miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Sperm Collection and Treatment
2.3. Primers Design
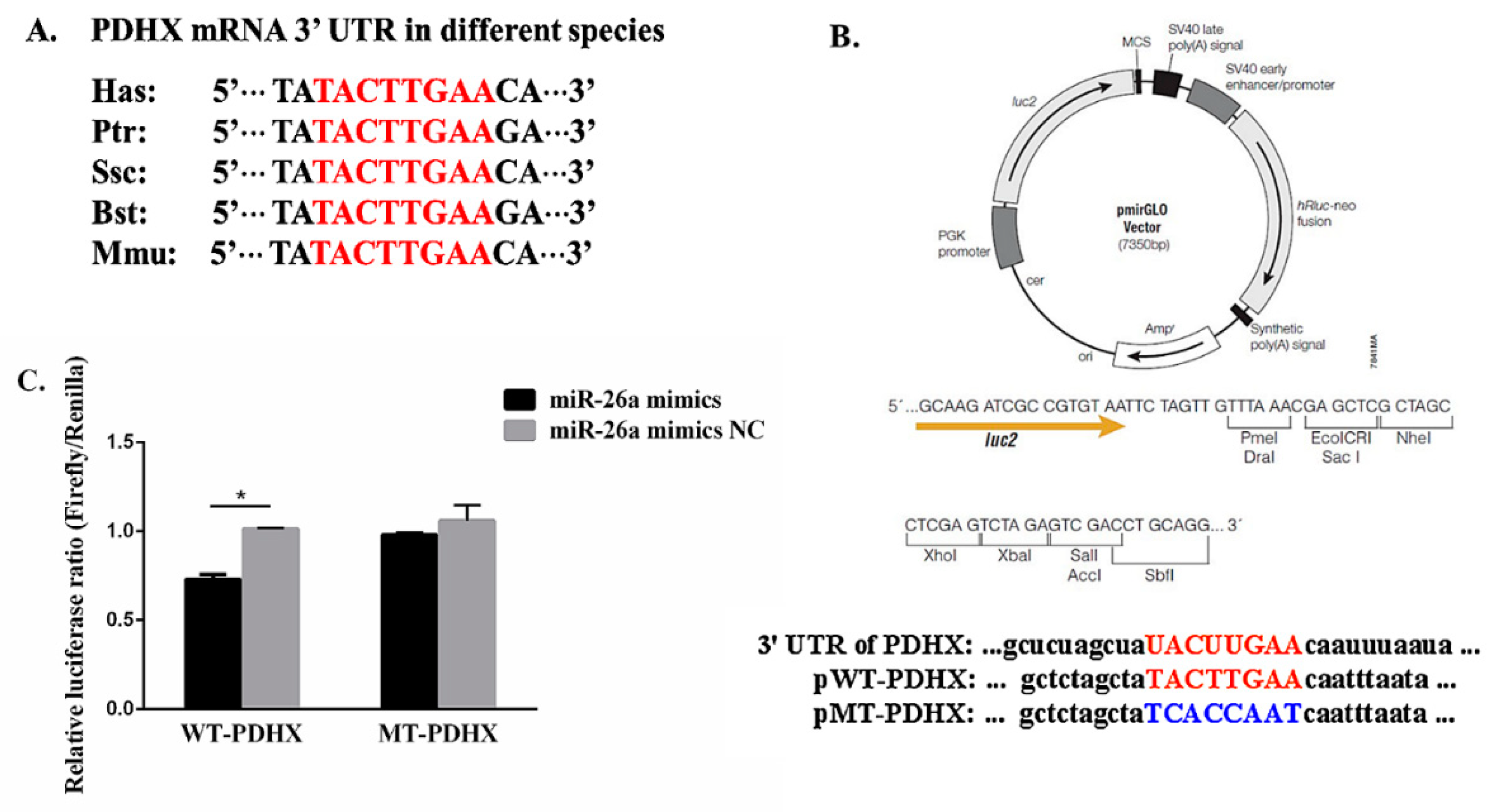
2.4. Target Prediction of miR-26a and Dual Luciferase Reporter Assay
2.5. Sperm Liquid Storage, Cryopreservation, and Electrotransfection
2.6. Sperm Viability Detection
2.7. Total RNA Extraction, cDNA Synthesis and RT-qPCR
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. PDHX is Direct Target Gene of miR-26a
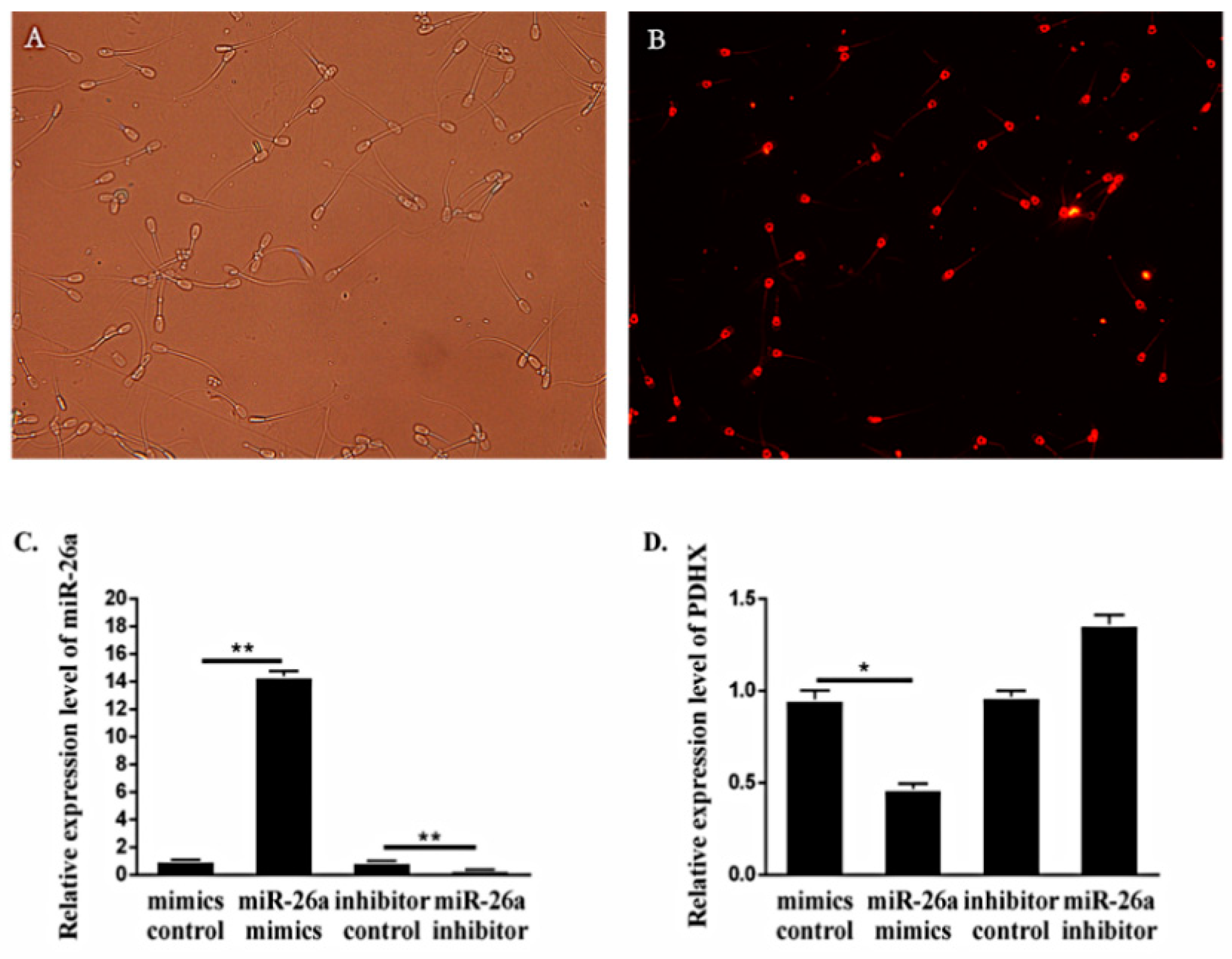
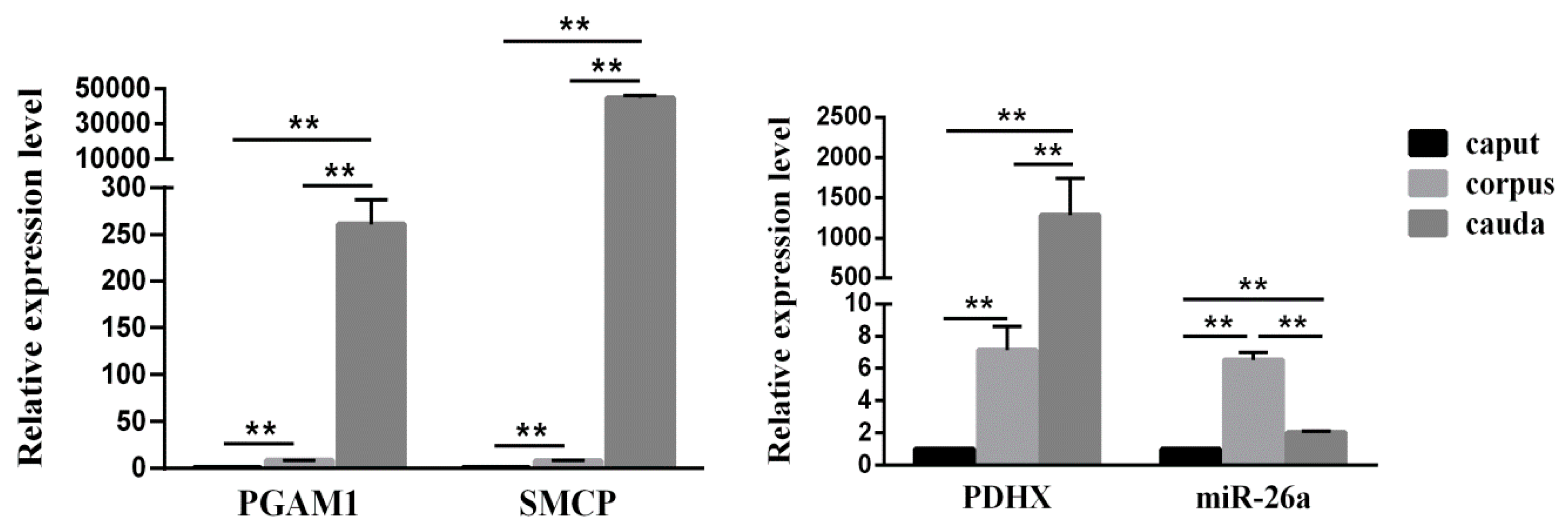
3.2. Expression of miR-26a and PDHX in Epididymal Sperm
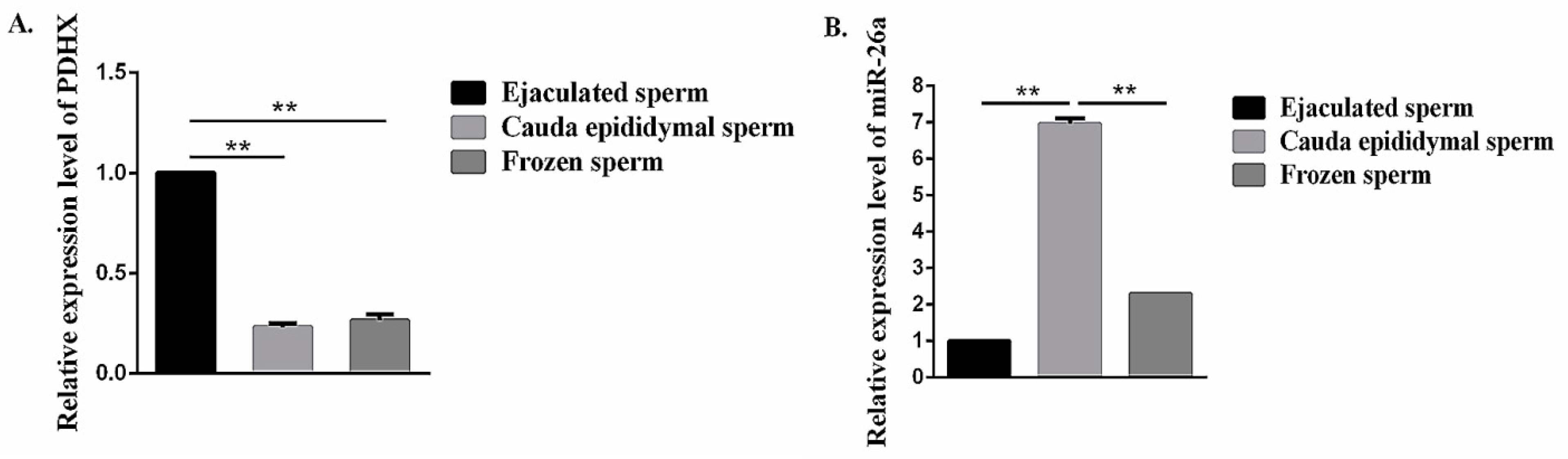
3.3. Comparison of miR-26a and PDHX Expression in Epididymal cauda, Ejaculated and Frozen-Thawed Boar Sperm
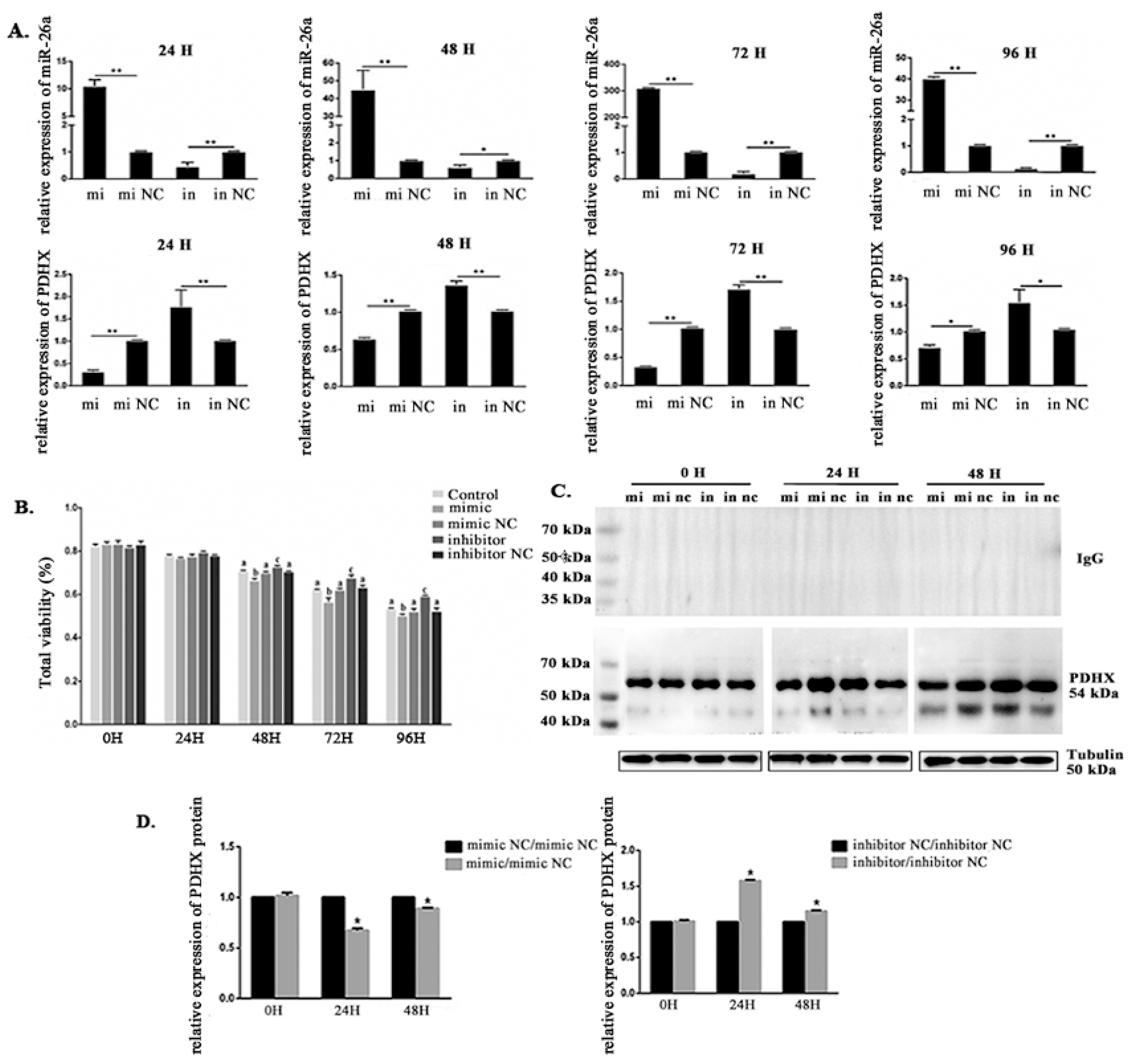
3.4. Effects of miR-26a Transfection on PDHX Expression and Boar Sperm Viability Under Liquid Preservation Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dacheux, J.L.; Dacheux, F. New insights into epididymal function in relation to sperm maturation. Reproduction 2013, 147, R27–R42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Update 2008, 15, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakošek Pipan, M.; Mrkun, J.; Nemec Svete, A.; Zrimšek, P. Improvement of liquid stored boar semen quality by removing low molecular weight proteins and supplementation with α-tocopherol. Anim. Reprod. Sci. 2017, 186, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Trzcińska, M.; Bryła, M.; Smorąg, Z. Apoptotic-like changes in the spermatozoa of fresh and stored boar semen and the quality of embryos produced in vivo. Anim. Reprod. Sci. 2011, 124, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.M.; Vazquez, J.M.; Martinez, E.A.; Roca, J.; Lucas, X.; Rodriguez-Martinez, H. Effects of holding time during cooling and of type of package on plasma membrane integrity, motility and in vitro oocyte penetration ability of frozen-thawed boar spermatozoa. Theriogenology 2001, 55, 1593–1605. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Impact of storage prior to cryopreservation on plasma membrane function and fertility of boar sperm. Theriogenology 2005, 63, 396–410. [Google Scholar] [CrossRef]
- Akyol, N.; Varışlı, Ö.; Kızıl, S.H. Effects of long-term storage on some spermatological parameters in cryopreserved bull semen. Cryoletters 2018, 39, 354–358. [Google Scholar]
- O’Dell, W.T.; Almquist, J.O. Freezing bovine semen. IV. Effect of freezing on the metabolic activity of bovine spermatozoa during and after storage at −79 °C. J. Dairy Sci. 1958, 41, 1792–1799. [Google Scholar] [CrossRef]
- Rodríguez-Gil, J.E.; Bonet, S. Current knowledge on boar sperm metabolism: Comparison with other mammalian species. Theriogenology 2016, 85, 4–11. [Google Scholar] [CrossRef]
