MRE11 Is Crucial for Malaria Parasite Transmission and Its Absence Affects Expression of Interconnected Networks of Key Genes Essential for Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals
2.3. Generation of Transgenic Parasites
2.4. Parasite Genotyping and Western Blotting
2.5. RNA-Seq Transcriptome Sequencing
2.6. Phenotypic Analysis
2.7. Electron Microscopy
2.8. Statistical Analyses
2.9. Data Availability
3. Results
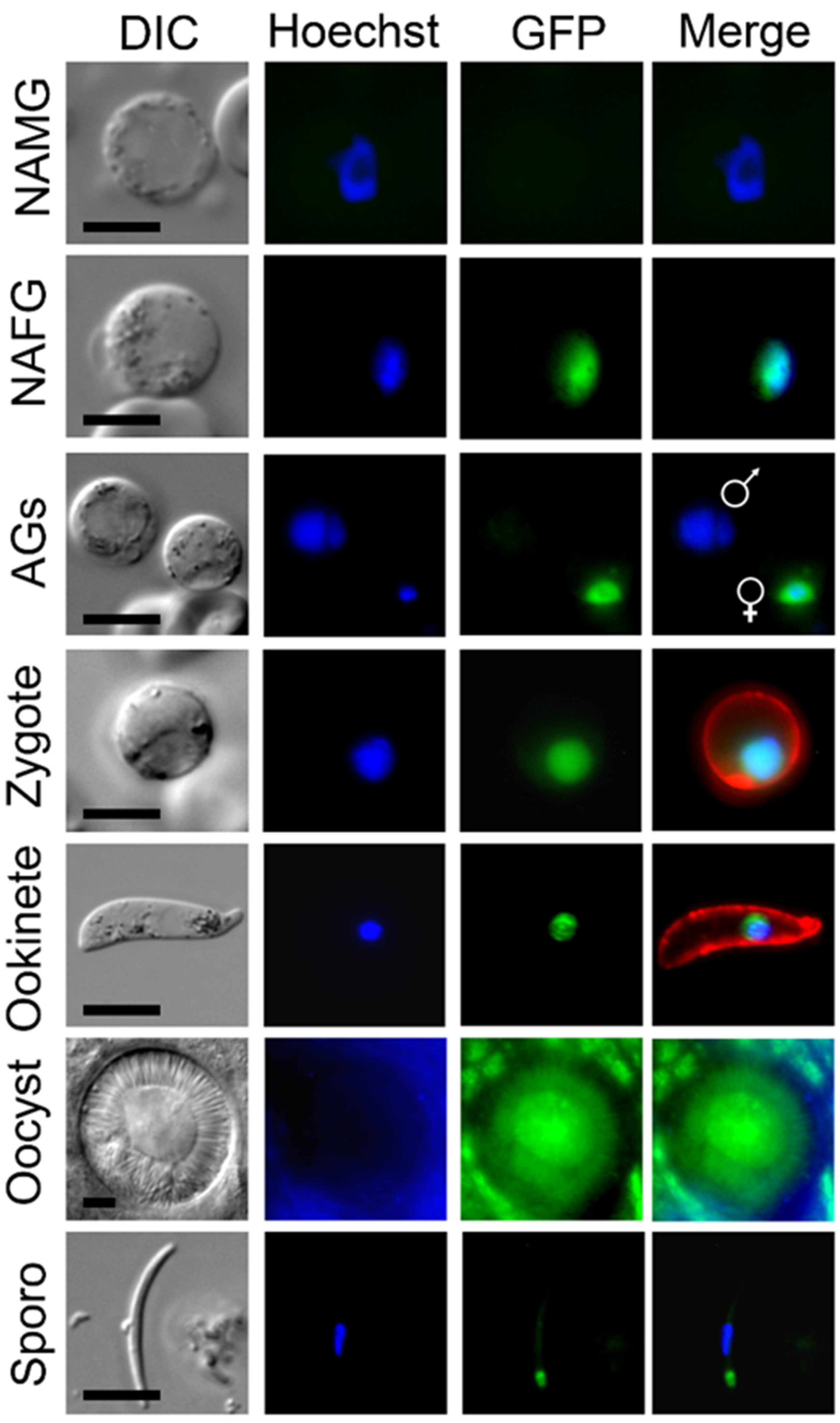
3.1. MRE11-GFP Has a Nuclear Location and Is Female Cell Lineage Specific
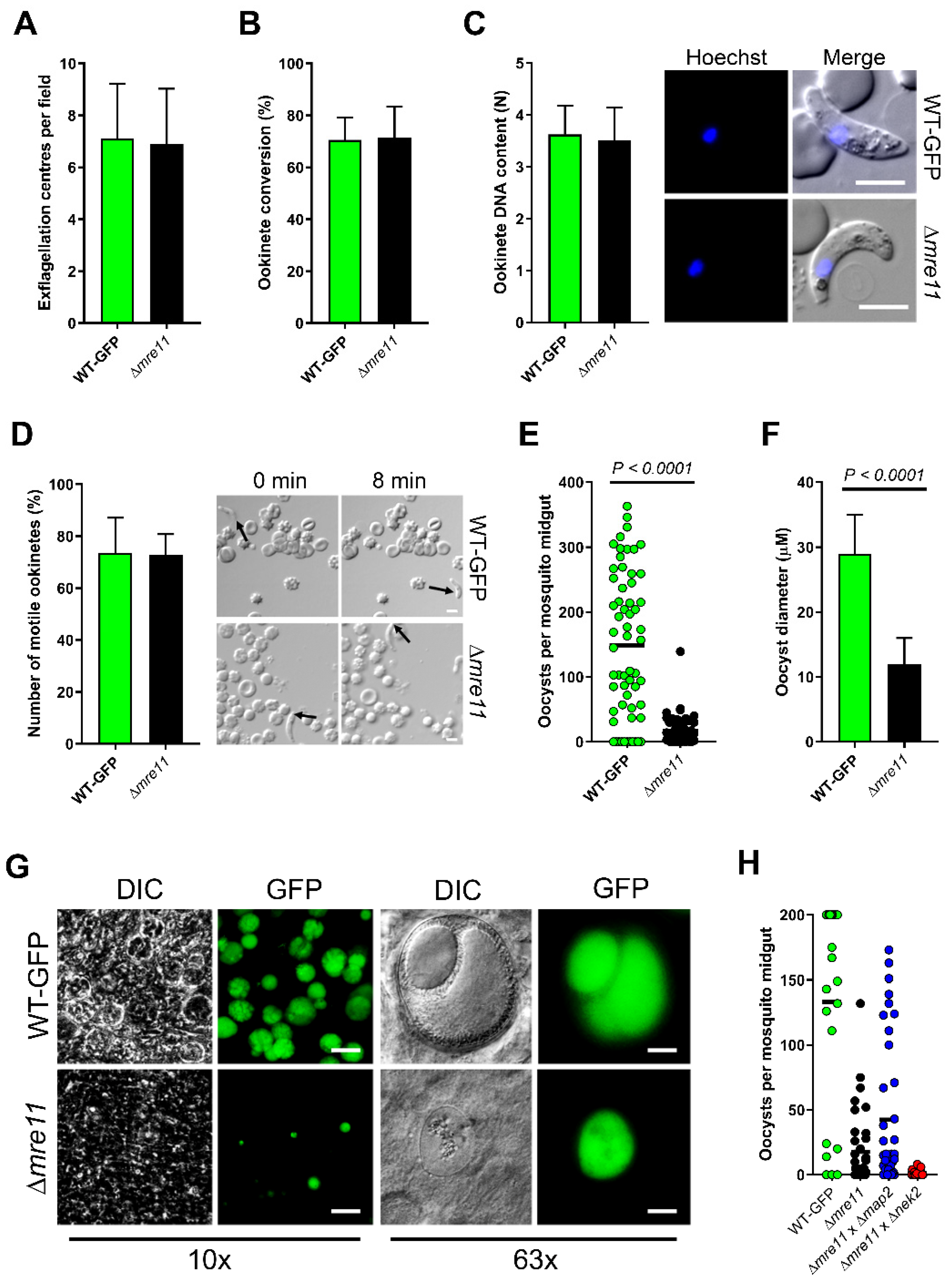
3.2. MRE11 Is Required for Oocyst Maturation and Sporogony in the Mosquito
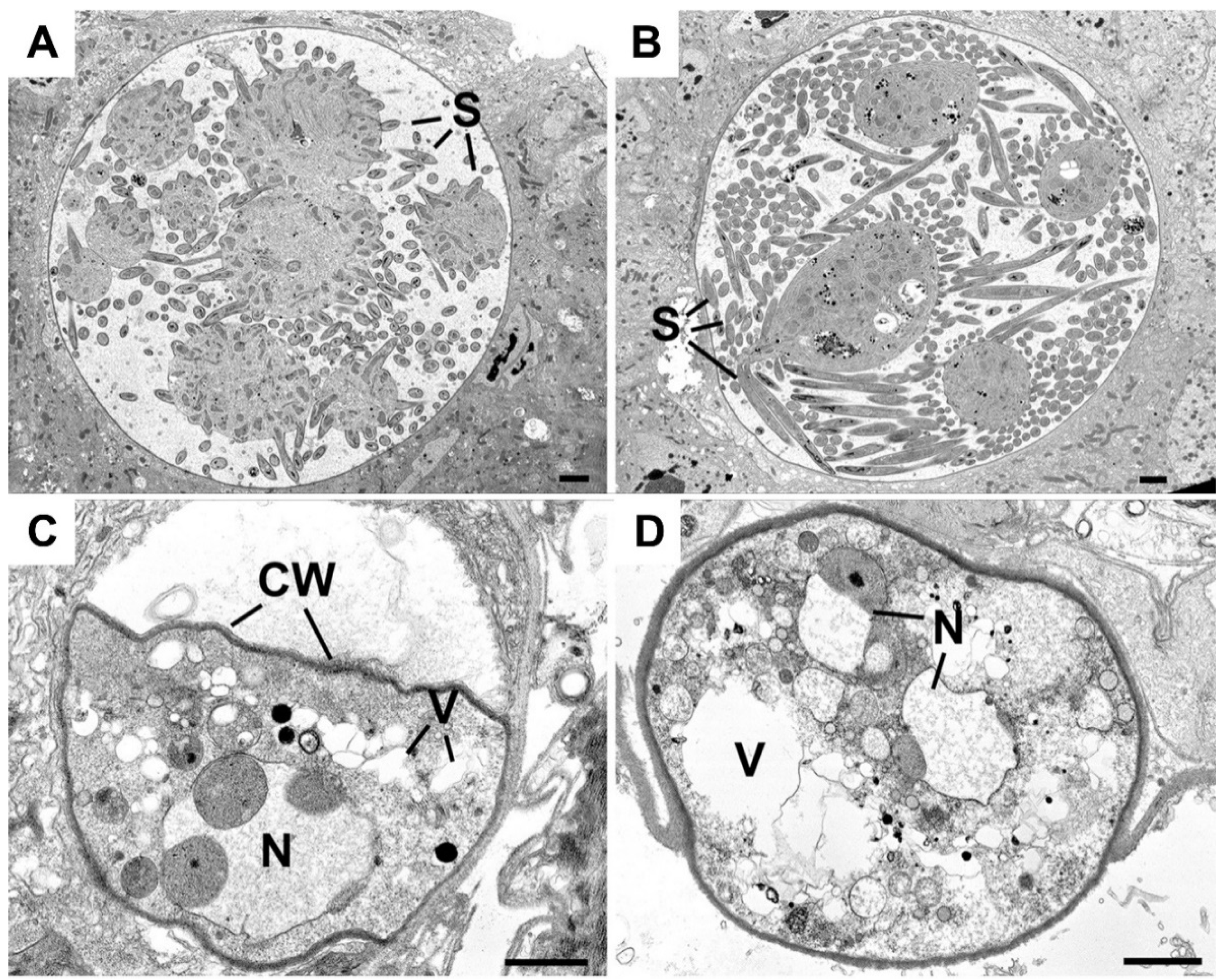
3.3. Ultrastructure Analysis Reveals Degenerative Cytoplasm and Complete Lack of Sporozoite Formation in Δmre11 Parasites
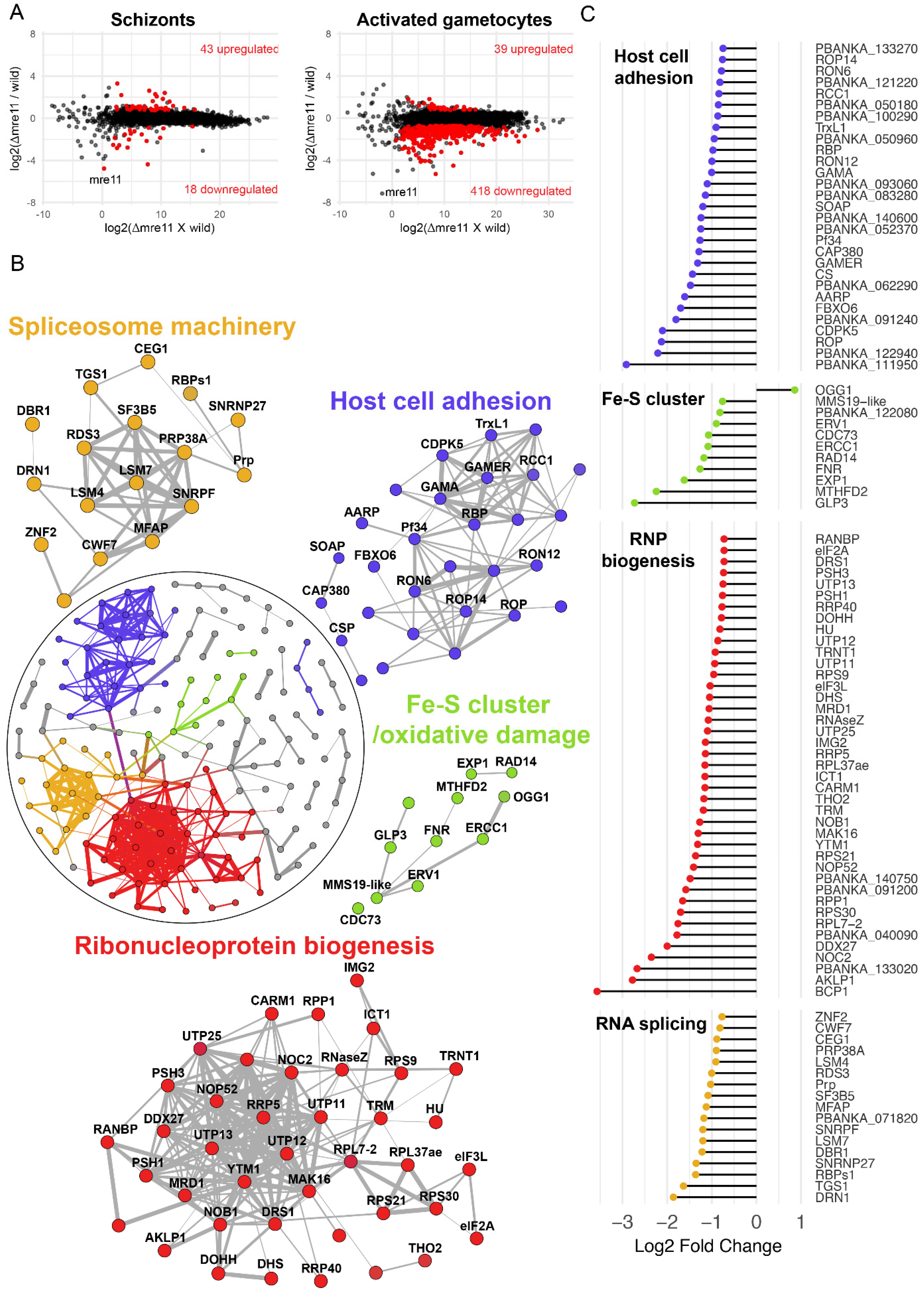
3.4. Absence of PbMRE11 Results in Transcriptional Downregulation of Key Genes Essential to All Eukaryotic Life
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Bannister, L.H.; Sherman, I.W. Malaria. In Els; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Guttery, D.S.; Roques, M.; Holder, A.A.; Tewari, R. Commit and transmit: Molecular players in plasmodium sexual development and zygote differentiation. Trends Parasitol. 2015, 31, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Symington, L.S.; Fidock, D.A. DNA repair mechanisms and their biological roles in the malaria parasite plasmodium falciparum. Microbiol. Mol. Biol. Rev. 2014, 78, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, L.A.; Lawrence, E.A.; Deitsch, K.W. Malaria parasites utilize both homologous recombination and alternative end joining pathways to maintain genome integrity. Nucleic Acids Res. 2014, 42, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Stracker, T.H.; Petrini, J.H. The mre11 complex: Starting from the ends. Nat. Rev. Mol. Cell Biol. 2011, 12, 90–103. [Google Scholar] [CrossRef]
