miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Sperm Collection and Treatment
2.3. Primers Design
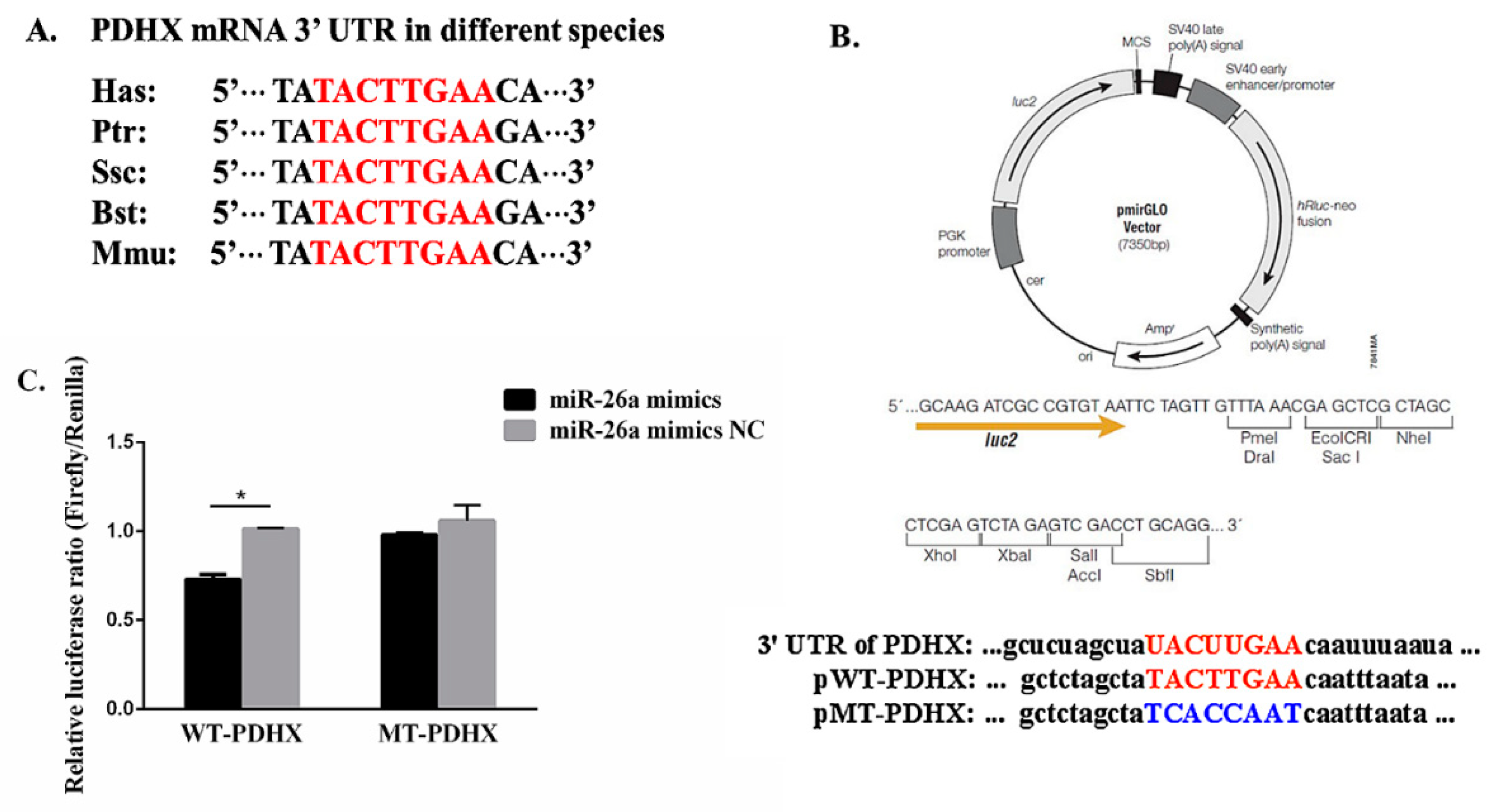
2.4. Target Prediction of miR-26a and Dual Luciferase Reporter Assay
2.5. Sperm Liquid Storage, Cryopreservation, and Electrotransfection
2.6. Sperm Viability Detection
2.7. Total RNA Extraction, cDNA Synthesis and RT-qPCR
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. PDHX is Direct Target Gene of miR-26a
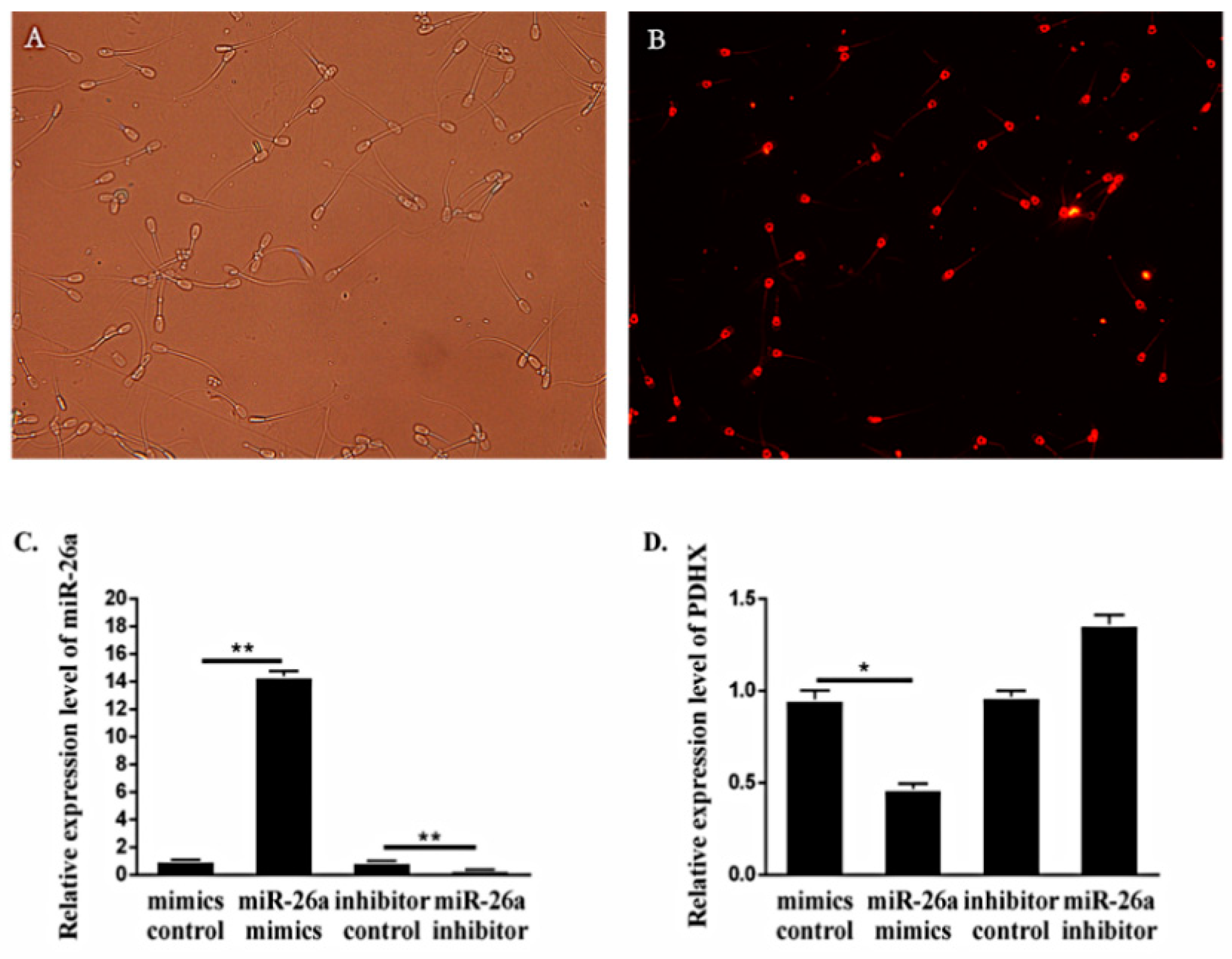
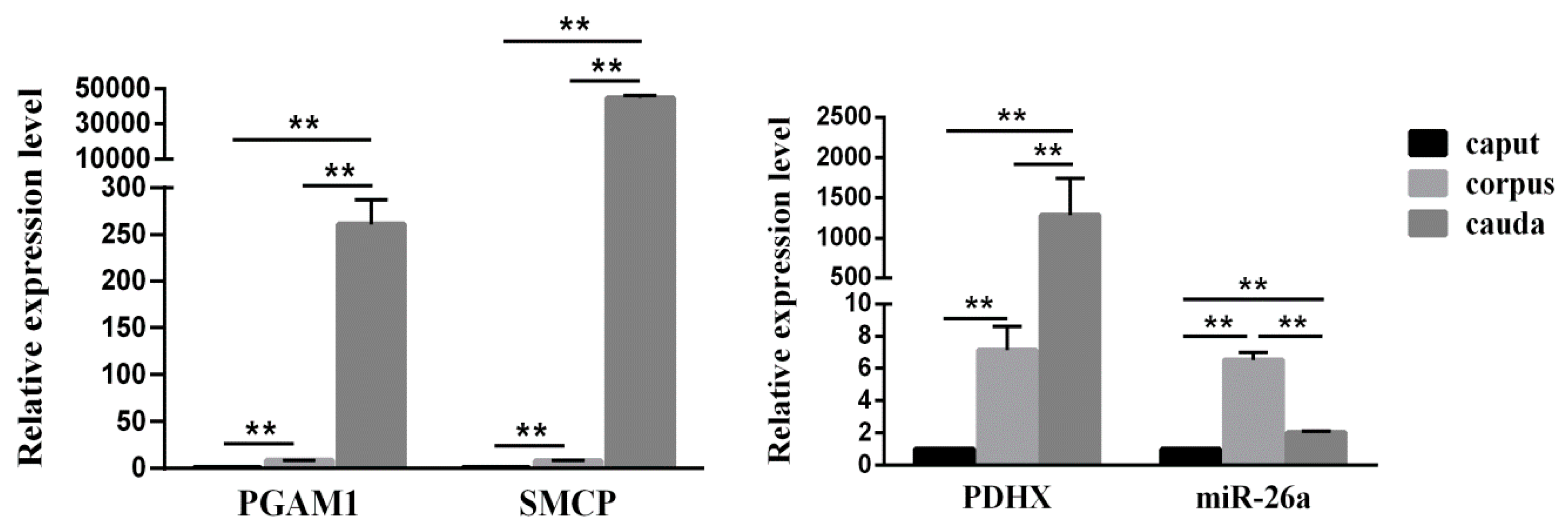
3.2. Expression of miR-26a and PDHX in Epididymal Sperm
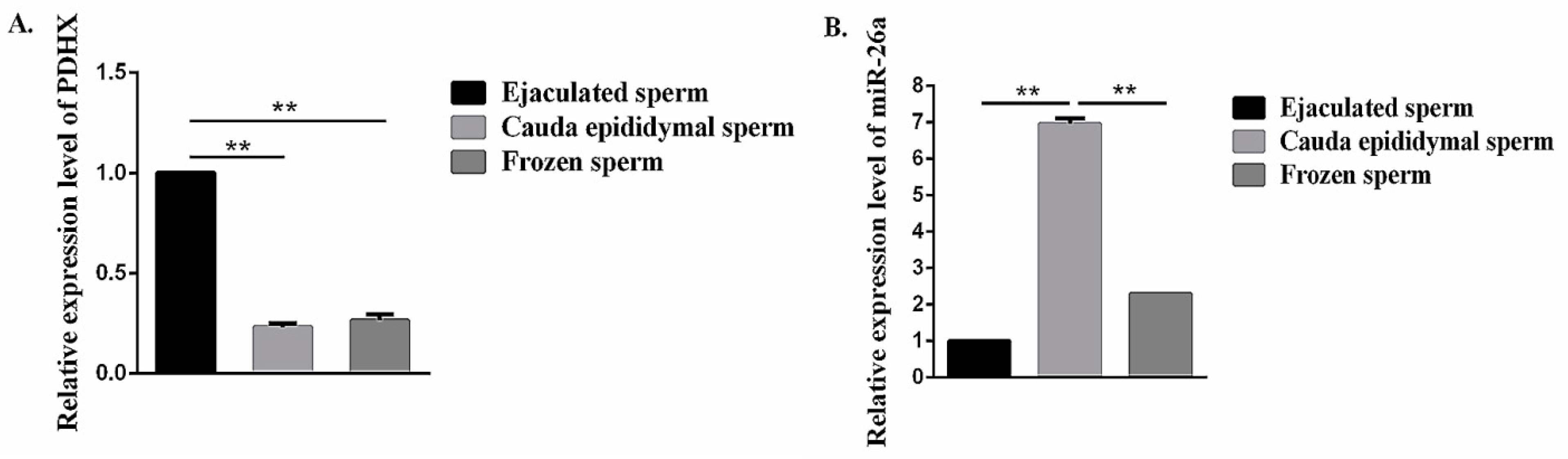
3.3. Comparison of miR-26a and PDHX Expression in Epididymal cauda, Ejaculated and Frozen-Thawed Boar Sperm
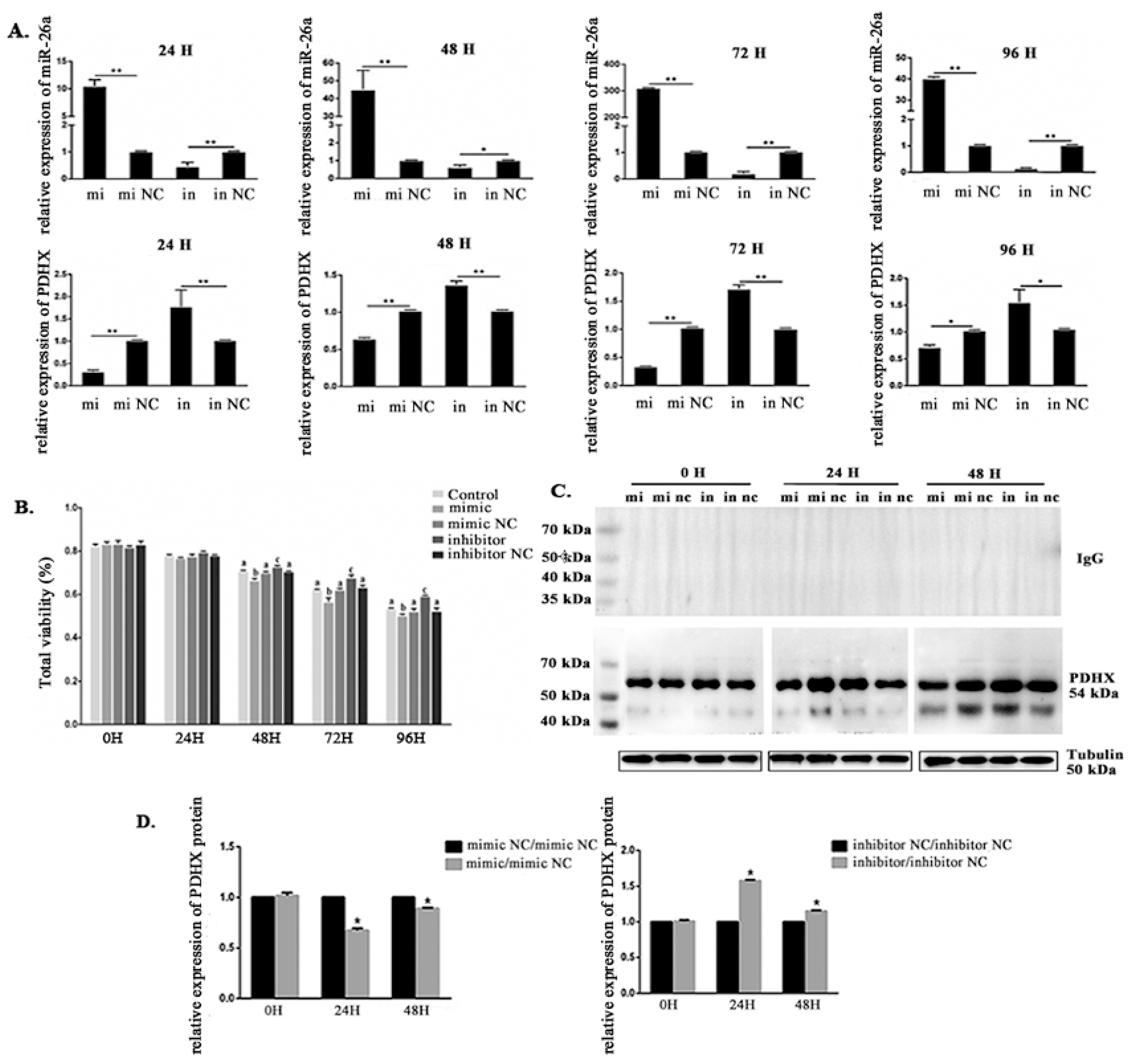
3.4. Effects of miR-26a Transfection on PDHX Expression and Boar Sperm Viability Under Liquid Preservation Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dacheux, J.L.; Dacheux, F. New insights into epididymal function in relation to sperm maturation. Reproduction 2013, 147, R27–R42. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Update 2008, 15, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Zakošek Pipan, M.; Mrkun, J.; Nemec Svete, A.; Zrimšek, P. Improvement of liquid stored boar semen quality by removing low molecular weight proteins and supplementation with α-tocopherol. Anim. Reprod. Sci. 2017, 186, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Trzcińska, M.; Bryła, M.; Smorąg, Z. Apoptotic-like changes in the spermatozoa of fresh and stored boar semen and the quality of embryos produced in vivo. Anim. Reprod. Sci. 2011, 124, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.M.; Vazquez, J.M.; Martinez, E.A.; Roca, J.; Lucas, X.; Rodriguez-Martinez, H. Effects of holding time during cooling and of type of package on plasma membrane integrity, motility and in vitro oocyte penetration ability of frozen-thawed boar spermatozoa. Theriogenology 2001, 55, 1593–1605. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Impact of storage prior to cryopreservation on plasma membrane function and fertility of boar sperm. Theriogenology 2005, 63, 396–410. [Google Scholar] [CrossRef]
- Akyol, N.; Varışlı, Ö.; Kızıl, S.H. Effects of long-term storage on some spermatological parameters in cryopreserved bull semen. Cryoletters 2018, 39, 354–358. [Google Scholar]
- O’Dell, W.T.; Almquist, J.O. Freezing bovine semen. IV. Effect of freezing on the metabolic activity of bovine spermatozoa during and after storage at −79 °C. J. Dairy Sci. 1958, 41, 1792–1799. [Google Scholar] [CrossRef]
- Rodríguez-Gil, J.E.; Bonet, S. Current knowledge on boar sperm metabolism: Comparison with other mammalian species. Theriogenology 2016, 85, 4–11. [Google Scholar] [CrossRef]