- Galantino-Homer, H.L.; Florman, H.M.; Storey, B.T.; Dobrinski, I.; Kopf, G.S. Bovine sperm capacitation: Assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol. Reprod. Dev. 2004, 67, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Connor, D.E. Fructose metabolism by mature boar spermatozoa. Reprod. Fertil. Dev. 2000, 12, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Rigau, T.; Rivera, M.; Palomo, M.J.; Fernández-Novell, J.M.; Moqas, T.; Ballester, J.; Peňa, A.; Otaegui, P.J.; Guinovart, J.J.; Rodríguez-Gil, J.E. Differential effects of glucose and fructose on hexose metabolism in dog spermatozoa. Reproduction 2002, 123, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, J.Z.; Xu, Z.; Xu, Y.; Xu, A.; Chen, W.; Miao, C.; Liu, S.; Wang, Z.; Jia, R. Metabolomic profiling of human spermatozoa in idiopathic asthenozoospermia patients using gas chromatography-mass spectrometry. BioMed Res. Int. 2018, 2018, 8327506. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.N.; Freund, M. Factors affecting fructose utilization and lactic acid formation by human semen. The role of glucose and pyruvic acid. Fertil. Steril. 1971, 22, 639–644. [Google Scholar] [CrossRef]
- Jones, A.R.; Connor, D.E. Control of glycolysis in mature boar spermatozoa: Effect of pH in vitro. Reprod. Fertil. Dev. 2004, 16, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Crocker, J. The Metabolic and Molecular Bases of Inherited Disease CD-ROM. Mol. Pathol. 1997, 50, 279. [Google Scholar] [CrossRef] [Green Version]
- Bricker, D.K.; Taylor, E.B.; Schell, J.C.; Orsak, T.; Boutron, A.; Chen, Y.C.; Cox, J.E.; Cardon, C.M.; Van Vranken, J.G.; Dephoure, N.; et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012, 337, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Aral, B.; Benelli, C.; Ait-Ghezala, G.; Amessou, M.; Fougue, F.; Maunoury, C.; Créau, N.; Kamoun, P.; Marsac, C. Mutations in PDX1, the human lipoyl-containing component X of the pyruvate dehydrogenase–complex gene on chromosome 11p1, in congenital lactic acidosis. Am. J. Hum. Genet. 1997, 61, 1318–1326. [Google Scholar] [CrossRef] [Green Version]
- Ling, M. Detection of a homozygous four base pair deletion in the protein X gene in a case of pyruvate dehydrogenase complex deficiency. Hum. Mol. Genet. 1998, 7, 501–505. [Google Scholar] [CrossRef] [Green Version]
- Miné, M.; Brivet, M.; Schiff, M.; de Baulny, H.O.; Chuzhanova, N.; Marsac, C. A novel gross deletion caused by non-homologous recombination of the PDHX gene in a patient with pyruvate dehydrogenase deficiency. Mol. Genet. Metab. 2006, 89, 106–110. [Google Scholar]