- Bressan, D.A.; Baxter, B.K.; Petrini, J.H. The mre11-rad50-xrs2 protein complex facilitates homologous recombination-based double-strand break repair in saccharomyces cerevisiae. Mol. Cell Biol. 1999, 19, 7681–7687. [Google Scholar] [CrossRef]
- Lavin, M.F. Atm and the mre11 complex combine to recognize and signal DNA double-strand breaks. Oncogene 2007, 26, 7749–7758. [Google Scholar] [CrossRef]
- Shiloh, Y.; Ziv, Y. The atm protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210. [Google Scholar] [CrossRef]
- Symington, L.S. Mechanism and regulation of DNA end resection in eukaryotes. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 195–212. [Google Scholar] [CrossRef]
- Tan, K.S.; Leal, S.T.; Cross, G.A. Trypanosoma brucei mre11 is non-essential but influences growth, homologous recombination and DNA double-strand break repair. Mol. Biochem. Parasit. 2002, 125, 11–21. [Google Scholar] [CrossRef]
- Pandey, R.; Mohmmed, A.; Pierrot, C.; Khalife, J.; Malhotra, P.; Gupta, D. Genome wide in silico analysis of plasmodium falciparum phosphatome. BMC Genomics 2014, 15, 1024. [Google Scholar] [CrossRef] [PubMed]
- Badugu, S.B.; Nabi, S.A.; Vaidyam, P.; Laskar, S.; Bhattacharyya, S.; Bhattacharyya, M.K. Identification of plasmodium falciparum DNA repair protein mre11 with an evolutionarily conserved nuclease function. PLoS ONE 2015, 10, e0125358. [Google Scholar] [CrossRef]
