Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models
Abstract
:1. Introduction
2. Establishment of Immunocompromised Mice
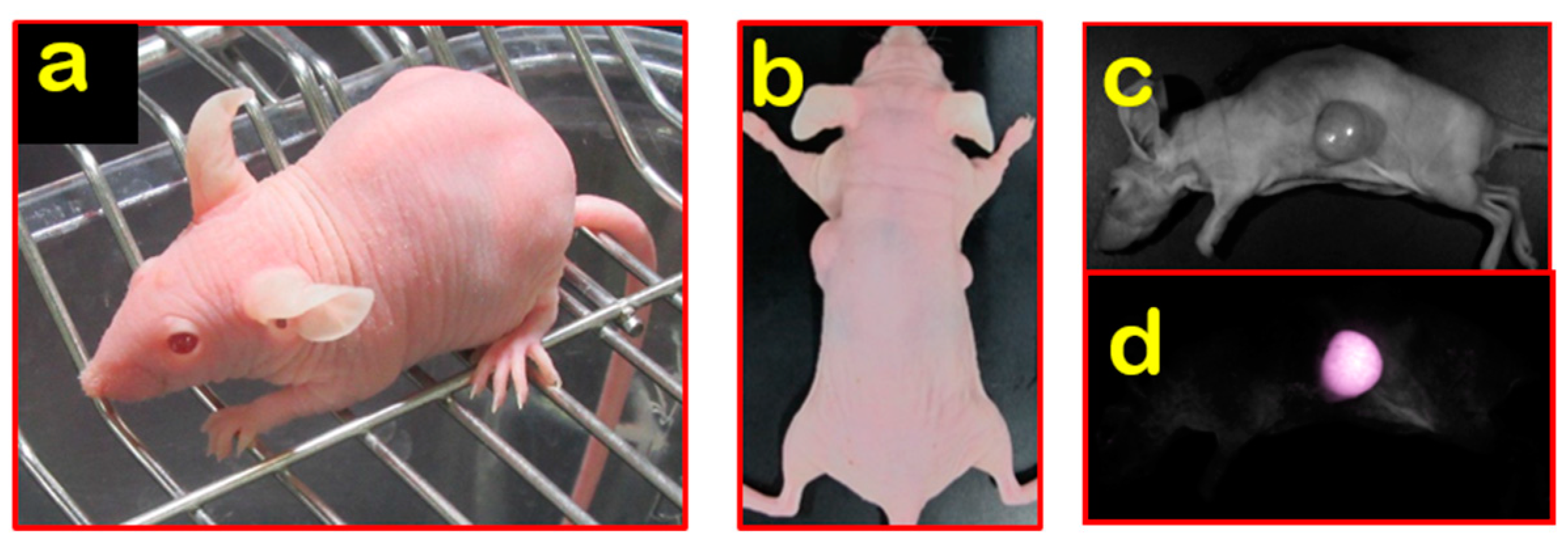
2.1. Nude Mice
2.2. Severe Combined Immunodeficient Mice
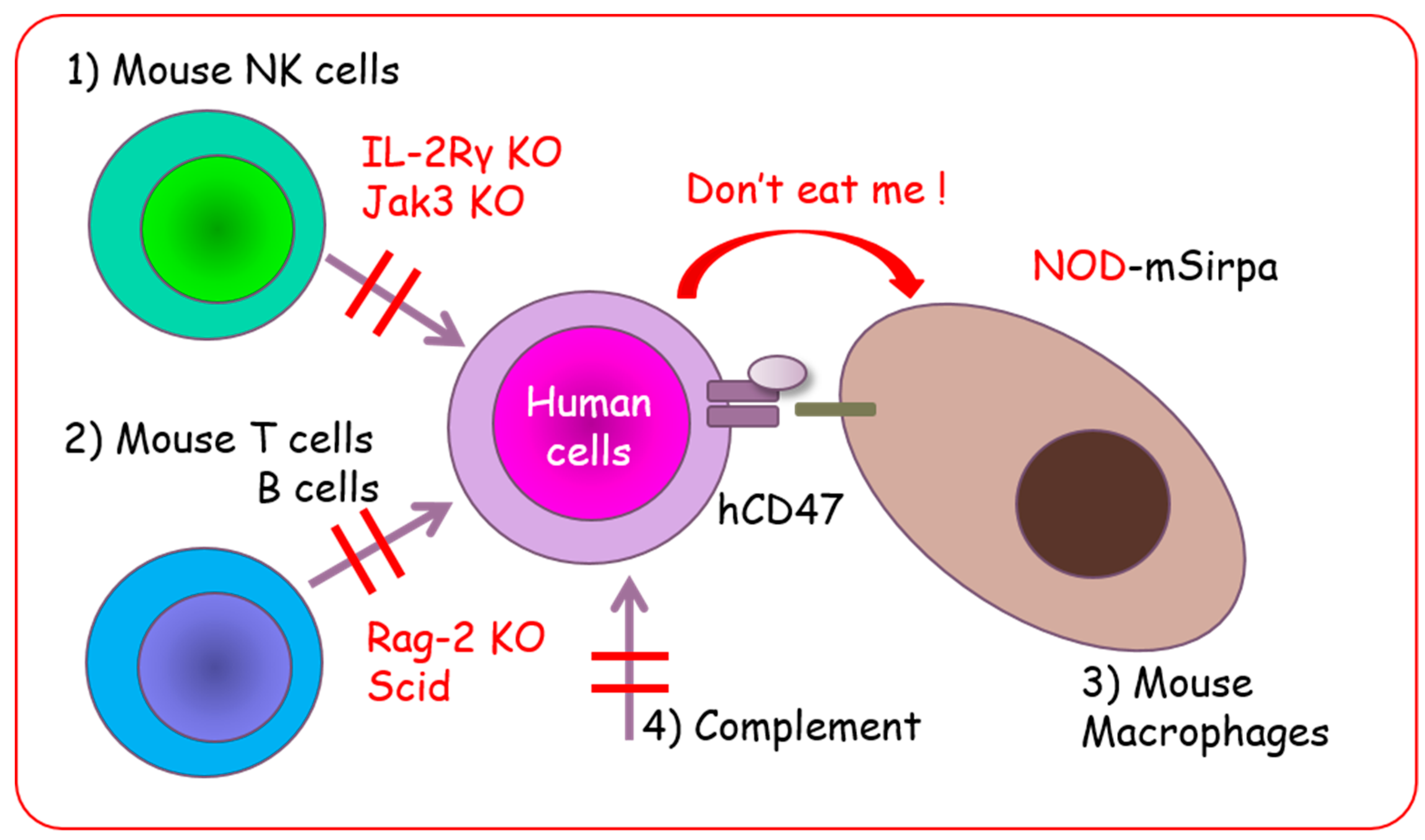
2.3. Non-Obese Diabetic/SCID Mice and NOD/SCID-Based Immunocompromised Mice
2.4. BALB/c Background Immunocompromised Mice
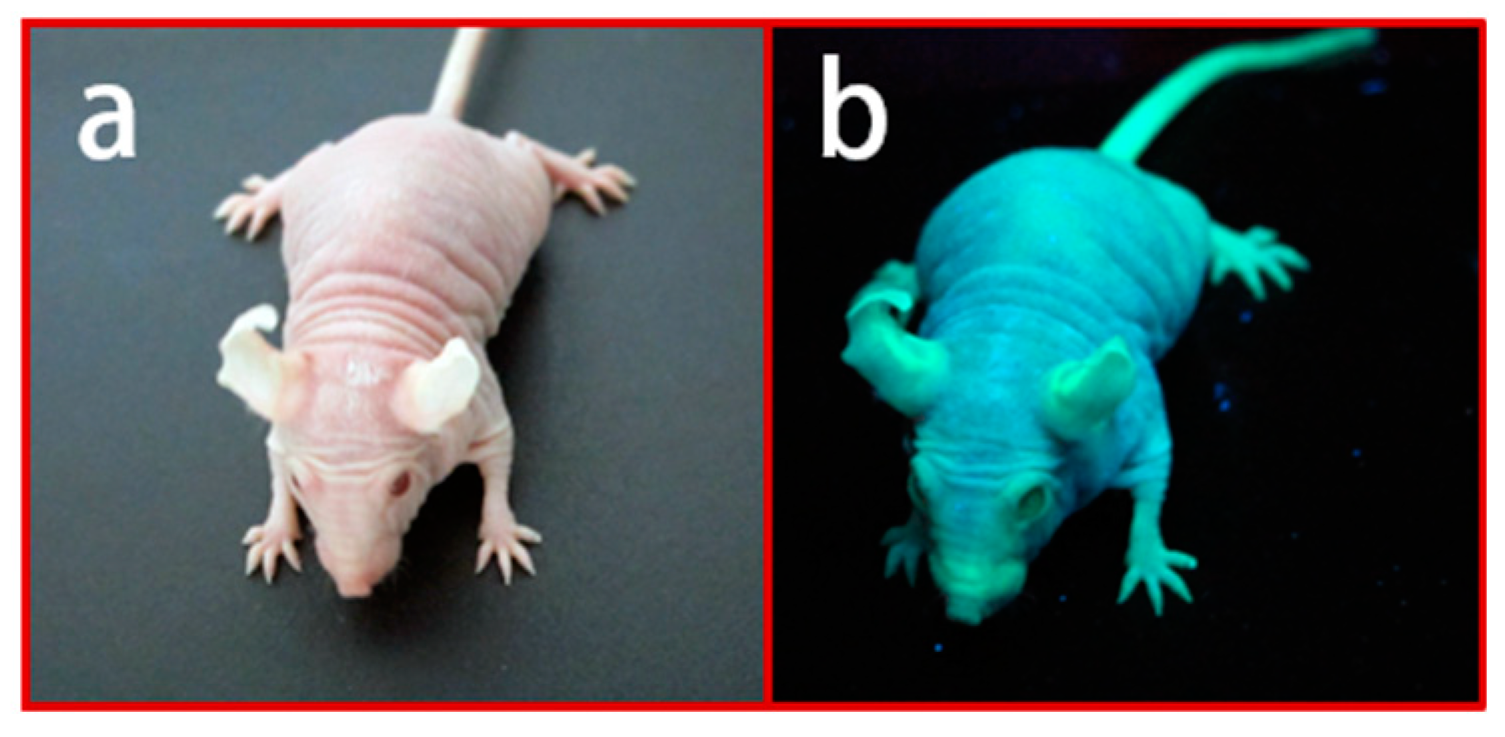
3. Establishment and Application of Nude/Hairless Immunocompromised Mice
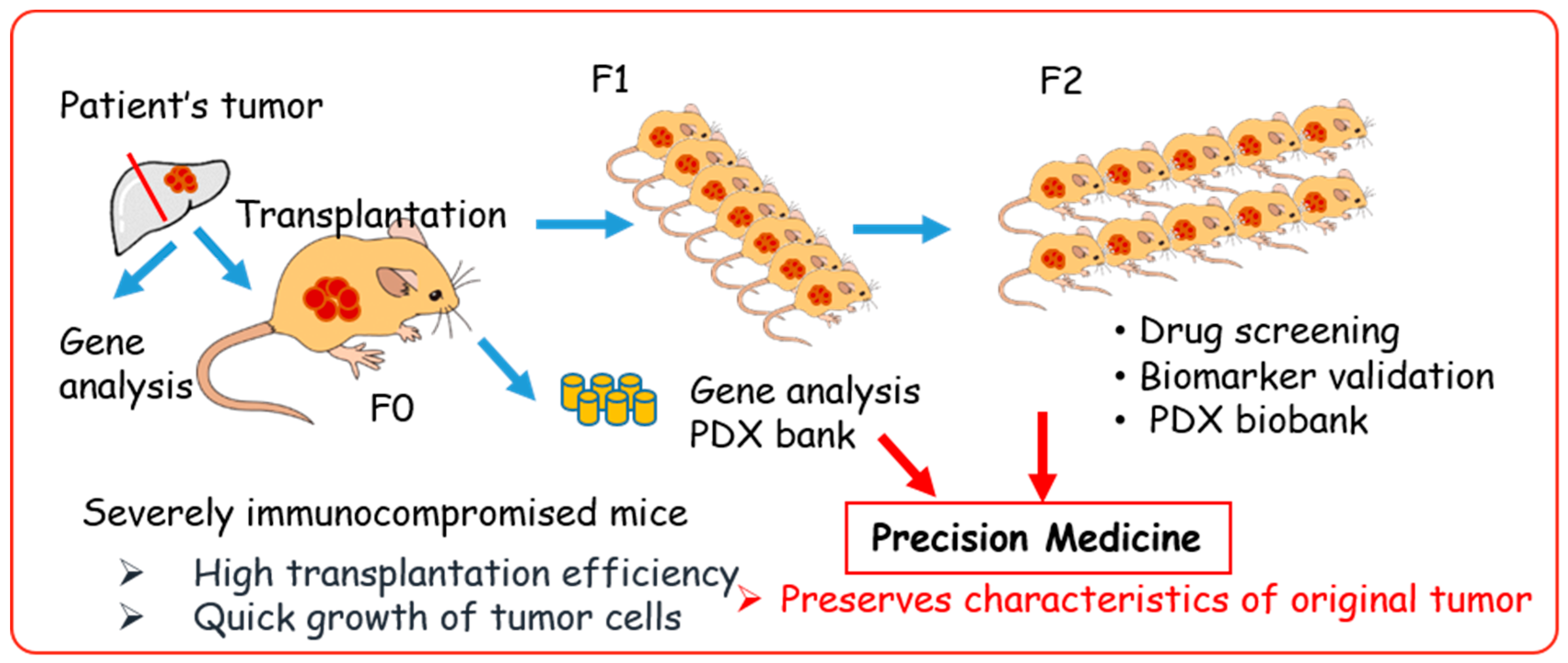
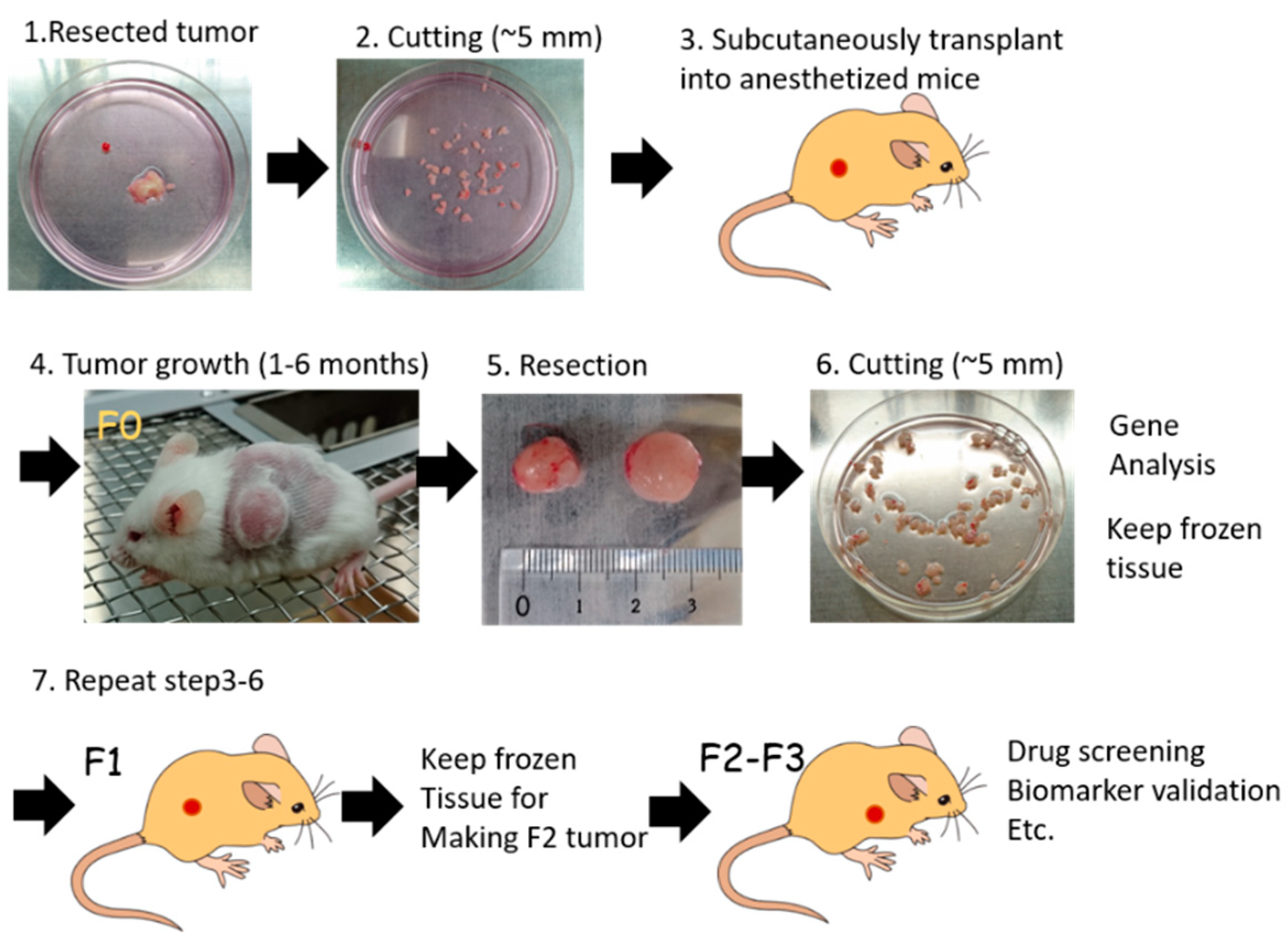
4. Establishment of PDX Models Using Various Immunocompromised Mice
5. Generation of PDX-Derived Cell Lines
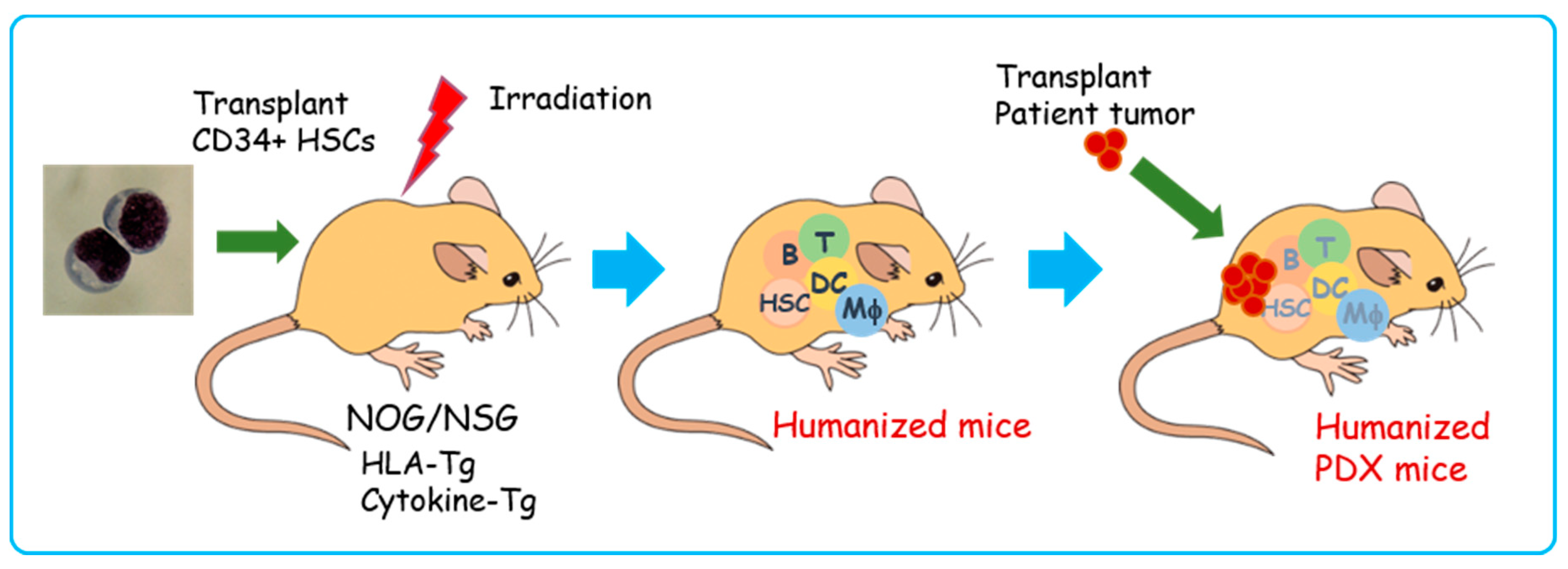
6. PDX in Humanized Mice
7. Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alteri, E.; Guizzaro, L. Be open about drug failures to speed up research. Nature 2018, 563, 317–319. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Reichert, J.M.; Feldman, L.; Malins, A. Clinical approval success rates for investigational cancer drugs. Clin. Pharmacol. Ther. 2013, 94, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Us cancer institute overhauls cell lines. Veteran cells to be replaced by human tumours grown in mice. Nature 2016, 530, 391. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Hayakawa, F.; Morishita, T.; Sugimoto, K.; Minamikawa, Y.; Iwase, M.; Yamamoto, H.; Hirano, D.; Imoto, N.; Shimada, K.; et al. Ym155 induces apoptosis through proteasome-dependent degradation of mcl-1 in primary effusion lymphoma. Pharmacol. Res. 2017, 120, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.P. ’Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966, 8, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Budzynski, W.; Radzikowski, C. Cytotoxic cells in immunodeficient athymic mice. Immunopharmacol. Immunotoxicol. 1994, 16, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, B.C.; Fogh, J. The nude mouse in cancer research. Adv. Cancer Res. 1985, 44, 69–120. [Google Scholar]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef]
- McCune, J.M.; Namikawa, R.; Kaneshima, H.; Shultz, L.D.; Lieberman, M.; Weissman, I.L. The scid-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science 1988, 241, 1632–1639. [Google Scholar] [CrossRef]
- Mosier, D.E.; Gulizia, R.J.; Baird, S.M.; Wilson, D.B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 1988, 335, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Taghian, A.; Budach, W.; Zietman, A.; Freeman, J.; Gioioso, D.; Ruka, W.; Suit, H.D. Quantitative comparison between the transplantability of human and murine tumors into the subcutaneous tissue of ncr/sed-nu/nu nude and severe combined immunodeficient mice. Cancer Res. 1993, 53, 5012–5017. [Google Scholar] [PubMed]
- Roder, J.; Duwe, A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature 1979, 278, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Mosier, D.E.; Stell, K.L.; Gulizia, R.J.; Torbett, B.E.; Gilmore, G.L. Homozygous scid/scid;beige/beige mice have low levels of spontaneous or neonatal t cell-induced b cell generation. J. Exp. Med. 1993, 177, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Galvani, S.; Canivet, C.; Kamar, N.; Bohler, T. Reconstitution of immunodeficient scid/beige mice with human cells: Applications in preclinical studies. Toxicology 2008, 246, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Kunimoto, K.; Muraoka, Y.; Mizushima, Y.; Katagiri, K.; Tochino, Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. Exp. Anim. 1980, 29, 1–13. [Google Scholar]
- Kikutani, H.; Makino, S. The murine autoimmune diabetes model: Nod and related strains. Adv. Immunol. 1992, 51, 285–322. [Google Scholar]
- Shultz, L.D.; Schweitzer, P.A.; Christianson, S.W.; Gott, B.; Schweitzer, I.B.; Tennent, B.; McKenna, S.; Mobraaten, L.; Rajan, T.V.; Greiner, D.L.; et al. Multiple defects in innate and adaptive immunologic function in nod/ltsz-scid mice. J. Immunol. 1995, 154, 180–191. [Google Scholar]
