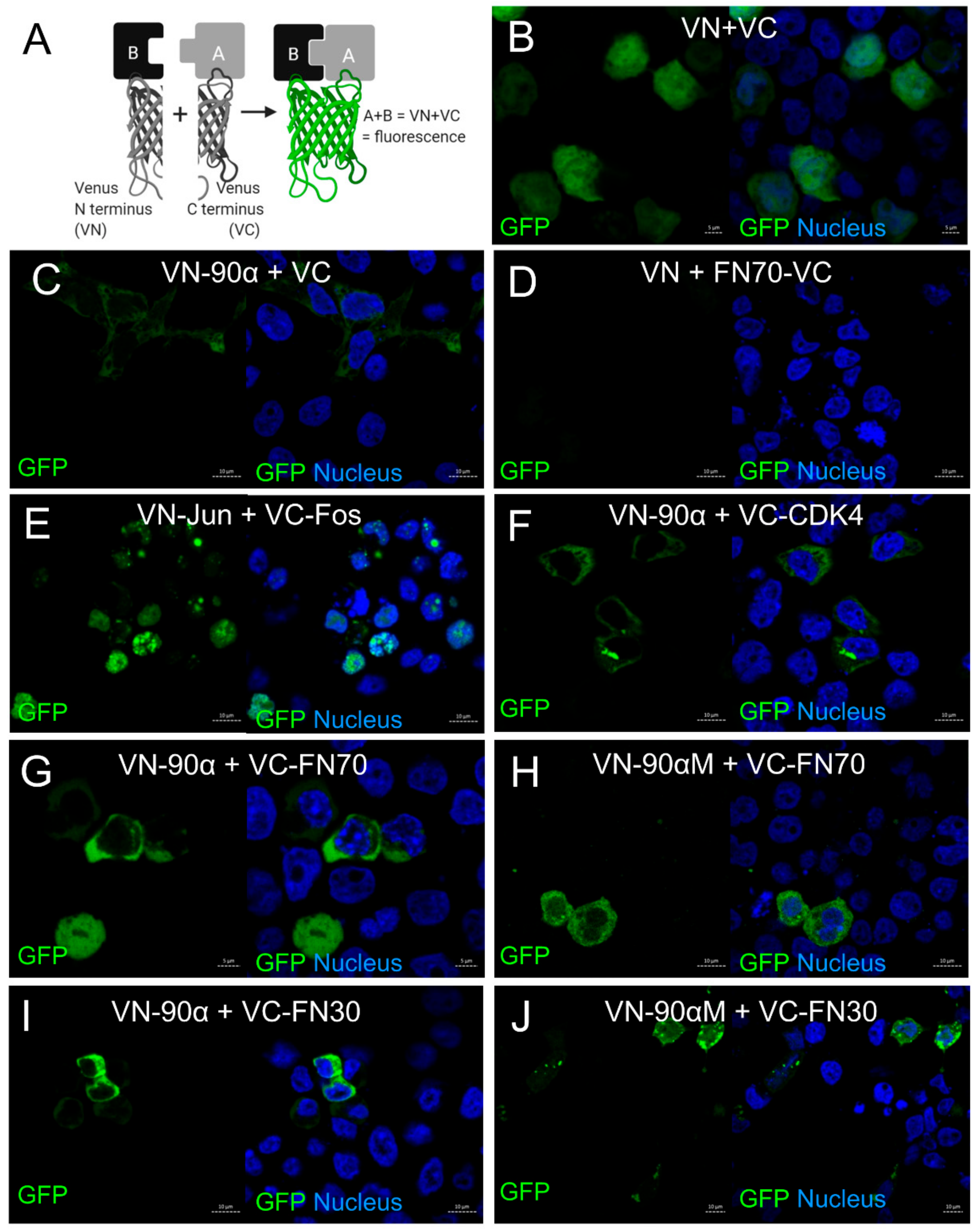
3.3. Analysis of FN and HSP90α Interaction by Biomolecular Fluorescence Complementation (BiFC) Assay
3.4. Analysis of the Effect of FN Fragment Stability on HSP90 Interaction
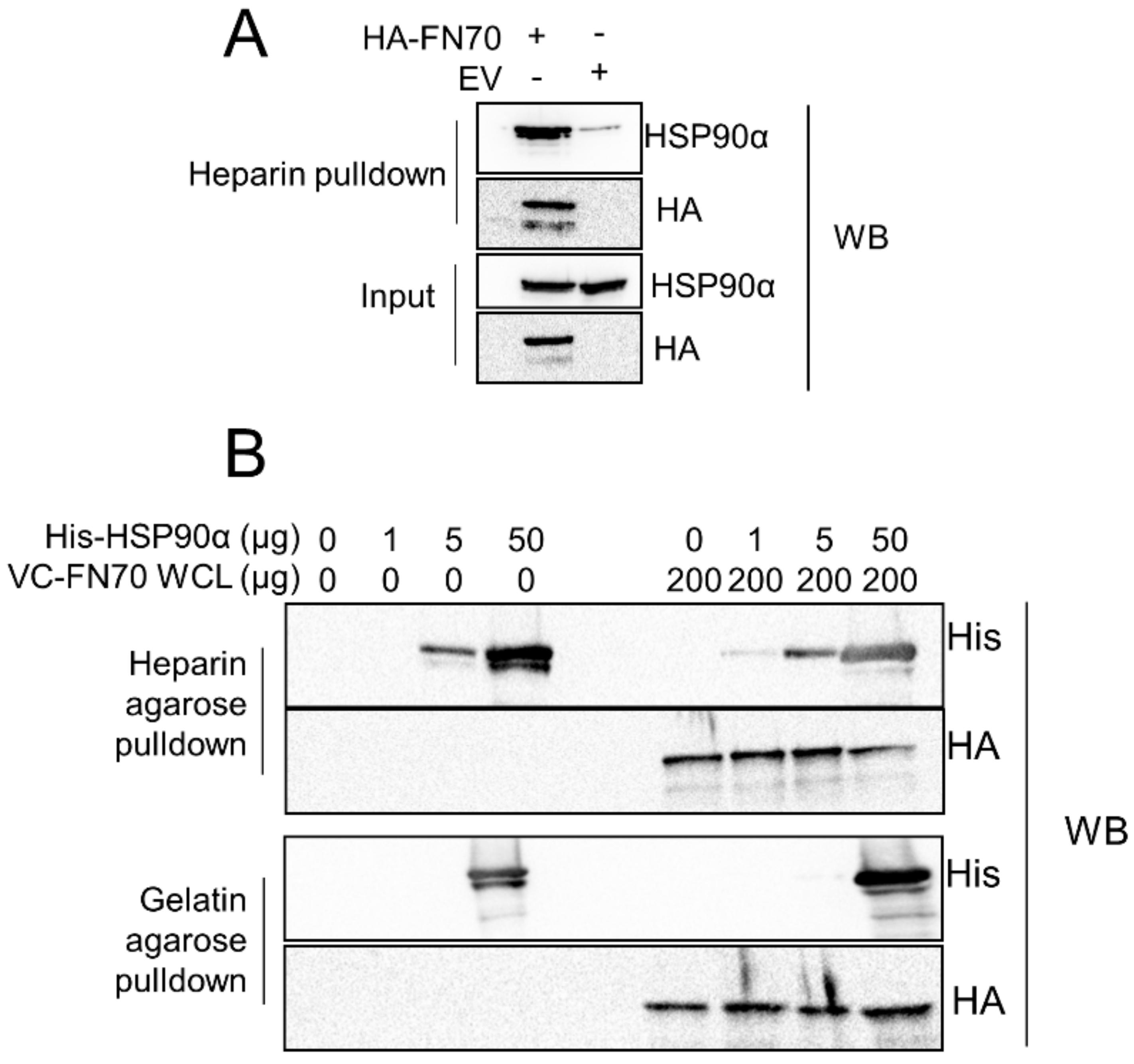
3.5. FN70 and HSP90 Interaction in the Presence of Heparin and Gelatin
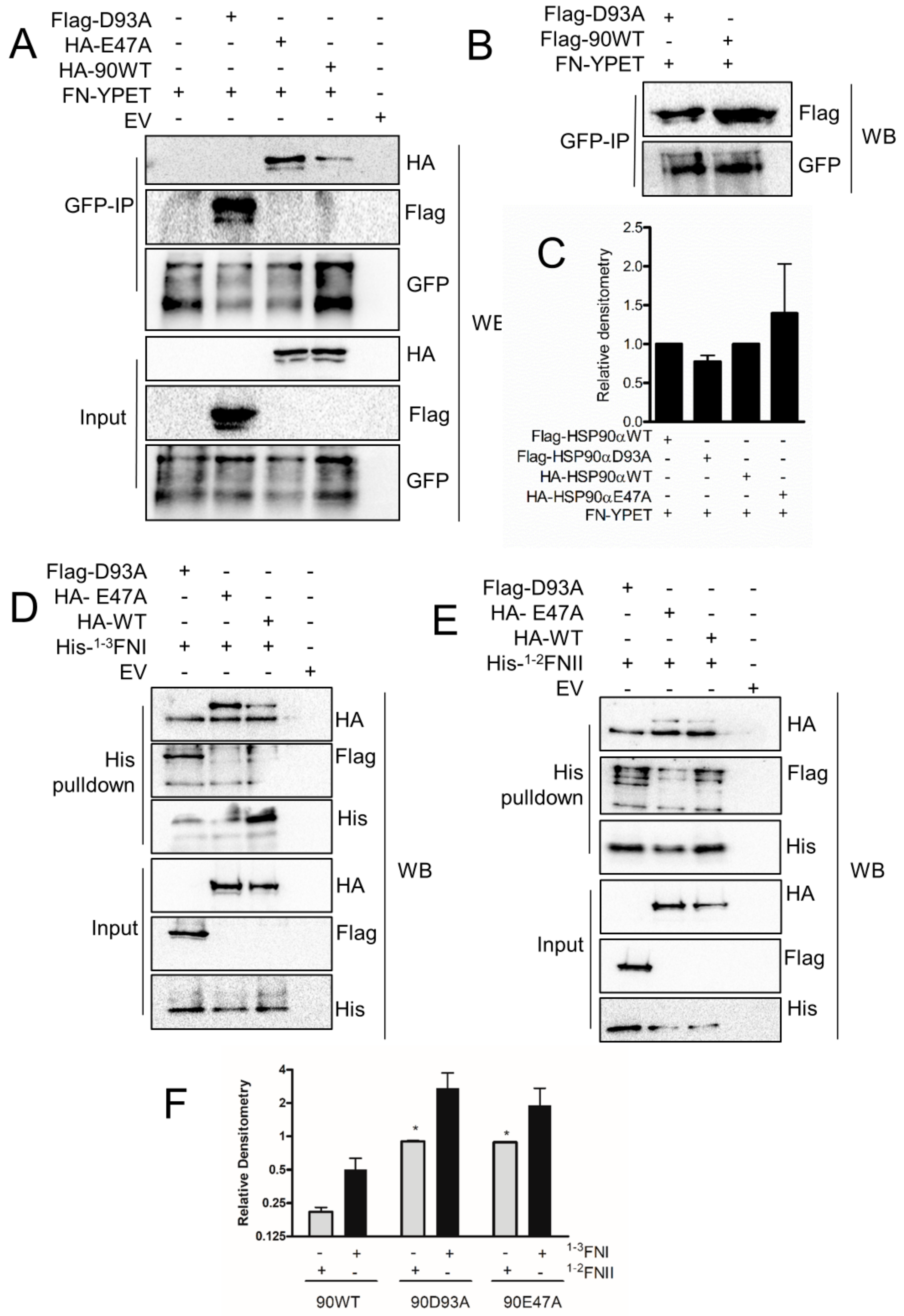
3.6. HSP90 and FN Interaction Involves Type-I FN Motifs
3.7. Interaction of FN with Conformationally Restricted HSP90 Mutants
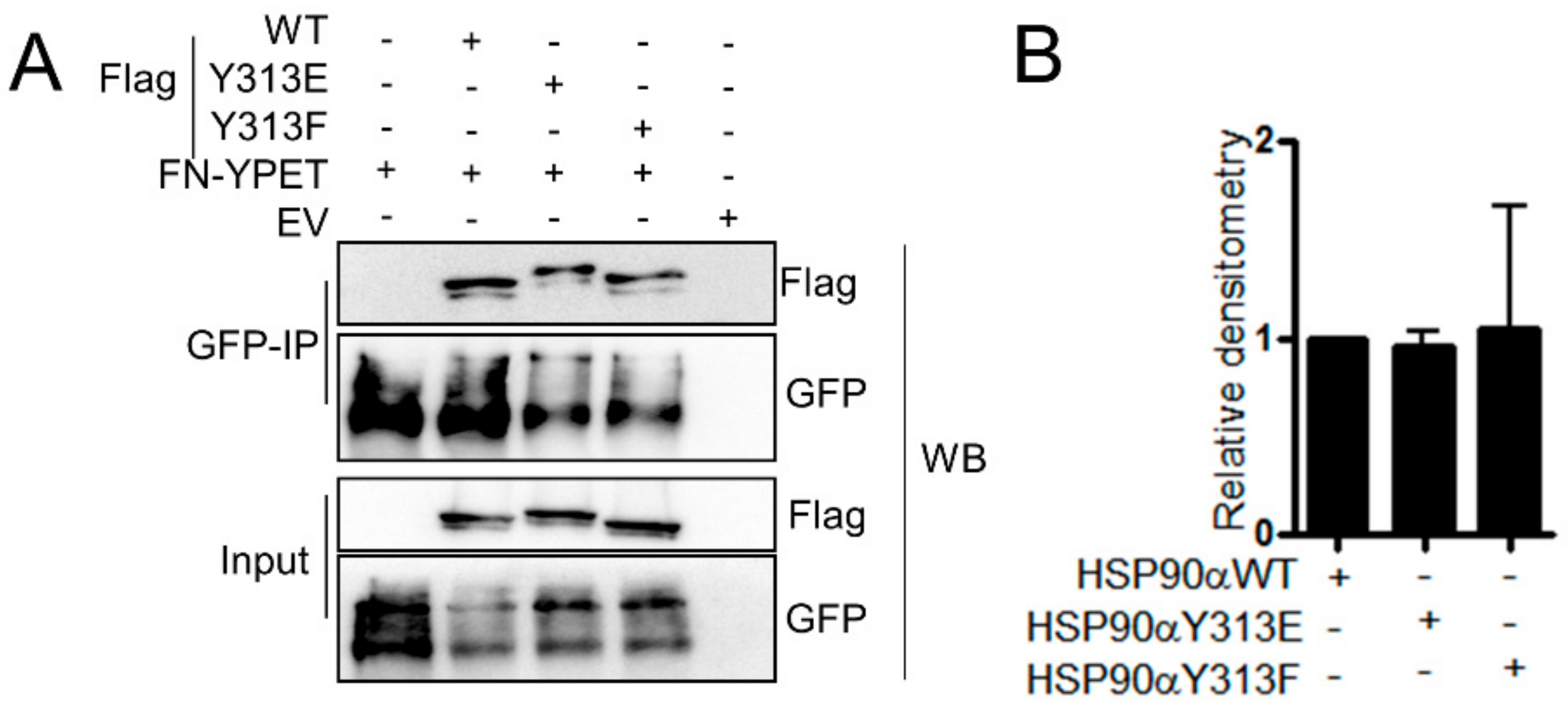
3.8. Phosphorylation of HSP90-Y313 does not Affect. HSP90–FN Interaction
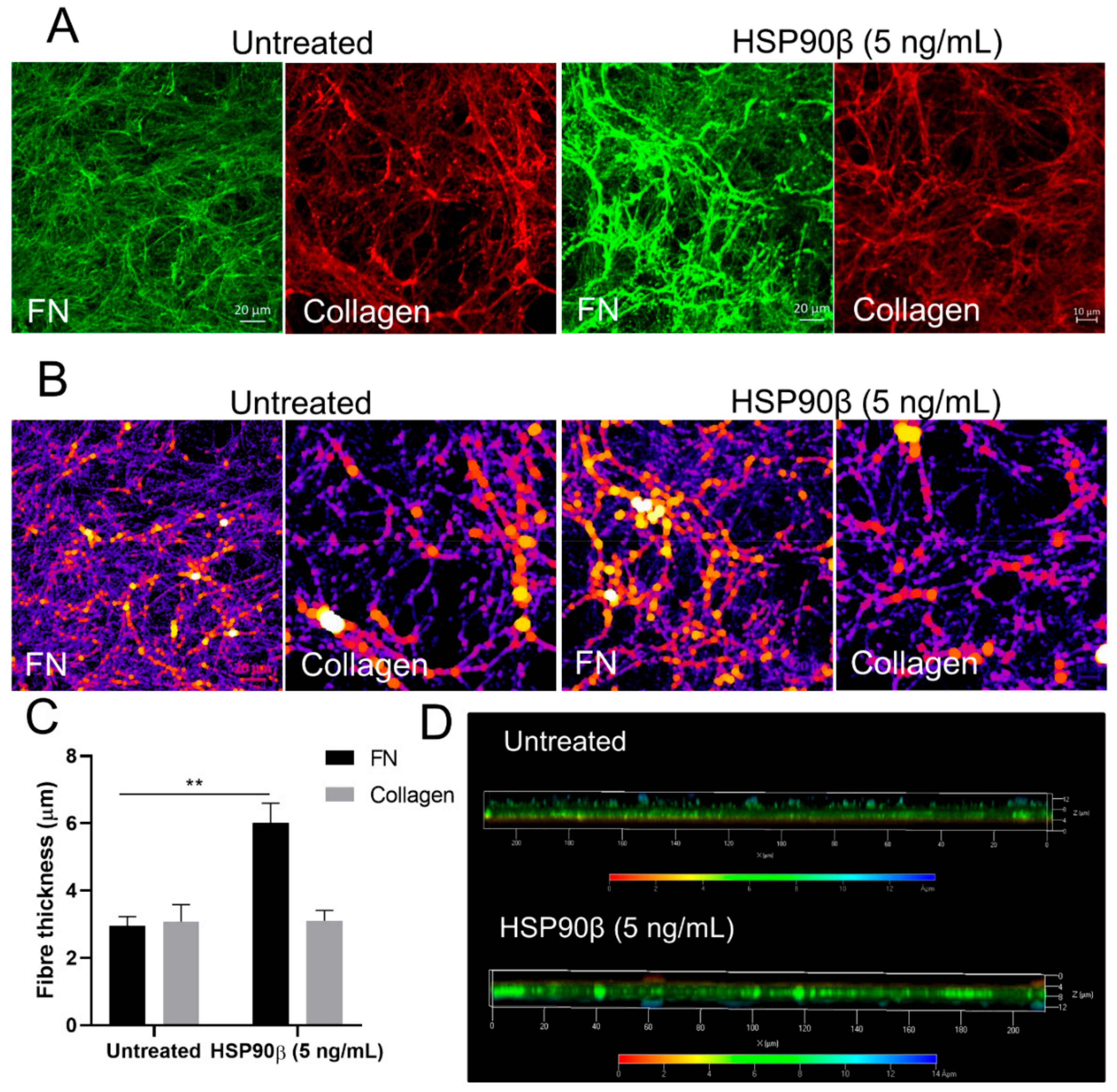
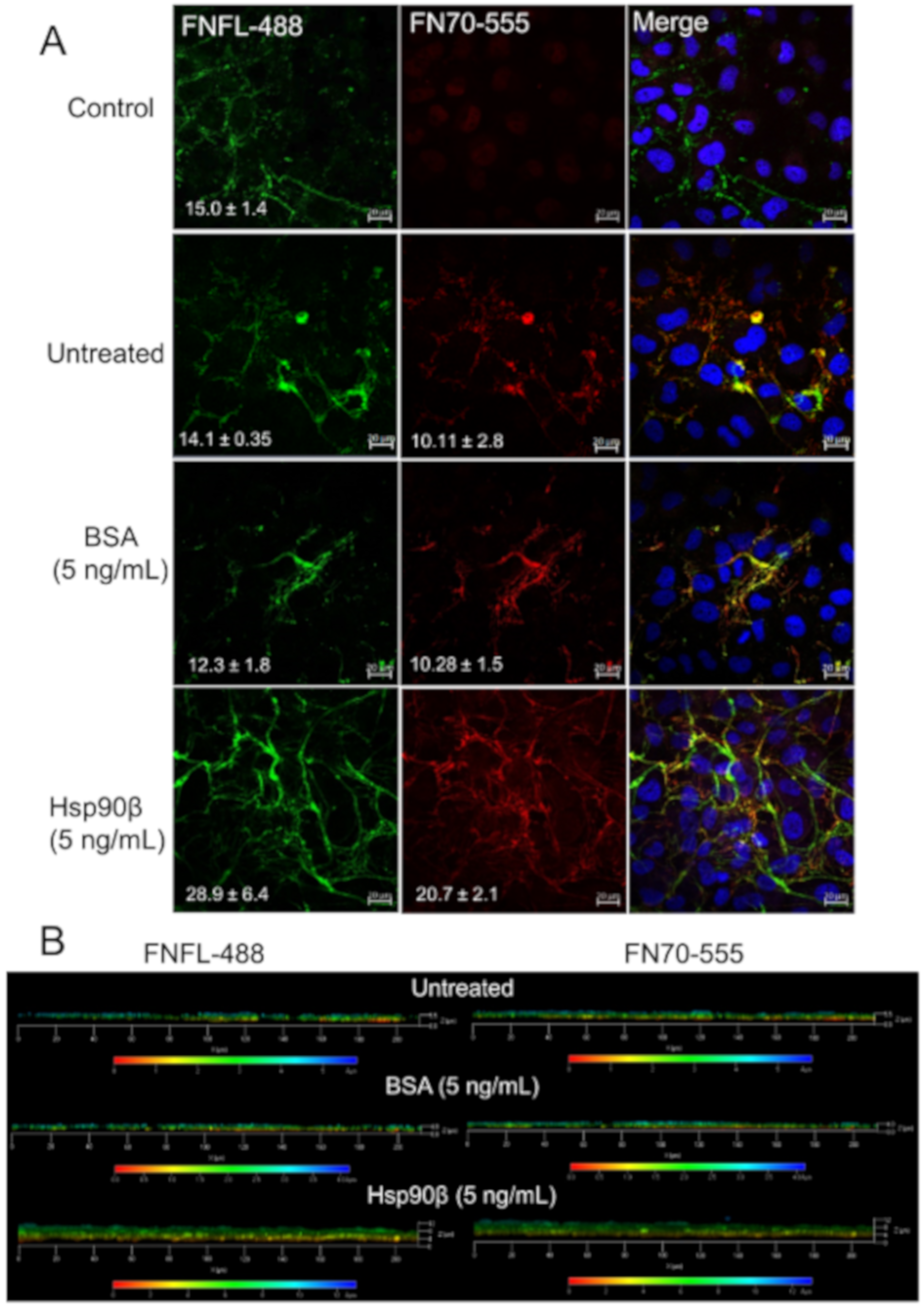
3.9. Extracellular HSP90 Increases the FN Matrix In Vitro
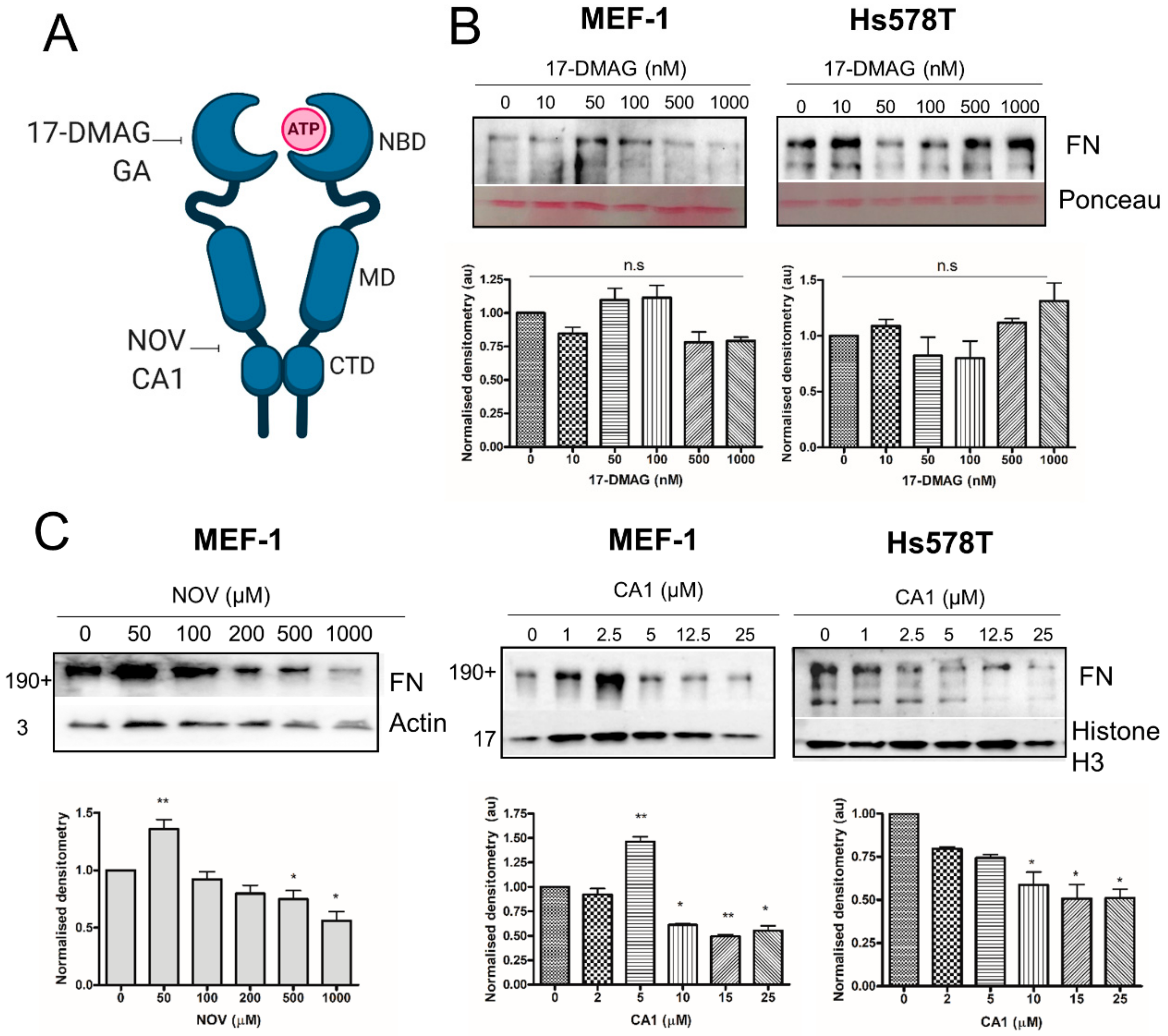
3.10. C-terminal, but not N-terminal, HSP90 Inhibitors Reduce Total FN Levels In Vitro
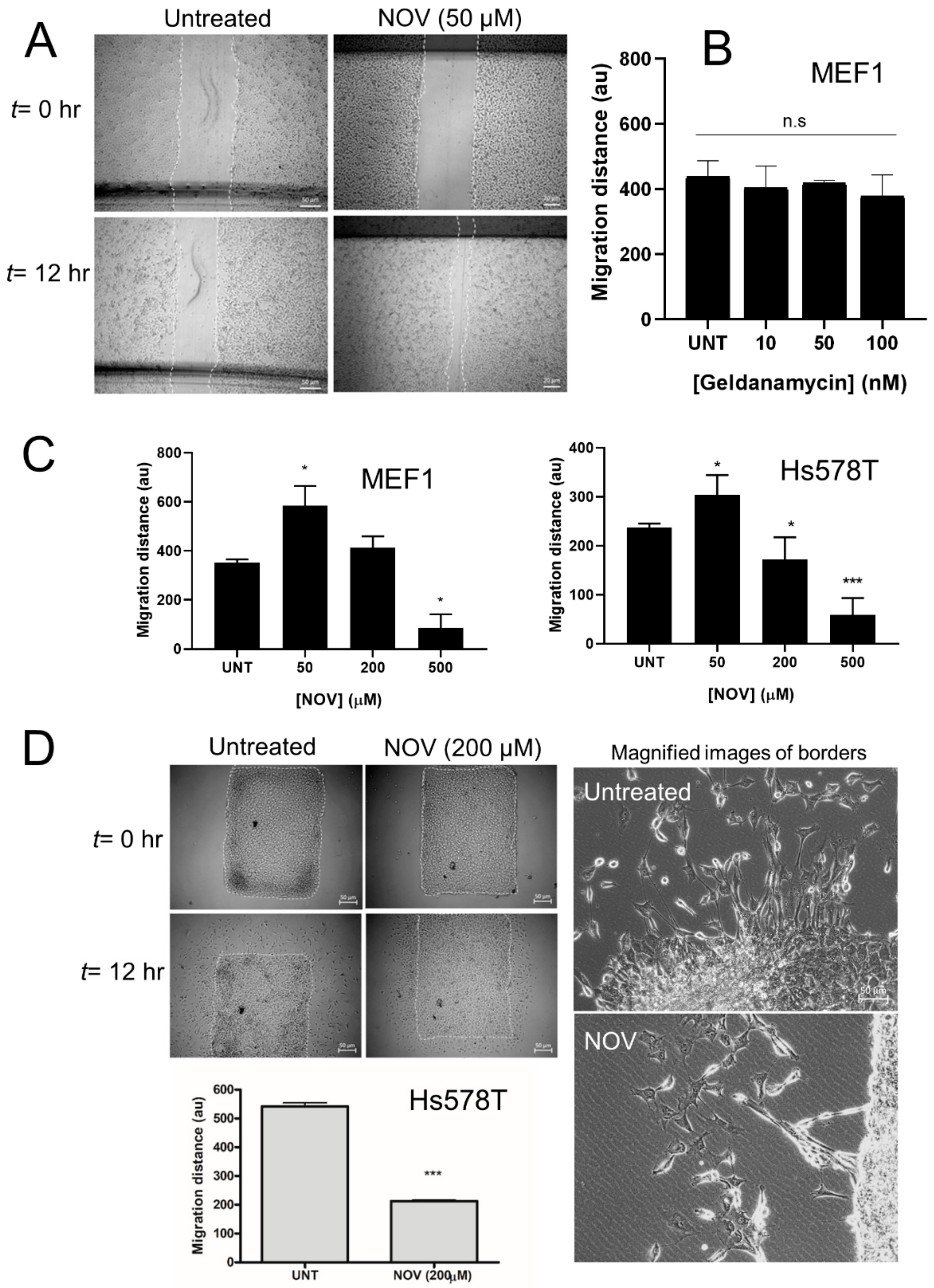
3.11. C-Terminal Hsp90 Inhibitors Alter Cell Migration In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef]
- Li, J.; Buchner, J. Structure, function and regulation of the Hsp90 machinery. Biomed. J. 2013, 36, 106–117. [Google Scholar] [PubMed]
- Hance, M.W.; Nolan, K.D.; Isaacs, J.S. The double-edged sword: Conserved functions of extracellular Hsp90 in wound healing and cancer. Cancers 2014, 6, 1065–1097. [Google Scholar] [CrossRef] [PubMed]
- Clare, D.K.; Saibil, H.R. ATP-driven molecular chaperone machines. Biopolymers 2013, 99, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Prodromou, C. The “active life” of Hsp90 complexes. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin. Ther. Targets 2009, 13, 469–478. [Google Scholar] [CrossRef]
- Bracher, A.; Hartl, F.U. Hsp90 structure: When two ends meet. Nat. Struct. Mol. Biol. 2006, 13, 478–480. [Google Scholar] [CrossRef]
- Röhl, A.; Rohrberg, J.; Buchner, J. The chaperone Hsp90: Changing partners for demanding clients. Trends Biochem. Sci. 2013, 38, 253–262. [Google Scholar] [CrossRef]
- Jackson, S.E.; Queitsch, C.; Toft, D. Hsp90: From structure to phenotype. Nat. Struct. Mol. Biol. 2004, 11, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Didenko, T.; Duarte, A.M.S.; Karagöz, G.E.; Rüdiger, S.G.D. Hsp90 structure and function studied by NMR spectroscopy. Biochim. Biophys. Acta 2012, 1823, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Hawle, P.; Siepmann, M.; Harst, A.; Siderius, M.; Reusch, H.P.; Obermann, W.M.J. The Middle Domain of Hsp90 Acts as a Discriminator between Different Types of Client Proteins. Mol. Cell. Biol. 2006, 26, 8385–8395. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Prodromou, C.; Hu, B.; Vaughan, C.; Roe, S.M.; Panaretou, B.; Piper, P.W.; Pearl, L.H. Structural and functional analysis of the middle segment of Hsp90: Implications for ATP hydrolysis and client protein and cochaperone interactions. Mol. Cell 2003, 11, 647–658. [Google Scholar] [CrossRef]