- Gupta, D.K.; Patra, A.T.; Zhu, L.; Gupta, A.P.; Bozdech, Z. DNA damage regulation and its role in drug-related phenotypes in the malaria parasites. Sci. Rep. 2016, 6, 23603. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA damage response in cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Bushell, E.; Gomes, A.R.; Sanderson, T.; Anar, B.; Girling, G.; Herd, C.; Metcalf, T.; Modrzynska, K.; Schwach, F.; Martin, R.E.; et al. Functional profiling of a plasmodium genome reveals an abundance of essential genes. Cell 2017, 170, 260–272.e268. [Google Scholar] [CrossRef] [PubMed]
- Stanway, R.R.; Bushell, E.; Chiappino-Pepe, A.; Roques, M.; Sanderson, T.; Franke-Fayard, B.; Caldelari, R.; Golomingi, M.; Nyonda, M.; Pandey, V.; et al. Genome-scale identification of essential metabolic processes for targeting the plasmodium liver stage. Cell 2019, 179, 1112–1128.e1126. [Google Scholar] [CrossRef]
- Tewari, R.; Straschil, U.; Bateman, A.; Böhme, U.; Cherevach, I.; Gong, P.; Pain, A.; Billker, O. The systematic functional analysis of plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 2010, 8, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Janse, C.J.; Franke-Fayard, B.; Mair, G.R.; Ramesar, J.; Thiel, C.; Engelmann, S.; Matuschewski, K.; van Gemert, G.J.; Sauerwein, R.W.; Waters, A.P. High efficiency transfection of plasmodium berghei facilitates novel selection procedures. Mol. Biochem. Parasit. 2006, 145, 60–70. [Google Scholar] [CrossRef]
- Guttery, D.S.; Poulin, B.; Ramaprasad, A.; Wall, R.J.; Ferguson, D.J.; Brady, D.; Patzewitz, E.M.; Whipple, S.; Straschil, U.; Wright, M.H.; et al. Genome-wide functional analysis of plasmodium protein phosphatases reveals key regulators of parasite development and differentiation. Cell Host Cell Host Microbe 2014, 16, 128–140. [Google Scholar] [CrossRef]
