Physiological Adaptation to Simultaneous Chronic Exposure to High-Fat Diet and Dichlorodipheniletylhene (DDE) in Wistar Rat Testis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Lipid Peroxidation
2.3. SOD and GPx Activity Assay
2.4. Electrophoresis and Western Blot Analysis
2.5. Immunohistochemistry
2.6. Quantitative Real-Time PCR Analysis
2.7. Histological Analysis
2.8. Serum Testosterone Levels
2.9. Statistical Analysis
3. Results
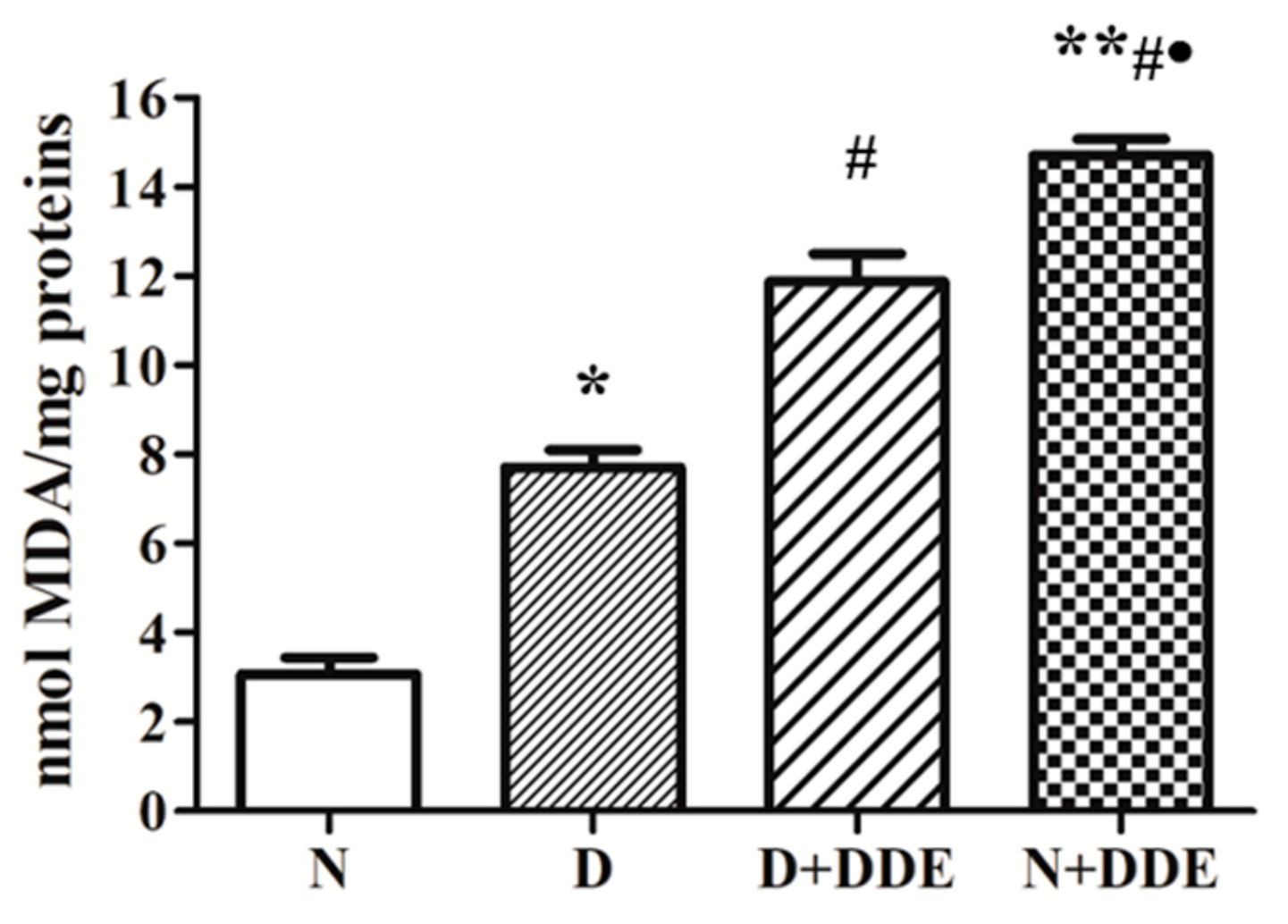
3.1. High-Fat Diet and DDE Induce Testicular Lipid Peroxidation
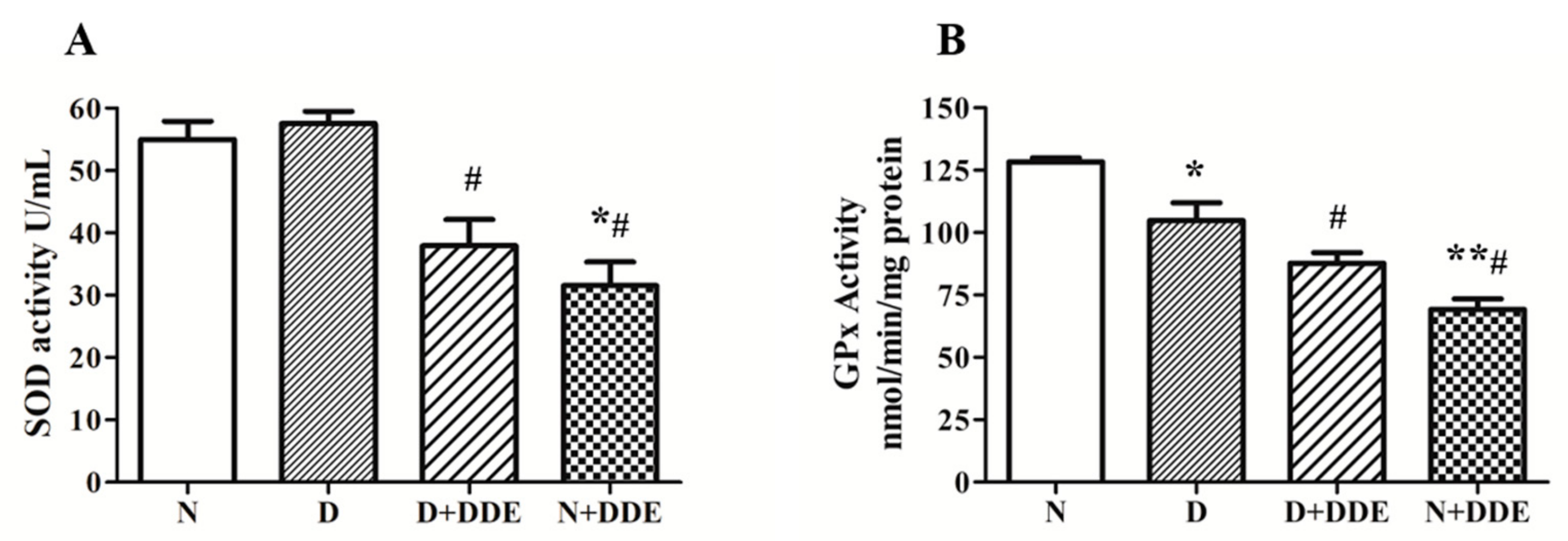
3.2. Modulation of Antioxidant Enzymes Activities: SOD and GPx
3.3. High-Fat Diet and DDE Induce Pro-Apoptotic Stimuli
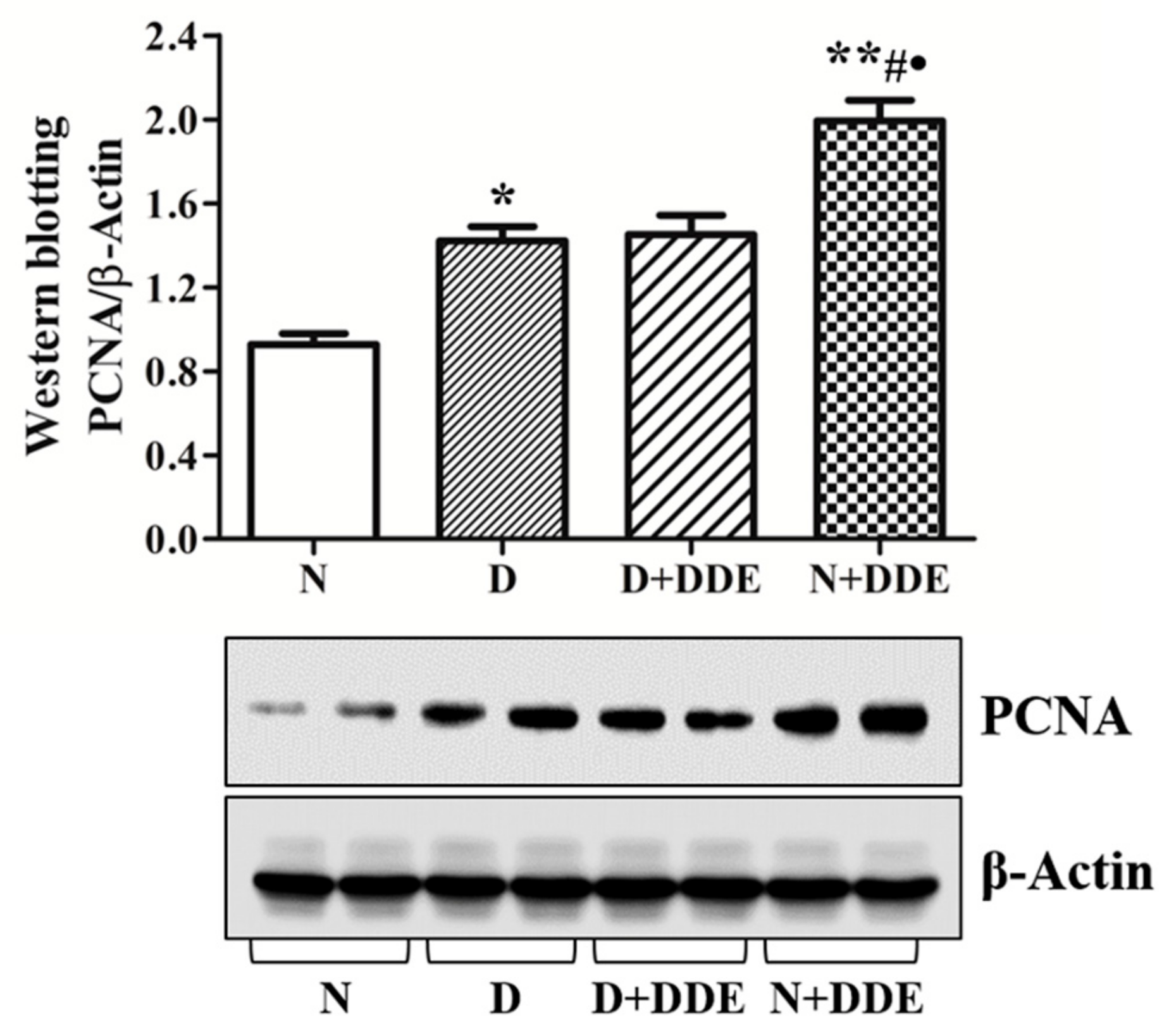
3.4. High-Fat Diet and DDE Induce PCNA Up-Regulation
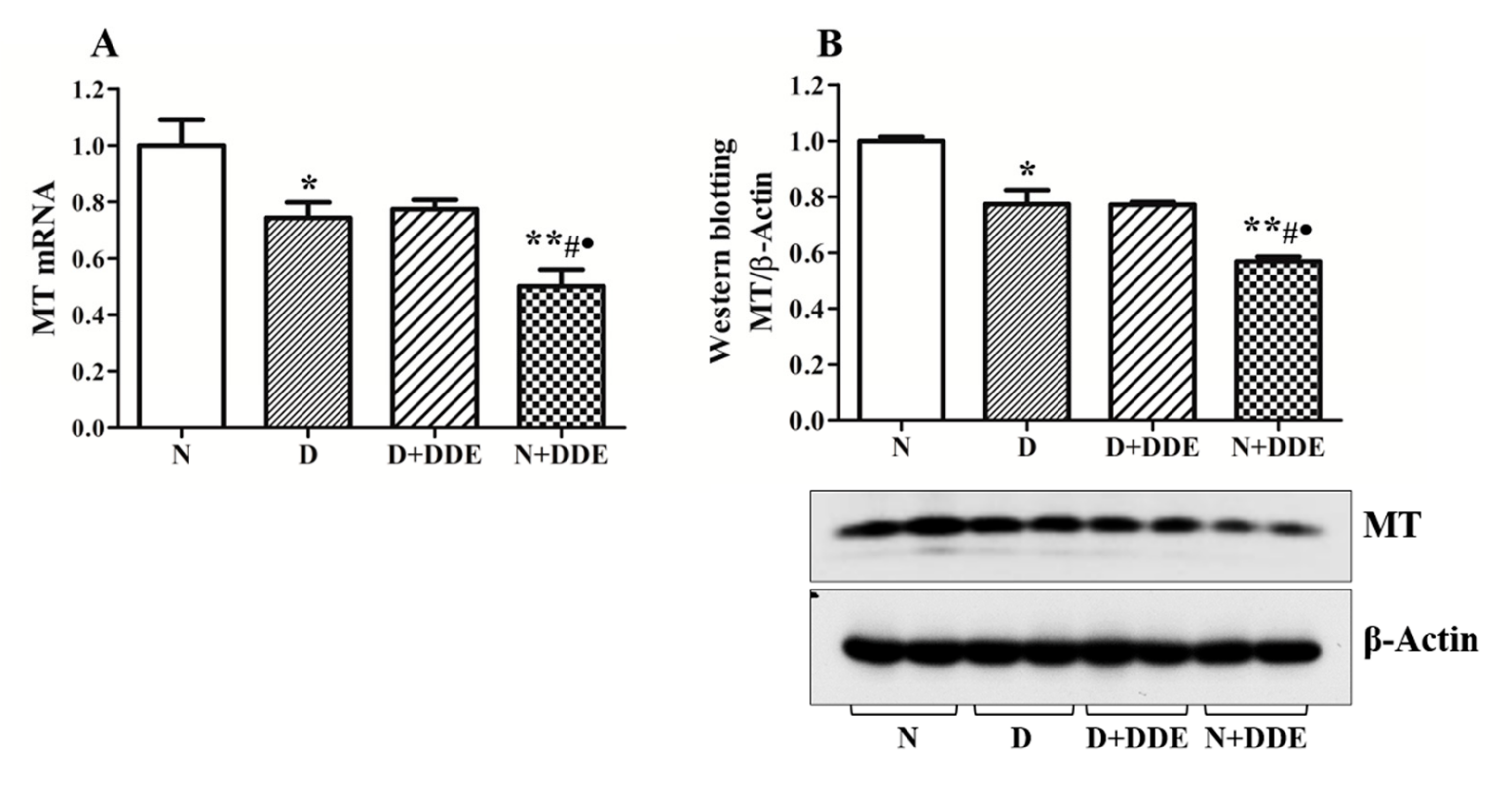
3.5. Changes of Metallothioneins Expression and Synthesis
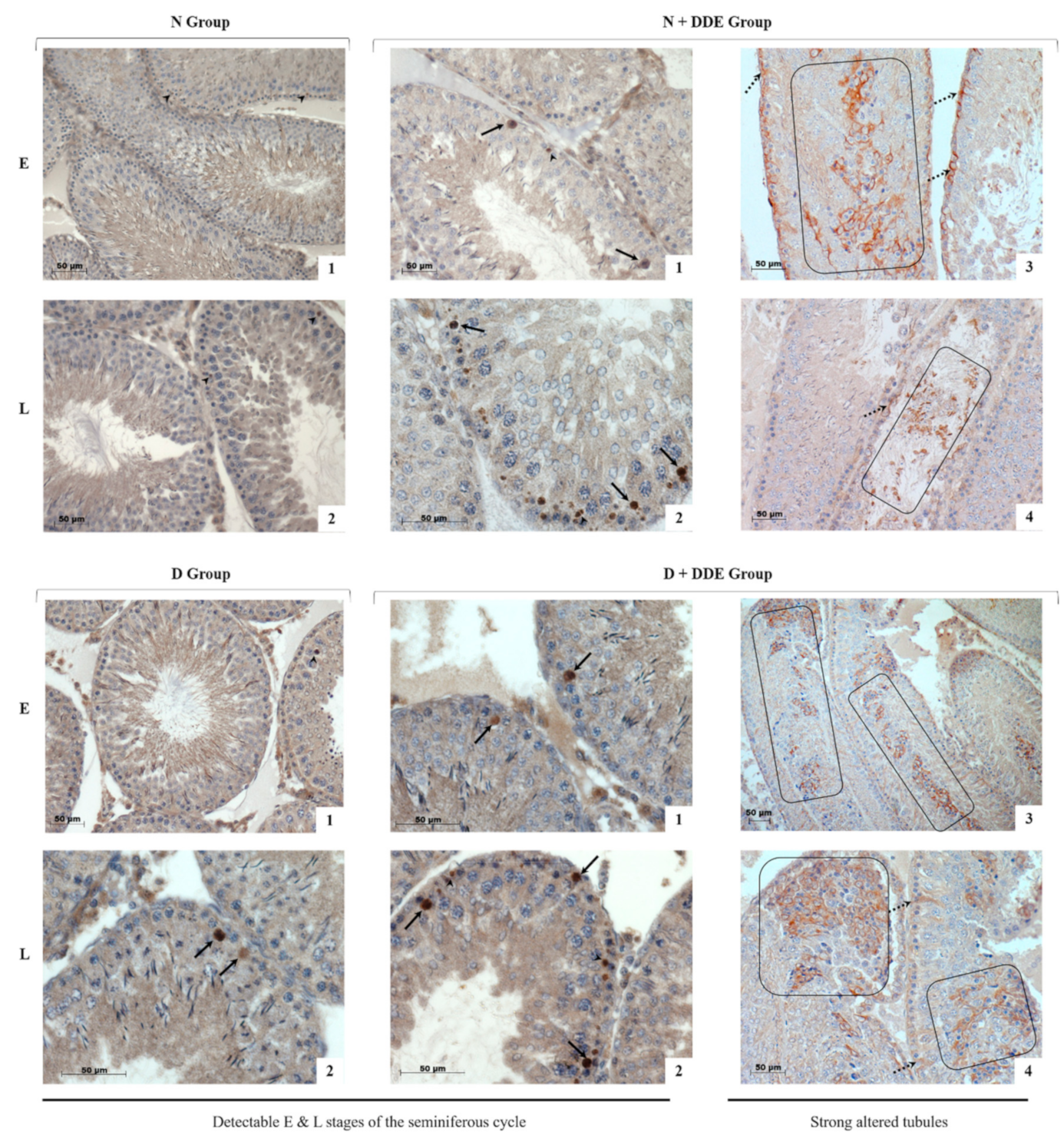
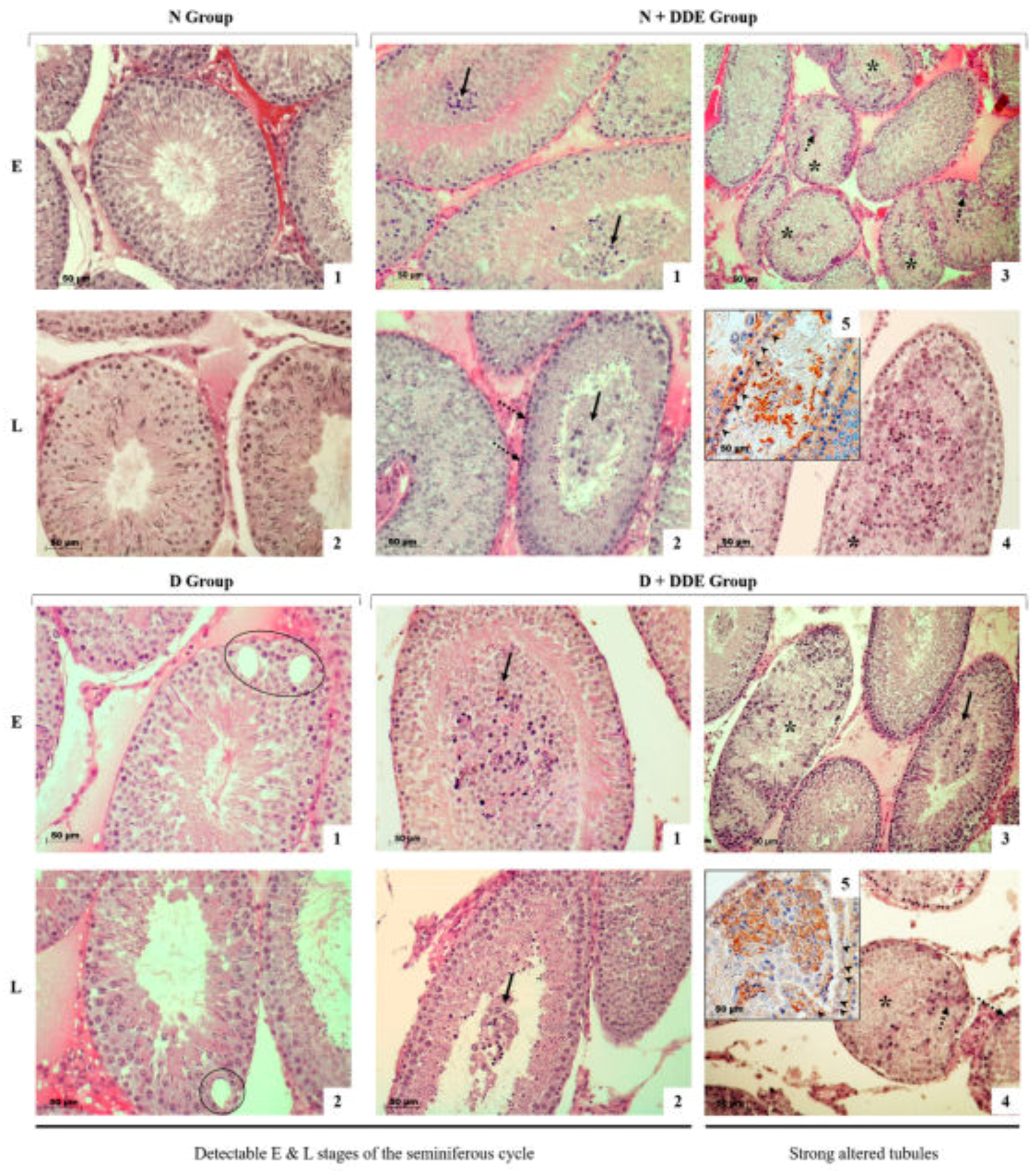
3.6. High-Fat Diet and DDE Induce Alterations in Testis Morphology
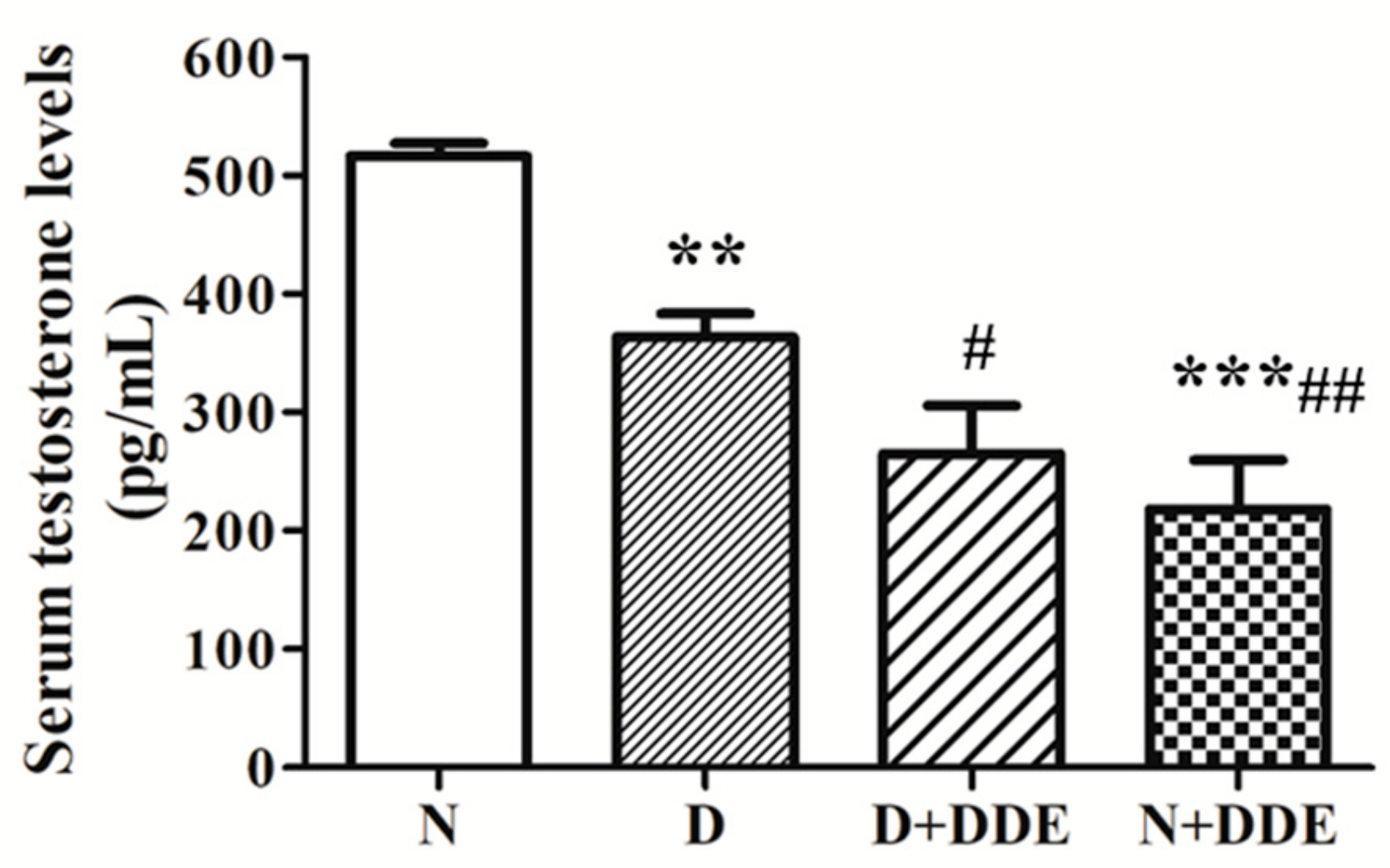
3.7. Serum Testosterone Levels
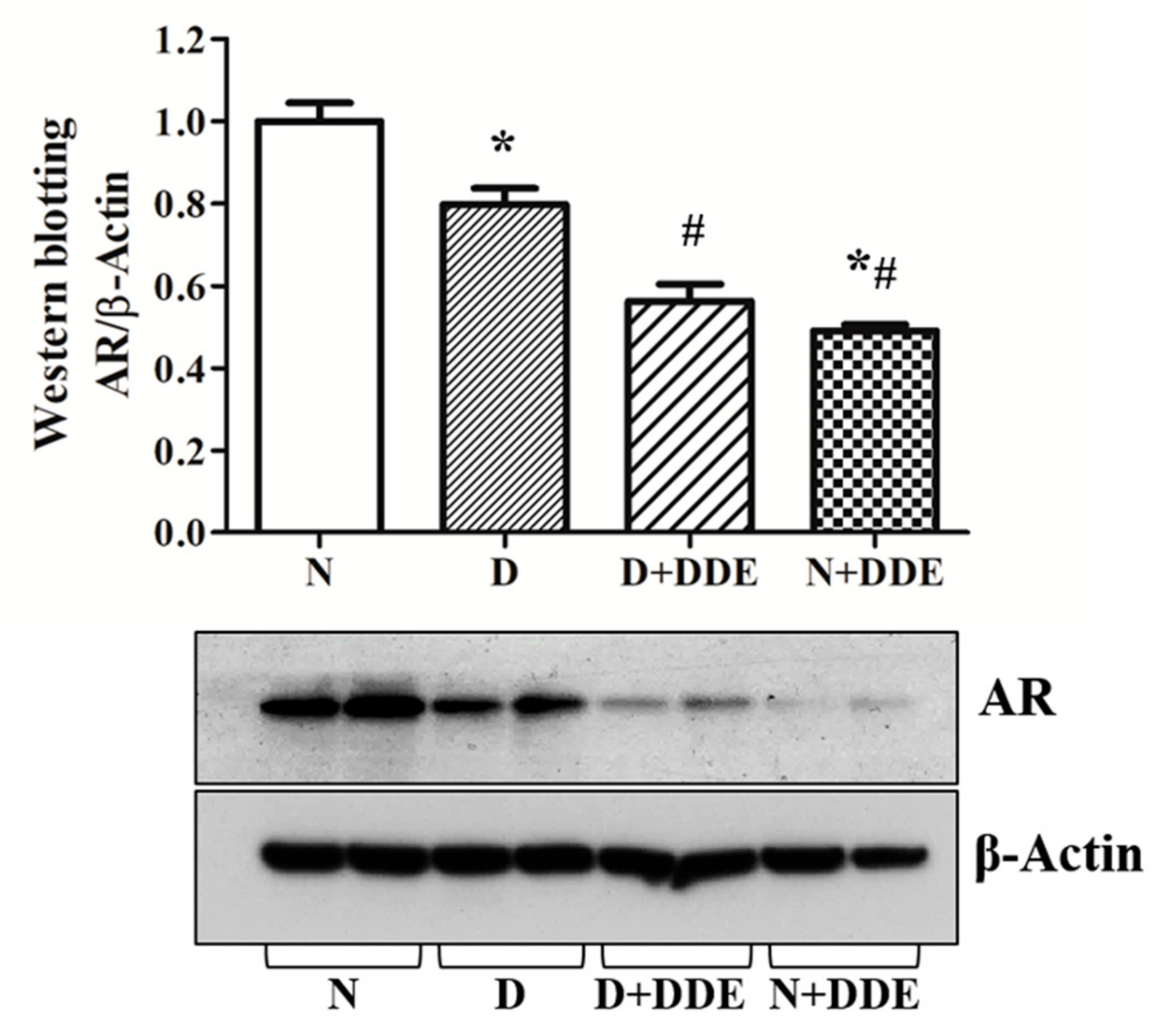
3.8. Androgen Receptor Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chavarro, J.E.; Toth, T.L.; Sadio, S.M.; Hauser, R. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Human Reprod. 2008, 23, 2584–2590. [Google Scholar] [CrossRef]
- Ghanayem, B.I.; Bai, R.; Kissling, G.E.; Travlos, G.; Hoffler, U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol. Reprod. 2010, 82, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Hauser, R. Exposure to Polychlorinated Biphenyls (PCBs) and male reproduction. Syst. Biol. Reprod. Med. 2010, 56, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.Y.; Kyung, M.; Kim, M.J.; Jung, B.Y.; Cho, M.C.; Choi, S.M.; Kim, Y.W.; Lim, S.K.; Lim, D.S.; Won, A.J.; et al. Human Risk Assessment of Endocrine-Disrupting Chemicals Derived from Plastic Food Containers. CRFSFS 2012, 11, 453–470. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Cavaco, J.E.; Oliveira, P.F. High-energy diets: A threat for male fertility? Obes. Rev. 2014, 15, 996–1007. [Google Scholar] [CrossRef]
