Grb7, a Critical Mediator of EGFR/ErbB Signaling, in Cancer Development and as a Potential Therapeutic Target
Abstract
1. Introduction
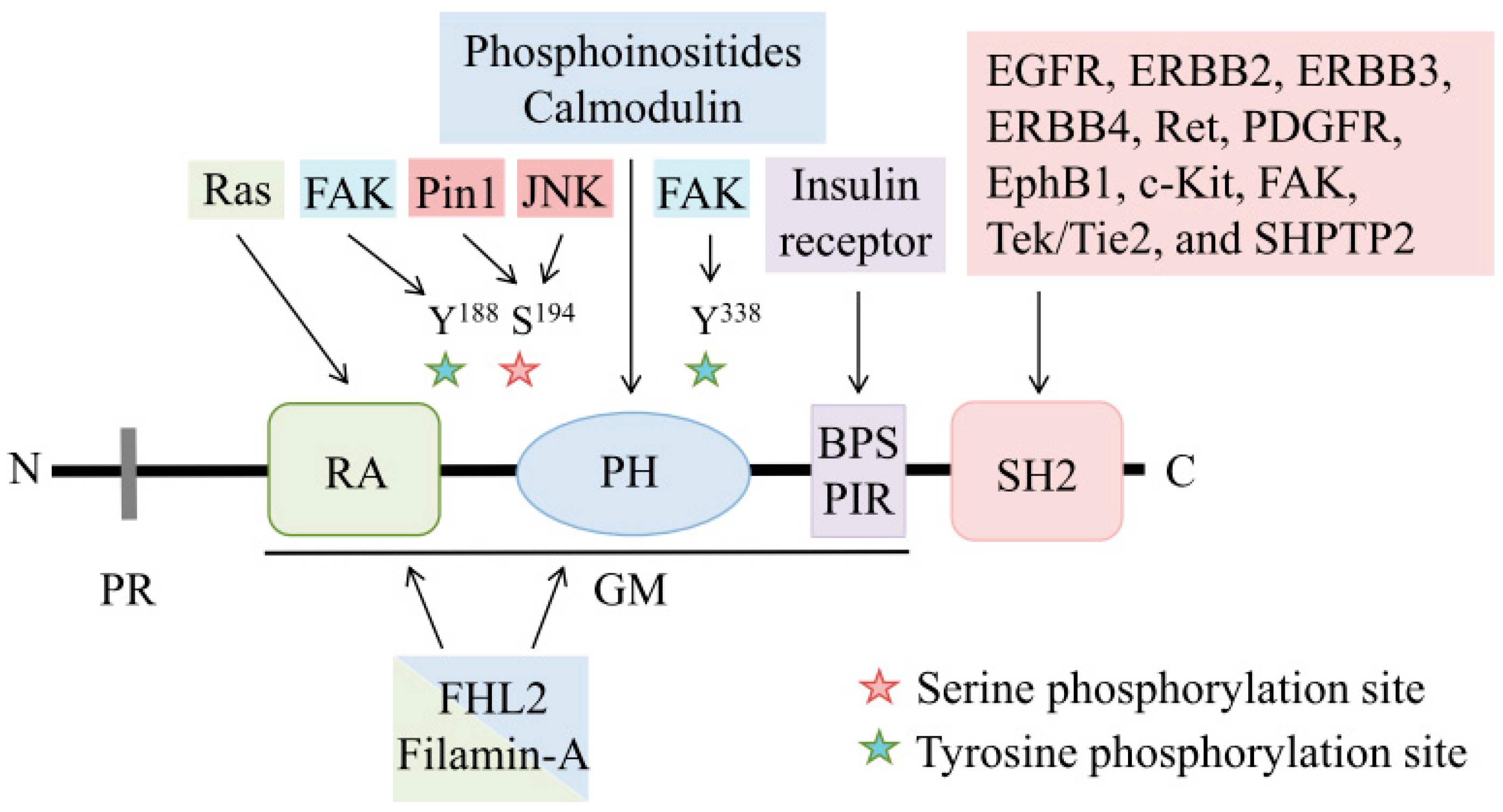
1.1. Structure of Grb7
1.1.1. Proline-Rich Region
1.1.2. GM Region
1.1.3. SH2 Domain
1.2. Regulatory Mechanisms of Grb7
1.2.1. Phosphorylation
1.2.2. Localization
1.2.3. Dimerization
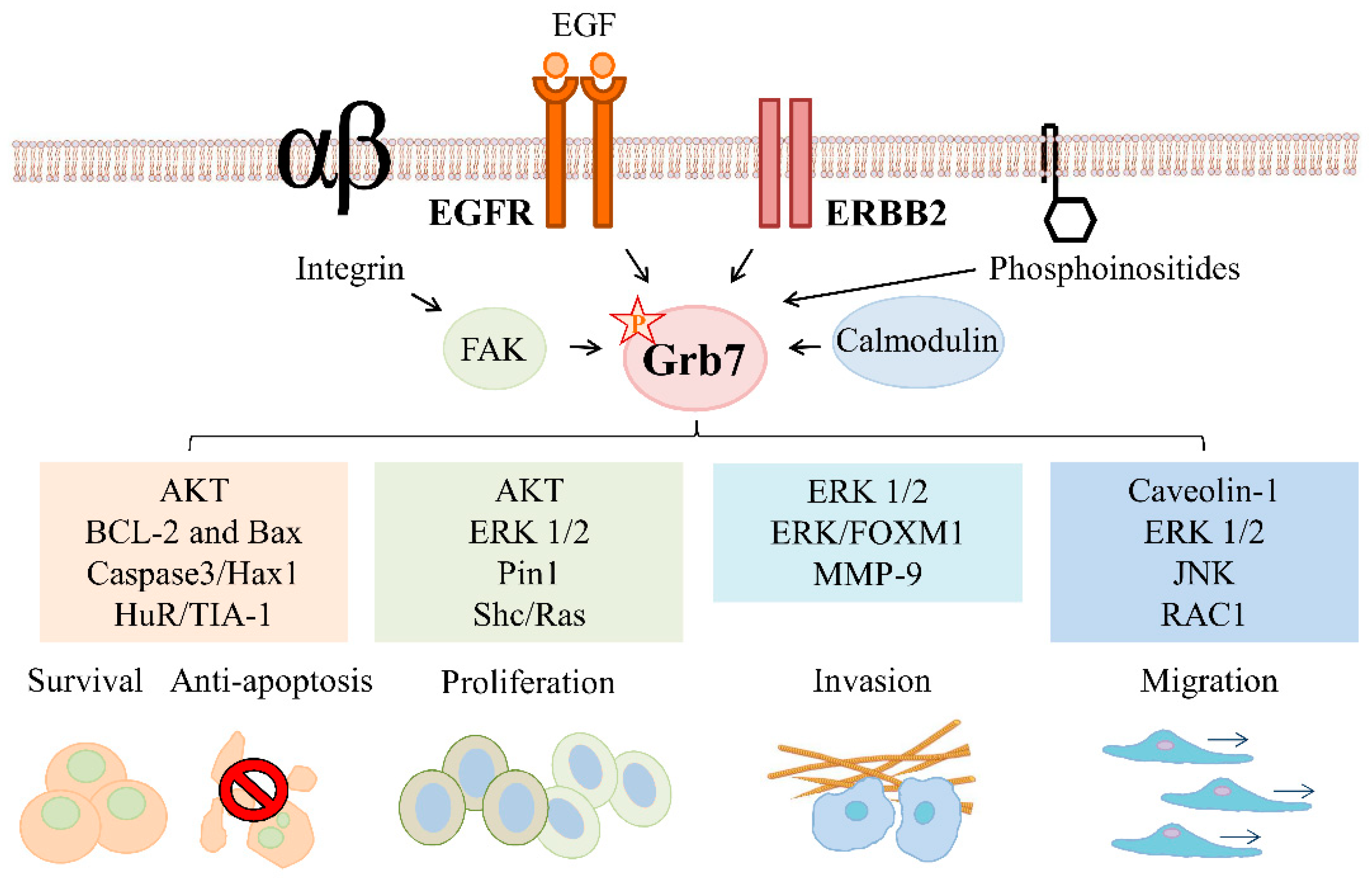
2. Grb7 Signaling in ERBB Family-Mediated Cancer Development
2.1. Grb7 Signaling in ERBB Family-Mediated Cancer Survival and Proliferation
2.1.1. Grb7 Signaling in ERBB Family-Mediated Cancer Survival
2.1.2. Grb7 Signaling in ERBB Family-Mediated Cancer Proliferation
2.2. Grb7 Signaling in ERBB Family-Mediated Cancer Migration, Invasion, and Metastasis
2.2.1. Grb7 Signaling in ERBB Family-Mediated Cancer Migration
2.2.2. Grb7 Signaling in ERBB Family-Mediated Cancer Invasion and Metastasis
3. Cooverexpression and Coamplification of the Grb7 and ERBB Family in Clinical Applications
3.1. Grb7 and Coamplification/Cooverexpression of Grb7 and the ERBB Family in Clinical Studies
3.2. Grb7 as a Prognostic Marker in Cancer
3.3. Grb7 as a Therapeutic Target in Cancer
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Margolis, B.; Silvennoinen, O.; Comoglio, F.; Roonprapunt, C.; Skolnik, E.; Ullrich, A.; Schlessinger, J. High-efficiency expression/cloning of epidermal growth factor-receptor-binding proteins with Src homology 2 domains. Proc. Natl. Acad. Sci. USA 1992, 89, 8894–8898. [Google Scholar] [CrossRef]
