Characterization of the Polysialylation Status in Ovaries of the Salmonid Fish Coregonus maraena and the Percid Fish Sander lucioperca
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Western Blotting
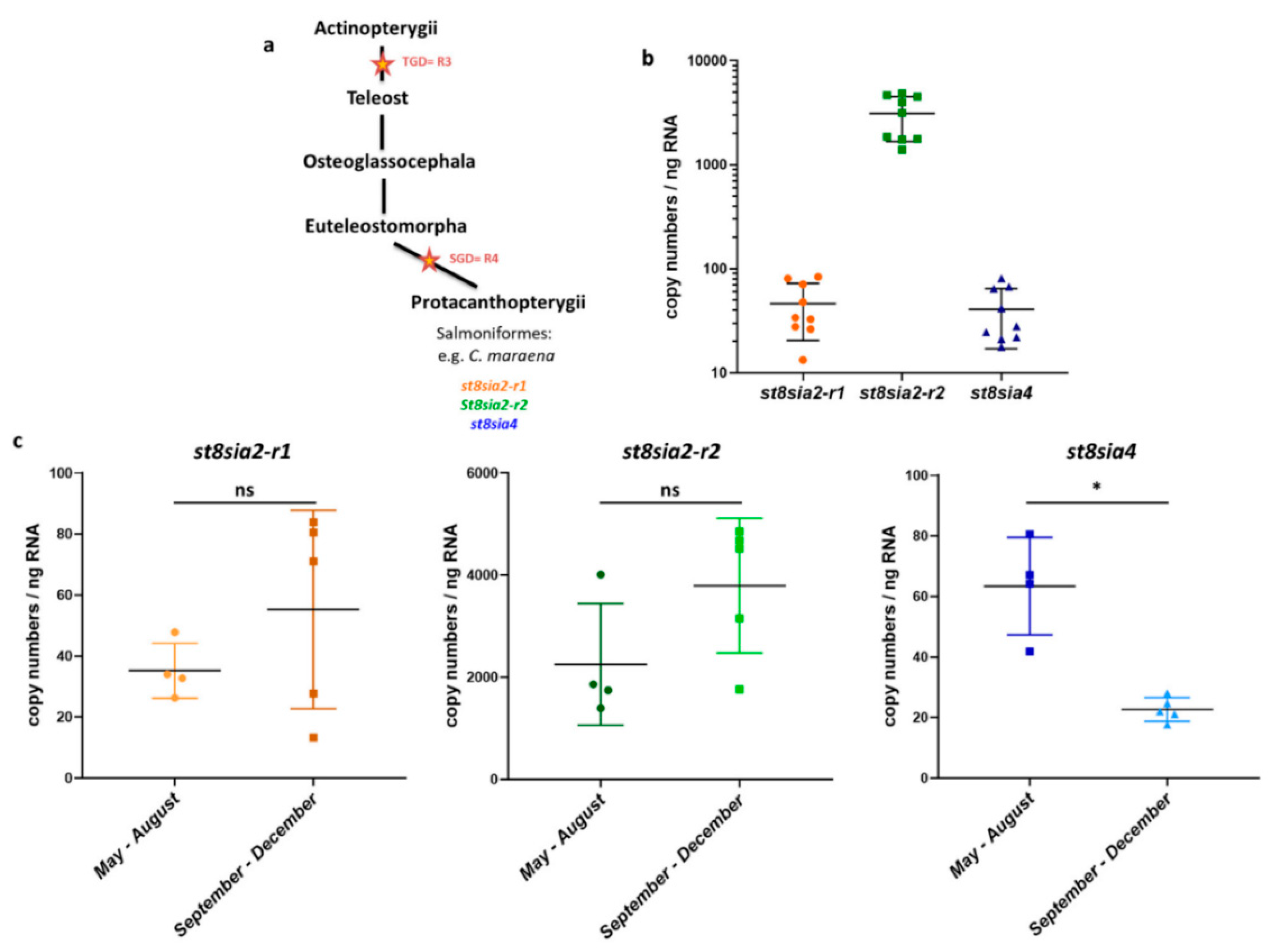
2.3. Real-Time Quantitative PCR (RT-qPCR)
- st8sia2 r-1:
- 5´AGCCTCATCAGGAAGAACATCC-3´ (sense)5´-TTCCCTACGATGGCACAGCGT-3´ (antisense)
- st8sia2 r-2:
- 5´-CGTTCAACAGGAGCCTCTCTAA-3´ (sense)5´-TTCCCTACGATGGCACAGCGC-3´ (antisense)
- st8sia4:
- 5´-ATGATAAGGAAGGACGTGCTGC-3´ (sense)5´-TGTTGAGCGTTCGGCGTCTGT-3´ (antisense)
- st8sia2:
- 5´-GAGGAAGAAACTGCAAATACTGG-3´ (sense)5´-AGTTGTTTGACGAGAGCTTGACA-3´ (antisense)
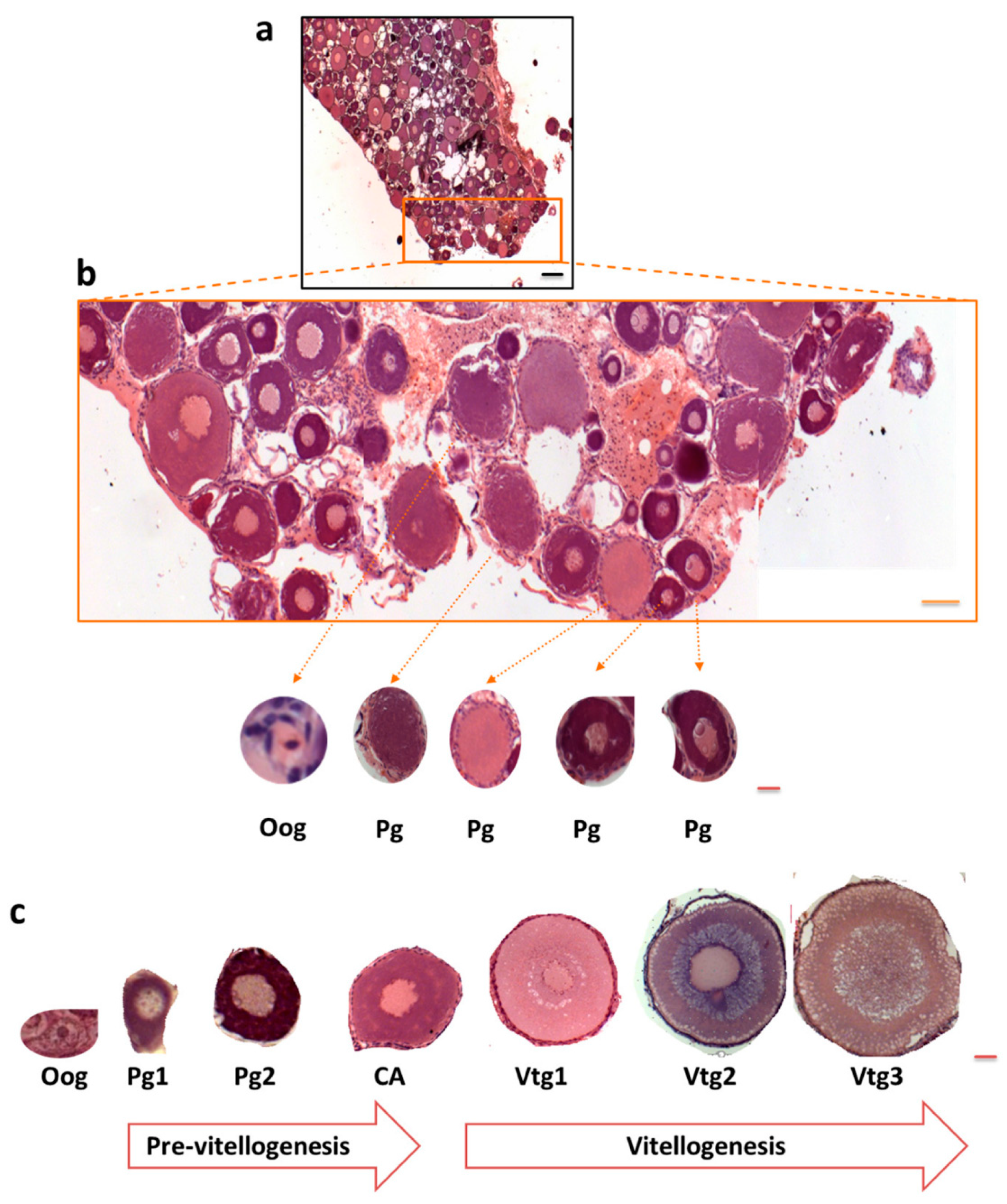
2.4. Histological Sample Preparation
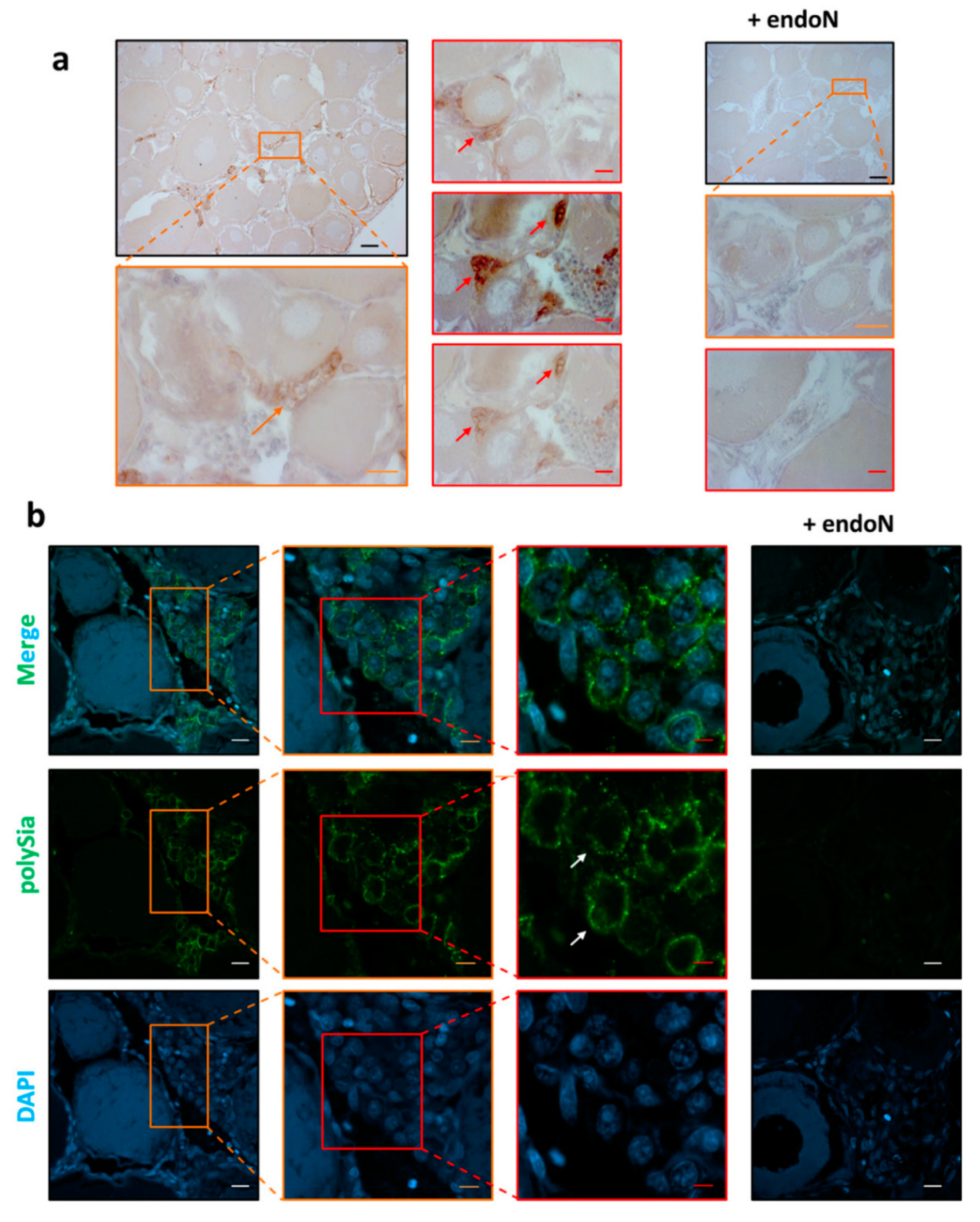
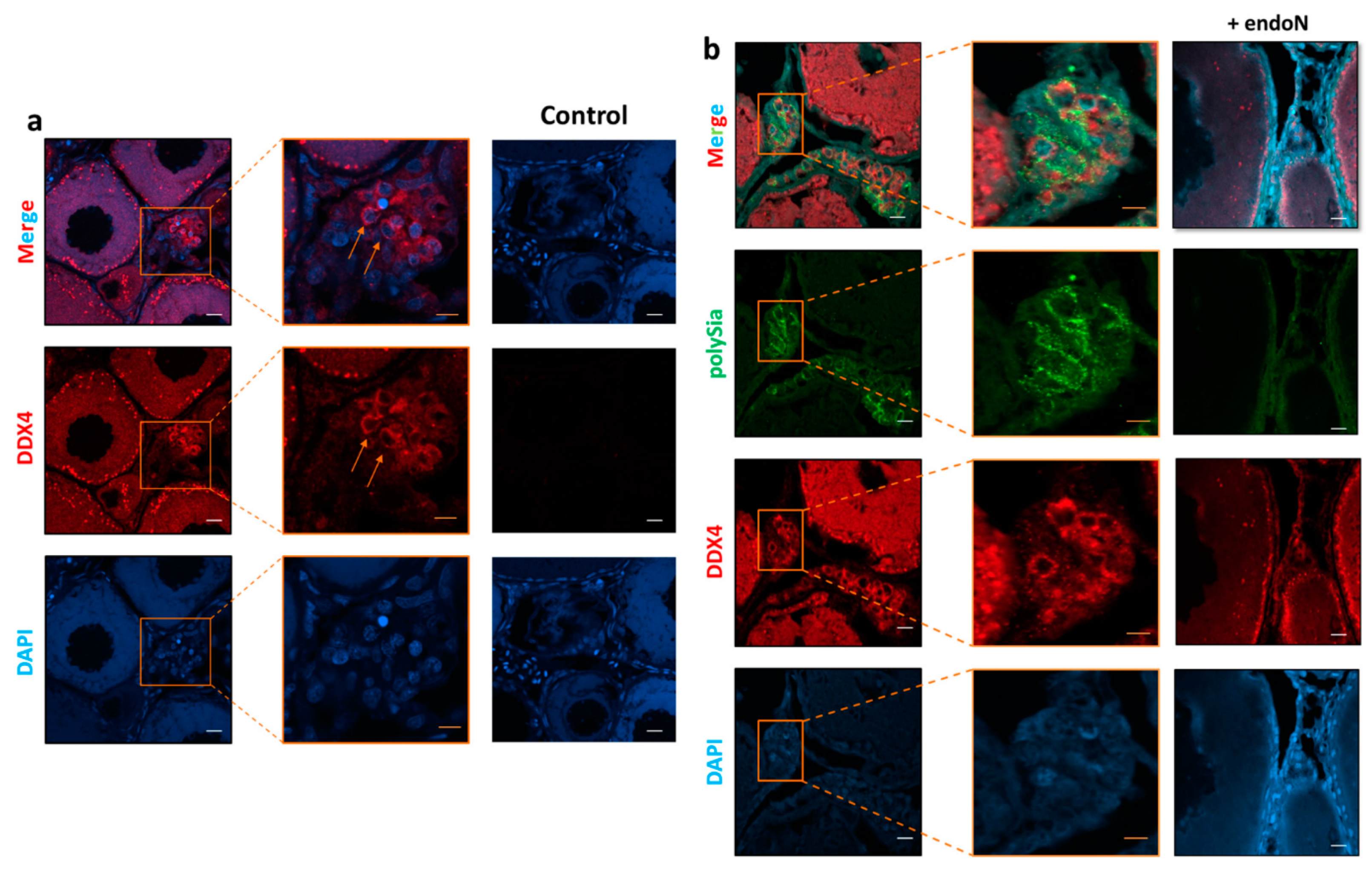
2.5. Immunohistochemistry Procedure
2.6. Fluorescent Staining
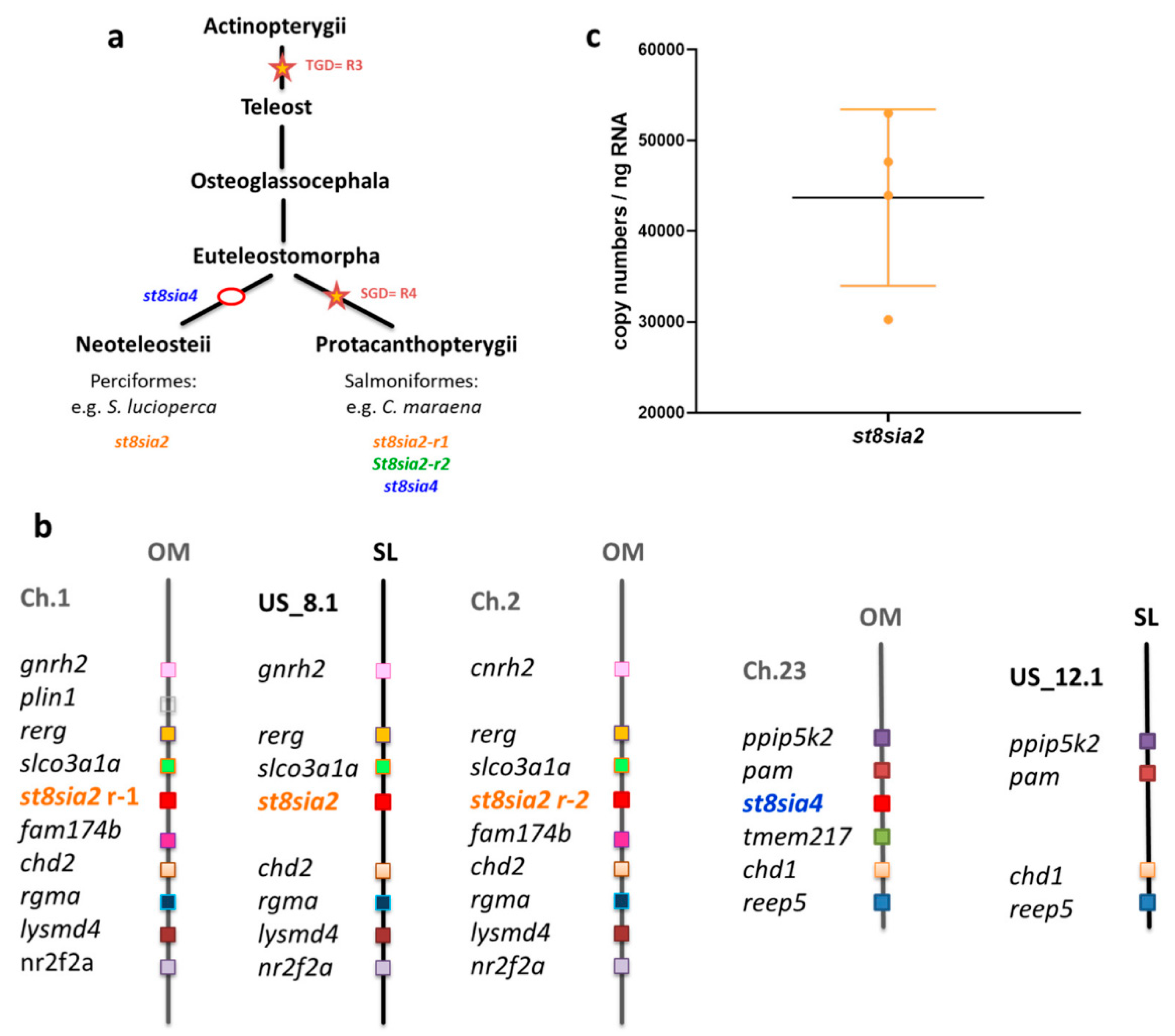
2.7. Synteny Analysis
2.8. Statistical Analysis
3. Results
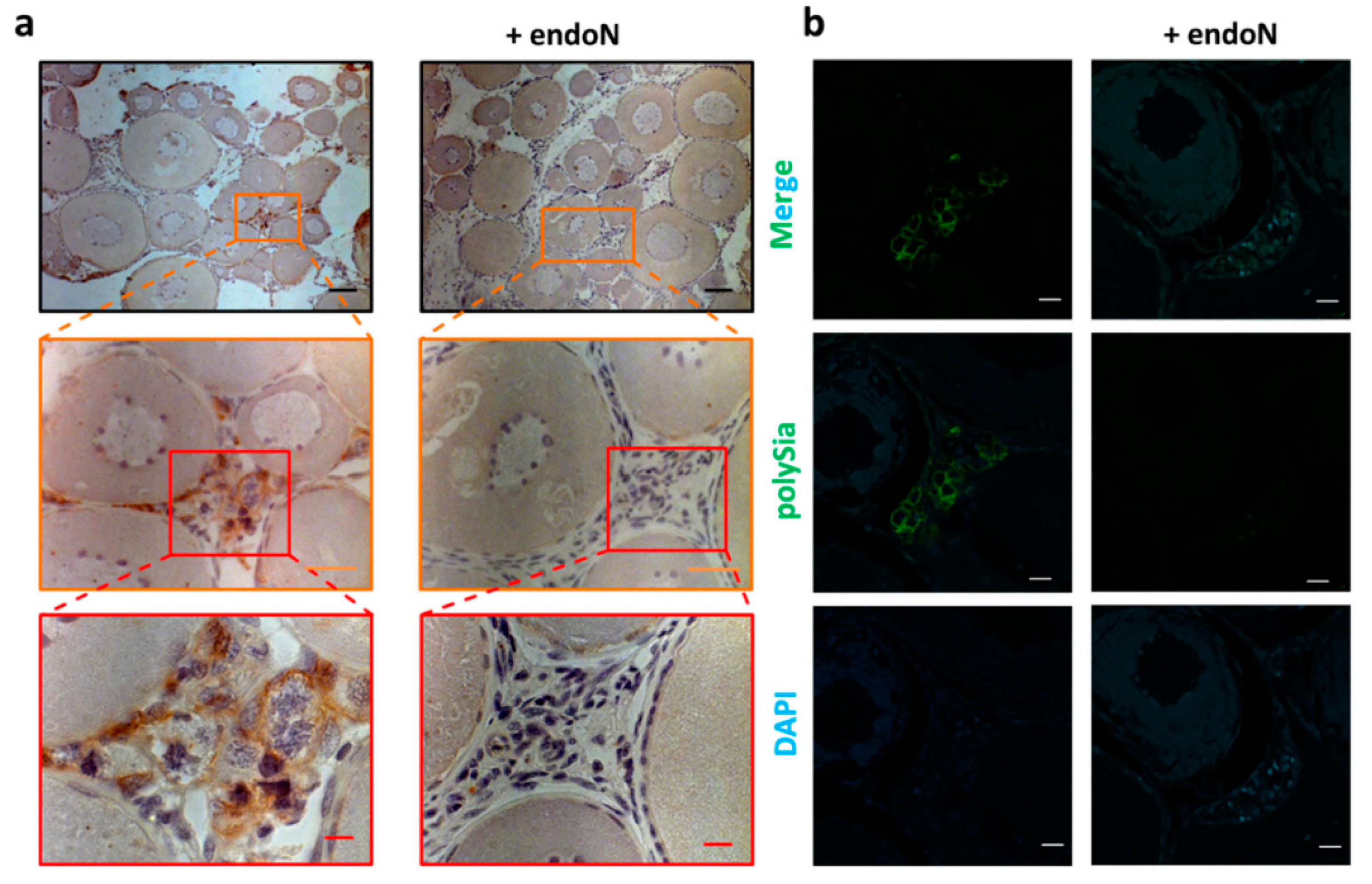
3.1. Polysialylation in Ovary of C. maraena
3.2. Expression Levels of PolySiaT Genes in C. maraena Ovaries
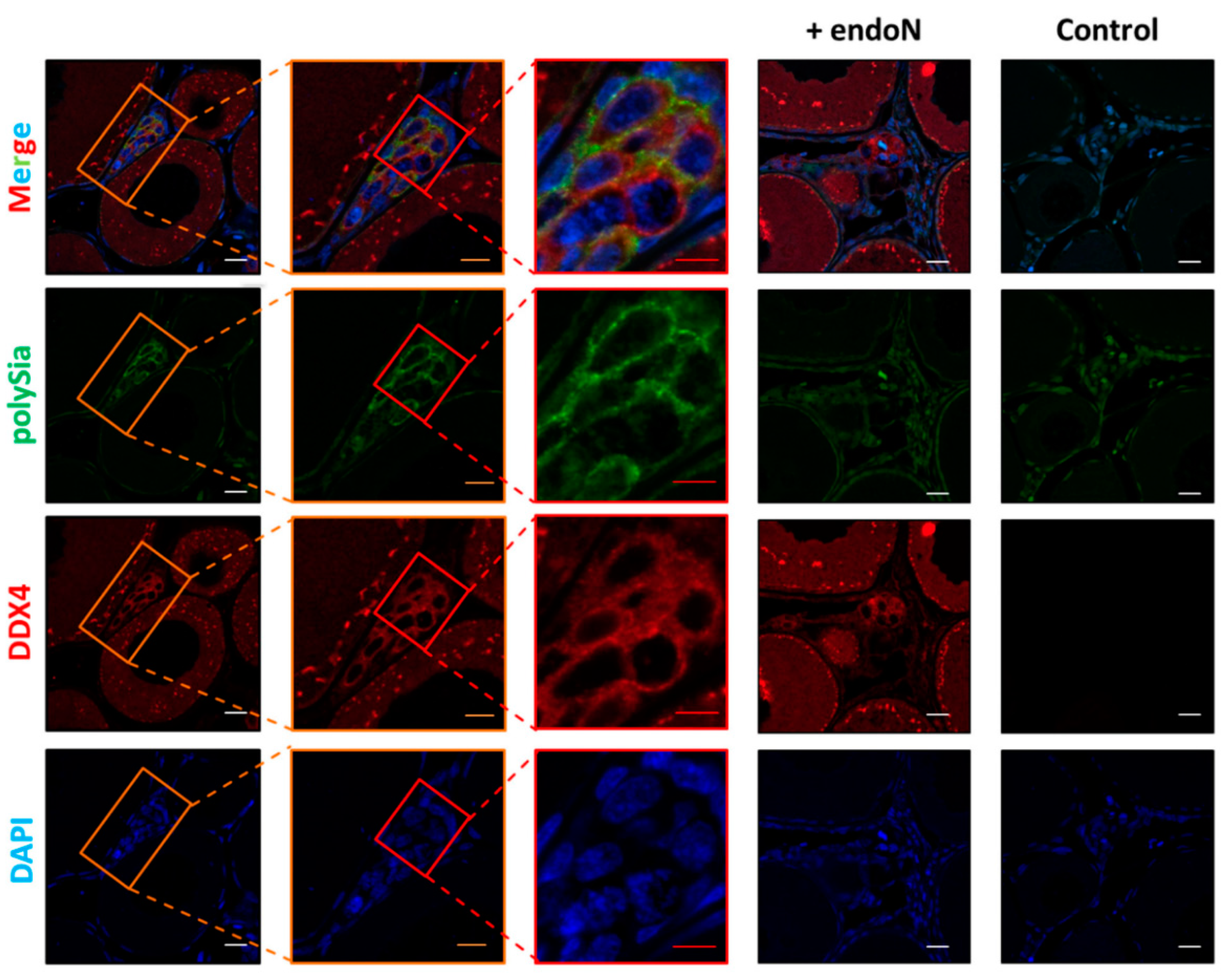
3.3. Localization of Polysia in Ovaries of Maraena Whitefish
3.4. Polysialylation in Ovaries of S. lucioperca
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angata, T.; Varki, A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem. Rev. 2002, 102, 439–469. [Google Scholar] [CrossRef] [PubMed]