- Galantino-Homer, H.L.; Florman, H.M.; Storey, B.T.; Dobrinski, I.; Kopf, G.S. Bovine sperm capacitation: Assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol. Reprod. Dev. 2004, 67, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Connor, D.E. Fructose metabolism by mature boar spermatozoa. Reprod. Fertil. Dev. 2000, 12, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Rigau, T.; Rivera, M.; Palomo, M.J.; Fernández-Novell, J.M.; Moqas, T.; Ballester, J.; Peňa, A.; Otaegui, P.J.; Guinovart, J.J.; Rodríguez-Gil, J.E. Differential effects of glucose and fructose on hexose metabolism in dog spermatozoa. Reproduction 2002, 123, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, J.Z.; Xu, Z.; Xu, Y.; Xu, A.; Chen, W.; Miao, C.; Liu, S.; Wang, Z.; Jia, R. Metabolomic profiling of human spermatozoa in idiopathic asthenozoospermia patients using gas chromatography-mass spectrometry. BioMed Res. Int. 2018, 2018, 8327506. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.N.; Freund, M. Factors affecting fructose utilization and lactic acid formation by human semen. The role of glucose and pyruvic acid. Fertil. Steril. 1971, 22, 639–644. [Google Scholar] [CrossRef]
- Jones, A.R.; Connor, D.E. Control of glycolysis in mature boar spermatozoa: Effect of pH in vitro. Reprod. Fertil. Dev. 2004, 16, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Crocker, J. The Metabolic and Molecular Bases of Inherited Disease CD-ROM. Mol. Pathol. 1997, 50, 279. [Google Scholar] [CrossRef][Green Version]
- Bricker, D.K.; Taylor, E.B.; Schell, J.C.; Orsak, T.; Boutron, A.; Chen, Y.C.; Cox, J.E.; Cardon, C.M.; Van Vranken, J.G.; Dephoure, N.; et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012, 337, 96–100. [Google Scholar] [CrossRef]
- Aral, B.; Benelli, C.; Ait-Ghezala, G.; Amessou, M.; Fougue, F.; Maunoury, C.; Créau, N.; Kamoun, P.; Marsac, C. Mutations in PDX1, the human lipoyl-containing component X of the pyruvate dehydrogenase–complex gene on chromosome 11p1, in congenital lactic acidosis. Am. J. Hum. Genet. 1997, 61, 1318–1326. [Google Scholar] [CrossRef]
- Ling, M. Detection of a homozygous four base pair deletion in the protein X gene in a case of pyruvate dehydrogenase complex deficiency. Hum. Mol. Genet. 1998, 7, 501–505. [Google Scholar] [CrossRef]
- Miné, M.; Brivet, M.; Schiff, M.; de Baulny, H.O.; Chuzhanova, N.; Marsac, C. A novel gross deletion caused by non-homologous recombination of the PDHX gene in a patient with pyruvate dehydrogenase deficiency. Mol. Genet. Metab. 2006, 89, 106–110. [Google Scholar]