- Patel, M.S.; Roche, T.E. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990, 4, 3224–3233. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.K.; Otero, L.J.; LeGris, M.; Brown, R.M. Pyruvate dehydrogenase deficiency. J. Med. Genet. 1994, 31, 875. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.H.; Mackay, N.; Petrovabenedict, R.; Ozalp, I.; Coskun, T.; Stacpoole, P.W. Defects in the E2 lipoyl transacetylase and the X-lipoyl containing component of the pyruvate dehydrogenase complex in patients with lactic acidemia. J. Clin. Investig. 1990, 85, 1821–1824. [Google Scholar] [CrossRef]
- Dey, R.; Aral, B.; Abitbol, M.; Marsac, C. Pyruvate dehydrogenase deficiency as a result of splice-site mutation in the PDX1 gene. Mol. Genet. Metab. 2002, 76, 344–347. [Google Scholar] [CrossRef]
- Brown, R.M.; Head, R.A.; Morris, A.A.; Raiman, J.A.; Walter, J.H.; Whitehouse, W.P.; Brown, G.K. Pyruvate dehydrogenase E3 binding protein (protein X) deficiency. Dev. Med. Child. Neurol. 2006, 48, 756. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Mine, M.; Desguerre, I.; Slama, A.; Van Den Berghe, L.; Brivet, M.; Aral, B.; Marsac, C. A new case of pyruvate dehydrogenase deficiency due to a novel mutation in the PDX1 gene. Ann. Neurol. 2003, 53, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, S.C.; Dong, S.; Ivan, C.; Alahari, S.K. Suppression of PDHX by microRNA-27b deregulates cell metabolism and promotes growth in breast cancer. Mol. Cancer 2018, 17, 100. [Google Scholar] [CrossRef]
- Rikmenspoel, R. The inhibition by amytal of respiration and motility of bull spermatozoa. Exp. Cell Res. 1965, 37, 312–326. [Google Scholar] [CrossRef]
- Windsor, D.P. Mitochondrial function and ram sperm fertility. Reprod. Fertil. Dev. 1997, 9, 279–284. [Google Scholar] [CrossRef]
- Frenkel, G.; Peterson, R.N.; Freund, M. Oxidative and glycolytic metabolism of semen components by washed guinea pig spermatozoa. Fertil. Steril. 1975, 26, 144–147. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Shi, L.Z.; Tam, J.; Chandsawangbhuwana, C.; Durrant, B.; Botvinick, E.L.; Berns, M.W. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J. Cell Physiol. 2008, 217, 745–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J. Biol. Chem. 2015, 290, 20613–20626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, S.; Chiang, K.; Bassilian, S.; Lee, W.N.; Boros, L.G.; Fernández-Novell, J.M.; Centelles, J.J.; Medrano, A.; Rodriguez-Gil, J.E.; Cascante, M. Metabolic strategy of boar spermatozoa revealed by a metabolomic characterization. FEBS Lett. 2003, 554, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Nevo, A.C.; Polge, C.; Frederick, G. Aerobic and Anaerobic Metabolism of Boar spermatozoa in Relation to Their Motility. Reproduction 1970, 22, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Erin, C.; Safranski, T.J.; Pratt, S.L. Differential expression of porcine sperm microRNAs and their association with sperm morphology and motility. Theriogenology 2011, 76, 1532–1539. [Google Scholar]