- Galuska, C.E.; Lütteke, T.; Galuska, S.P. Is polysialylated NCAM not only a regulator during brain development but also during the formation of other organs? Biology 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Colley, K.J.; Kitajima, K.; Sato, C. Polysialic acid: Biosynthesis, novel functions and applications. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 498–532. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Elkashef, S.M.; Loadman, P.M.; Patterson, L.H.; Falconer, R.A. Recent advances in the analysis of polysialic acid from complex biological systems. Carbohydr. Polym. 2019, 224, 115145. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Feuerstacke, C.; Kaese, M.; Saboor, F.; Middendorff, R.; Galuska, S.P. Polysialylation of NCAM characterizes the proliferation period of contractile elements during postnatal development of the epididymis. PLoS ONE 2015, 10, e0123960. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Iwasaki, M. Isolation of a novel glycoprotein from the eggs of rainbow trout: Occurrence of disialosyl groups on all carbohydrate chains. Biochem. Biophys. Res. Commun. 1978, 83, 1018–1023. [Google Scholar] [CrossRef]
- Inoue, S.; Kitajima, K.; Inoue, Y.; Kudo, S. Localization of Polysialoglycoprotein as a Major Glycoprotein Component in Cortical Alveoli of the Unfertilized Eggs of Salmo-Gairdneri. Dev. Biol. 1987, 123, 442–454. [Google Scholar] [CrossRef]
- Iwasaki, M.; Inoue, S. Structures of the carbohydrate units of polysialoglycoproteins isolated from the eggs of four species of salmonid fishes. Glycoconj. J. 1985, 2, 209–228. [Google Scholar] [CrossRef]