- Patel, M.S.; Roche, T.E. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990, 4, 3224–3233. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.K.; Otero, L.J.; LeGris, M.; Brown, R.M. Pyruvate dehydrogenase deficiency. J. Med. Genet. 1994, 31, 875. [Google Scholar] [CrossRef]
- Robinson, B.H.; Mackay, N.; Petrovabenedict, R.; Ozalp, I.; Coskun, T.; Stacpoole, P.W. Defects in the E2 lipoyl transacetylase and the X-lipoyl containing component of the pyruvate dehydrogenase complex in patients with lactic acidemia. J. Clin. Investig. 1990, 85, 1821–1824. [Google Scholar] [CrossRef]
- Dey, R.; Aral, B.; Abitbol, M.; Marsac, C. Pyruvate dehydrogenase deficiency as a result of splice-site mutation in the PDX1 gene. Mol. Genet. Metab. 2002, 76, 344–347. [Google Scholar] [CrossRef]
- Brown, R.M.; Head, R.A.; Morris, A.A.; Raiman, J.A.; Walter, J.H.; Whitehouse, W.P.; Brown, G.K. Pyruvate dehydrogenase E3 binding protein (protein X) deficiency. Dev. Med. Child. Neurol. 2006, 48, 756. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Mine, M.; Desguerre, I.; Slama, A.; Van Den Berghe, L.; Brivet, M.; Aral, B.; Marsac, C. A new case of pyruvate dehydrogenase deficiency due to a novel mutation in the PDX1 gene. Ann. Neurol. 2003, 53, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, S.C.; Dong, S.; Ivan, C.; Alahari, S.K. Suppression of PDHX by microRNA-27b deregulates cell metabolism and promotes growth in breast cancer. Mol. Cancer 2018, 17, 100. [Google Scholar] [CrossRef]
- Rikmenspoel, R. The inhibition by amytal of respiration and motility of bull spermatozoa. Exp. Cell Res. 1965, 37, 312–326. [Google Scholar] [CrossRef]
- Windsor, D.P. Mitochondrial function and ram sperm fertility. Reprod. Fertil. Dev. 1997, 9, 279–284. [Google Scholar] [CrossRef]
- Frenkel, G.; Peterson, R.N.; Freund, M. Oxidative and glycolytic metabolism of semen components by washed guinea pig spermatozoa. Fertil. Steril. 1975, 26, 144–147. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Shi, L.Z.; Tam, J.; Chandsawangbhuwana, C.; Durrant, B.; Botvinick, E.L.; Berns, M.W. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J. Cell Physiol. 2008, 217, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 2015, 290, 20613–20626. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Chiang, K.; Bassilian, S.; Lee, W.N.; Boros, L.G.; Fernández-Novell, J.M.; Centelles, J.J.; Medrano, A.; Rodriguez-Gil, J.E.; Cascante, M. Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett. 2003, 554, 342–346. [Google Scholar] [CrossRef]
- Nevo, A.C.; Polge, C.; Frederick, G. Aerobic and Anaerobic Metabolism of Boar spermatozoa in Relation to Their Motility. Reproduction 1970, 22, 109–118. [Google Scholar] [CrossRef][Green Version]
