Similar Population of CD133+ and DDX4+ VSEL-Like Stem Cells Sorted from Human Embryonic Stem Cell, Ovarian, and Ovarian Cancer Ascites Cell Cultures: The Real Embryonic Stem Cells?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.2. Cell Cultures
2.3. Magnetic-Activated Cell Sorting Based on CD133 Expression
2.4. Exposure of Sorted Cells to Valproic Acid and FSH for Activation
2.5. Immunocytochemistry for CD133, DDX4, SSEA4, PRDM14, and S100 Expression
2.6. qPCR Analysis of CD133 and DDX4 Expression
2.7. In Vitro Differentiation of Cells into Adipogenic, Osteogenic and Neural lineages
3. Results
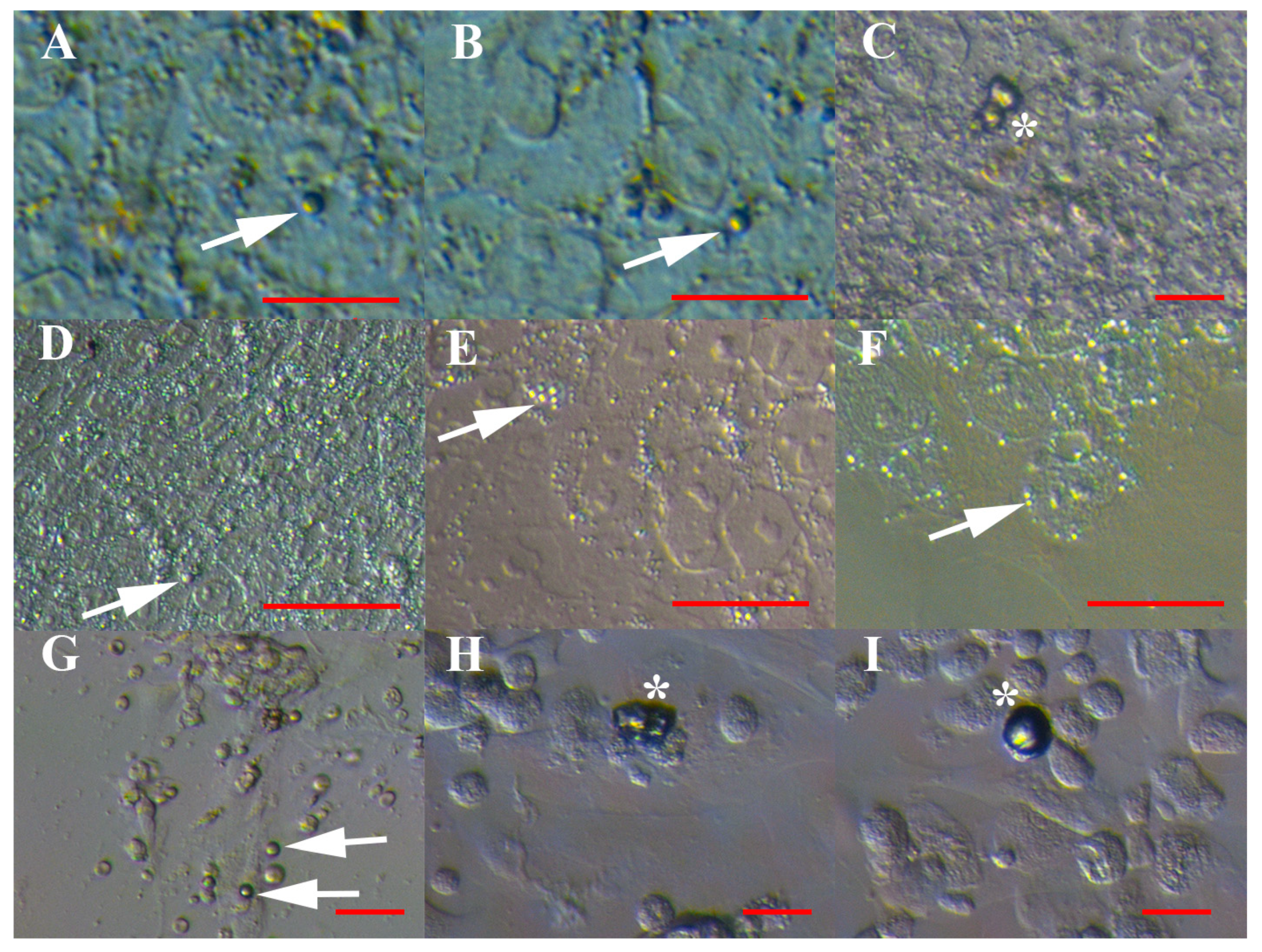
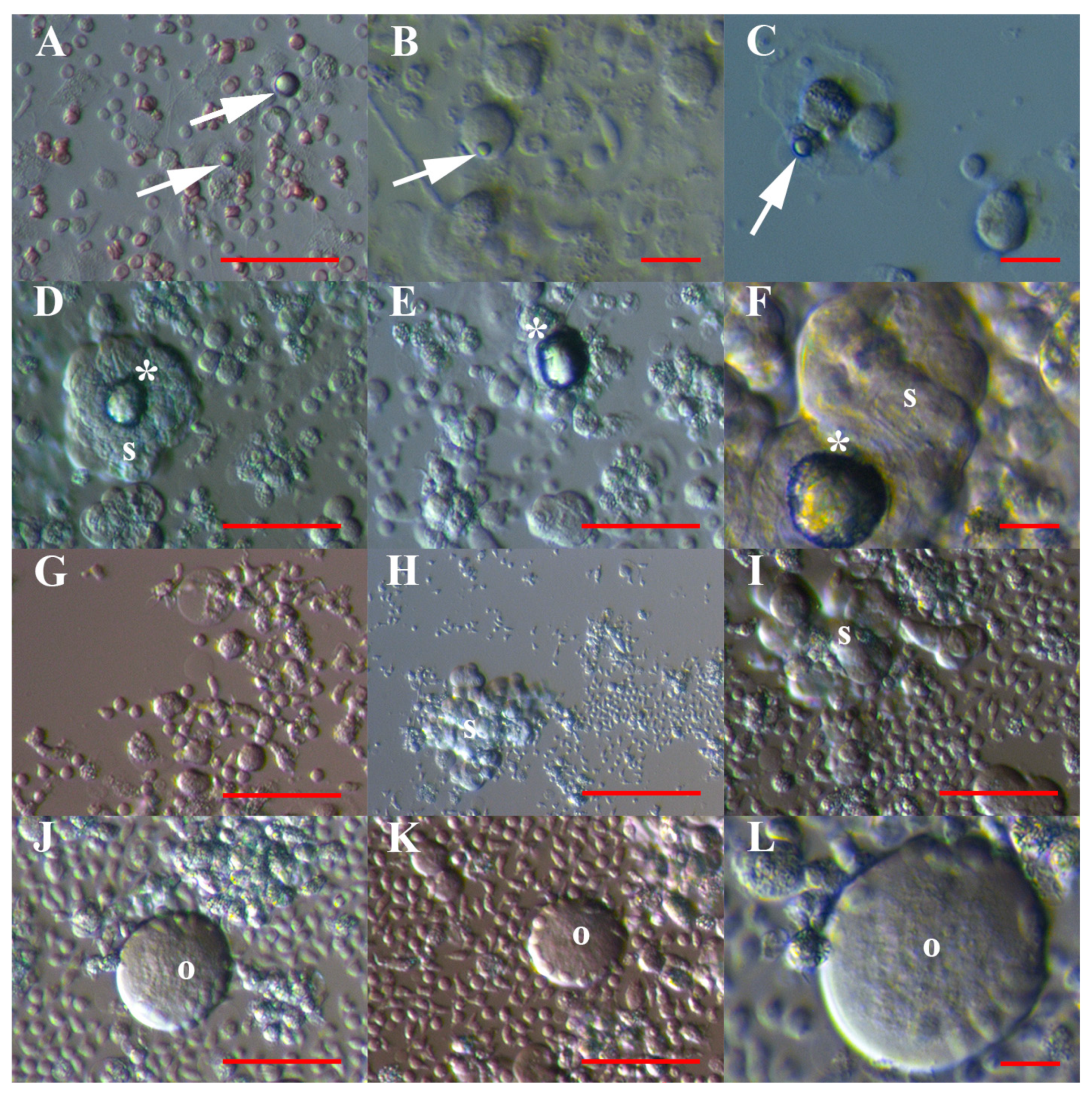
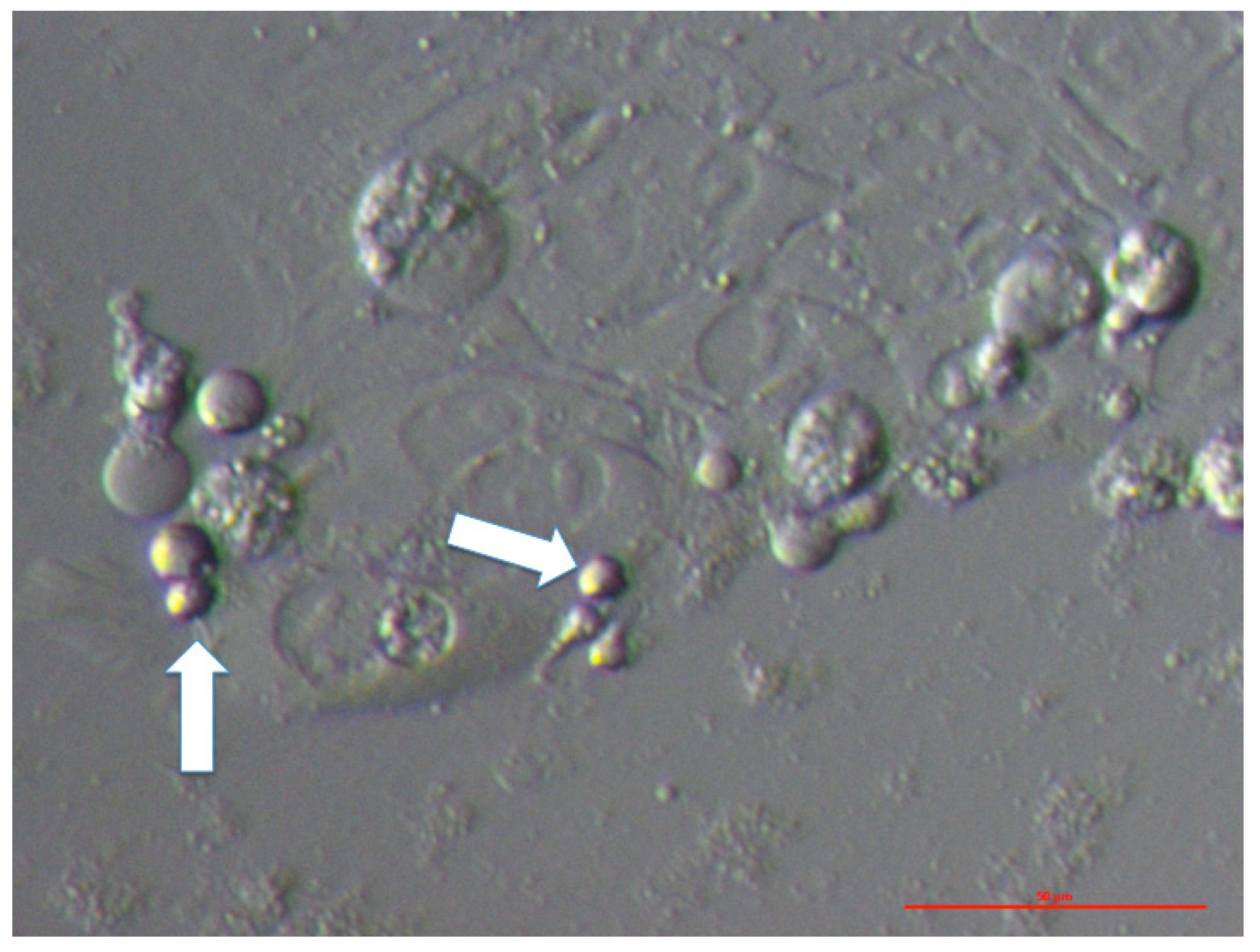
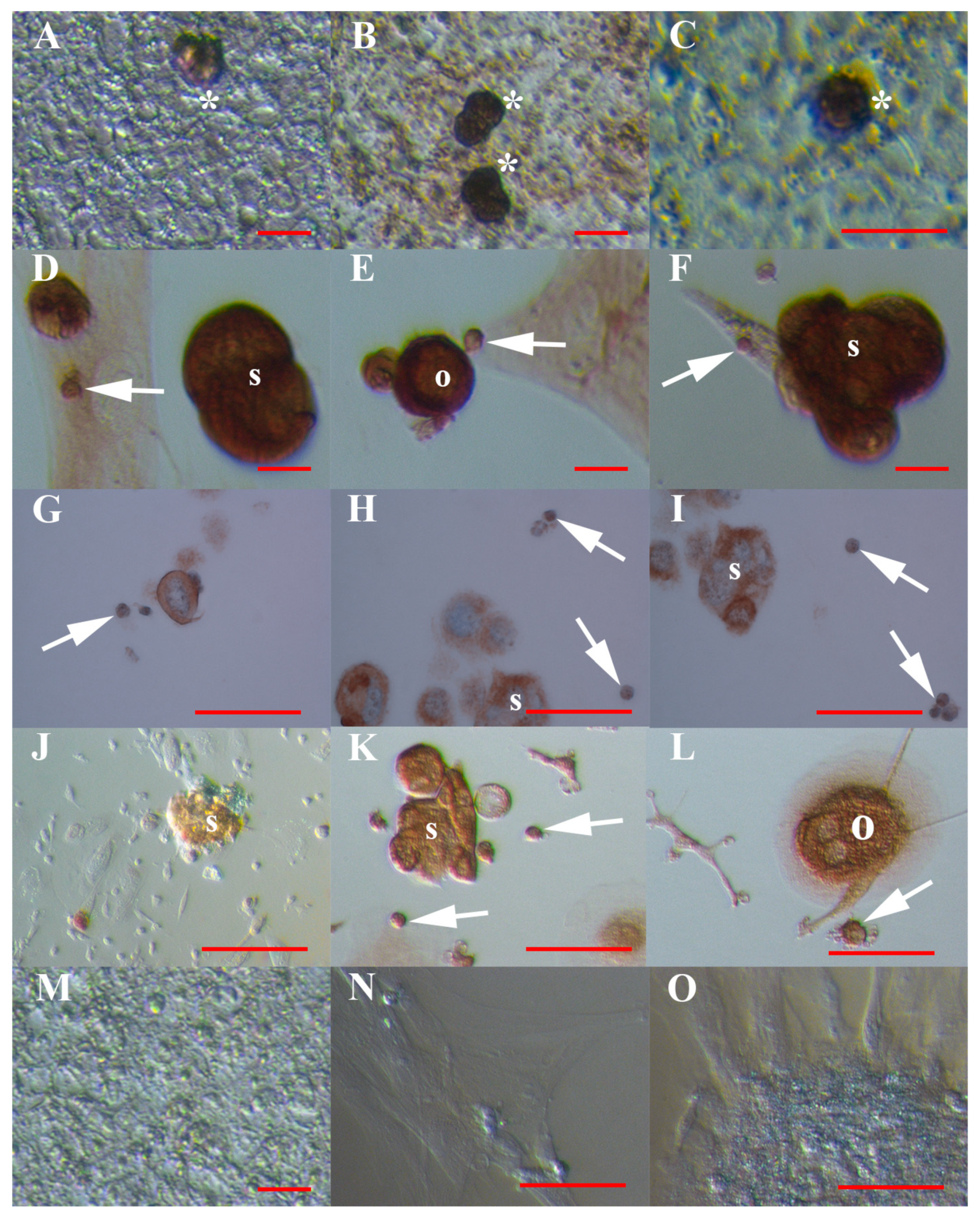
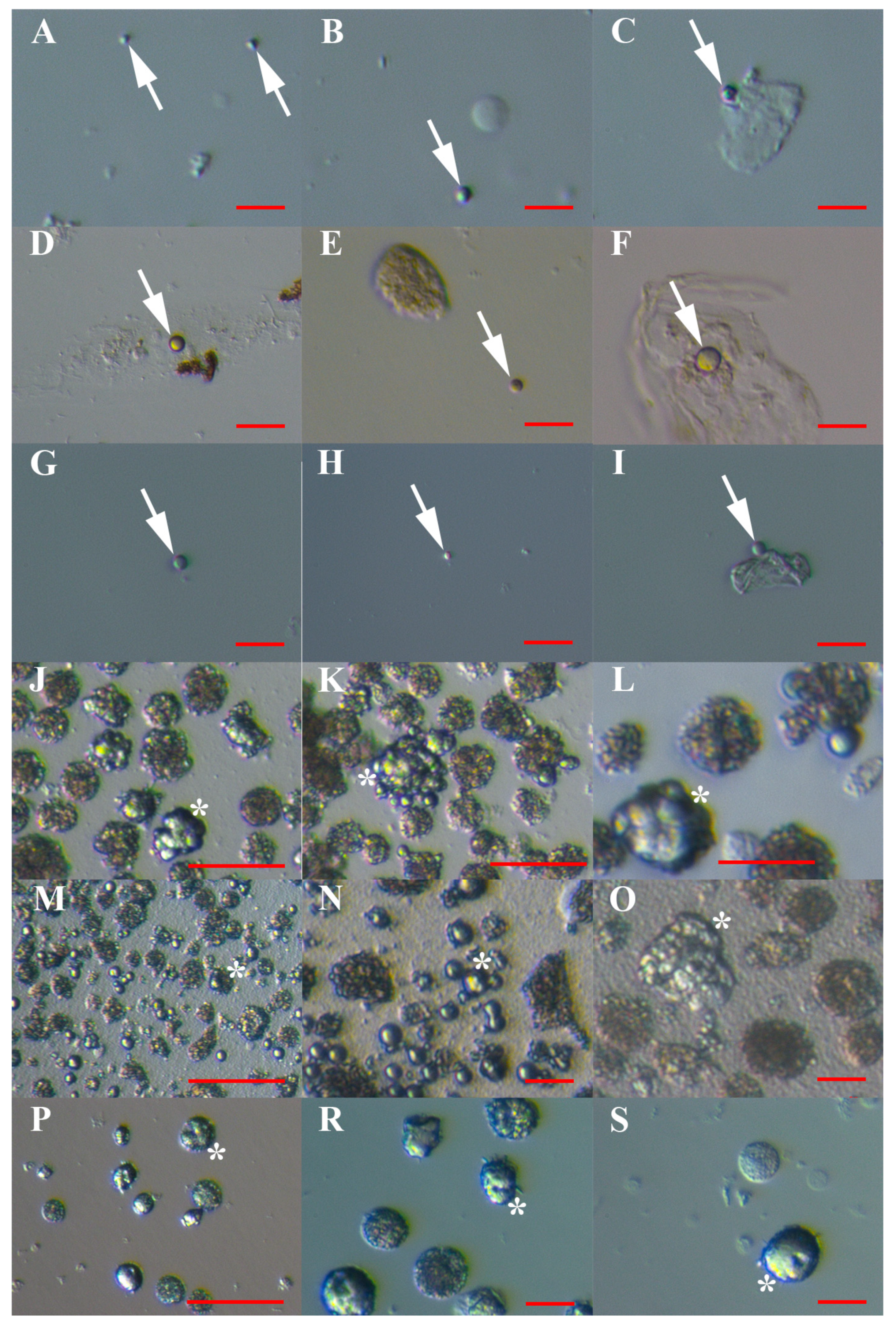
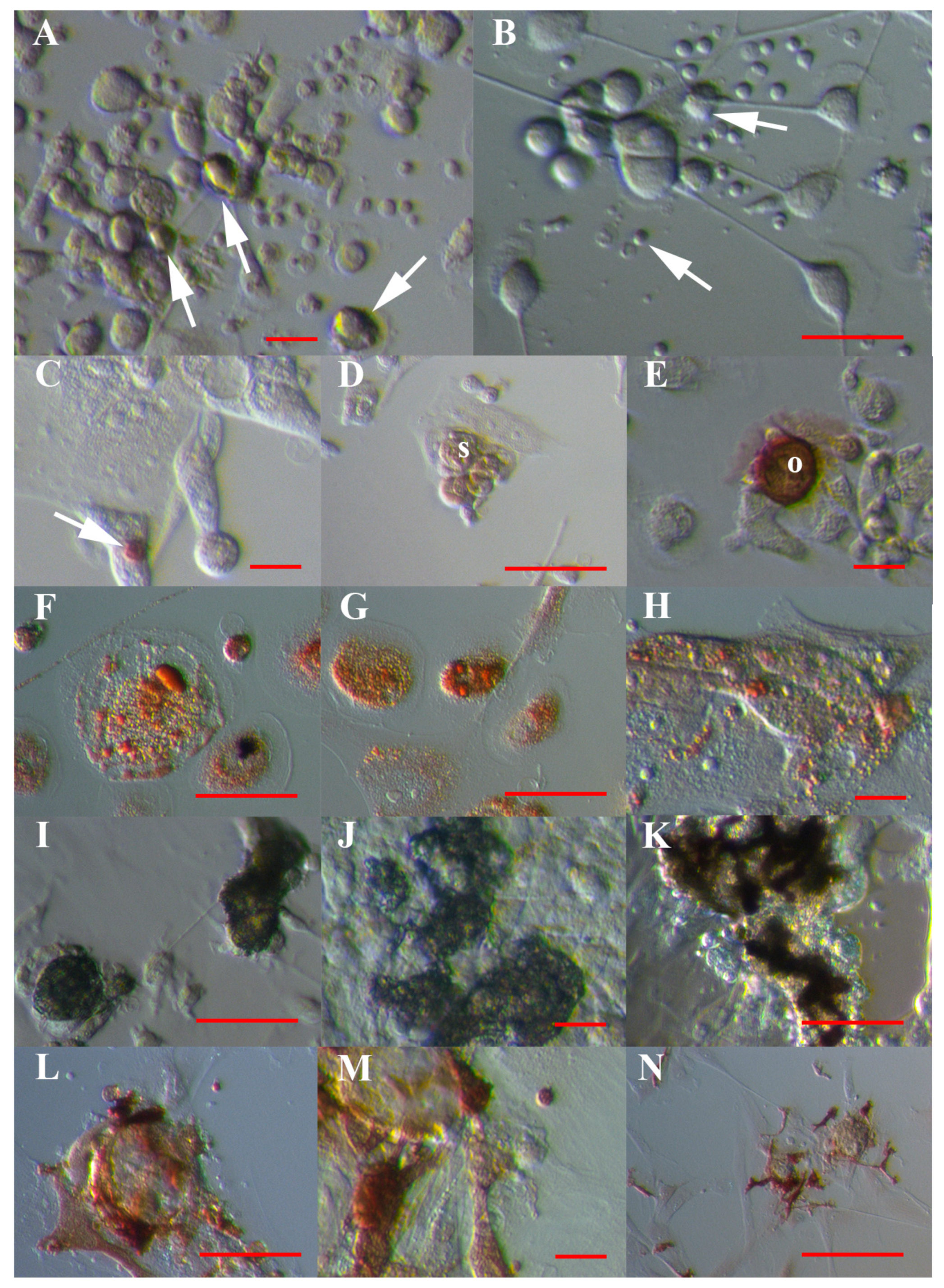
3.1. VSEL-Like Stem Cells in Cell Cultures
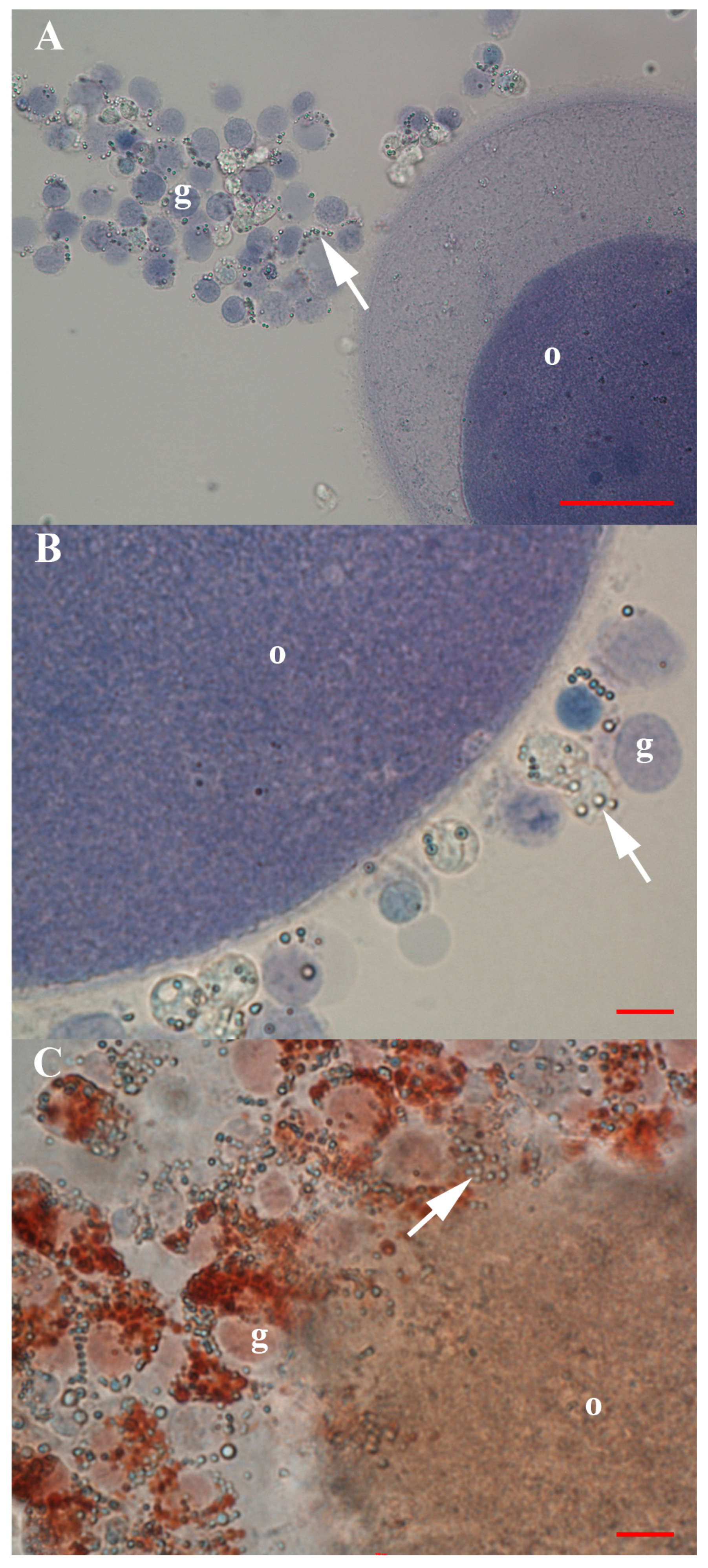
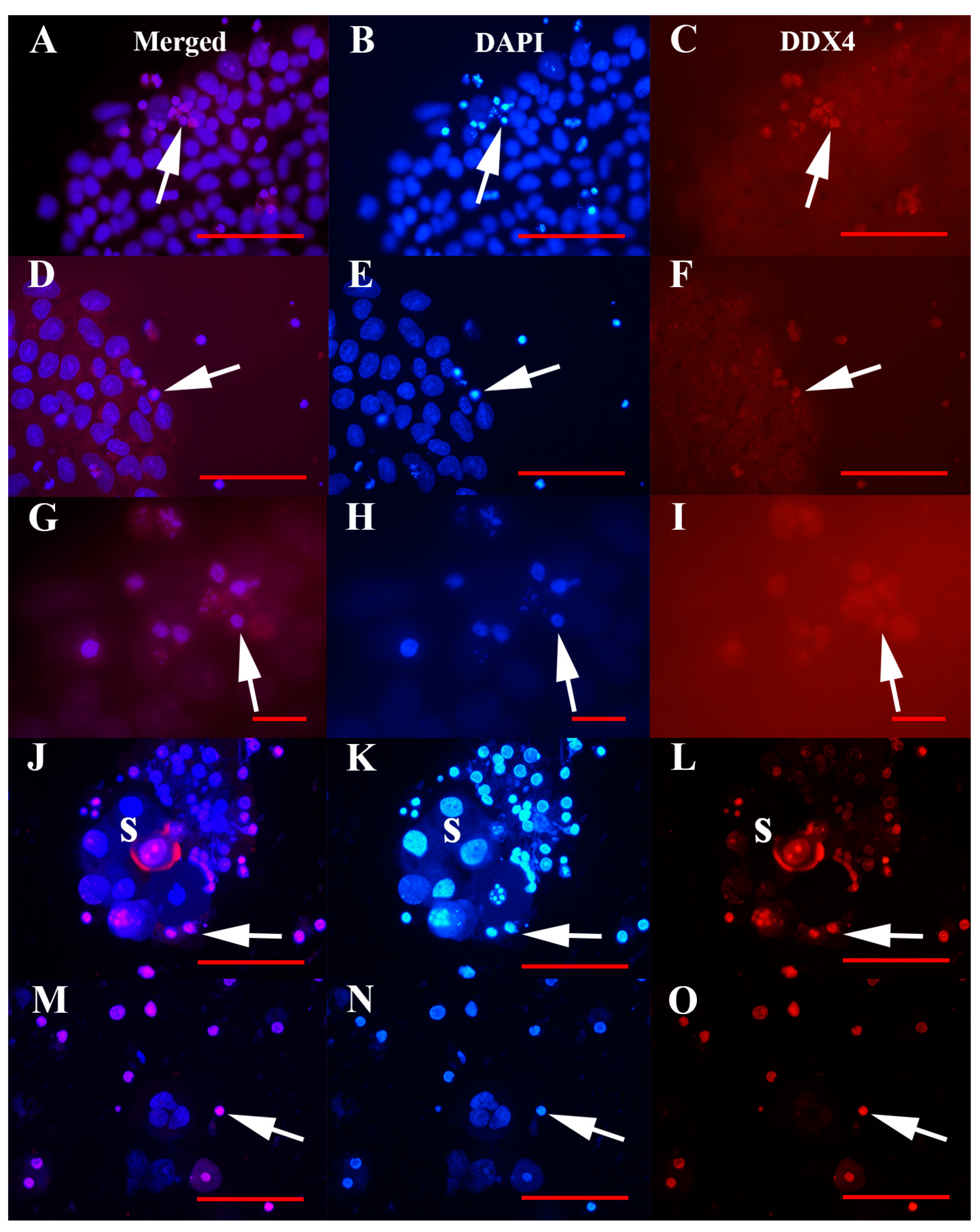
3.2. Positivity of VSEL-Like Stem Cells, Spheroids and Oocyte-Like Cells for the Germinal Lineage-Related Markers DDX4 and PRDM14
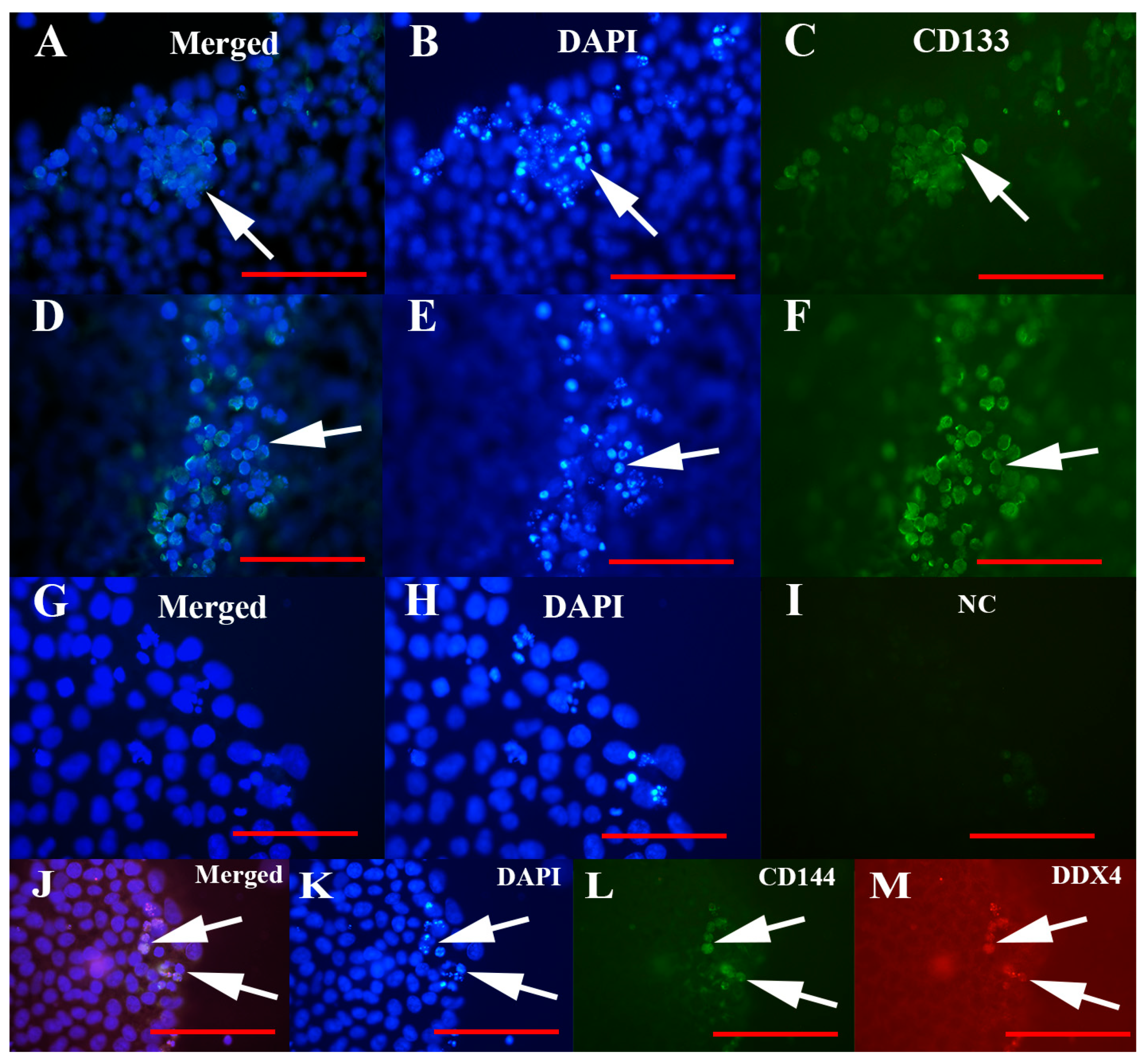
3.3. Positivity of Small VSEL-Like Cells for CD133 and Coexpression with the Germinal Marker DDX4
3.4. MACS Sorting of CD133+ Cells
3.5. Artificial Expansion of VSEL-Like Stem Cells after CD133-based MACS Sorting Using Valproic Acid and FSH
3.6. Differentiation of Sorted and Artificially Activated CD133+ VSEL-Like Stem Cells into Adipogenic, Osteogenic and Neural Lineages
3.7. qPCR Analysis of the Expression of the CD133 and DDX4 Genes in Cell Cultures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kucia, M.; Reca, R.; Campbell, F.R.; Zuba-Surma, E.; Majka, M.; Ratajczak, J.; Ratajczak, M.Z. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 2006, 20, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Halasa, M.; Wysoczynski, M.; Baskiewicz-Masiuk, M.; Moldenhawer, S.; Zuba-Surma, E.; Czajka, R.; Wojakowski, W.; Machalinski, B.; Ratajczak, M.Z. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: Preliminary report. Leukemia 2007, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.L.; Rossi, E.; Saubamea, B.; Cras, A.; Mignon, V.; Silvestre, J.S.; Smadja, D.M. Human very Small Embryonic-like Cells Support Vascular Maturation and Therapeutic Revascularization Induced by Endothelial Progenitor Cells. Stem Cell Rev. 2017, 13, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M. Bone Marrow Very Small Embryonic-Like Stem Cells: New Generation of Autologous Cell Therapy Soon Ready for Prime Time? Stem Cell Rev. 2017, 13, 198–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guerin, C.L.; Loyer, X.; Vilar, J.; Cras, A.; Mirault, T.; Gaussem, P.; Silvestre, J.S.; Smadja, D.M. Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: Evidence of vasculogenic potential. Thromb. Haemost. 2015, 113, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X.; Lui, M.; Chu, P.J.; Yoo, J.; Chang, M.; Yen, Y. Identification of a distinct small cell population from human bone marrow reveals its multipotency in vivo and in vitro. PLoS ONE 2014, 9, e85112. [Google Scholar] [CrossRef]
- Gharib, S.A.; Khalyfa, A.; Kucia, M.J.; Dayyat, E.A.; Kim, J.; Clair, H.B.; Gozal, D. Transcriptional landscape of bone marrow-derived very small embryonic-like stem cells during hypoxia. Respir. Res. 2011, 12, 63. [Google Scholar] [CrossRef][Green Version]
- Sovalat, H.; Scrofani, M.; Eidenschenk, A.; Pasquet, S.; Rimelen, V.; Hénon, P. Identification and isolation from either adult human bone marrow or G-CSF-mobilized peripheral blood of CD34(+)/CD133(+)/CXCR4(+)/Lin(-)CD45(-) cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp. Hematol. 2011, 39, 495–505. [Google Scholar]
- Gounari, E.; Daniilidis, A.; Tsagias, N.; Michopoulou, A.; Kouzi, K.; Koliakos, G. Isolation of a novel embryonic stem cell cord blood-derived population with in vitro hematopoietic capacity in the presence of Wharton’s jelly-derived mesenchymal stromal cells. Cytotherapy 2019, 21, 246–259. [Google Scholar] [CrossRef]
- Lahlil, R.; Scrofani, M.; Barbet, R.; Tancredi, C.; Aries, A.; Hénon, P. VSELs Maintain their Pluripotency and Competence to Differentiate after Enhanced Ex Vivo Expansion. Stem Cell Rev. 2018, 14, 510–524. [Google Scholar] [CrossRef]
- Monti, M.; Imberti, B.; Bianchi, N.; Pezzotta, A.; Morigi, M.; Del Fante, C.; Redi, C.A.; Perotti, C. A Novel Method for Isolation of Pluripotent Stem Cells from Human Umbilical Cord Blood. Stem Cells Dev. 2017, 26, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Halasa, M.; Baskiewicz-Masiuk, M.; Dabkowska, E.; Machalinski, B. An efficient two-step method to purify very small embryonic-like (VSEL) stem cells from umbilical cord blood (UCB). Folia Histochem. Cytobiol. 2008, 46, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Chhabria, S.; Jadhav, V.; Bhartiya, D.; Tripathi, A. Stem Cells and Progenitors in Human Peripheral Blood Get Activated by Extremely Active Resveratrol (XARTM). Stem Cell Rev. 2018, 14, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Sovalat, H.; Scrofani, M.; Eidenschenk, A.; Hénon, P. Human Very Small Embryonic-Like Stem Cells Are Present in Normal Peripheral Blood of Young, Middle-Aged, and Aged Subjects. Stem Cells Int. 2016, 2016, 7651645. [Google Scholar] [CrossRef] [PubMed]