- Sato, C.; Kitajima, K.; Tazawa, I.; Inoue, Y.; Inoue, S.; Troy, F.A. Structural diversity in the Alpha-2-]8-Linked polysialic acid chains in salmonid fish egg glycoproteins-occurrence of poly(Neu5ac), poly(Neu5gc), poly(Neu5ac, Neu5gc), poly(Kdn), and their partially acetylated forms. J. Biol. Chem. 1993, 268, 23675–23684. [Google Scholar] [PubMed]
- Ravasio, V.; Damiati, E.; Zizioli, D.; Orizio, F.; Giacopuzzi, E.; Manzoni, M.; Bresciani, R.; Borsani, G.; Monti, E. Genomic and biochemical characterization of sialic acid acetylesterase (siae) in zebrafish. Glycobiology 2017, 27, 938–946. [Google Scholar] [CrossRef]
- Kitajima, K.; Nomoto, H.; Inoue, Y.; Iwasaki, M.; Inoue, S. Fish egg polysialoglycoproteins: Circular dichroism and proton nuclear magnetic resonance studies of novel oligosaccharide units containing one sialidase-resistant N-glycolylneuraminic acid residue in each molecule. Biochemistry 1984, 23, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Harduin-Lepers, A.; Petit, D.; Mollicone, R.; Delannoy, P.; Petit, J.M.; Oriol, R. Evolutionary history of the alpha2,8-sialyltransferase (ST8Sia) gene family: Tandem duplications in early deuterostomes explain most of the diversity found in the vertebrate ST8Sia genes. BMC Evol. Biol. 2008, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Petit, D.; Teppa, E.; Cenci, U.; Ball, S.; Harduin-Lepers, A. Reconstruction of the sialylation pathway in the ancestor of eukaryotes. Sci. Rep. 2018, 8, 2946. [Google Scholar] [CrossRef]