- Notarangelo, L.D.; Giliani, S.; Mazza, C.; Mella, P.; Savoldi, G.; Rodriguez-Perez, C.; Mazzolari, E.; Fiorini, M.; Duse, M.; Plebani, A.; et al. Of genes and phenotypes: The immunological and molecular spectrum of combined immune deficiency. Defects of the gamma(c)-jak3 signaling pathway as a model. Immunol. Rev. 2000, 178, 39–48. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakajima, H.; Saito, Y.; Saito, T.; Leonard, W.J.; Iwamoto, I. Janus kinase 3 (jak3) is essential for common cytokine receptor gamma chain (gamma(c))-dependent signaling: Comparative analysis of gamma(c), jak3, and gamma(c) and jak3 double-deficient mice. Int. Immunol. 2000, 12, 123–132. [Google Scholar] [CrossRef]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. Nod/scid/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human lymphoid and myeloid cell development in nod/ltsz-scid il2r gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005, 174, 6477–6489. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Harada, H.; Ito, T.; Saito, T.; Suzu, S. Early development of human hematopoietic and acquired immune systems in new born nod/scid/jak3null mice intrahepatic engrafted with cord blood-derived cd34 + cells. Int. J. Hematol. 2008, 88, 476–482. [Google Scholar] [CrossRef] [PubMed]
- McDermott, S.P.; Eppert, K.; Lechman, E.R.; Doedens, M.; Dick, J.E. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood 2010, 116, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagatani, M.; Kodera, T.; Suzuki, D.; Igura, S.; Fukunaga, Y.; Kanemitsu, H.; Nakamura, D.; Mochizuki, M.; Kemi, M.; Tamura, K.; et al. Comparison of biological features between severely immuno-deficient nod/shi-scid il2rg(null) and nod/ltsz-scid il2rg(null) mice. Exp. Anim. 2019. [Google Scholar] [CrossRef]
- Navarro-Alvarez, N.; Yang, Y.G. Cd47: A new player in phagocytosis and xenograft rejection. Cell. Mol. Immunol. 2011, 8, 285–288. [Google Scholar] [CrossRef]
- Takenaka, K.; Prasolava, T.K.; Wang, J.C.; Mortin-Toth, S.M.; Khalouei, S.; Gan, O.I.; Dick, J.E.; Danska, J.S. Polymorphism in sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007, 8, 1313–1323. [Google Scholar] [CrossRef]
- Yamauchi, T.; Takenaka, K.; Urata, S.; Shima, T.; Kikushige, Y.; Tokuyama, T.; Iwamoto, C.; Nishihara, M.; Iwasaki, H.; Miyamoto, T.; et al. Polymorphic sirpa is the genetic determinant for nod-based mouse lines to achieve efficient human cell engraftment. Blood 2013, 121, 1316–1325. [Google Scholar] [CrossRef]
- Traggiai, E.; Chicha, L.; Mazzucchelli, L.; Bronz, L.; Piffaretti, J.C.; Lanzavecchia, A.; Manz, M.G. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 2004, 304, 104–107. [Google Scholar] [CrossRef]
- Ono, A.; Hattori, S.; Kariya, R.; Iwanaga, S.; Taura, M.; Harada, H.; Suzu, S.; Okada, S. Comparative study of human hematopoietic cell engraftment into balb/c and c57bl/6 strain of rag-2/jak3 double-deficient mice. J. Biomed Biotechnol. 2011, 2011, 539748. [Google Scholar] [CrossRef]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Establishment of a patient-derived tumor xenograft model and application for precision cancer medicine. Chem. Pharm. Bull. 2018, 66, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, C.; Takenaka, K.; Urata, S.; Yamauchi, T.; Shima, T.; Kuriyama, T.; Daitoku, S.; Saito, Y.; Miyamoto, T.; Iwasaki, H.; et al. The balb/c-specific polymorphic sirpa enhances its affinity for human cd47, inhibiting phagocytosis against human cells to promote xenogeneic engraftment. Exp. Hematol. 2014, 42, 163–171.e161. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Kariya, R.; Matsuda, K.; Kudo, E.; Katano, H.; Okada, S. A potential role of the nod genetic background in mouse peritoneal macrophages for the development of primary effusion lymphoma. Leuk Res. 2016, 42, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Kojima, Y.; Matsuda, K.; Kariya, R.; Taura, M.; Kuwahara, K.; Nagai, H.; Katano, H.; Okada, S. Efficacy of anti-cd47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur. J. Cancer 2014, 50, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Strowig, T.; Rongvaux, A.; Rathinam, C.; Takizawa, H.; Borsotti, C.; Philbrick, W.; Eynon, E.E.; Manz, M.G.; Flavell, R.A. Transgenic expression of human signal regulatory protein alpha in rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc. Natl. Acad. Sci. USA 2011, 108, 13218–13223. [Google Scholar] [CrossRef] [PubMed]
- Kariya, R.; Matsuda, K.; Gotoh, K.; Vaeteewoottacharn, K.; Hattori, S.; Okada, S. Establishment of nude mice with complete loss of lymphocytes and nk cells and application for in vivo bio-imaging. Vivo 2014, 28, 779–784. [Google Scholar]
- Tanaka, A.; Takeda, S.; Kariya, R.; Matsuda, K.; Urano, E.; Okada, S.; Komano, J. A novel therapeutic molecule against htlv-1 infection targeting provirus. Leukemia 2013, 27, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Benavides, F.; Oberyszyn, T.M.; VanBuskirk, A.M.; Reeve, V.E.; Kusewitt, D.F. The hairless mouse in skin research. J. Dermatol. Sci. 2009, 53, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiniger, H.J.; Meier, H.; Kaliss, N.; Cherry, M.; Chen, H.W.; Stoner, R.D. Hereditary immunodeficiency and leukemogenesis in hrs-j mice. Cancer Res. 1974, 34, 201–211. [Google Scholar] [PubMed]