- Jahn, M.; Rehn, A.; Pelz, B.; Hellenkamp, B.; Richter, K.; Riefa, M.; Buchner, J.; Hugel, T. The charged linker of the molecular chaperone Hsp90 modulates domain contacts and biological function. Proc. Natl. Acad. Sci. USA 2014, 111, 17881–17886. [Google Scholar] [CrossRef]
- Hainzl, O.; Lapina, M.C.; Buchner, J.; Richter, K. The charged linker region is an important regulator of Hsp90 function. J. Biol. Chem. 2009, 284, 22559–22567. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Mollapour, M.; Prodromou, C.; Lee, C.-T.; Panaretou, B.; Yoshida, S.; Mayer, M.P.; Neckers, L.M. Charged linker sequence modulates eukaryotic heat shock protein 90 (Hsp90) chaperone activity. Proc. Natl. Acad. Sci. 2012, 109, 2937–2942. [Google Scholar] [CrossRef]
- Jego, G.; Hazoumé, A.; Seigneuric, R.; Garrido, C. Targeting heat shock proteins in cancer. Cancer Lett. 2013, 332, 275–285. [Google Scholar] [CrossRef]
- Mayer, M.P.; Le Breton, L. Hsp90: Breaking the symmetry. Mol. cell 2015, 58, 8–20. [Google Scholar] [CrossRef]
- Wong, D.S.; Jay, D.G. Emerging Roles of Extracellular Hsp90 in Cancer, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 129, ISBN 9780128022900. [Google Scholar]
- Tsutsumi, S.; Neckers, L. Extracellular heat shock protein 90: A role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007, 98, 1536–1539. [Google Scholar] [CrossRef]
- Baker-Williams, A.J.; Hashmi, F.; Budzyński, M.A.; Woodford, M.R.; Gleicher, S.; Himanen, S.V.; Makedon, A.M.; Friedman, D.; Cortes, S.; Namek, S.; et al. Co-chaperones TIMP2 and AHA1 Competitively Regulate Extracellular HSP90:Client MMP2 Activity and Matrix Proteolysis. Cell Rep. 2019, 28, 1894–1906.e6. [Google Scholar] [CrossRef]
- Ullrich, S.J.; Robinson, E.A.; Law, L.W.; Willingham, M.; Appella, E. A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc. Natl. Acad. Sci. USA 1986, 83, 3121–3125. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tsen, F.; Sahu, D.; Bhatia, A.; Chen, M.; Multhoff, G.; Woodley, D.T. Extracellular Hsp90 (eHsp90) as the Actual Target in Clinical Trials. Intentionally or Unintentionally. Int. Rev. Cell Mol. Biol. 2013, 303, 203–235. [Google Scholar] [PubMed]
- Stellas, D.; El Hamidieh, A.; Patsavoudi, E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol. 2010, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, X.; Zhuo, W.; Shi, H.; Feng, D.; Sun, Y.; Liang, Y.; Fu, Y.; Zhou, D.; Luo, Y. The regulatory mechanism of extracellular Hsp90α on matrix metalloproteinase-2 processing and tumor angiogenesis. J. Biol. Chem. 2010, 285, 40039–40049. [Google Scholar] [CrossRef]