- Force, A.; Lynch, M.; Pickett, F.B.; Amores, A.; Yan, Y.L.; Postlethwait, J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [PubMed]
- Asahina, S.; Sato, C.; Matsuno, M.; Matsuda, T.; Colley, K.; Kitajima, K. Involvement of the alpha2,8-polysialyltransferases II/STX and IV/PST in the biosynthesis of polysialic acid chains on the O-linked glycoproteins in rainbow trout ovary. J. Biochem. 2006, 140, 687–701. [Google Scholar] [CrossRef]
- Venuto, M.T.; Decloquement, M.; Martorell Ribera, J.; Noel, M.; Rebl, A.; Cogez, V.; Petit, D.; Galuska, S.P.; Harduin-Lepers, A. Vertebrate Alpha2,8-Sialyltransferases (ST8Sia): A Teleost Perspective. Int. J. Mol. Sci. 2020, 21, 513. [Google Scholar] [CrossRef]
- Kitazume, S.; Kitajima, K.; Inoue, S.; Inoue, Y.; Troy, F.A. Developmental Expression of Trout Egg Polysialoglycoproteins and the Prerequisite Alpha-2,6-Sialyl, and Alpha-2,8-Sialyl and Alpha-2,8-Polysialyltransferase Activities Required for Their Synthesis during Oogenesis. J. Biol. Chem. 1994, 269, 10330–10340. [Google Scholar]
- Kanamori, A.; Inoue, S.; Iwasaki, M.; Kitajima, K.; Kawai, G.; Yokoyama, S.; Inoue, Y. Deaminated Neuraminic Acid-Rich Glycoprotein of Rainbow-Trout Egg Vitelline Envelope-Occurrence of a Novel Alpha-2,8-Linked Oligo(Deaminated Neuraminic Acid) Structure in O-Linked Glycan Chains. J. Biol. Chem. 1990, 265, 21811–21819. [Google Scholar]
- Kitajima, K.; Inoue, Y.; Inoue, S. Polysialoglycoproteins of Salmonidae fish eggs. Complete structure of 200-kDa polysialoglycoprotein from the unfertilized eggs of rainbow trout (Salmo gairdneri). J. Biol. Chem. 1986, 261, 5262–5269. [Google Scholar]