- Crottes, D.; Rapetti-Mauss, R.; Alcaraz-Perez, F.; Tichet, M.; Gariano, G.; Martial, S.; Guizouarn, H.; Pellissier, B.; Loubat, A.; Popa, A.; et al. Sigmar1 regulates membrane electrical activity in response to extracellular matrix stimulation to drive cancer cell invasiveness. Cancer Res. 2016, 76, 607–618. [Google Scholar] [CrossRef]
- Smee, D.F.; Dagley, A.; Downs, B.; Hagloch, J.; Tarbet, E.B. Enhanced efficacy of cidofovir combined with vaccinia immune globulin in treating progressive cutaneous vaccinia virus infections in immunosuppressed hairless mice. Antimicrob. Agents Chemother. 2015, 59, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Wei, X.; Lin, S.; Qin, L.; Cheng, L.; Li, P. Current status and perspectives of patient-derived xenograft models in cancer research. J. Hematol. Oncol. 2017, 10, 106. [Google Scholar] [CrossRef]
- Hoffman, R. Green fluorescent protein imaging of tumour growth, metastasis, and angiogenesis in mouse models. Lancet. Oncol. 2002, 3, 546–556. [Google Scholar] [CrossRef]
- Yang, M.; Reynoso, J.; Jiang, P.; Li, L.; Moossa, A.R.; Hoffman, R.M. Transgenic nude mouse with ubiquitous green fluorescent protein expression as a host for human tumors. Cancer Res. 2004, 64, 8651–8656. [Google Scholar] [CrossRef]
- Niclou, S.P.; Danzeisen, C.; Eikesdal, H.P.; Wiig, H.; Brons, N.H.; Poli, A.M.; Svendsen, A.; Torsvik, A.; Enger, P.O.; Terzis, J.A.; et al. A novel egfp-expressing immunodeficient mouse model to study tumor-host interactions. FASEB J. 2008, 22, 3120–3128. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, K.; Kariya, R.; Matsuda, K.; Hattori, S.; Vaeteewoottacharn, K.; Okada, S. A novel egfp-expressing nude mice with complete loss of lymphocytes and nk cells to study tumor-host interactions. Biosci. Trends 2014, 8, 202–205. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Bouvet, M. Imaging the microenvironment of pancreatic cancer patient-derived orthotopic xenografts (pdox) growing in transgenic nude mice expressing gfp, rfp, or cfp. Cancer Lett. 2016, 380, 349–355. [Google Scholar] [CrossRef]
- Vaeteewoottacharn, K.; Kariya, R.; Dana, P.; Fujikawa, S.; Matsuda, K.; Ohkuma, K.; Kudo, E.; Kraiklang, R.; Wongkham, C.; Wongkham, S.; et al. Inhibition of carbonic anhydrase potentiates bevacizumab treatment in cholangiocarcinoma. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 9023–9035. [Google Scholar] [CrossRef]
- Tentler, J.J.; Tan, A.C.; Weekes, C.D.; Jimeno, A.; Leong, S.; Pitts, T.M.; Arcaroli, J.J.; Messersmith, W.A.; Eckhardt, S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012, 9, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, J.W.; Caldas, C.; Bruna, A. Maintaining tumor heterogeneity in patient-derived tumor xenografts. Cancer Res. 2015, 75, 2963–2968. [Google Scholar] [CrossRef]
- Wang, D.; Pham, N.A.; Tong, J.; Sakashita, S.; Allo, G.; Kim, L.; Yanagawa, N.; Raghavan, V.; Wei, Y.; To, C.; et al. Molecular heterogeneity of non-small cell lung carcinoma patient-derived xenografts closely reflect their primary tumors. Int. J. Cancer 2017, 140, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G. Next-generation in vivo modeling of human cancers. Front. Oncol. 2018, 8, 429. [Google Scholar] [CrossRef]
- Collins, A.T.; Lang, S.H. A systematic review of the validity of patient derived xenograft (pdx) models: The implications for translational research and personalised medicine. PeerJ 2018, 6, e5981. [Google Scholar] [CrossRef]
- Bleijs, M.; van de Wetering, M.; Clevers, H.; Drost, J. Xenograft and organoid model systems in cancer research. EMBO J. 2019, 38, e101654. [Google Scholar] [CrossRef]
- Gao, H.; Korn, J.M.; Ferretti, S.; Monahan, J.E.; Wang, Y.; Singh, M.; Zhang, C.; Schnell, C.; Yang, G.; Zhang, Y.; et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015, 21, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Pompili, L.; Porru, M.; Caruso, C.; Biroccio, A.; Leonetti, C. Patient-derived xenografts: A relevant preclinical model for drug development. J. Exp. Clin. Cancer Res. Cr 2016, 35, 189. [Google Scholar] [CrossRef]
- Vaeteewoottacharn, K.; Pairojkul, C.; Kariya, R.; Muisuk, K.; Imtawil, K.; Chamgramol, Y.; Bhudhisawasdi, V.; Khuntikeo, N.; Pugkhem, A.; Saeseow, O.T.; et al. Establishment of highly transplantable cholangiocarcinoma cell lines from a patient-derived xenograft mouse model. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Teng, L.; Shen, Y.; He, K.; Xu, Z.; Li, G. Patient-derived human tumour tissue xenografts in immunodeficient mice: A systematic review. Clin. Transl. Oncol. 2010, 12, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Chijiwa, T.; Kawai, K.; Noguchi, A.; Sato, H.; Hayashi, A.; Cho, H.; Shiozawa, M.; Kishida, T.; Morinaga, S.; Yokose, T.; et al. Establishment of patient-derived cancer xenografts in immunodeficient nog mice. Int. J. Oncol. 2015, 47, 61–70. [Google Scholar] [CrossRef]