- McCready, J.; Sims, J.D.; Chan, D.; Jay, D.G. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: A role for plasminogen activation. BMC Cancer 2010, 10, 294. [Google Scholar] [CrossRef]
- Karagöz, G.E.; Duarte, A.M.S.; Akoury, E.; Ippel, H.; Biernat, J.; Morán Luengo, T.; Radli, M.; Didenko, T.; Nordhues, B.A.; Veprintsev, D.B.; et al. Hsp90-tau complex reveals molecular basis for specificity in chaperone action. Cell 2014, 156, 963–974. [Google Scholar] [CrossRef]
- Taipale, M.; Krykbaeva, I.; Koeva, M.; Kayatekin, C.; Westover, K.D.; Karras, G.I.; Lindquist, S. Quantitative analysis of Hsp90-client interactions reveals principles of substrate recognition. Cell 2012, 150, 987–1001. [Google Scholar] [CrossRef]
- Hunter, M.C.; O’Hagan, K.L.; Kenyon, A.; Dhanani, K.C.H.; Prinsloo, E.; Edkins, A.L. Hsp90 binds directly to fibronectin (FN) and inhibition reduces the extracellular fibronectin matrix in breast cancer cells. PLoS ONE 2014, 9, e86842. [Google Scholar] [CrossRef]
- Boel, N.M.E.; Hunter, M.C.; Edkins, A.L. LRP1 is required for novobiocin-mediated fibronectin turnover. Sci. Rep. 2018, 8, 11438. [Google Scholar] [CrossRef]
- Bae, Y.K.; Kim, A.; Kim, M.K.; Choi, J.E.; Kang, S.H.; Lee, S.J. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Human Pathol. 2013, 44, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Multhaupt, H.A.B.; Leitinger, B.; Gullberg, D.; Couchman, J.R. Extracellular matrix component signaling in cancer. Adv. Drug Deliv. Rev. 2016, 97, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, B.; Eiró, N.; Marín, L.; González-Reyes, S.; González, L.O.; Lamelas, M.L.; Vizoso, F.J. Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology 2014, 64, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.L.; Sikes, R.A. Insidious changes in stromal matrix fuel cancer progression. Mol. cancer Res. 2014, 12, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.K.; Olden, K.; Yamada, K.M. Fibronectin and integrins in invasion and metastasis. Cancer Metast. Rev. 1995, 14, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.K.; Gillis, J.L.; Johnson, I.R.D.; Nassar, Z.D.; Moldovan, M.; Levrier, C.; Sadowski, M.C.; Chin, M.Y.; Tomlinson Guns, E.S.; Tarulli, G.; et al. Dysregulated fibronectin trafficking by Hsp90 inhibition restricts prostate cancer cell invasion. Sci. Rep. 2018, 8, 2090. [Google Scholar] [CrossRef]