- James, K.; Bhartiya, D.; Ganguly, R.; Kaushik, A.; Gala, K.; Singh, P.; Metkari, S.M. Gonadotropin and steroid hormones regulate pluripotent very small embryonic-like stem cells in adult mouse uterine endometrium. J. Ovarian Res. 2018, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Patel, H.; Bhartiya, D. Chemoablated mouse seminiferous tubular cells enriched for very small embryonic-like stem cells undergo spontaneous spermatogenesis in vitro. Reprod. Biol. Endocrinol. 2015, 13, 33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Gao, L.; Zuba-Surma, E.K.; Peng, X.; Kucia, M.; Ratajczak, M.Z.; Wang, W.; Enzmann, V.; Kaplan, H.J.; Dean, D.C. Identification of small Sca-1(+), Lin(−), CD45(−) multipotential cells in the neonatal murine retina. Exp. Hematol. 2009, 37, 1096–1107. [Google Scholar] [CrossRef]
- Nakatsuka, R.; Iwaki, R.; Matsuoka, Y.; Sumide, K.; Kawamura, H.; Fujioka, T.; Sasaki, Y.; Uemura, Y.; Asano, H.; Kwon, A.H.; et al. Identification and Characterization of Lineage(−)CD45(-)Sca-1(+) VSEL Phenotypic Cells Residing in Adult Mouse Bone Tissue. Stem Cells Dev. 2016, 25, 27–42. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Shin, D.M.; Liu, R.; Marlicz, W.; Tarnowski, M.; Ratajczak, J.; Kucia, M. Epiblast/germ line hypothesis of cancer development revisited: Lesson from the presence of Oct-4+ cells in adult tissues. Stem Cell Rev. 2010, 6, 307–316. [Google Scholar] [CrossRef]
- Shin, D.M.; Liu, R.; Klich, I.; Wu, W.; Ratajczak, J.; Kucia, M.; Ratajczak, M.Z. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia 2010, 24, 1450–1461. [Google Scholar] [CrossRef]
- Skirecki, T.; Mikaszewska-Sokolewicz, M.; Godlewska, M.; Dołęgowska, B.; Czubak, J.; Hoser, G.; Kawiak, J.; Zielińska-Borkowska, U. Mobilization of Stem and Progenitor Cells in Septic Shock Patients. Sci. Rep. 2019, 9, 3289. [Google Scholar] [CrossRef] [PubMed]
- Golipoor, Z.; Mehraein, F.; Zafari, F.; Alizadeh, A.; Ababzadeh, S.; Baazm, M. Migration of Bone Marrow-Derived Very Small Embryonic-Like Stem Cells toward An Injured Spinal Cord. Cell J. 2016, 17, 639–647. [Google Scholar]