- Gabrielsen, J.S.; Tanrikut, C. Chronic exposures and male fertility: The impacts of environment, diet, and drug use on spermatogenesis. Andrology 2016, 4, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Heitmann, B.L.; Blomberg, J.M.; Halldorsson, T.I.; Andersson, A.M.; Skakkebæk, N.E.; Joensen, U.N.; Lauritsen, M.P.; Christiansen, P.; Dalgård, C.; et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am. J. Clin. Nutr. 2013, 97, 411–418. [Google Scholar] [CrossRef]
- De Vriese, S.R.; Christophe, A.B. Fatty acid remodelling during sperm maturation: Variation of docosahexaenoic acid content. In Male Fertility and Lipid Metabolism; The American Oil Chemists Society: Urbana, IL, USA, 2003; pp. 96–117. [Google Scholar]
- Dagher, S.M.; Talhouk, R.S.; Nasrallah, S.S.; Tannous, R.I.; Mroueh, S.M. Relationship of dietary intake to DDE residues in breast milk of nursing mothers in Beirut. Food Add. Cont. 1999, 16, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.T.; Lee, T.K.M.; Chen, K.; Wong, H.L.; Zheng, J.S.; Giesy, J.P.; Lo, K.K.W.; Yamashita, N.; Lam, P.K.S. Human health risk assessment of organochlorines associated with fish consumption in a coastal city in China. Environ. Pollut. 2005, 136, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ostby, J.; Monosson, E.; Kelce, W.R.; Gray, L.E. Environmental antiandrogens: Low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol. Ind. Health. 1999, 15, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Spira, A.; Multigner, L. Contribution of environmental factors to the risk of male infertility. Human Reprod. 2001, 16, 1768–1776. [Google Scholar] [CrossRef]
- Sikka, S.C. Relative impact of oxidative stress on male reproductive function. Curr. Med. Chem. 2001, 8, 851–862. [Google Scholar] [CrossRef] [PubMed]
- De Jager, C.; Farias, P.; Barraza-Villarreal, A.; Avila, M.H.; Ayotte, P.; Dewailly, E.; Dombrowski, C.; Rousseau, F.; Sanchez, V.D.; Bailey, J.L. Reduced seminal parameters associated with environmental DDT exposure and p,p′-DDE concentrations in men in Chiapas, Mexico: A cross-sectional study. J. Androl. 2006, 27, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 117, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Streit, B. Bioaccumulation processes in ecosystems. Cell. Mol. Life Sci. 1992, 48, 955–970. [Google Scholar] [CrossRef]