- Shultz, L.D.; Goodwin, N.; Ishikawa, F.; Hosur, V.; Lyons, B.L.; Greiner, D.L. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb. Protoc. 2014, 2014, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M.; Xue, A.; Mittal, A.; Samra, J.S.; Smith, R.; Hugh, T.J. Patient-derived xenograft models of colorectal cancer in pre-clinical research: A systematic review. Oncotarget 2016, 7, 66212–66225. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, X.; Liu, P.; Li, M.; Luo, F. Patient-derived xenograft mouse models: A high fidelity tool for individualized medicine. Oncol. Lett. 2019, 17, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sittithumcharee, G.; Suppramote, O.; Vaeteewoottacharn, K.; Sirisuksakun, C.; Jamnongsong, S.; Lapanuwat, P.; Suntiparpluacha, M.; Matha, A.; Chusorn, P.; Buraphat, P.; et al. Dependency of cholangiocarcinoma on cyclin d-dependent kinase activity. Hepatology 2019. [Google Scholar] [CrossRef] [PubMed]
- Murayama, T.; Gotoh, N. Patient-derived xenograft models of breast cancer and their application. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer 2015, 15, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Revelo, M.P.; Sudilovsky, D.; Cao, M.; Chen, W.G.; Goetz, L.; Xue, H.; Sadar, M.; Shappell, S.B.; Cunha, G.R.; et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate 2005, 64, 149–159. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kang, W.; Han, J.Y.; Min, S.; Kang, J.; Lee, A.; Kwon, J.Y.; Lee, C.; Park, H. An integrative approach to precision cancer medicine using patient-derived xenografts. Mol. Cells 2016, 39, 77–86. [Google Scholar]
- Whittle, J.R.; Lewis, M.T.; Lindeman, G.J.; Visvader, J.E. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. Bcr 2015, 17, 17. [Google Scholar] [CrossRef]
- Centenera, M.M.; Hickey, T.E.; Jindal, S.; Ryan, N.K.; Ravindranathan, P.; Mohammed, H.; Robinson, J.L.; Schiewer, M.J.; Ma, S.; Kapur, P.; et al. A patient-derived explant (pde) model of hormone-dependent cancer. Mol. Oncol. 2018, 12, 1608–1622. [Google Scholar] [CrossRef]
- Oyama, R.; Takahashi, M.; Yoshida, A.; Sakumoto, M.; Takai, Y.; Kito, F.; Shiozawa, K.; Qiao, Z.; Arai, Y.; Shibata, T.; et al. Generation of novel patient-derived cic- dux4 sarcoma xenografts and cell lines. Sci. Rep. 2017, 7, 4712. [Google Scholar] [CrossRef]
- Borodovsky, A.; McQuiston, T.J.; Stetson, D.; Ahmed, A.; Whitston, D.; Zhang, J.; Grondine, M.; Lawson, D.; Challberg, S.S.; Zinda, M.; et al. Generation of stable pdx derived cell lines using conditional reprogramming. Mol. Cancer 2017, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Shima, K.; Mizuma, M.; Hayashi, H.; Nakagawa, K.; Okada, T.; Sakata, N.; Omura, N.; Kitamura, Y.; Motoi, F.; Rikiyama, T.; et al. Potential utility of egfp-expressing nog mice (nog-egfp) as a high purity cancer sampling system. J. Exp. Clin. Cancer Res. Cr 2012, 31, 55. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A. Immunotherapy: Past, present and future. Nat. Med. 2003, 9, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chen, J. Current status and future directions of cancer immunotherapy. J. Cancer 2018, 9, 1773–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Tonomura, N.; Shimizu, A.; Wang, S.; Yang, Y.G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and cd34+ cell transplantation. Blood 2006, 108, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.J.; Bird, G.; Refaeli, Y.; Jimeno, A. Humanized mouse xenograft models: Narrowing the tumor-microenvironment gap. Cancer Res. 2016, 76, 6153–6158. [Google Scholar] [CrossRef] [PubMed]
- De La Rochere, P.; Guil-Luna, S.; Decaudin, D.; Azar, G.; Sidhu, S.S.; Piaggio, E. Humanized mice for the study of immuno-oncology. Trends Immunol. 2018, 39, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Billerbeck, E.; Barry, W.T.; Mu, K.; Dorner, M.; Rice, C.M.; Ploss, A. Development of human cd4+foxp3+ regulatory t cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing nod-scid il2rgamma(null) humanized mice. Blood 2011, 117, 3076–3086. [Google Scholar] [CrossRef]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Doi, T.; Sone, A.; Suzuki, N.; Fujiwara, H.; et al. Generation of functional human t-cell subsets with hla-restricted immune responses in hla class i expressing nod/scid/il2r gamma(null) humanized mice. Proc. Natl. Acad. Sci. USA 2010, 107, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liu, B.; Rubio, M.T.; Wang, X.; Ojcius, D.M.; Tang, R.; Durrbach, A.; Ru, Z.; Zhou, Y.; Lone, Y.C. Creation of an immunodeficient hla-transgenic mouse (humamice) and functional validation of human immunity after transfer of hla-matched human cells. PLoS ONE 2017, 12, e0173754. [Google Scholar] [CrossRef] [PubMed]