- Said, T.M.; Gaglani, A.; Agarwal, A. Implication of apoptosis in sperm cryoinjury. Reprod. Biomed. Online 2010, 21, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Findlay, J.K.; Hutt, K.J.; Kerr, J.B. Apoptosis in the germ line. Reproduction 2011, 141, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Grunewald, S.; Paasch, U.; Said, T.M.; Sharma, R.K.; Agarwal, A.; Glander, H.J. Modulation of mitochondrial mediated apoptosis in ejaculated human spermatozoa and its impact on sperm motility. Fertil. Steril. 2004, 82, S285. [Google Scholar] [CrossRef]
- Li, D.H.; Yuan, D.; Zhang, L.Y.; Qiao, P.; Liang, X.; Chang, B. Increase of apoptosis and decrease of sperm motility induced by oxidative stress after exposed to butyl p-hydroxybenzoate. J. Hyg. Res. 2017, 46, 196–200. [Google Scholar]
- Perdichizzi, A.; Nicoletti, F.; La Vignera, S.; Barone, N.; D’Agata, R.; Vicari, E.; Calogero, A.E. Effects of Tumour Necrosis Factor-alpha on human sperm motility and apoptosis. J. Clin. Immunol. 2007, 27, 152–162. [Google Scholar] [CrossRef]
- Ma, J.D.; Fan, Y.; Zhang, J.W. Testosterone-dependent miR-26a-5p and let-7g-5p act as signaling mediators to regulate sperm apoptosis via targeting PTEN and PMAIP1. Int. J. Mol. Sci. 2018, 19, 1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Dai, D.H.; Li, Y.; Zhang, Y.; Zhang, M.; Zhou, G.B.; Zeng, C.J. Differences in the expression of microRNAs and their predicted gene targets between cauda epididymal and ejaculated boar sperm. Theriogenology 2016, 86, 2162–2171. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Y.; Du, G.; Han, L.; Zheng, S.; Liang, J.; Huang, X.; Qin, Y.; Wu, W.; Chen, M.; et al. Down-regulated let-7b-5p represses glycolysis metabolism by targeting AURKB in asthenozoospermia. Gene 2018, 663, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.O.; Sharbati, S.; Rodríguez-Alvarez, L.L.; Cox, J.F.; Hultschig, C.; Einspanier, R. MicroRNA expression profiling of elongated cloned and in vitro–fertilized bovine embryos. Theriogenology 2010, 73, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.K.; Gagan, J.; Yan, Z.; Dutta, A. miR-26a is required for skeletal muscle differentiation and regeneration in mice. Gene Dev. 2012, 26, 2180–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.M.; Ju, Z.H.; Li, Q.L.; Hou, Q.; Wang, C.; Li, J.; Li, R.; Wang, L.; Sun, T.; Hang, S.; et al. Solexa sequencing of novel and differentially expressed microRNAs in testicular and ovarian tissues in Holstein cattle. Int. J. Biol. Sci. 2011, 7, 1016–1026. [Google Scholar] [CrossRef]