- Jones, K.C.; de Voogt, P. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Quinete, N.; Schettgen, T.; Bertram, J.; Kraus, T. Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 11951–11972. [Google Scholar] [PubMed]
- Bishopp, F.C. Insect Problems in World War II with Special References to the Insecticide DDT. Am. J. Public Health Nations Health 1945, 35, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Blus, L.J.; Wiemeyer, S.N.; Bunck, C.M. Clarification of effects of DDE on shell thickness, size, mass, and shape of avian eggs. Environ. Pollut. 1997, 95, 67–74. [Google Scholar] [CrossRef]
- Tiemann, U. In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: A review. Reprod. Toxicol. 2008, 25, 316–326. [Google Scholar] [CrossRef]
- Norén, K.; Lunden, A.; Pettersson, E.; Bergman, A. Methylsulfonyl metabolites of PCBs and DDE in human milk in Sweden, 1972-1992. Environ. Health Perspect. 1996, 104, 766–772. [Google Scholar] [PubMed]
- Weistrand, C.; Norén, K. Methylsulfonyl metabolites of PCBs and DDE in human tissues. Environ. Health Persp. 1997, 105, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Ropstad, E.; Oskam, I.C.; Lyche, J.L.; Larsen, H.J.; Lie, E.; Haave, M.; Dahl, E.; Wiger, R.; Skaare, J.U. Endocrine disruption induced by organochlorines (OCs): Field studies and experimental models. J. Toxicol. Environ. Health A 2006, 8, 53–76. [Google Scholar] [CrossRef]
- Wang, X.P.; Gong, P.; Yao, T.D.; Jones, K.C. Passive Air Sampling of Organochlorine Pesticides, Polychlorinated Biphenyls, and Polybrominated Diphenyl Ethers Across the Tibetan Plateau. Environ. Sci. Technol. 2010, 44, 2988–2993. [Google Scholar] [CrossRef] [PubMed]
- Rehwagen, R. WHO recommends DDT to control malaria. BMJ 2006, 333, 622. [Google Scholar] [CrossRef]