- Erin, C.; Safranski, T.J.; Pratt, S.L. Differential expression of porcine sperm microRNAs and their association with sperm morphology and motility. Theriogenology 2011, 76, 1532–1539. [Google Scholar]
- Said, T.M.; Gaglani, A.; Agarwal, A. Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 2010, 21, 456–462. [Google Scholar] [CrossRef]
- Aitken, R.J.; Findlay, J.K.; Hutt, K.J.; Kerr, J.B. Apoptosis in the germ line. Reproduction 2011, 141, 139–150. [Google Scholar] [CrossRef]
- Grunewald, S.; Paasch, U.; Said, T.M.; Sharma, R.K.; Agarwal, A.; Glander, H.J. Modulation of mitochondrial mediated apoptosis in ejaculated human spermatozoa and its impact on sperm motility. Fertil. Steril. 2004, 82, S285. [Google Scholar] [CrossRef]
- Li, D.H.; Yuan, D.; Zhang, L.Y.; Qiao, P.; Liang, X.; Chang, B. Increase of apoptosis and decrease of sperm motility induced by oxidative stress after exposed to butyl p-hydroxybenzoate. J. Hyg. Res. 2017, 46, 196–200. [Google Scholar]
- Perdichizzi, A.; Nicoletti, F.; La Vignera, S.; Barone, N.; D’Agata, R.; Vicari, E.; Calogero, A.E. Effects of Tumour Necrosis Factor-alpha on human sperm motility and apoptosis. J. Clin. Immunol. 2007, 27, 152–162. [Google Scholar] [CrossRef]
- Ma, J.D.; Fan, Y.; Zhang, J.W. Testosterone-dependent miR-26a-5p and let-7g-5p act as signaling mediators to regulate sperm apoptosis via targeting PTEN and PMAIP1. Int. J. Mol. Sci. 2018, 19, 1233. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Dai, D.H.; Li, Y.; Zhang, Y.; Zhang, M.; Zhou, G.B.; Zeng, C.J. Differences in the expression of microRNAs and their predicted gene targets between cauda epididymal and ejaculated boar sperm. Theriogenology 2016, 86, 2162–2171. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Y.; Du, G.; Han, L.; Zheng, S.; Liang, J.; Huang, X.; Qin, Y.; Wu, W.; Chen, M.; et al. Down-regulated let-7b-5p represses glycolysis metabolism by targeting AURKB in asthenozoospermia. Gene 2018, 663, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.O.; Sharbati, S.; Rodríguez-Alvarez, L.L.; Cox, J.F.; Hultschig, C.; Einspanier, R. MicroRNA expression profiling of elongated cloned and in vitro–fertilized bovine embryos. Theriogenology 2010, 73, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.K.; Gagan, J.; Yan, Z.; Dutta, A. miR-26a is required for skeletal muscle differentiation and regeneration in mice. Gene Dev. 2012, 26, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; Ju, Z.H.; Li, Q.L.; Hou, Q.; Wang, C.; Li, J.; Li, R.; Wang, L.; Sun, T.; Hang, S.; et al. Solexa sequencing of novel and differentially expressed microRNAs in testicular and ovarian tissues in Holstein cattle. Int. J. Biol. Sci. 2011, 7, 1016–1026. [Google Scholar] [CrossRef]