- Roques, M.; Wall, R.J.; Douglass, A.P.; Ramaprasad, A.; Ferguson, D.J.; Kaindama, M.L.; Brusini, L.; Joshi, N.; Rchiad, Z.; Brady, D.; et al. Plasmodium p-type cyclin cyc3 modulates endomitotic growth during oocyst development in mosquitoes. PLoS Pathog. 2015, 11, e1005273. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. String v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Howick, V.M.; Russell, A.J.C.; Andrews, T.; Heaton, H.; Reid, A.J.; Natarajan, K.; Butungi, H.; Metcalf, T.; Verzier, L.H.; Rayner, J.C.; et al. The malaria cell atlas: Single parasite transcriptomes across the complete plasmodium life cycle. Science 2019, 365, eaaw2619. [Google Scholar] [CrossRef] [PubMed]
- Patzewitz, E.M.; Guttery, D.S.; Poulin, B.; Ramakrishnan, C.; Ferguson, D.J.; Wall, R.J.; Brady, D.; Holder, A.A.; Szoor, B.; Tewari, R. An ancient protein phosphatase, shlp1, is critical to microneme development in plasmodium ookinetes and parasite transmission. Cell Rep. 2013, 3, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Dorin, D.; Moon, R.; Doerig, C.; Billker, O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol. Microbiol. 2005, 58, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Kehr, S.; Sturm, N.; Rahlfs, S.; Przyborski, J.M.; Becker, K. Compartmentation of redox metabolism in malaria parasites. PLoS Pathog. 2010, 6, e1001242. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Adinolfi, S.; Pastore, A. Ferredoxin, in conjunction with nadph and ferredoxin-nadp reductase, transfers electrons to the iscs/iscu complex to promote iron-sulfur cluster assembly. Biochim. Biophys. Acta 2015, 1854, 1113–1117. [Google Scholar] [CrossRef]