- Kelce, W.R.; Stone, C.R.; Laws, S.C.; Gray, L.E.; Kemppainen, J.A.; Wilson, E.M. Persistent DDT metabolite p,p’–DDE is a potent androgen receptor antagonist. Nature 1995, 375, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.N.; Tanabe, S.; Tatsukawa, R.; Saito, S.; Miyazaki, N. Reduction in the testosterone levels by PCBs and DDE in Dall’s porpoises of northwestern North Pacific. Mar. Pollut. Bull. 1987, 18, 643–646. [Google Scholar] [CrossRef]
- Daxenberger, A. Pollutants with androgen-disrupting potency. Eur. J. Lipid Sci. Technol. 2002, 104, 124–130. [Google Scholar] [CrossRef]
- Rogan, W.J.; Chen, A. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). Lancet 2005, 366, 763–773. [Google Scholar] [CrossRef]
- Laird, B.D.; Goncharov, A.B.; Chan, H.M. Body burden of metals and persistent organic pollutants among Inuit in the Canadian Arctic. Environ. Int. 2013, 59, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.E., Jr.; Kelce, W.R. Latent effects of pesticides and toxic substances on sexual differentiation of rodents. Toxicol. Ind. Health 1996, 12, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.C.; Sun, H.; Chen, J.F.; Bian, Q.; Song, L.; Wang, X.R. Androgen receptor activities of p,p’-DDE, fenvalerate and phoxim detected by androgen receptor reporter gene assay. Toxicol. Lett. 2006, 160, 151–157. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Li, H.W.; Wang, Y.P.; Liu, C.J.; Yang, K.D. p,p′-DDE induces apoptosis and mRNA expression of apoptosis-associated genes in testes of pubertal rats. Environ. Toxicol. 2013, 28, 31–41. [Google Scholar] [CrossRef]
- Song, Y.; Liang, X.; Hu, Y.; Wang, Y.; Yu, H.; Yang, K. p,p′-DDE induces mitochondria-mediated apoptosis of cultured rat Sertoli cells. Toxicology 2008, 253, 53–61. [Google Scholar] [CrossRef]
- Fiorini, C.; Gilleron, J.; Carette, D.; Valette, A.; Tilloy, A.; Chevalier, S.; Segretain, D.; Pointis, G. Accelerated internalization of junctional membrane proteins (connexin 43, N-cadherin and ZO-1) within endocytic vacuoles: An early event of DDT carcinogenicity. Biochim. Biophys. Acta 2008, 1778, 56–67. [Google Scholar] [CrossRef]
- Tebourbi, O.; Driss, M.R.; Sakly, M.; Rhouma, K.B. Metabolism of DDT in different tissues of young rats. J. Environ. Sci. Health B 2006, 41, 167–176. [Google Scholar] [CrossRef]