- Lucas-Fernandez, E.; Garcia-Palmero, I.; Villalobo, A. Genomic organization and control of the grb7 gene family. Curr. Genomics 2008, 9, 60–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shen, T.L.; Guan, J.L. Grb7 in intracellular signaling and its role in cell regulation. Front. Biosci. 2004, 9, 192–200. [Google Scholar] [CrossRef]
- Han, D.C.; Shen, T.L.; Guan, J.L. The Grb7 family proteins: Structure, interactions with other signaling molecules and potential cellular functions. Oncogene 2001, 20, 6315–6321. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.Y.; Li, T.K.; Ding, S.T.; Lai, I.R.; Shen, T.L. EGF-induced Grb7 recruits and promotes Ras activity essential for the tumorigenicity of Sk-Br3 breast cancer cells. J. Biol. Chem. 2010, 285, 29279–29285. [Google Scholar] [CrossRef]
- Tanaka, S.; Pero, S.C.; Taguchi, K.; Shimada, M.; Mori, M.; Krag, D.N.; Arii, S. Specific peptide ligand for Grb7 signal transduction protein and pancreatic cancer metastasis. J. Natl. Cancer Inst. 2006, 98, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Pero, S.C.; Oligino, L.; Daly, R.J.; Soden, A.L.; Liu, C.; Roller, P.P.; Li, P.; Krag, D.N. Identification of novel non-phosphorylated ligands, which bind selectively to the SH2 domain of Grb7. J. Biol. Chem. 2002, 277, 11918–11926. [Google Scholar] [CrossRef]
- Stein, D.; Wu, J.; Fuqua, S.A.; Roonprapunt, C.; Yajnik, V.; D’Eustachio, P.; Moskow, J.J.; Buchberg, A.M.; Osborne, C.K.; Margolis, B. The SH2 domain protein GRB-7 is co-amplified, overexpressed and in a tight complex with HER2 in breast cancer. EMBO J. 1994, 13, 1331–1340. [Google Scholar] [CrossRef]