- Bellaye, P.S.; Shimbori, C.; Yanagihara, T.; Carlson, D.A.; Hughes, P.; Upagupta, C.; Sato, S.; Wheildon, N.; Haystead, T.; Ask, K.; et al. Synergistic role of HSP90α and HSP90β to promote myofibroblast persistence in lung fibrosis. Eur. Respir. J. 2018, 51, 1700386. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Schwarzbauer, J.E.; DeSimone, D.W. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef]
- Mao, Y.; Schwarzbauer, J.E. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005, 24, 389–399. [Google Scholar] [CrossRef]
- Maurer, L.M.; Ma, W.; Mosher, D.F. Dynamic structure of plasma fibronectin. C Rev. Biochem. Mol. Biol. 2016, 51, 213–227. [Google Scholar] [CrossRef]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of Fibronectin Extracellular Matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef] [PubMed]
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenes. Tissue Repair 2011, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; Fulton, D.; Chen, Y.; Fairchild, T.A.; McCabe, T.J.; Fujita, N.; Tsuruo, T.; Sessa, W.C. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 2002, 90, 866–873. [Google Scholar] [CrossRef]
- Ohashi, T.; Erickson, H.P. Fibronectin aggregation and assembly: The unfolding of the second fibronectin type III domain. J. Biol. Chem. 2011, 286, 39188–39199. [Google Scholar] [CrossRef] [PubMed]
- Shyu, Y.J.; Liu, H.; Deng, X.; Hu, C.D. Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. BioTechniques 2006, 40, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Suslova, E.; Briand, P.A. 2-color photobleaching experiments reveal distinct intracellular dynamics of two components of the Hsp90 complex. Exp. Cell Res. 2006, 312, 3949–3958. [Google Scholar] [CrossRef]
- Xu, W.; Beebe, K.; Chavez, J.D.; Boysen, M.; Lu, Y.Y.; Zuehlke, A.D.; Keramisanou, D.; Trepel, J.B.; Prodromou, C.; Mayer, M.P.; et al. Hsp90 middle domain phosphorylation initiates a complex conformational program to recruit the ATPase-stimulating cochaperone Aha1. Nat. Commun. 2019, 10, 2574. [Google Scholar] [CrossRef]
- Prince, T.L.; Kijima, T.; Tatokoro, M.; Lee, S.; Tsutsumi, S.; Yim, K.; Rivas, C.; Alarcon, S.; Schwartz, H.; Khamit-Kush, K.; et al. Client proteins and small molecule inhibitors display distinct binding preferences for constitutive and stress-induced hsp90 isoforms and their conformationally restricted mutants. PLoS ONE 2015, 10, e0141786. [Google Scholar] [CrossRef]
- Woodford, M.R.; Sager, R.A.; Marris, E.; Dunn, D.M.; Blanden, A.R.; Murphy, R.L.; Rensing, N.; Shapiro, O.; Panaretou, B.; Prodromou, C.; et al. Tumor suppressor Tsc1 is a new Hsp90 co-chaperone that facilitates folding of kinase and non-kinase clients. EMBO J. 2017, 36, 3650–3665. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Partch, C.L. Analysis of protein stability and ligand interactions by thermal shift assay. Curr. Protoc. Protein Sci. 2015, 79, 28.9.1–28.9.14. [Google Scholar] [CrossRef]