- Brietzke, A.; Borchel, A.; Altmann, S.; Nipkow, M.; Rebl, A.; Brunner, R.M.; Goldammer, T. Transcriptome sequencing of maraena whitefish (Coregonus maraena). Mar. Genom. 2016, 29, 27–29. [Google Scholar] [CrossRef]
- Frosch, M.; Gorgen, I.; Boulnois, G.J.; Timmis, K.N.; Bitter-Suermann, D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: Isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc. Natl. Acad. Sci. USA 1985, 82, 1194–1198. [Google Scholar] [CrossRef]
- Stummeyer, K.; Dickmanns, A.; Muhlenhoff, M.; Gerardy-Schahn, R.; Ficner, R. Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat. Struct. Mol. Biol. 2005, 12, 90–96. [Google Scholar] [CrossRef]
- Welinder, C.; Ekblad, L. Coomassie staining as loading control in western blot analysis. J. Proteome Res. 2011, 10, 1416–1419. [Google Scholar] [CrossRef]
- Nguinkal, J.A.; Brunner, R.M.; Verleih, M.; Rebl, A.; Ríos-Pérez, L.d.l.; Schäfer, N.; Hadlich, F.; Stüeken, M.; Wittenburg, D.; Goldammer, T. The first highly contiguous genome assembly of pikeperch (sander lucioperca), an emerging aquaculture species in europe. Genes 2019, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Altmann, S.; Rebl, A.; Kuhn, C.; Goldammer, T. Identification and de novo sequencing of housekeeping genes appropriate for gene expression analyses in farmed maraena whitefish (Coregonus maraena) during crowding stress. Fish Physiol. Biochem. 2015, 41, 397–412. [Google Scholar] [CrossRef]