- Huang, J.M.; Guo, F.; Zhang, Z.B.; Zhang, Y.; Wang, X.; Ju, Z.; Yang, C.; Wang, C.; Hou, M.; Zhong, J. PCK1 is negatively regulated by bta-miR-26a, and a single-nucleotide polymorphism in the 3′ untranslated region is involved in semen quality and longevity of holstein bulls. Mol. Reprod. Dev. 2016, 83, 217–225. [Google Scholar] [CrossRef]
- Bissonnette, N.; Lévesque-Sergerie, J.P.; Thibault, C.; Boissonneault, G. Spermatozoal transcriptome profiling for bull sperm motility: A potential tool to evaluate semen quality. Reproduction 2009, 138, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Capra, E.; Turri, F.; Lazzari, B.; Cremonesi, P.; Gliozzi, T.M.; Fojadelli, I.; Stella, A.; Pizzi, F. Small RNA sequencing of cryopreserved semen from single bull revealed altered miRNAs and piRNAs expression between High- and Low-motile sperm populations. BMC Genom. 2017, 18, 14. [Google Scholar] [CrossRef] [Green Version]
- Saenz, J.R.; Guerrero, C.A.; Paccamonti, D.; Eilts, B.E.; Bondioli, K.R.; Godke, R.A. Processing of postmortem bovine epididymal sperm after cooling the testes for 24 hours. Reprod. Fertil. Dev. 2008, 20, 126. [Google Scholar] [CrossRef]
- Lewis, B.P.; Shih, I.H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of mammalian microRNA targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef] [Green Version]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human microRNA targets. PLoS Biol. 2005, 3, e264. [Google Scholar] [CrossRef] [Green Version]
- He, S.M.; Zeng, S.M.; Zhou, Z.W.; He, Z.X.; Zhou, S.F. Hsa-microRNA-181a is a regulator of a number of cancer genes and a biomarker for endometrial carcinoma in patients: A bioinformatic and clinical study and the therapeutic implication. Drug Des. Dev. Ther. 2015, 9, 1103–1175. [Google Scholar]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.J.; Tang, K.Y.; He, L.; Peng, W.P.; Ding, L.; Fang, D.H.; Zhang, Y. Effects of glycerol on apoptotic signaling pathways during boar spermatozoa cryopreservation. Cryobiology 2014, 68, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, X.; Li, H.X.; Cui, Q.W.; Yu, J.; Wang, G.L. Delivery of catSper2 siRNA into rat sperms by electroporation repressed Ca2+ influx during sperm hyperactivation. Agric. Sci. China 2011, 10, 1958–1967. [Google Scholar] [CrossRef]
- Bianchi, E.; Stermer, A.; Boekelheide, K.; Sigman, M.; Hall, S.J.; Reyes, G.; Dere, E.; Hwang, K. High-quality human and rat spermatozoal RNA isolation for functional genomic studies. Andrology 2018, 6, 374–383. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhu, Z.; Umehara, T.; Okazaki, T.; Goto, M.; Fujita, Y.; Hogue, S.A.M.; Kawai, T.; Zeng, W.; Shimada, M. Gene Expression and protein synthesis in mitochondria enhance the duration of high-speed linear motility in boar sperm. Front. Physiol. 2019, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Liu, Y.L.; Jin, X.W.; Lu, W.; Liu, J.; Xia, Z.; Yuan, Q.; Zhao, X.; Xu, N.; Liang, S. MicroRNA-26a regulates glucose metabolism by direct targeting PDHX in colorectal cancer cells. BMC Cancer 2014, 14, 443. [Google Scholar] [CrossRef] [Green Version]