- Huang, J.M.; Guo, F.; Zhang, Z.B.; Zhang, Y.; Wang, X.; Ju, Z.; Yang, C.; Wang, C.; Hou, M.; Zhong, J. PCK1 is negatively regulated by bta-miR-26a, and a single-nucleotide polymorphism in the 3′ untranslated region is involved in semen quality and longevity of holstein bulls. Mol. Reprod. Dev. 2016, 83, 217–225. [Google Scholar] [CrossRef]
- Bissonnette, N.; Lévesque-Sergerie, J.P.; Thibault, C.; Boissonneault, G. Spermatozoal transcriptome profiling for bull sperm motility: A potential tool to evaluate semen quality. Reproduction 2009, 138, 65–80. [Google Scholar] [CrossRef]
- Capra, E.; Turri, F.; Lazzari, B.; Cremonesi, P.; Gliozzi, T.M.; Fojadelli, I.; Stella, A.; Pizzi, F. Small RNA sequencing of cryopreserved semen from single bull revealed altered miRNAs and piRNAs expression between High- and Low-motile sperm populations. BMC Genom. 2017, 18, 14. [Google Scholar] [CrossRef]
- Saenz, J.R.; Guerrero, C.A.; Paccamonti, D.; Eilts, B.E.; Bondioli, K.R.; Godke, R.A. Processing of postmortem bovine epididymal sperm after cooling the testes for 24 hours. Reprod. Fertil. Dev. 2008, 20, 126. [Google Scholar] [CrossRef]
- Lewis, B.P.; Shih, I.H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of mammalian microRNA targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human microRNA targets. PLoS Biol. 2005, 3, e264. [Google Scholar] [CrossRef]
- He, S.M.; Zeng, S.M.; Zhou, Z.W.; He, Z.X.; Zhou, S.F. Hsa-microRNA-181a is a regulator of a number of cancer genes and a biomarker for endometrial carcinoma in patients: A bioinformatic and clinical study and the therapeutic implication. Drug Des. Dev. Ther. 2015, 9, 1103–1175. [Google Scholar]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.J.; Tang, K.Y.; He, L.; Peng, W.P.; Ding, L.; Fang, D.H.; Zhang, Y. Effects of glycerol on apoptotic signaling pathways during boar spermatozoa cryopreservation. Cryobiology 2014, 68, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, X.; Li, H.X.; Cui, Q.W.; Yu, J.; Wang, G.L. Delivery of catSper2 siRNA into rat sperms by electroporation repressed Ca2+ influx during sperm hyperactivation. Agric. Sci. China 2011, 10, 1958–1967. [Google Scholar] [CrossRef]
- Bianchi, E.; Stermer, A.; Boekelheide, K.; Sigman, M.; Hall, S.J.; Reyes, G.; Dere, E.; Hwang, K. High-quality human and rat spermatozoal RNA isolation for functional genomic studies. Andrology 2018, 6, 374–383. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhu, Z.; Umehara, T.; Okazaki, T.; Goto, M.; Fujita, Y.; Hogue, S.A.M.; Kawai, T.; Zeng, W.; Shimada, M. Gene Expression and protein synthesis in mitochondria enhance the duration of high-speed linear motility in boar sperm. Front. Physiol. 2019, 10, 252. [Google Scholar] [CrossRef]