- Rissler, M.; Wiedemann, N.; Pfannschmidt, S.; Gabriel, K.; Guiard, B.; Pfanner, N.; Chacinska, A. The essential mitochondrial protein erv1 cooperates with mia40 in biogenesis of intermembrane space proteins. J. Mol. Biol. 2005, 353, 485–492. [Google Scholar] [CrossRef]
- Stehling, O.; Vashisht, A.A.; Mascarenhas, J.; Jonsson, Z.O.; Sharma, T.; Netz, D.J.; Pierik, A.J.; Wohlschlegel, J.A.; Lill, R. Mms19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 2012, 337, 195–199. [Google Scholar] [CrossRef]
- Stolc, V.; Altman, S. Rpp1, an essential protein subunit of nuclear rnase p required for processing of precursor trna and 35s precursor rrna in saccharomyces cerevisiae. Genes Dev. 1997, 11, 2926–2937. [Google Scholar] [CrossRef]
- Lindner, S.E.; Swearingen, K.E.; Shears, M.J.; Walker, M.P.; Vrana, E.N.; Hart, K.J.; Minns, A.M.; Sinnis, P.; Moritz, R.L.; Kappe, S.H.I. Transcriptomics and proteomics reveal two waves of translational repression during the maturation of malaria parasite sporozoites. Nat. Commun. 2019, 10, 4964. [Google Scholar] [CrossRef]
- Mair, G.R.; Braks, J.A.; Garver, L.S.; Wiegant, J.C.; Hall, N.; Dirks, R.W.; Khan, S.M.; Dimopoulos, G.; Janse, C.J.; Waters, A.P. Regulation of sexual development of plasmodium by translational repression. Science 2006, 313, 667–669. [Google Scholar] [CrossRef]
- Mair, G.R.; Lasonder, E.; Garver, L.S.; Franke-Fayard, B.M.; Carret, C.K.; Wiegant, J.C.; Dirks, R.W.; Dimopoulos, G.; Janse, C.J.; Waters, A.P. Universal features of post-transcriptional gene regulation are critical for plasmodium zygote development. PLoS Pathog. 2010, 6, e1000767. [Google Scholar] [CrossRef]
- Matthews, H.; Duffy, C.W.; Merrick, C.J. Checks and balances? DNA replication and the cell cycle in plasmodium. Parasit Vectors 2018, 11, 216. [Google Scholar] [CrossRef]