- Drukala, J.; Paczkowska, E.; Kucia, M.; Mlynska, E.; Krajewski, A.; Machalinski, B.; Madeja, Z.; Ratajczak, M.Z. Stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients after skin burn injury. Stem Cell Rev. 2012, 8, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Marlicz, W.; Zuba-Surma, E.; Kucia, M.; Blogowski, W.; Starzynska, T.; Ratajczak, M.Z. Various types of stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients with Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, A.; Zuba-Surma, E.K.; Ziada, K.M.; Kucia, M.; Cohen, D.A.; Kaplan, A.M.; Van Zant, G.; Selim, S.; Smyth, S.S.; Ratajczak, M.Z. Evidence of mobilization of pluripotent stem cells into peripheral blood of patients with myocardial ischemia. Exp. Hematol. 2010, 38, 1131–1142.e1. [Google Scholar] [CrossRef]
- Wojakowski, W.; Ratajczak, M.Z.; Tendera, M. Mobilization of very small embryonic-like stem cells in acute coronary syndromes and stroke. Herz 2010, 35, 467–472. [Google Scholar] [CrossRef]
- Abouzaripour, M.; Ragerdi Kashani, I.; Pasbakhsh, P.; Atlasy, N. Intravenous transplantation of very small embryonic like stem cells in treatment of diabetes mellitus. Avicenna J. Med. Biotechnol. 2015, 7, 22–31. [Google Scholar]
- Chen, Z.H.; Lv, X.; Dai, H.; Liu, C.; Lou, D.; Chen, R.; Zou, G.M. Hepatic regenerative potential of mouse bone marrow very small embryonic-like stem cells. J. Cell Physiol. 2015, 230, 1852–1861. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, S.H.; Kim, Y.I.; Hwang, S.; Kwon, P.M.; Han, I.S.; Kwon, B.S. Adult stem cells from the hyaluronic acid-rich node and duct system differentiate into neuronal cells and repair brain injury. Stem Cells Dev. 2014, 23, 2831–2840. [Google Scholar] [CrossRef]
- Kassmer, S.H.; Jin, H.; Zhang, P.X.; Bruscia, E.M.; Heydari, K.; Lee, J.H.; Kim, C.F.; Kassmer, S.H.; Krause, D.S. Very small embryonic-like stem cells from murine bone marrow differentiate into epithelial cells of lung. Stem Cells 2013, 31, 2759–2766. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.J.; Qian, H.Y.; Wang, H.; Xu, H. Very small embryonic-like stem cells (VSELs)-a new promising candidate for use in cardiac regeneration. Ageing Res. Rev. 2011, 10, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Zuba-Surma, E.; Kucia, M.; Reca, R.; Wojakowski, W.; Ratajczak, J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia 2006, 20, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; Schneider, G.; Ratajczak, J.; Suszynska, M.; Kucia, M.; Ratajczak, M.Z. The cell cycle- and insulin-signaling-inhibiting miRNA expression pattern of very small embryonic-like stem cells contributes to their quiescent state. Exp. Biol. Med. (Maywood) 2015, 240, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.M.; Zuba-Surma, E.K.; Wu, W.; Ratajczak, J.; Wysoczynski, M.; Ratajczak, M.Z.; Kucia, M. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia 2009, 23, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.; Nagvenkar, P.; Pethe, P.; Hinduja, I.; Bhartiya, D. Molecular and phenotypic characterization of CD133 and SSEA4 enriched very small embryonic-like stem cells in human cord blood. Leukemia 2015, 29, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Mierzejewska, K.; Ratajczak, J.; Kucia, M. CD133 Expression Strongly Correlates with the Phenotype of Very Small Embryonic-/Epiblast-Like Stem Cells. Adv. Exp. Med. Biol. 2013, 777, 125–141. [Google Scholar]
- Klemba, A.; Purzycka-Olewiecka, J.K.; Wcisło, G.; Czarnecka, A.M.; Lewicki, S.; Lesyng, B.; Szczylik, C.; Kieda, C. Surface markers of cancer stem-like cells of ovarian cancer and their clinical relevance. Contemp. Oncol. (Pozn) 2018, 22, 48–55. [Google Scholar] [CrossRef]
- Zhao, L.; Li, J.; Liu, M.; Zhou, H.; Zou, H.; Wei, Y.; Sun, K.; Li, G.; Li, S.; Pang, L. The clinicopathological parameters significance of CD133 and Nestin in epithelial ovarian cancer: A meta-analysis. Future Oncol. 2017, 13, 2555–2570. [Google Scholar] [CrossRef]
- Onisim, A.; Iancu, M.; Vlad, C.; Kubelac, P.; Fetica, B.; Fulop, A.; Achimas-Cadariu, A.; Achimas-Cadariu, P. Expression of Nestin and CD133 in serous ovarian carcinoma. J. BUON. 2016, 21, 1168–1175. [Google Scholar]
- Lee, Y.J.; Wu, C.C.; Li, J.W.; Ou, C.C.; Hsu, S.C.; Tseng, H.H.; Kao, M.C.; Liu, J.Y. A rational approach for cancer stem-like cell isolation and characterization using CD44 and prominin-1(CD133) as selection markers. Oncotarget 2016, 7, 78499–78515. [Google Scholar] [CrossRef][Green Version]
- Virant-Klun, I.; Zech, N.; Rozman, P.; Vogler, A.; Cvjeticanin, B.; Klemenc, P.; Malicev, E.; Meden-Vrtovec, H. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation 2008, 76, 843–856. [Google Scholar] [CrossRef]
- Virant-Klun, I.; Rozman, P.; Cvjeticanin, B.; Vrtacnik-Bokal, E.; Novakovic, S.; Rülicke, T.; Dovc, P.; Meden-Vrtovec, H. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem Cells Dev. 2009, 18, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Skutella, T.; Hren, M.; Gruden, K.; Cvjeticanin, B.; Vogler, A.; Sinkovec, J. Isolation of small SSEA-4-positive putative stem cells from the ovarian surface epithelium of adult human ovaries by two different methods. Biomed. Res. Int. 2013, 2013, 690415. [Google Scholar] [CrossRef] [PubMed]