- Nashine, V.C.; Kroetsch, A.M.; Sahin, E.; Zhou, R.; Adams, M.L. Orthogonal high-throughput thermal scanning method for rank ordering protein formulations. AAPS PharmSciTech 2013, 14, 1360–1366. [Google Scholar] [CrossRef][Green Version]
- Castelló-Cros, R.; Khan, D.R.; Simons, J.; Valianou, M.; Cukierman, E. Staged stromal extracellular 3D matrices differentially regulate breast cancer cell responses through PI3K and beta1-integrins. BMC Cancer 2009, 9, 1–19. [Google Scholar] [CrossRef]
- Rose, R.H.; Briddon, S.J.; Holliday, N.D. Bimolecular fluorescence complementation: Lighting up seven transmembrane domain receptor signalling networks. Br. J. Pharmacol. 2010, 159, 738–750. [Google Scholar] [CrossRef]
- Snigireva, A.V.; Vrublevskaya, V.V.; Afanasyev, V.N.; Morenkov, O.S. Cell surface heparan sulfate proteoglycans are involved in the binding of Hsp90α and Hsp90β to the cell plasma membrane. Cell Adhes. Migr. 2015, 9, 460–468. [Google Scholar] [CrossRef]
- Doube, M.; Klosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Bradshaw, A.D. The Extracellular Matrix. In Encyclopedia of Cell Biology; Elsevier Inc: Amsterdam, The Netherlands, 2016; Volume 2, pp. 694–703. ISBN 9780123944474. [Google Scholar]
- Golden, M. Cell biology of extracellular matrix. J. Vasc. Surg. 1993, 18, 142. [Google Scholar] [CrossRef][Green Version]
- Rishikof, D.C.; Ricupero, D.A.; Goldstein, R.H. Extracellular matrix. In Idiopathic Pulmonary Fibrosis; CRC Press: Boca Raton, FL, USA, 2003; pp. 481–506. ISBN 9780203913444. [Google Scholar]
- Hirschi, S.D.; Gray, S.D.; Thibeault, S.L. Fibronectin. J. Voice 2002, 16, 310–316. [Google Scholar] [CrossRef]
- Ohashi, T.; Erickson, H.P. Revisiting the mystery of fibronectin multimers: The fibronectin matrix is composed of fibronectin dimers cross-linked by non-covalent bonds. Matrix Biol. 2009, 28, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.K.; Mosher, D.F. Fibronectin: Structure, assembly, and cardiovascular implications. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1363–1370. [Google Scholar] [CrossRef]
- Sottile, J.; Chandler, J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol. Biol. Cell 2005, 16, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Sottile, J. MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J. Cell Sci. 2011, 124, 4039–4050. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Fibronectin in cell adhesion and invasion. Cancer Metast. Rev. 1984, 3, 43–51. [Google Scholar] [CrossRef]