- Borde, V. The multiple roles of the mre11 complex for meiotic recombination. Chromosome Res. 2007, 15, 551–563. [Google Scholar] [CrossRef]
- Mlambo, G.; Coppens, I.; Kumar, N. Aberrant sporogonic development of dmc1 (a meiotic recombinase) deficient plasmodium berghei parasites. PLoS ONE 2012, 7, e52480. [Google Scholar] [CrossRef]
- Thomson, E.; Ferreira-Cerca, S.; Hurt, E. Eukaryotic ribosome biogenesis at a glance. J. Cell. Sci. 2013, 126, 4815–4821. [Google Scholar] [CrossRef]
- Canning, E.U.; Sinden, R.E. The organization of the ookinete and observations on nuclear division in oocysts of plasmodium berghei. Parasitology 1973, 67, 29–40. [Google Scholar] [CrossRef]
- Baker, D.A. Malaria gametocytogenesis. Mol. Biochem. Parasitol. 2010, 172, 57–65. [Google Scholar] [CrossRef]
- Missbach, S.; Weis, B.L.; Martin, R.; Simm, S.; Bohnsack, M.T.; Schleiff, E. 40s ribosome biogenesis co-factors are essential for gametophyte and embryo development. PLoS ONE 2013, 8, e54084. [Google Scholar] [CrossRef]
- Will, C.L.; Luhrmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect Biol. 2011, 3, a003707. [Google Scholar] [CrossRef]
- Adamson, B.; Smogorzewska, A.; Sigoillot, F.D.; King, R.W.; Elledge, S.J. A genome-wide homologous recombination screen identifies the rna-binding protein rbmx as a component of the DNA-damage response. Nat. Cell Biol. 2012, 14, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, M.; Sanjiv, K.; Helleday, T.; Herr, P.; Mortusewicz, O. The spliceosome u2 snrnp factors promote genome stability through distinct mechanisms; transcription of repair factors and r-loop processing. Oncogenesis 2016, 5, e280. [Google Scholar] [CrossRef] [PubMed]