- Nagae, M.; Ikeda, A.; Hane, M.; Hanashima, S.; Kitajima, K.; Sato, C.; Yamaguchi, Y. Crystal structure of anti-polysialic acid antibody single chain Fv fragment complexed with octasialic acid: Insight into the binding preference for polysialic acid. J. Biol. Chem. 2013, 288, 33784–33796. [Google Scholar] [CrossRef] [PubMed]
- Finne, J.; Makela, P.H. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J. Biol. Chem. 1985, 260, 1265–1270. [Google Scholar]
- Mühlenhoff, M.; Eckhardt, M.; Bethe, A.; Frosch, M.; Gerardy-Schahn, R. Polysialylation of NCAM by a single enzyme. Curr. Biol. 1996, 6, 1188–1191. [Google Scholar] [CrossRef]
- Kuhnle, A.; Veelken, R.; Galuska, C.E.; Saftenberger, M.; Verleih, M.; Schuppe, H.C.; Rudloff, S.; Kunz, C.; Galuska, S.P. Polysialic acid interacts with lactoferrin and supports its activity to inhibit the release of neutrophil extracellular traps. Carbohydr. Polym. 2019, 208, 32–41. [Google Scholar] [CrossRef]
- Naito-Matsui, Y.; Davies, L.R.; Takematsu, H.; Chou, H.H.; Tangvoranuntakul, P.; Carlin, A.F.; Verhagen, A.; Heyser, C.J.; Yoo, S.W.; Choudhury, B.; et al. physiological exploration of the long term evolutionary selection against expression of n-glycolylneuraminic acid in the brain. J. Biol. Chem. 2017, 292, 2557–2570. [Google Scholar] [CrossRef]
- Davies, L.R.; Pearce, O.M.; Tessier, M.B.; Assar, S.; Smutova, V.; Pajunen, M.; Sumida, M.; Sato, C.; Kitajima, K.; Finne, J.; et al. Metabolism of vertebrate amino sugars with N-glycolyl groups: Resistance of alpha2-8-linked N-glycolylneuraminic acid to enzymatic cleavage. J. Biol. Chem. 2012, 287, 28917–28931. [Google Scholar] [CrossRef]