- Theocharides, A.P.; Rongvaux, A.; Fritsch, K.; Flavell, R.A.; Manz, M.G. Humanized hemato-lymphoid system mice. Haematologica 2016, 101, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Ojima, H.; Yoshikawa, D.; Ino, Y.; Shimizu, H.; Miyamoto, M.; Kokubu, A.; Hiraoka, N.; Morofuji, N.; Kondo, T.; Onaya, H.; et al. Establishment of six new human biliary tract carcinoma cell lines and identification of mageh1 as a candidate biomarker for predicting the efficacy of gemcitabine treatment. Cancer Sci. 2010, 101, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Cavalloni, G.; Peraldo-Neia, C.; Sassi, F.; Chiorino, G.; Sarotto, I.; Aglietta, M.; Leone, F. Establishment of a patient-derived intrahepatic cholangiocarcinoma xenograft model with kras mutation. Bmc Cancer 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Merino-Trigo, A.; Lacroix, L.; Pocard, M.; Goere, D.; Mariani, P.; Landron, S.; Bigot, L.; Nemati, F.; Dartigues, P.; et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin. Cancer Res. 2012, 18, 5314–5328. [Google Scholar] [CrossRef] [PubMed]
- Bertotti, A.; Migliardi, G.; Galimi, F.; Sassi, F.; Torti, D.; Isella, C.; Cora, D.; Di Nicolantonio, F.; Buscarino, M.; Petti, C.; et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies her2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011, 1, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Fitzgibbon, M.P.; Mortales, C.L.; Towlerton, A.M.; Upton, M.P.; Yeung, R.S.; McIntosh, M.W.; Warren, E.H. Phenotypic and transcriptional fidelity of patient-derived colon cancer xenografts in immune-deficient mice. PLoS ONE 2013, 8, e79874. [Google Scholar] [CrossRef]
- Garrido-Laguna, I.; Uson, M.; Rajeshkumar, N.V.; Tan, A.C.; de Oliveira, E.; Karikari, C.; Villaroel, M.C.; Salomon, A.; Taylor, G.; Sharma, R.; et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin. Cancer Res. 2011, 17, 5793–5800. [Google Scholar] [CrossRef]
- Mattie, M.; Christensen, A.; Chang, M.S.; Yeh, W.; Said, S.; Shostak, Y.; Capo, L.; Verlinsky, A.; An, Z.; Joseph, I.; et al. Molecular characterization of patient-derived human pancreatic tumor xenograft models for preclinical and translational development of cancer therapeutics. Neoplasia 2013, 15, 1138–1150. [Google Scholar] [CrossRef]
- Guo, S.; Gao, S.; Liu, R.; Shen, J.; Shi, X.; Bai, S.; Wang, H.; Zheng, K.; Shao, Z.; Liang, C.; et al. Oncological and genetic factors impacting pdx model construction with nsg mice in pancreatic cancer. FASEB J. 2019, 33, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, J.; Tang, J.; Chen, S.; He, K.; Jiang, X.; Jiang, W.; Teng, L. Establishment of patient-derived gastric cancer xenografts: A useful tool for preclinical evaluation of targeted therapies involving alterations in her-2, met and fgfr2 signaling pathways. BMC Cancer 2017, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tian, T.; Li, Z.; Tang, Z.; Wang, L.; Wu, J.; Li, Y.; Dong, B.; Li, N.; Zou, J.; et al. Establishment and characterization of patient-derived tumor xenograft using gastroscopic biopsies in gastric cancer. Sci. Rep. 2015, 5, 8542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Zhang, L.; Fan, S.; Zhang, M.; Fu, H.; Liu, Y.; Yin, X.; Chen, H.; Xie, L.; Zhang, J.; et al. Patient-derived gastric carcinoma xenograft mouse models faithfully represent human tumor molecular diversity. PLoS ONE 2015, 10, e0134493. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Lee, J.E.; Kim, H.; Sim, M.H.; Kim, K.K.; Lee, G.; Kim, H.I.; An, J.Y.; Hyung, W.J.; Kim, C.B.; et al. Establishment and characterisation of patient-derived xenografts as paraclinical models for gastric cancer. Sci. Rep. 2016, 6, 22172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keysar, S.B.; Astling, D.P.; Anderson, R.T.; Vogler, B.W.; Bowles, D.W.; Morton, J.J.; Paylor, J.J.; Glogowska, M.J.; Le, P.N.; Eagles-Soukup, J.R.; et al. A patient tumor transplant model of squamous cell cancer identifies pi3k inhibitors as candidate therapeutics in defined molecular bins. Mol. Oncol. 2013, 7, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Kimple, R.J.; Harari, P.M.; Torres, A.D.; Yang, R.Z.; Soriano, B.J.; Yu, M.; Armstrong, E.A.; Blitzer, G.C.; Smith, M.A.; Lorenz, L.D.; et al. Development and characterization of hpv-positive and hpv-negative head and neck squamous cell carcinoma tumorgrafts. Clin. Cancer Res. 2013, 19, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, E.; Vincent-Salomon, A.; Auger, N.; Degeorges, A.; Assayag, F.; de Cremoux, P.; de Plater, L.; Guyader, C.; De Pinieux, G.; Judde, J.G.; et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin. Cancer Res. 2007, 13, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Cottu, P.; Marangoni, E.; Assayag, F.; de Cremoux, P.; Vincent-Salomon, A.; Guyader, C.; de Plater, L.; Elbaz, C.; Karboul, N.; Fontaine, J.J.; et al. Modeling of response to endocrine therapy in a panel of human luminal breast cancer xenografts. Breast Cancer Res. Treat. 2012, 133, 595–606. [Google Scholar] [CrossRef] [PubMed]