- Naro, C.; Bielli, P.; Pagliarini, V.; Sette, C. The interplay between DNA damage response and rna processing: The unexpected role of splicing factors as gatekeepers of genome stability. Front. Genet. 2015, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.S.; Hawley, B.R.; Wilczynska, A.; Bushell, M. The roles of rna in DNA double-strand break repair. Br. J. Cancer 2020, 122, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, V.O.; Venkitaraman, A.R. Rna processing and genome stability: Cause and consequence. Mol. Cell 2016, 61, 496–505. [Google Scholar] [CrossRef]
- Veatch, J.R.; McMurray, M.A.; Nelson, Z.W.; Gottschling, D.E. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell 2009, 137, 1247–1258. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Zhou, X.; Zhuang, Z.; Wang, W.; He, L.; Wu, H.; Cao, Y.; Pan, F.; Zhao, J.; Hu, Z.; Sekhar, C.; et al. Ogg1 is essential in oxidative stress induced DNA demethylation. Cell Signal. 2016, 28, 1163–1171. [Google Scholar] [CrossRef]
- Scott, A.D.; Neishabury, M.; Jones, D.H.; Reed, S.H.; Boiteux, S.; Waters, R. Spontaneous mutation, oxidative DNA damage, and the roles of base and nucleotide excision repair in the yeast saccharomyces cerevisiae. Yeast 1999, 15, 205–218. [Google Scholar] [CrossRef]
- Guzder, S.N.; Torres-Ramos, C.; Johnson, R.E.; Haracska, L.; Prakash, L.; Prakash, S. Requirement of yeast rad1-rad10 nuclease for the removal of 3’-blocked termini from DNA strand breaks induced by reactive oxygen species. Genes Dev. 2004, 18, 2283–2291. [Google Scholar] [CrossRef]