- Nagahama, Y. 6 The functional morphology of teleost gonads. In Fish Physiology; Hoar, W.S., Randall, D.J., Donaldson, E.M., Eds.; Academic Press: Cambridge, MA, USA, 1983; Volume 9, pp. 223–275. [Google Scholar]
- Rocha MJ, R.E. Morphofunctional aspects of reproduction from synchronous to asynchronous fishes–an overview. Fish Endocrinol. 2006, 2, 571–624. [Google Scholar]
- De Felici, M.; Klinger, F.G.; Farini, D.; Scaldaferri, M.L.; Iona, S.; Lobascio, M. Establishment of oocyte population in the fetal ovary: Primordial germ cell proliferation and oocyte programmed cell death. Reprod. Biomed. Online 2005, 10, 182–191. [Google Scholar] [CrossRef]
- Nakamura, S.; Kobayashi, K.; Nishimura, T.; Tanaka, M. Ovarian Germline Stem Cells in the Teleost Fish, Medaka (Oryzias latipes). Int. J. Biol. Sci. 2011, 7, 403–409. [Google Scholar] [CrossRef]
- Costa, E. Reproductive Strategies of Marine Fishes from the Southwest Atlantic Ocean: An Application of Histological and Image Processing Techniques. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2015. [Google Scholar] [CrossRef]
- Erler, P.; Sweeney, A.; Monaghan, J.R. Regulation of Injury-Induced Ovarian Regeneration by Activation of Oogonial Stem Cells. Stem Cells 2017, 35, 236–247. [Google Scholar] [CrossRef]