- DeRose, Y.S.; Wang, G.; Lin, Y.C.; Bernard, P.S.; Buys, S.S.; Ebbert, M.T.; Factor, R.; Matsen, C.; Milash, B.A.; Nelson, E.; et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 2011, 17, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Claerhout, S.; Prat, A.; Dobrolecki, L.E.; Petrovic, I.; Lai, Q.; Landis, M.D.; Wiechmann, L.; Schiff, R.; Giuliano, M.; et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013, 73, 4885–4897. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Bizzaro, F.; Cesca, M.; Guffanti, F.; Ganzinelli, M.; Decio, A.; Ghilardi, C.; Perego, P.; Fruscio, R.; Buda, A.; et al. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014, 74, 6980–6990. [Google Scholar] [CrossRef] [PubMed]
- Heo, E.J.; Cho, Y.J.; Cho, W.C.; Hong, J.E.; Jeon, H.K.; Oh, D.Y.; Choi, Y.L.; Song, S.Y.; Choi, J.J.; Bae, D.S.; et al. Patient-derived xenograft models of epithelial ovarian cancer for preclinical studies. Cancer Res. Treat. 2017, 49, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, Z.C.; Katre, A.A.; Steg, A.D.; Erickson, B.K.; Shah, M.M.; Alvarez, R.D.; Conner, M.G.; Schneider, D.; Chen, D.; Landen, C.N. Using heterogeneity of the patient-derived xenograft model to identify the chemoresistant population in ovarian cancer. Oncotarget 2014, 5, 8750–8764. [Google Scholar] [CrossRef] [PubMed]
- Weroha, S.J.; Becker, M.A.; Enderica-Gonzalez, S.; Harrington, S.C.; Oberg, A.L.; Maurer, M.J.; Perkins, S.E.; AlHilli, M.; Butler, K.A.; McKinstry, S.; et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin. Cancer Res. 2014, 20, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.D.; Hartley, L.; Cook, M.; Heong, V.; Boehm, E.; McShane, L.; Pyman, J.; McNally, O.; Ananda, S.; Harrell, M.; et al. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol. Oncol. 2014, 8, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, I.; Rolff, J.; Soong, R.; Hoffmann, J.; Hammer, S.; Sommer, A.; Becker, M.; Merk, J. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin. Cancer Res. 2008, 14, 6456–6468. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Guan, J.; English, J.C.; Flint, J.; Yee, J.; Evans, K.; Murray, N.; Macaulay, C.; Ng, R.T.; Gout, P.W.; et al. Patient-derived first generation xenografts of non-small cell lung cancers: Promising tools for predicting drug responses for personalized chemotherapy. Clin. Cancer Res. 2010, 16, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, R.; Wang, L.; Correa, A.M.; Pataer, A.; Xu, Y.; Zhang, X.; Ren, C.; Wu, S.; Meng, Q.H.; et al. Tumor characteristics associated with engraftment of patient-derived non-small cell lung cancer xenografts in immunocompromised mice. Cancer 2019. [Google Scholar] [CrossRef]
- Brabetz, S.; Leary, S.E.S.; Grobner, S.N.; Nakamoto, M.W.; Seker-Cin, H.; Girard, E.J.; Cole, B.; Strand, A.D.; Bloom, K.L.; Hovestadt, V.; et al. A biobank of patient-derived pediatric brain tumor models. Nat. Med. 2018, 24, 1752–1761. [Google Scholar] [CrossRef]
- Priolo, C.; Agostini, M.; Vena, N.; Ligon, A.H.; Fiorentino, M.; Shin, E.; Farsetti, A.; Pontecorvi, A.; Sicinska, E.; Loda, M. Establishment and genomic characterization of mouse xenografts of human primary prostate tumors. Am. J. Pathol. 2010, 176, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Wetterauer, C.; Vlajnic, T.; Schuler, J.; Gsponer, J.R.; Thalmann, G.N.; Cecchini, M.; Schneider, J.; Zellweger, T.; Pueschel, H.; Bachmann, A.; et al. Early development of human lymphomas in a prostate cancer xenograft program using triple knock-out immunocompromised mice. Prostate 2015, 75, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Beraud, C.; Bethry, A.; Danilin, S.; Lindner, V.; Coquard, C.; Rothhut, S.; Massfelder, T. Establishment of a large panel of patient-derived preclinical models of human renal cell carcinoma. Oncotarget 2016, 7, 59336–59359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivanand, S.; Pena-Llopis, S.; Zhao, H.; Kucejova, B.; Spence, P.; Pavia-Jimenez, A.; Yamasaki, T.; McBride, D.J.; Gillen, J.; Wolff, N.C.; et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci. Transl. Med. 2012, 4, 137ra175. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Manley, B.J.; Becerra, M.F.; Redzematovic, A.; Casuscelli, J.; Tennenbaum, D.M.; Reznik, E.; Han, S.; Benfante, N.; Chen, Y.B.; et al. Tumor xenografts of human clear cell renal cell carcinoma but not corresponding cell lines recapitulate clinical response to sunitinib: Feasibility of using biopsy samples. Eur. Urol. Focus 2017, 3, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Einarsdottir, B.O.; Bagge, R.O.; Bhadury, J.; Jespersen, H.; Mattsson, J.; Nilsson, L.M.; Truve, K.; Lopez, M.D.; Naredi, P.; Nilsson, O.; et al. Melanoma patient-derived xenografts accurately model the disease and develop fast enough to guide treatment decisions. Oncotarget 2014, 5, 9609–9618. [Google Scholar] [CrossRef] [PubMed]
- Krepler, C.; Sproesser, K.; Brafford, P.; Beqiri, M.; Garman, B.; Xiao, M.; Shannan, B.; Watters, A.; Perego, M.; Zhang, G.; et al. A comprehensive patient-derived xenograft collection representing the heterogeneity of melanoma. Cell Rep. 2017, 21, 1953–1967. [Google Scholar] [CrossRef]
- Zhao, Y.; Shuen, T.W.H.; Toh, T.B.; Chan, X.Y.; Liu, M.; Tan, S.Y.; Fan, Y.; Yang, H.; Lyer, S.G.; Bonney, G.K.; et al. Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut 2018, 67, 1845–1854. [Google Scholar] [CrossRef]
- Yao, L.C.; Aryee, K.E.; Cheng, M.; Kaur, P.; Keck, J.G.; Brehm, M.A. Creation of pdx-bearing humanized mice to study immuno-oncology. Methods Mol. Biol. 2019, 1953, 241–252. [Google Scholar]
- Buque, A.; Galluzzi, L. Modeling tumor immunology and immunotherapy in mice. Trends Cancer 2018, 4, 599–601. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Kim, K.; Kim, S.H.; Chung, Y.J.; Lee, C. Studying cancer immunotherapy using patient-derived xenografts (pdxs) in humanized mice. Exp. Mol. Med. 2018, 50, 99. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Saito, M.; Tanaka, K.; Iwanaga, S.; Ali, S.N.; Seki, T.; Okada, S.; Kohara, M.; Harada, S.; Kai, C.; et al. Evaluation of a recombinant measles virus expressing hepatitis c virus envelope proteins by infection of human pbl-nod/scid/jak3null mouse. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, e81–e88. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Suzu, S.; Ito, T.; Okada, S. Selective expansion and engraftment of human cd16+ nk cells in nod/scid mice. Eur. J. Immunol. 2005, 35, 3599–3609. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Matsuda, K.; Srikoon, P.; Kariya, R.; Hattori, S.; Taura, M.; Katano, H.; Okada, S. Potent antitumor activity of zoledronic acid-induced vgamma9vdelta2 t cells against primary effusion lymphoma. Cancer Lett. 2013, 331, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chanana, P.; Davila, J.I.; Hou, X.; Zanfagnin, V.; McGehee, C.D.; Goode, E.L.; Polley, E.C.; Haluska, P.; Weroha, S.J.; et al. Gene expression differences between matched pairs of ovarian cancer patient tumors and patient-derived xenografts. Sci. Rep. 2019, 9, 6314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-David, U.; Ha, G.; Tseng, Y.Y.; Greenwald, N.F.; Oh, C.; Shih, J.; McFarland, J.M.; Wong, B.; Boehm, J.S.; Beroukhim, R.; et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 2017, 49, 1567–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio, S.; Hidalgo, M.; Kung, A.L. Examining the utility of patient-derived xenograft mouse models. Nat. Rev. Cancer 2015, 15, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinska, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Maelandsmo, G.M.; et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.T.; Alferez, D.G.; Amant, F.; Annibali, D.; Arribas, J.; Biankin, A.V.; Bruna, A.; Budinska, E.; Caldas, C.; Chang, D.K.; et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 2017, 17, 254–268. [Google Scholar] [CrossRef]
| Mice | NOD/SCID | NOG | NSG | NOJ |
|---|---|---|---|---|
| Strain | NOD.Cg-Prkdcscid | NOD.Cg-PrkdcscidIl2rgtm1Sug/Jic | NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ | NOD.Cg-PrkdcscidJak3tm1card |
| Genetic defects | SCID | SCID, IL-2γ Partial deficiency | SCID, IL-2Rγ Complete deficiency | SCID, Jak3 deficiency |
| Developer | CIEA 1, Jackson Laboratory | CIEA 1 | Jackson Laboratory | Kumamoto University |
| Supplier | Japan Clea Charles River | Japan Clea | Charles River | Kumamoto University |
| NK activities | NK cell dysfunction | Complete loss of NK cells | Complete loss of NK cells | Complete loss of NK cells |
| Reference | [18] | [21] | [22] | [23] |
| Mice | SCID | Rag-1/Rag-2 Knock Out Mice |