- Cetkovic-Cvrlje, M.; Olson, M.; Schindler, B.; Gong, H.K. Exposure to DDT metabolite p,p′-DDE increases autoimmune type 1 diabetes incidence in NOD mouse model. J. Immunotoxicol. 2015, 13, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Salgado, B.; Olivero-Verbel, J.; Guerrero-Castilla, A. Direct effect of p,p′- DDT on mice liver. Braz. J. Pharm. Sci. 2016, 52, 287–298. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Wang, Y.P.; Song, Y.; Li, H.W.; Liu, C.J.; Wu, Z.G.; Yang, K.D. p,p-DDE induces testicular apoptosis in prepubertal rats via the Fas/FasL pathway. Toxicol. Lett. 2010, 193, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, M.P.; Klebanoff, M.A.; Zhou, H.; Brock, J.W. Association between maternal serum concentration of DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet 2001, 14, 110–114. [Google Scholar] [CrossRef]
- Auer, T.; Khoschsorur, G.A.; Rabl, H.; Iberer, F.; Petutschnigg, B.; Wasler, A.; Tscheliessnigg, K.H. Detection of lipid peroxidation products by malondialdehyde (MDA-TBA reaction) in organ transplantation. Transplant. Proc. 1995, 27, 2749–2751. [Google Scholar] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. The enzymatic antioxidant system of human spermatozoa. Adv. Androl. 2014, 2014, 1–15. [Google Scholar]
- Makita, Y.; Matsuura, T.; Ogata, R.; Romero, Y.; Omura, M.; Tanaka, A.; Hirata, M.; Inoue, N. Systemic effects of orally administered p, p’-dde on immature male wistar rats during pubertal period. J. Occup. Health 2003, 45, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Mollica, M.P.; Lionetti, L.; Moreno, M.; Lombardi, A.; De Lange, P.; Antonelli, A.; Lanni, A.; Cavaliere, G.; Barletta, A.; Goglia, F. 3,5-diiodo-l-thyronine, by modulating mitochondrial functions, reverses hepatic fat accumulation in rats fed a high-fat diet. J. Hepatol. 2009, 51, 363–370. [Google Scholar] [CrossRef]
- Makita, Y.; Matsuura, T.; Ogata, R.; Omura, M.; Tanaka, A.; Hirata, M.; Inoue, N. Systemic toxicity of p,p′-DDE in aged male Wistar rats following oral administration. Fukuoka Igaku Zasshi. 2003, 94, 59–65. [Google Scholar]