- Kumra, H.; Reinhardt, D.P. Fibronectin-targeted drug delivery in cancer. Adv. Drug Deliv. Rev. 2016, 97, 101–110. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Dhanani, K.C.H.; Samson, W.J.; Edkins, A.L. Fibronectin is a stress responsive gene regulated by HSF1 in response to geldanamycin. Sci. Rep. 2017, 7, 17617. [Google Scholar] [CrossRef]
- Panaretou, B.; Siligardi, G.; Meyer, P.; Maloney, A.; Sullivan, J.K.; Singh, S.; Millson, S.H.; Clarke, P.A.; Naaby-Hansen, S.; Stein, R.; et al. Activation of the ATPase activity of Hsp90 by the stress-regulated cochaperone Aha1. Mol. Cell 2002, 10, 1307–1318. [Google Scholar] [CrossRef]
- Buchner, J. Hsp90 and Co.—A holding for folding. Trends Biochem. Sci. 1999, 24, 136–141. [Google Scholar] [CrossRef]
- Wayne, N.; Bolon, D.N. Charge-Rich Regions Modulate the Anti-Aggregation Activity of Hsp90. J. Mol. Biol. 2010, 401, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Pursell, N.W.; Mishra, P.; Bolon, D.N.A. Solubility-promoting function of Hsp90 Contributes to client maturation and robust cell growth. Eukaryot. Cell 2012, 11, 1033–1041. [Google Scholar] [CrossRef][Green Version]
- Kijima, T.; Prince, T.L.; Tigue, M.L.; Yim, K.H.; Schwartz, H.; Beebe, K.; Lee, S.; Budzynski, M.A.; Williams, H.; Trepel, J.B.; et al. HSP90 inhibitors disrupt a transient HSP90-HSF1 interaction and identify a noncanonical model of HSP90-mediated HSF1 regulation. Sci. Rep. 2018, 8, 6976. [Google Scholar] [CrossRef]
- Luo, Q.; Boczek, E.E.; Wang, Q.; Buchner, J.; Kaila, V.R.I. Hsp90 dependence of a kinase is determined by its conformational landscape. Sci. Rep. 2017, 7, 43996. [Google Scholar] [CrossRef]
- Jez, J.M.; Chen, J.C.H.; Rastelli, G.; Stroud, R.M.; Santi, D.V. Crystal structure and molecular modeling of 17-DMAG in complex with human Hsp90. Chem. Biol. 2003, 10, 361–368. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, S.C.; Kim, O.H.; Kim, K.H.; Song, K.Y.; Lee, S.K.; Choi, B.J.; Jeong, W.; Kim, S.J. HSP90 inhibitor 17-DMAG exerts anticancer effects against gastric cancer cells principally by altering oxidant-antioxidant balance. Oncotarget 2017, 8, 56473–56489. [Google Scholar] [CrossRef][Green Version]
- Mellatyar, H.; Talaei, S.; Pilehvar-Soltanahmadi, Y.; Barzegar, A.; Akbarzadeh, A.; Shahabi, A.; Barekati-Mowahed, M.; Zarghami, N. Targeted cancer therapy through 17-DMAG as an Hsp90 inhibitor: Overview and current state of the art. Biomed. Pharmacother. 2018, 102, 608–617. [Google Scholar] [CrossRef]
- Sullivan, W.P.; Owen, B.A.L.; Toft, D.O. The influence of ATP and p23 on the conformation of hsp90. J. Biol. Chem. 2002, 277, 45942–45948. [Google Scholar] [CrossRef]