- Elkouby, Y.M.; Jamieson-Lucy, A.; Mullins, M.C. Oocyte polarization is coupled to the chromosomal bouquet, a conserved polarized nuclear configuration in meiosis. PLoS Biol. 2016, 14, e1002335. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- Rutishauser, U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008, 9, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Kitajima, K. Polysialylation and disease. Mol. Asp. Med. 2020. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venuto, M.T.; Martorell-Ribera, J.; Bochert, R.; Harduin-Lepers, A.; Rebl, A.; Galuska, S.P. Characterization of the Polysialylation Status in Ovaries of the Salmonid Fish Coregonus maraena and the Percid Fish Sander lucioperca. Cells 2020, 9, 2391. https://doi.org/10.3390/cells9112391
Venuto MT, Martorell-Ribera J, Bochert R, Harduin-Lepers A, Rebl A, Galuska SP. Characterization of the Polysialylation Status in Ovaries of the Salmonid Fish Coregonus maraena and the Percid Fish Sander lucioperca. Cells. 2020; 9(11):2391. https://doi.org/10.3390/cells9112391
Chicago/Turabian StyleVenuto, Marzia Tindara, Joan Martorell-Ribera, Ralf Bochert, Anne Harduin-Lepers, Alexander Rebl, and Sebastian Peter Galuska. 2020. "Characterization of the Polysialylation Status in Ovaries of the Salmonid Fish Coregonus maraena and the Percid Fish Sander lucioperca" Cells 9, no. 11: 2391. https://doi.org/10.3390/cells9112391
APA StyleVenuto, M. T., Martorell-Ribera, J., Bochert, R., Harduin-Lepers, A., Rebl, A., & Galuska, S. P. (2020). Characterization of the Polysialylation Status in Ovaries of the Salmonid Fish Coregonus maraena and the Percid Fish Sander lucioperca. Cells, 9(11), 2391. https://doi.org/10.3390/cells9112391