- Yeung, C.H.; Cooper, T.G. Developmental changes in signalling transduction factors in maturing sperm during epididymal transit. Cell. Mol. Biol. 2003, 49, 341–349. [Google Scholar] [PubMed]
- Di Santo, M.; Tarozzi, N.; Nadalini, M.; Borini, A. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv. Neurol. 2012, 2012, 854837. [Google Scholar] [CrossRef] [PubMed]
- da Silva, Z.; de Souza, A.P.; Pandolfi, J.R.C.; da Fonseca, F.N.; da Veiga Lima-Rosa, C.A.; Margues, M.G. Comparison between electroporation and polyfection in pig sperm: Efficiency and cell viability implications. Zygote 2018, 26, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.; Chen, S.X.; Luo, W.J.; Jiang, H.H.; Zhang, P.F.; Yi, H. Proteomic analysis of secreted proteins of non-small cell lung cancer. Chin. J. Cancer 2006, 25, 1361–1367. [Google Scholar]
- Hitosugi, T.; Zhou, L.; Elf, S.; Fan, J.; Kang, H.B.; Seo, J.H.; Shan, C.; Dai, Q.; Zhang, L.; Xie, J.; et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell 2012, 22, 585–600. [Google Scholar] [CrossRef] [Green Version]
- Maiorino, M.; Roveri, A.; Benazzi, L.; Bosello, V.; Mauri, P.; Toppo, S.; Tosatto, S.C.; Ursini, F. Functional interaction of phospholipid hydroperoxide glutathione peroxidase with sperm mitochondrion-associated cysteine-rich protein discloses the adjacent cysteine motif as a new substrate of the selenoperoxidase. J. Biol. Chem. 2005, 280, 38395–38402. [Google Scholar] [CrossRef] [Green Version]
- Nayernia, K.; Adham, I.M.; Burkhardt-Göttges, E.; Neesen, J.; Rieche, M.; Wolf, S.; Sancken, U.; Kleene, K.; Engel, W. Asthenozoospermia in mice with targeted deletion of the sperm mitochondrion-associated cysteine-rich protein (Smcp) Gene. Mol. Cell. Biol. 2002, 22, 3046–3052. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.H.; Li, Y.W.; Xie, H.L.; Li, Q.; Dong, H.B.; Sun, M.J.; Gao, W.Q.; Tan, J.H. Effects of glucose metabolism pathways on sperm motility and oxidative status during long-term liquid storage of goat semen. Theriogenology 2016, 86, 839–849. [Google Scholar] [CrossRef]
- du Plessis, S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235. [Google Scholar] [CrossRef]
- Gatti, J.L.; Métayer, S.; Belghazi, M.; Dacheux, F.; Dacheux, J.L. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol. Reprod. 2005, 72, 1452–1465. [Google Scholar] [CrossRef]