|---|---|---|
| Chromosome | Chr.16 | Chr.11 p13 |
| Mutated gene | Prkdc | Recombination-activation gene-1/-2 |
| Mutation | Natural mutant | Homologous recombination |
| Immunological phenotype | Deficiency of mature B and T lymphocytes NK cells are normal | Deficiency of mature B and T lymphocytes NK cells were normal |
| Radiation sensitivity | Sensitive (Lethal dose < 3 Gy) | Normal (Lethal dose 9 Gy) |
| Leakiness | Leaky | None |
| Mice | Hairless | Nude | SCID Hairless | Nude R/J | |
|---|---|---|---|---|---|
| Strain | BALB/c | BALB/c | CB17.Cg/ICR | BALB/c | |
| Gene abnormality | Hairless | Foxn1 | Hairless, SCID | Foxn1, Rag-2, Jak3 | |
| Immune system | T cells | + | − | − | − |
| B cells | + | + | − | − | |
| NK cells | + | + | + | − | |
| Hair coat | None | None | None | None | |
| Mouse Strain | Phenotype | Advantage | Disadvantage/Consideration | Success Rate of PDX |
|---|---|---|---|---|
| Nude | No thymus, no coat of hair | Well characterized, easy to detect s.c. tumor | Functional B and NK cells, increased T cell leakage with age | Low |
| SCID | No mature T and B cells | Better engraftment compared with nude | Functional NK cell, leakage of T cells, radiosensitive | Low |
| SCID/Beige | No mature T and B cells, impaired Mφ and NK function | Better engraftment compared with SCID | Leakage of T cells, radiosensitive | Moderate |
| NOD/SCID | No mature T and B cells Impaired NK function Impaired Mφ & DC | Better engraftment | Spontaneous lymphoma Short life span (av. 36wks) Radiosensitive | Moderate |
| NOG/NSG/NOJ | No mature T and B cells, no NK cells, impaired Mφ and DC | Excellent engraftment of PDX including hematopoietic malignancies | Need strict SPF conditions, breeding is not easy, expensive | High |
| BALB/c Rag2null/IL2Rγnull (BRG) Rag-2 null/Jak3 null (BRJ) | No mature T and B cells, no NK cells | Excellent engraftment of PDX, resistant to stress, easy breeding, radio resistant | High |
| Tumor Type | Mice Strain | Implantation Site | Number of Sample | Engraftment Ratio | References |
|---|---|---|---|---|---|
| Cholangiocarcinoma | SCID NOD/SCID BRJ | s.c. * s.c. s.c. | 55 20 16 | 34.5% 5.8% 75% | Ojima, 2010 [84] Cavalloni, 2016 [85] Vaeteewoottacharn, 2019 [57] |
| Colorectal cancer | Nude NOD/SCID NSG | s.c. s.c. s.c | 85 85 27 | 63.5% 87% 54% | Julien, 2012 [86] Bertolini, 2011 [87] Chou, 2013 [88] |
| Pancreatic cancer | Nude SCID NSG | s.c. s.c. s.c | 69 12 121 | 61% 67% 71.1% | Garrido-Laguna, 2011 [89] Mattie, 2013 [90] Guo, 2019 [91] |
| Gastric cancer | Nude NOD/SCID Nude/SCID Nude/NOG | s.c. s.c. s.c s.c | 32 185 83/119 62 | 73.7% 34.1% 16.9%/26.9% 24.2% | Wang, 2017 [92] Zhu, 2015 [93] Zhang, 2015 [94] Choi, 2016 [95] |
| Head and neck cancer | Nude NSG | s.c. s.c. | 46 26 | 54% 84.6% | Keysar, 2013 [96] Kimple, 2013 [97] |
| Breast cancer | Nude Nude Nude NOD/SCID SCID/Beige NSG | s.c. fat pad ** fat pad fat pad s.c. s.c. | 200 314 109 49 162 32 | 12.5% 2.5% (ER+) 24.3% (ER−) 27% 19% 31.3% | Marangoni, 2007 [98] Cottu, 2012 [99] DeRose, 2011 [100] Zhang, 2013 [101] |
| Ovarian cancer | Nude Nude SCID SCID NSG | s.c. r.c. *** s.c. s.c. s.c. | 138 45 34 168 12 | 25% 48.8% 50% 74% 83% | Ricci, 2014 [102] Heo, 2017 [103] Dobbin, 2014 [104] Weroha, 2014 [105] Topp, 2014 [106] |
| Non-small lung cancer | NOD/SCID NOD/SCID NOD/SCID NSG | s.c. r.c. s.c. s.c. | 102 527 308 441 | 25% 90% 26% 28.7% | Fichtner, 2008 [107] Dong, 2010 [108] Chen, 2019 [109] Wang, 2017 [51] |
| Glioblastoma | NSG | orthotopic | 100 | 30% | Brabetz, 2018 [110] |
| Prostate | Nude NOD/SCID SCID SCID SCID NSG | s.c. s.c. s.c. orthotopic r.c. s.c. | 23 23 86 57 122 27 | 39% 48% 58.1% 71.9% 93.4% 37% | Priolo, 2010 [111] Wang, 2005 [66] Wetterauer, 2015 [112] |
| Renal cell carcinoma | Nude NOD/SCID NSG | s.c. r.c. s.c. | 336 94 74 | 8.9% 37.2% 45% | Lang, 2016 [113] Sivanand, 2013 [114] Dong, 2017 [115] |
| Melanoma | NOG NSG | s.c. s.c. | 26 694 | 88.4% 65.8% | Einarsdottir, 2014 [116] Krepler, 2017 [117] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models. Cells 2019, 8, 889. https://doi.org/10.3390/cells8080889
Okada S, Vaeteewoottacharn K, Kariya R. Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models. Cells. 2019; 8(8):889. https://doi.org/10.3390/cells8080889
Chicago/Turabian StyleOkada, Seiji, Kulthida Vaeteewoottacharn, and Ryusho Kariya. 2019. "Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models" Cells 8, no. 8: 889. https://doi.org/10.3390/cells8080889
APA StyleOkada, S., Vaeteewoottacharn, K., & Kariya, R. (2019). Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models. Cells, 8(8), 889. https://doi.org/10.3390/cells8080889