- Laemmli, V.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Scudiero, R.; Cigliano, L.; Verderame, M. Age-related changes of metallothionein 1/2 and metallothionein 3 expression in rat brain. C. R. Biol. 2017, 340, 13–17. [Google Scholar] [CrossRef]
- Spagnuolo, M.S.; Mollica, M.P.; Maresca, B.; Cavaliere, G.; Cefaliello, C.; Trinchese, G.; Scudiero, R.; Crispino, M.; Cigliano, L. High Fat Diet and Inflammation – Modulation of Haptoglobin Level in Rat Brain. Fronti. Cell. Neurosci. 2015, 9, 1–9. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Leblond, C.P.; Clermont, Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad. Sci. 1952, 55, 548–573. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, V.; Lionetti, L.; Putti, R.; Sica, R.; Scudiero, R. Combined effects of DDE and hyperlipidic diet on metallothionein expression and synthesis in rat tissues. Environ. Toxicol. 2019, 34, 283–293. [Google Scholar] [PubMed]
- Liu, D.; Xu, Y. p53, oxidative stress, and aging. Antioxid. Redox. Signal. 2011, 15, 1669–1678. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct Activation of Bax by p53 Mediates Mitochondrial Membrane Permeabilization and Apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.H.; Mu, Y.; Wang, B.A.; Li, X.L.; Lu, J.M.; Li, J.Y.; Pan, C.Y.; Yanase, T.; Nawata, H. Saturated free fatty acids palmitic acid and stearic acid induce apoptosis by stimulation of ceramide generation in rat testicular Leydig cells. Biochem. Biophys. Res. Commun. 2003, 303, 1002–1007. [Google Scholar] [CrossRef]
- Siddighi, S.; Patton, W.C.; Jacobson, J.D.; King, A.; Chan, P.J. Correlation of sperm parameters with apoptosis assessed by dual fluorescence dna integrity assay. Arch. Androl. 2009, 50, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Franca, L.R.; Avelar, G.F.; Almeida, F.F. Spermatogenesis and sperm transit through the epi-didymis in mammals with emphasis on pigs. Theriogenology 2005, 63, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Shaha, C.; Tripathi, R.; Mishra, D.P. Male germ cell apoptosis: Regulation and biology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1501–1515. [Google Scholar] [CrossRef]
- Lizama, C.; Alfaro, I.; Reyes, J.G.; Moreno, R.D. Up-regulation of CD95 (Apo-1/Fas) is associated with spermatocyte apoptosis during the first round of spermatogenesis in the rat. Apoptosis 2007, 12, 499–512. [Google Scholar] [CrossRef]