- Guillet, M.; Boiteux, S. Endogenous DNA abasic sites cause cell death in the absence of apn1, apn2 and rad1/rad10 in saccharomyces cerevisiae. EMBO J. 2002, 21, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Barillas-Mury, C. Plasmodium oocysts: Overlooked targets of mosquito immunity. Trends Parasitol. 2016, 32, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Kugou, K.; Sasanuma, H.; Matsumoto, K.; Shirahige, K.; Ohta, K. Mre11 mediates gene regulation in yeast spore development. Genes Genet. Syst. 2007, 82, 21–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guttery, D.S.; Ramaprasad, A.; Ferguson, D.J.P.; Zeeshan, M.; Pandey, R.; Brady, D.; Holder, A.A.; Pain, A.; Tewari, R. MRE11 Is Crucial for Malaria Parasite Transmission and Its Absence Affects Expression of Interconnected Networks of Key Genes Essential for Life. Cells 2020, 9, 2590. https://doi.org/10.3390/cells9122590
Guttery DS, Ramaprasad A, Ferguson DJP, Zeeshan M, Pandey R, Brady D, Holder AA, Pain A, Tewari R. MRE11 Is Crucial for Malaria Parasite Transmission and Its Absence Affects Expression of Interconnected Networks of Key Genes Essential for Life. Cells. 2020; 9(12):2590. https://doi.org/10.3390/cells9122590
Chicago/Turabian StyleGuttery, David S., Abhinay Ramaprasad, David J. P. Ferguson, Mohammad Zeeshan, Rajan Pandey, Declan Brady, Anthony A. Holder, Arnab Pain, and Rita Tewari. 2020. "MRE11 Is Crucial for Malaria Parasite Transmission and Its Absence Affects Expression of Interconnected Networks of Key Genes Essential for Life" Cells 9, no. 12: 2590. https://doi.org/10.3390/cells9122590
APA StyleGuttery, D. S., Ramaprasad, A., Ferguson, D. J. P., Zeeshan, M., Pandey, R., Brady, D., Holder, A. A., Pain, A., & Tewari, R. (2020). MRE11 Is Crucial for Malaria Parasite Transmission and Its Absence Affects Expression of Interconnected Networks of Key Genes Essential for Life. Cells, 9(12), 2590. https://doi.org/10.3390/cells9122590