- Sullivan, W.; Stensgard, B.; Caucutt, G.; Bartha, B.; McMahon, N.; Alnemri, E.S.; Litwack, G.; Toft, D. Nucleotides and two functional states of hsp90. J. Biol. Chem. 1997, 272, 8007–8012. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Prince, T.; Manjarrez, J.R.; Scroggins, B.T.; Matts, R.L. Characterization of the interaction of Aha1 with components of the Hsp90 chaperone machine and client proteins. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1092–1101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oroz, J.; Kim, J.H.; Chang, B.J.; Zweckstetter, M. Mechanistic basis for the recognition of a misfolded protein by the molecular chaperone Hsp90. Nat. Struct. Mol. Biol. 2017, 24, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Keramisanou, D.; Aboalroub, A.; Zhang, Z.; Liu, W.; Marshall, D.; Diviney, A.; Larsen, R.W.; Landgraf, R.; Gelis, I. Molecular Mechanism of Protein Kinase Recognition and Sorting by the Hsp90 Kinome-Specific Cochaperone Cdc37. Mol. Cell 2016, 62, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Saibil, H. Europe PMC Funders Group Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2015, 14, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Radli, M.; Rüdiger, S.G.D. Dancing with the Diva: Hsp90–Client Interactions. J. Mol. Biol. 2018, 430, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, O.R.; Freiburger, L.; Rutz, D.A.; Krause, M.; Zierer, B.K.; Alvira, S.; Cuéllar, J.; Valpuesta, J.; Madl, T.; Sattler, M.; et al. Modulation of the Hsp90 chaperone cycle by a stringent client protein. Mol. Cell 2014, 53, 941–953. [Google Scholar] [CrossRef]
- Pearl, L.H.; Prodromou, C. Structure and in vivo function of Hsp90. Annu. Rev. Biochem. 2000, 10, 46–51. [Google Scholar] [CrossRef]
- Lackie, R.E.; Maciejewski, A.; Ostapchenko, V.G.; Marques-Lopes, J.; Choy, W.Y.; Duennwald, M.L.; Prado, V.F.; Prado, M.A.M. The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Front. Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef]
- Jaeger, A.M.; Whitesell, L. HSP90: Enabler of Cancer Adaptation. Annu. Rev. Cancer Biol. 2019, 3, 275–297. [Google Scholar] [CrossRef]
- Verba, K.A.; Wang, R.Y.R.; Arakawa, A.; Liu, Y.; Shirouzu, M.; Yokoyama, S.; Agard, D.A. Atomic structure of Hsp90-Cdc37-Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 2016, 352, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, E.; Goswami, D.; Southworth, D.; Griffin, P.R.; Agard, D.A. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 2014, 157, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, K.D.; Demady, D.R.; Stancato, L.F.; Krishna, P.; Pratt, W.B. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor·hsp90 heterocomplexes formed by hsp90·p60·hsp70. J. Biol. Chem. 1997, 272, 21213–21220. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Campbell, I.D. Fibronectin structure and assembly. Curr. Opin. Cell Biol. 1994, 6, 648–655. [Google Scholar] [CrossRef]