- Chen, B.; Liu, Y.L.; Jin, X.W.; Lu, W.; Liu, J.; Xia, Z.; Yuan, Q.; Zhao, X.; Xu, N.; Liang, S. MicroRNA-26a regulates glucose metabolism by direct targeting PDHX in colorectal cancer cells. BMC Cancer 2014, 14, 443. [Google Scholar] [CrossRef]
- Yeung, C.H.; Cooper, T.G. Developmental changes in signalling transduction factors in maturing sperm during epididymal transit. Cell. Mol. Biol. 2003, 49, 341–349. [Google Scholar] [PubMed]
- Di Santo, M.; Tarozzi, N.; Nadalini, M.; Borini, A. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv. Neurol. 2012, 2012, 854837. [Google Scholar] [CrossRef] [PubMed]
- da Silva, Z.; de Souza, A.P.; Pandolfi, J.R.C.; da Fonseca, F.N.; da Veiga Lima-Rosa, C.A.; Margues, M.G. Comparison between electroporation and polyfection in pig sperm: Efficiency and cell viability implications. Zygote 2018, 26, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.; Chen, S.X.; Luo, W.J.; Jiang, H.H.; Zhang, P.F.; Yi, H. Proteomic analysis of secreted proteins of non-small cell lung cancer. Chin. J. Cancer 2006, 25, 1361–1367. [Google Scholar]
- Hitosugi, T.; Zhou, L.; Elf, S.; Fan, J.; Kang, H.B.; Seo, J.H.; Shan, C.; Dai, Q.; Zhang, L.; Xie, J.; et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell 2012, 22, 585–600. [Google Scholar] [CrossRef]
- Maiorino, M.; Roveri, A.; Benazzi, L.; Bosello, V.; Mauri, P.; Toppo, S.; Tosatto, S.C.; Ursini, F. Functional interaction of phospholipid hydroperoxide glutathione peroxidase with sperm mitochondrion-associated cysteine-rich protein discloses the adjacent cysteine motif as a new substrate of the selenoperoxidase. J. Biol. Chem. 2005, 280, 38395–38402. [Google Scholar] [CrossRef]
- Nayernia, K.; Adham, I.M.; Burkhardt-Göttges, E.; Neesen, J.; Rieche, M.; Wolf, S.; Sancken, U.; Kleene, K.; Engel, W. Asthenozoospermia in mice with targeted deletion of the sperm mitochondrion-associated cysteine-rich protein (Smcp) Gene. Mol. Cell. Biol. 2002, 22, 3046–3052. [Google Scholar] [CrossRef]
- Qiu, J.H.; Li, Y.W.; Xie, H.L.; Li, Q.; Dong, H.B.; Sun, M.J.; Gao, W.Q.; Tan, J.H. Effects of glucose metabolism pathways on sperm motility and oxidative status during long-term liquid storage of goat semen. Theriogenology 2016, 86, 839–849. [Google Scholar] [CrossRef]
- du Plessis, S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Gatti, J.L.; Métayer, S.; Belghazi, M.; Dacheux, F.; Dacheux, J.L. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol. Reprod. 2005, 72, 1452–1465. [Google Scholar] [CrossRef]