- Stimpfel, M.; Skutella, T.; Cvjeticanin, B.; Meznaric, M.; Dovc, P.; Novakovic, S.; Cerkovnik, P.; Vrtacnik-Bokal, E.; Virant-Klun, I. Isolation, characterization and differentiation of cells expressing pluripotetnt/multipotent markers from adult human ovaries. Cell Tissue Res. 2013, 354, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I. Functional Testing of Primitive Oocyte-like Cells Developed in Ovarian Surface Epithelium Cell Culture from Small VSEL-like Stem Cells: Can They Be Fertilized One Day ? Stem Cell Rev. 2018, 14, 715–721. [Google Scholar] [CrossRef]
- Virant-Klun, I.; Skutella, T.; Kubista, M.; Vogler, A.; Sinkovec, J.; Meden-Vrtovec, H. Expression of pluripotency and oocyte-related genes in single putative stem cells from human adult ovarian surface epithelium cultured in vitro in the presence of follicular fluid. Biomed. Res. Int. 2013, 2013, 861460. [Google Scholar]
- Parte, S.; Bhartiya, D.; Telang, J.; Daithankar, V.; Salvi, V.; Zaveri, K.; Hinduja, I. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 2011, 20, 1451–1464. [Google Scholar] [CrossRef]
- Silvestris, E.; Cafforio, P.; D’Oronzo, S.; Felici, C.; Silvestris, F.; Loverro, G. In vitro differentiation of human oocyte-like cells from oogonial stem cells: Single-cell isolation and molecular characterization. Hum. Reprod. 2018, 33, 464–473. [Google Scholar] [CrossRef]
- Patel, H.; Bhartiya, D.; Parte, S. Further characterization of adult sheep ovarian stem cells and their involvement in neo-oogenesis and follicle assembly. J. Ovarian Res. 2018, 11, 3. [Google Scholar] [CrossRef]
- Bui, H.T.; Van Thuan, N.; Kwon, D.N.; Choi, Y.J.; Kang, M.H.; Han, J.W.; Kim, T.; Kim, J.H. Identification and characterization of putative stem cells in the adult pig ovary. Development 2014, 141, 2235–2244. [Google Scholar] [CrossRef][Green Version]
- Virant-Klun, I.; Stimpfel, M. Novel population of small tumour-initiating stem cells in the ovaries of women with borderline ovarian cancer. Sci. Rep. 2016, 6, 34730. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Kenda-Suster, N.; Smrkolj, S. Small putative NANOG, SOX2, and SSEA-4-positive stem cells resembling very small embryonic-like stem cells in sections of ovarian tissue in patients with ovarian cancer. J. Ovarian Res. 2016, 9, 12. [Google Scholar] [CrossRef]
- Latifi, A.; Luwor, R.B.; Bilandzic, M.; Nazaretian, S.; Stenvers, K.; Pyman, J.; Zhu, H.; Thompson, E.W.; Quinn, M.A.; Findlay, J.K.; et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: Molecular phenotype of chemoresistant ovarian tumors. PLoS One 2012, 7, e46858. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Djedaini, M.; Hoffman, R. Ex Vivo Expansion of Hematopoietic Stem Cells from Human Umbilical Cord Blood-Derived CD34+ Cells Using Valproic Acid. J. Vis. Exp. 