- da Silveira, J.C.; de Ávila, A.C.F.C.M.; Garrett, H.; Bruemmer, J.E.; Winger, Q.A.; Bouma, G.J. Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. J. Endocrinol. 2018, 236, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Barkalina, N.; Jones, C.; Wood, M.J.; Coward, K. Extracellular vesicle-mediated delivery of molecular compounds into gametes and embryos: Learning from nature. Hum. Reprod. Update 2015, 21, 627–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belleannée, C. Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J. Androl. 2015, 17, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Saez, F.; Girouard, J.; Frenette, G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol. Dis. 2005, 35, 1–10. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, R.; Frenette, G.; Girouard, J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 2007, 9, 483–491. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in sperm during epididymal maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef]
- Nixon, B.; Stanger, S.J.; Mihalas, B.P.; Reilly, J.N.; Anderson, A.L.; Tyagi, S.; Holt, J.E.; McLaughlin, E.A. The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol. Reprod. 2015, 93, 91. [Google Scholar] [CrossRef]
- Reilly, J.N.; McLaughlin, E.A.; Stanger, S.J.; Anderson, A.L.; Hutcheon, K.; Church, K.; Mihalas, B.P.; Tyagi, S.; Holt, J.E.; Eamens, A.L.; et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 2016, 6, 31794. [Google Scholar] [CrossRef]
- Chen, H.; Yang, P.; Chu, X.; Huang, Y.; Liu, T.; Zhang, Q.; Li, Q.; Hu, L.; Wagas, Y.; Ahmed, N.; et al. Cellular evidence for nano-scale exosome secretion and interactions with spermatozoa in the epididymis of the Chinese soft-shelled turtle, Pelodiscus sinensis. Oncotarget 2016, 7, 19242–19250. [Google Scholar] [CrossRef] [Green Version]
- Nixon, B.; De Iuliis, G.N.; Hart, H.M.; Zhou, W.; Mathe, A.; Bernstein, I.R.; Anderson, A.L.; Stanger, S.J.; Skerrett-Byrne, D.A.; Jamaluddin, M.F.B.; et al. Proteomic profiling of mouse epididymosomes reveals their contributions to post-testicular sperm maturation. Mol. Cell. Proteom. 2019, 18, S91–S108. [Google Scholar] [CrossRef] [Green Version]
- Saez, F.; Frenette, G.; Sullivan, R. Epididymosomes and prostasomes: Their roles in post-testicular maturation of the sperm cells. J. Androl. 2003, 24, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, G. Prostasomes are mediators of intercellular communication: From basic research to clinical implications. J. Intern. Med. 2012, 271, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Arienti, G. The motility of human spermatozoa as influenced by prostasomes at various ph levels. Biol. Cell 1999, 91, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Johansson, L.; Lundkvist, O.; Ronguist, G. Enhanced recruitment of motile spermatozoa by prostasome inclusion in swim-up medium. Hum. Reprod. 1994, 9, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R. miRNA and Methylation: A multifaceted liaison. Chembiochem 2015, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Berulava, T.; Rahmann, S.; Rademacher, K.; Klein-Hitpass, L.; Horsthemke, B. N6-Adenosine Methylation in miRNAs. PLoS ONE 2015, 10, e0118438. [Google Scholar] [CrossRef]