- da Silveira, J.C.; de Ávila, A.C.F.C.M.; Garrett, H.; Bruemmer, J.E.; Winger, Q.A.; Bouma, G.J. Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. J. Endocrinol. 2018, 236, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Barkalina, N.; Jones, C.; Wood, M.J.; Coward, K. Extracellular vesicle-mediated delivery of molecular compounds into gametes and embryos: Learning from nature. Hum. Reprod. Update 2015, 21, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Belleannée, C. Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J. Androl. 2015, 17, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Saez, F.; Girouard, J.; Frenette, G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol. Dis. 2005, 35, 1–10. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef]
- Sullivan, R.; Frenette, G.; Girouard, J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 2007, 9, 483–491. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in sperm during epididymal maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef]
- Nixon, B.; Stanger, S.J.; Mihalas, B.P.; Reilly, J.N.; Anderson, A.L.; Tyagi, S.; Holt, J.E.; McLaughlin, E.A. The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol. Reprod. 2015, 93, 91. [Google Scholar] [CrossRef]
- Reilly, J.N.; McLaughlin, E.A.; Stanger, S.J.; Anderson, A.L.; Hutcheon, K.; Church, K.; Mihalas, B.P.; Tyagi, S.; Holt, J.E.; Eamens, A.L.; et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 2016, 6, 31794. [Google Scholar] [CrossRef]
- Chen, H.; Yang, P.; Chu, X.; Huang, Y.; Liu, T.; Zhang, Q.; Li, Q.; Hu, L.; Wagas, Y.; Ahmed, N.; et al. Cellular evidence for nano-scale exosome secretion and interactions with spermatozoa in the epididymis of the Chinese soft-shelled turtle, Pelodiscus sinensis. Oncotarget 2016, 7, 19242–19250. [Google Scholar] [CrossRef]
- Nixon, B.; De Iuliis, G.N.; Hart, H.M.; Zhou, W.; Mathe, A.; Bernstein, I.R.; Anderson, A.L.; Stanger, S.J.; Skerrett-Byrne, D.A.; Jamaluddin, M.F.B.; et al. Proteomic profiling of mouse epididymosomes reveals their contributions to post-testicular sperm maturation. Mol. Cell. Proteom. 2019, 18, S91–S108. [Google Scholar] [CrossRef]
- Saez, F.; Frenette, G.; Sullivan, R. Epididymosomes and prostasomes: Their roles in post-testicular maturation of the sperm cells. J. Androl. 2003, 24, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, G. Prostasomes are mediators of intercellular communication: From basic research to clinical implications. J. Intern. Med. 2012, 271, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Arienti, G. The motility of human spermatozoa as influenced by prostasomes at various ph levels. Biol. Cell 1999, 91, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Johansson, L.; Lundkvist, O.; Ronguist, G. Enhanced recruitment of motile spermatozoa by prostasome inclusion in swim-up medium. Hum. Reprod. 1994, 9, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R. miRNA and Methylation: A multifaceted liaison. Chembiochem 2015, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Berulava, T.; Rahmann, S.; Rademacher, K.; Klein-Hitpass, L.; Horsthemke, B. N6-Adenosine Methylation in miRNAs. PLoS ONE 2015, 10, e0118438. [Google Scholar] [CrossRef]