- Vidal, J.D.; Whitney, K.M. Morphologic manifestations of testicular and epididymal toxicity. Spermatogenesis 2014, 4, 1–17. [Google Scholar] [CrossRef]
- Creasy, D.M. Pathogenesis of Male Reproductive Toxicity. Toxicol. Pathol. 2001, 29, 64–76. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Xue, K.; Gu, G.; Fan, W.; Xu, Y.; Ding, Z. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS ONE 2015, 10, e0120775. [Google Scholar]
- Arrebola, J.P.; Cuellar, M.; Claure, E.; Quevedo, M.; Antelo, S.R.; Mutch, E.; Ramirez, E.; Fernandez, M.F.; Olea, N.; Mercado, L.A. Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and adipose tissue from Bolivia. Environ. Res. 2012, 112, 40–47. [Google Scholar] [CrossRef]
- Migliaccio, V.; Scudiero, R.; Sica, R.; Lionetti, L.; Putti, R. Oxidative stress and mitochondrial uncoupling protein 2 expression in hepatic steatosis induced by exposure to xenobiotic DDE and high fat diet in male Wistar rats. PLoS ONE 2019, 14, e0215955. [Google Scholar] [CrossRef]
- Marouani, N.; Hallegue, D.; Sakly, M.; Benkhalifa, M.; Rhouma, K.B.; Tebourbi, O. p,p′-DDT induces testicular oxidative stress-induced apoptosis in adult rats. Reprod. Biol. Endocrinol. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, J.; Jin, X.; Li, Z.; Zhao, M.; Liu, W. p,p′-Dichlorodiphenyldichloroethylene induces colorectal adenocarcinoma cell proliferation through oxidative stress. PLoS ONE 2014, 9, e112700. [Google Scholar] [CrossRef]
- Penna-Videau, S.; Bustos-Obregon, E.; Cermeno-Vivas, J.R.; Chirino, D. Malathion affects spermatogenic proliferation in mouse. Int. J. Morphol. 2012, 30, 1399–1407. [Google Scholar] [CrossRef]
- Lebda, M.; Gad, S.; Gaafar, H. Effects of lipoic acid on acrylamide induced testicular damage. Mater. Sociomed. 2014, 26, 208–212. [Google Scholar] [CrossRef]
- Türedi, S.; Yuluğ, E.; Alver, A.; Kutlu, O.; Kahraman, C. Effects of resveratrol on doxorubicin induced testicular damage in rats. Experim. Toxicol. Pathol. 2015, 67, 229–235. [Google Scholar] [CrossRef]