- Yuan, S.; Tang, H.; Xing, J.; Fan, X.; Cai, X.; Li, Q.; Han, P.; Luo, Y.; Zhang, Z.; Jiang, B.; et al. Methylation by NSun2 represses the levels and function of microRNA 125b. Mol. Cell. Biol. 2014, 34, 3630–3641. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.Z.; Yang, F.; Zhou, C.C.; Liu, F.; Yuan, J.H.; Wang, F.; Wang, T.T.; Xu, Q.G.; Zhou, W.P.; Sun, S.H. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N6-methyladenosine-dependent primary microRNA processing. Hepatology 2017, 65, 529–543. [Google Scholar] [CrossRef]
- Alarcón, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N6-methyladenosine marks primary microRNAs for processing. Nature 2015, 519, 482–485. [Google Scholar] [CrossRef]
- Chu, C.; Zheng, G.Y.; Hu, S.G.; Zhang, J.; Xie, S.; Ma, W.; Ni, M.; Tang, C.; Zhou, L.; Zhou, Y.; et al. Epididymal region-specific miRNA expression and DNA methylation and their roles in controlling gene expression in rats. PLoS ONE 2015, 10, e0124450. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence (5′→3′) | Tm (°C) | Size (bp) | GenBank Accession |
|---|---|---|---|---|
| GAPDH | F: ACTCACTCTTCTACCTTTGATGCT | 60 | 100 | AF017079 |
| R: TGTTGCTGTAGCCAAATTCA | ||||
| PDHX | F: TCACAGCACGCAGTCTCTTT | 60 | 132 | XM_003122869.3 |
| R: AAGGCATCTCCAGCACTCAC | ||||
| SMCP | F: CTGAGTGCACCTGCCTGAATA | 60 | 106 | AY788095.1 |
| R: TTGGACCCCTGTCTTGGACT | ||||
| PGAM1 | F: GCGAGAGCCTGAAGGACACTATTG | 60 | 138 | XM_003483535.4 |
| R: CCTCCAGATGCTTGACGATGCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liang, K.; Chang, Y.; Ran, M.; Zhang, Y.; Ali, M.A.; Dai, D.; Qazi, I.H.; Zhang, M.; Zhou, G.; et al. miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX. Cells 2020, 9, 146. https://doi.org/10.3390/cells9010146
Wang W, Liang K, Chang Y, Ran M, Zhang Y, Ali MA, Dai D, Qazi IH, Zhang M, Zhou G, et al. miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX. Cells. 2020; 9(1):146. https://doi.org/10.3390/cells9010146
Chicago/Turabian StyleWang, Wencan, Kai Liang, Yu Chang, Mingxia Ran, Yan Zhang, Malik Ahsan Ali, Dinghui Dai, Izhar Hyder Qazi, Ming Zhang, Guangbin Zhou, and et al. 2020. "miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX" Cells 9, no. 1: 146. https://doi.org/10.3390/cells9010146
APA StyleWang, W., Liang, K., Chang, Y., Ran, M., Zhang, Y., Ali, M. A., Dai, D., Qazi, I. H., Zhang, M., Zhou, G., Yang, J., Angel, C., & Zeng, C. (2020). miR-26a is Involved in Glycometabolism and Affects Boar Sperm Viability by Targeting PDHX. Cells, 9(1), 146. https://doi.org/10.3390/cells9010146