- Yuan, S.; Tang, H.; Xing, J.; Fan, X.; Cai, X.; Li, Q.; Han, P.; Luo, Y.; Zhang, Z.; Jiang, B.; et al. Methylation by NSun2 represses the levels and function of microRNA 125b. Mol. Cell. Biol. 2014, 34, 3630–3641. [Google Scholar] [CrossRef]
- Ma, J.Z.; Yang, F.; Zhou, C.C.; Liu, F.; Yuan, J.H.; Wang, F.; Wang, T.T.; Xu, Q.G.; Zhou, W.P.; Sun, S.H. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N6-methyladenosine-dependent primary microRNA processing. Hepatology 2017, 65, 529–543. [Google Scholar] [CrossRef]
- Alarcón, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N6-methyladenosine marks primary microRNAs for processing. Nature 2015, 519, 482–485. [Google Scholar] [CrossRef]
- Chu, C.; Zheng, G.Y.; Hu, S.G.; Zhang, J.; Xie, S.; Ma, W.; Ni, M.; Tang, C.; Zhou, L.; Zhou, Y.; et al. Epididymal region-specific miRNA expression and DNA methylation and their roles in controlling gene expression in rats. PLoS ONE 2015, 10, e0124450. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence (5′→3′) | Tm (°C) | Size (bp) | GenBank Accession |
|---|---|---|---|---|
| GAPDH | F: ACTCACTCTTCTACCTTTGATGCT | 60 | 100 | AF017079 |
| R: TGTTGCTGTAGCCAAATTCA | ||||
| PDHX | F: TCACAGCACGCAGTCTCTTT | 60 | 132 | XM_003122869.3 |
| R: AAGGCATCTCCAGCACTCAC | ||||
| SMCP | F: CTGAGTGCACCTGCCTGAATA | 60 | 106 | AY788095.1 |
| R: TTGGACCCCTGTCTTGGACT | ||||
| PGAM1 | F: GCGAGAGCCTGAAGGACACTATTG | 60 | 138 | XM_003483535.4 |
| R: CCTCCAGATGCTTGACGATGCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liang, K.; Chang, Y.; Ran, M.; Zhang, Y.; Ali, M.A.; Dai, D.; Qazi, I.H.; Zhang, M.; Zhou, G.; et al. miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX. Cells 2020, 9, 146. https://doi.org/10.3390/cells9010146
Wang W, Liang K, Chang Y, Ran M, Zhang Y, Ali MA, Dai D, Qazi IH, Zhang M, Zhou G, et al. miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX. Cells. 2020; 9(1):146. https://doi.org/10.3390/cells9010146
Chicago/Turabian StyleWang, Wencan, Kai Liang, Yu Chang, Mingxia Ran, Yan Zhang, Malik Ahsan Ali, Dinghui Dai, Izhar Hyder Qazi, Ming Zhang, Guangbin Zhou, and et al. 2020. "miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX" Cells 9, no. 1: 146. https://doi.org/10.3390/cells9010146
APA StyleWang, W., Liang, K., Chang, Y., Ran, M., Zhang, Y., Ali, M. A., Dai, D., Qazi, I. H., Zhang, M., Zhou, G., Yang, J., Angel, C., & Zeng, C. (2020). miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX. Cells, 9(1), 146. https://doi.org/10.3390/cells9010146