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Al-Sowayan, B.; Keogh, R.J.; Abumaree, M.; Georgiou, H.M.; Kalionis, B. Valproic acid stimulates in vitro migration of the placenta-derived mesenchymal/stromal cell line CMSC29. Stem Cell Investig. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Ren, Q.; Zuo, W.; Jia, R.; Xie, L.; Lin, R.; Zhao, H.; Chen, J.; Lei, Y.; Wang, P.; et al. Valproic acid exhibits anti-tumor activity selectively against EGFR/ErbB2/ErbB3-coexpressing pancreatic cancer via induction of ErbB family members-targeting microRNAs. J. Exp. Clin. Cancer Res. 2019, 38, 150. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, F.; Shen, W.W.; D’Ocon, P.; Romero, A.; Álamo, C. A History of the Pharmacological Treatment of Bipolar Disorder. Int. J. Mol. Sci. 2018, 19, 2143. [Google Scholar] [CrossRef] [PubMed]
- Vatzaki, E.; Straus, S.; Dogne, J.M.; Garcia Burgos, J.; Girard, T.; Martelletti, P. Latest clinical recommendations on valproate use for migraine prophylaxis in women of childbearing age: Overview from European Medicines Agency and European Headache Federation. J. Headache Pain 2018, 19, 68. [Google Scholar] [CrossRef]
- Parte, S.; Bhartiya, D.; Manjramkar, D.D.; Chauhan, A.; Joshi, A. Stimulation of ovarian stem cells by follicle stimulating hormone and basic fibroblast growth factor. J. Ovarian Res. 2013, 6, 20. [Google Scholar] [CrossRef]
- Patel, H.; Bhartiya, D.; Parte, S.; Gunjal, P.; Yedurkar, S.; Bhatt, M. Follicle stimulating hormone modulates ovarian stem cells through alternately spliced receptor variant FSH-R3. J. Ovarian Res. 2013, 6, 52. [Google Scholar] [CrossRef]
- Bhartiya, D.; Sriraman, K.; Gunjal, P.; Modak, H. Gonadotropin treatment augments postnatal oogenesis and primordial follicle assembly in adult mouse ovaries? J. Ovarian Res. 2012, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, Y.L.; McLaughlin, M.; Waterfall, M.; Dunlop, C.E.; Skehel, P.A.; Anderson, R.A.; Telfer, E.E. Initial characterization of adult human ovarian cell populations isolated by DDX4 expression and aldehyde dehydrogenase activity. Sci. Rep. 2018, 8, 6953. [Google Scholar] [CrossRef] [PubMed]
- He, Q.Z.; Luo, X.Z.; Wang, K.; Zhou, Q.; Ao, H.; Yang, Y.; Li, S.X.; Li, Y.; Zhu, H.T.; Duan, T. Isolation and characterization of cancer stem cells from high-grade serous ovarian carcinomas. Cell Physiol. Biochem. 2014, 33, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Hirohashi, Y.; Torigoe, T.; Yasuda, K.; Takahashi, A.; Asanuma, H.; Morita, R.; Mariya, T.; Asano, T.; Mizuuchi, M.; et al. ALDH1-high ovarian cancer stem-like cells can be isolated from serous and clear cell adenocarcinoma cells, and ALDH1 high expression is associated with poor prognosis. PLoS ONE 2013, 8, e65158. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, C.A.; Prinzler, J.; Sinn, B.V.; Budczies, J.; Denkert, C.; Noske, A.; Sehouli, J.; Braicu, E.I.; Dietel, M.; Darb-Esfahani, S. Aldehyde dehydrogenase 1/epidermal growth factor receptor coexpression is characteristic of a highly aggressive, poor-prognosis subgroup of high-grade serous ovarian carcinoma. Hum. Pathol. 2013, 44, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
| Types of Cell Cultures | Statistical Significance | ||||
|---|---|---|---|---|---|
| hESCs | Normal Ovaries | Ascites | Fibroblasts (Negative Control) | ||
| Mean proportion of VSEL-like stem cells/field | 35% ῶ (min. 10%–max. 50%) | 17% * (min. 0%–max. 67%) | 75% *ῶ (min. 33% –max. 75%) | 0% | *ῶ p < 0.0001 |
| Proportion of VSEL-like stem cells/counted cells | 32% ⌘ | 10% υ | 86% υ⌘ | 0% | υ⌘p < 0.01 |
| Cell Cultures | Gene Expression Level (± Standard Deviation) | |
|---|---|---|
| CD133 | DDX4 | |
| Normal ovary | 1.00 ± 0.10 | 1.00 ± 0.16 |
| Ovarian cancer ascites | 8.58 ± 0.80 | 9.55 ± 2.15 |
| hESCs | 12.00 ± 1.21 | 3.61 ± 0.67 |
| HT-19 | 8.20 ± 0.74 | ND |
| Fibroblasts | ND | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virant-Klun, I.; Skerl, P.; Novakovic, S.; Vrtacnik-Bokal, E.; Smrkolj, S. Similar Population of CD133+ and DDX4+ VSEL-Like Stem Cells Sorted from Human Embryonic Stem Cell, Ovarian, and Ovarian Cancer Ascites Cell Cultures: The Real Embryonic Stem Cells? Cells 2019, 8, 706. https://doi.org/10.3390/cells8070706
Virant-Klun I, Skerl P, Novakovic S, Vrtacnik-Bokal E, Smrkolj S. Similar Population of CD133+ and DDX4+ VSEL-Like Stem Cells Sorted from Human Embryonic Stem Cell, Ovarian, and Ovarian Cancer Ascites Cell Cultures: The Real Embryonic Stem Cells? Cells. 2019; 8(7):706. https://doi.org/10.3390/cells8070706
Chicago/Turabian StyleVirant-Klun, Irma, Petra Skerl, Srdjan Novakovic, Eda Vrtacnik-Bokal, and Spela Smrkolj. 2019. "Similar Population of CD133+ and DDX4+ VSEL-Like Stem Cells Sorted from Human Embryonic Stem Cell, Ovarian, and Ovarian Cancer Ascites Cell Cultures: The Real Embryonic Stem Cells?" Cells 8, no. 7: 706. https://doi.org/10.3390/cells8070706
APA StyleVirant-Klun, I., Skerl, P., Novakovic, S., Vrtacnik-Bokal, E., & Smrkolj, S. (2019). Similar Population of CD133+ and DDX4+ VSEL-Like Stem Cells Sorted from Human Embryonic Stem Cell, Ovarian, and Ovarian Cancer Ascites Cell Cultures: The Real Embryonic Stem Cells? Cells, 8(7), 706. https://doi.org/10.3390/cells8070706