- Citri, A.; Harari, D.; Shohat, G.; Ramakrishnan, P.; Gan, J.; Lavi, S.; Eisenstein, M.; Kimchi, A.; Wallach, D.; Pietrokovski, S.; et al. Hsp90 recognizes a common surface on client kinases. J. Biol. Chem. 2006, 281, 14361–14369. [Google Scholar] [CrossRef]
- Peysselon, F.; Xue, B.; Uversky, V.N.; Ricard-Blum, S. Intrinsic disorder of the extracellular matrix. Mol. Biosyst. 2011, 7, 3353–3365. [Google Scholar] [CrossRef]
- Tomasini-Johansson, B.R.; Annis, D.S.; Mosher, D.F. The N-terminal 70-kDa fragment of fibronectin binds to cell surface fibronectin assembly sites in the absence of intact fibronectin. Matrix Biology 2006, 25, 282–293. [Google Scholar] [CrossRef]
- Schwarzbauer, J.E. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J. Cell Biol. 1991, 113, 1463–1473. [Google Scholar] [CrossRef]
- Quade, B.J.; McDonald, J.A. Fibronectin’s amino-terminal matrix assembly site is located within the 29-kDa amino-terminal domain containing five type I repeats. J. Biol. Chem. 1988, 263, 19602–19609. [Google Scholar]
- Sottile, J.; Schwarzbauer, J.; Selegue, J.; Mosher, D.F. Five type I modules of fibronectin form a functional unit that binds to fibroblasts and Staphylococcus aureus. J. Biol. Chem. 1991, 266, 12840–12843. [Google Scholar]

| Average Tm of Unfolding ± SD (°C) (n = 3) | |||
|---|---|---|---|
| FN30 | FN45 | FN70 | FL-FN |
| 74.9 ± 1.7 | 77.6 ± 2.7 | 80.0 ± 4.5 | 76.4 ± 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, A.; Boel, N.M.-E.; Edkins, A.L. HSP90 Interacts with the Fibronectin N-terminal Domains and Increases Matrix Formation. Cells 2020, 9, 272. https://doi.org/10.3390/cells9020272
Chakraborty A, Boel NM-E, Edkins AL. HSP90 Interacts with the Fibronectin N-terminal Domains and Increases Matrix Formation. Cells. 2020; 9(2):272. https://doi.org/10.3390/cells9020272
Chicago/Turabian StyleChakraborty, Abir, Natasha Marie-Eraine Boel, and Adrienne Lesley Edkins. 2020. "HSP90 Interacts with the Fibronectin N-terminal Domains and Increases Matrix Formation" Cells 9, no. 2: 272. https://doi.org/10.3390/cells9020272
APA StyleChakraborty, A., Boel, N. M.-E., & Edkins, A. L. (2020). HSP90 Interacts with the Fibronectin N-terminal Domains and Increases Matrix Formation. Cells, 9(2), 272. https://doi.org/10.3390/cells9020272