- Fiorini, C.; Tilloy-Ellul, A.; Chevalier, S.; Charuel, C.; Pointis, G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicantsl reprod. Toxicolology 2004, 18, 413–421. [Google Scholar]
- Jandacek, R.J.; Heubi, J.E.; Buckley, D.D.; Khoury, J.C.; Turner, W.E.; Sjödin, A.; Olson, J.R.; Shelton, C.; Helms, K.; Bailey, T.D.; et al. Reduction of the Body Burden of PCBs and DDE by Dietary Intervention in a Randomized Trial. J. Nutr. Biochem. 2014, 25, 483–488. [Google Scholar]
- Pareek, T.K.; Joshi, A.R.; Sanyal, A.; Dighe, R.R. Insights into male germ cell apoptosis due to depletion of gonadotropins caused by GnRH antagonists. Apoptosis 2007, 12, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Danzo, B.J. Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ. Health Perspect. 1997, 105, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Luccio-Camelo, D.C.; Prins, G.S. Disruption of androgen receptor signaling in males by environmental chemicals. J. Steroid Biochem. Mol. Biol. 2011, 127, 74–82. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliaccio, V.; Sica, R.; Scudiero, R.; Simoniello, P.; Putti, R.; Lionetti, L. Physiological Adaptation to Simultaneous Chronic Exposure to High-Fat Diet and Dichlorodipheniletylhene (DDE) in Wistar Rat Testis. Cells 2019, 8, 443. https://doi.org/10.3390/cells8050443
Migliaccio V, Sica R, Scudiero R, Simoniello P, Putti R, Lionetti L. Physiological Adaptation to Simultaneous Chronic Exposure to High-Fat Diet and Dichlorodipheniletylhene (DDE) in Wistar Rat Testis. Cells. 2019; 8(5):443. https://doi.org/10.3390/cells8050443
Chicago/Turabian StyleMigliaccio, Vincenzo, Raffaella Sica, Rosaria Scudiero, Palma Simoniello, Rosalba Putti, and Lillà Lionetti. 2019. "Physiological Adaptation to Simultaneous Chronic Exposure to High-Fat Diet and Dichlorodipheniletylhene (DDE) in Wistar Rat Testis" Cells 8, no. 5: 443. https://doi.org/10.3390/cells8050443
APA StyleMigliaccio, V., Sica, R., Scudiero, R., Simoniello, P., Putti, R., & Lionetti, L. (2019). Physiological Adaptation to Simultaneous Chronic Exposure to High-Fat Diet and Dichlorodipheniletylhene (DDE) in Wistar Rat Testis. Cells, 8(5), 443. https://doi.org/10.3390/cells8050443