- Kauraniemi, P.; Barlund, M.; Monni, O.; Kallioniemi, A. New amplified and highly expressed genes discovered in the ERBB2 amplicon in breast cancer by cDNA microarrays. Cancer Res. 2001, 61, 8235–8240. [Google Scholar]
- Varis, A.; Wolf, M.; Monni, O.; Vakkari, M.L.; Kokkola, A.; Moskaluk, C.; Frierson, H., Jr.; Powell, S.M.; Knuutila, S.; Kallioniemi, A.; et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002, 62, 2625–2629. [Google Scholar]
- Bivin, W.W.; Yergiyev, O.; Bunker, M.L.; Silverman, J.F.; Krishnamurti, U. GRB7 Expression and Correlation with HER2 Amplification in Invasive Breast Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 553–558. [Google Scholar] [CrossRef]
- Frantz, J.D.; Giorgetti-Peraldi, S.; Ottinger, E.A.; Shoelson, S.E. Human GRB-IRbeta/GRB10. Splice variants of an insulin and growth factor receptor-binding protein with PH and SH2 domains. J. Biol. Chem. 1997, 272, 2659–2667. [Google Scholar] [CrossRef] [PubMed]
- Manser, J.; Roonprapunt, C.; Margolis, B.C. elegans cell migration gene mig-10 shares similarities with a family of SH2 domain proteins and acts cell nonautonomously in excretory canal development. Dev. Biol. 1997, 184, 150–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manser, J.; Wood, W.B. Mutations Affecting Embryonic-Cell Migrations in Caenorhabditis-Elegans. Dev. Genet. 1990, 11, 49–64. [Google Scholar] [CrossRef]
- Wojcik, J.; Girault, J.A.; Labesse, G.; Chomilier, J.; Mornon, J.P.; Callebaut, I. Sequence analysis identifies a ras-associating (RA)-like domain in the N-termini of band 4.1/JEF domains and in the Grb7/10/14 adapter family. Biochem. Biophys. Res. Commun. 1999, 259, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Siamakpour-Reihani, S.; Argiros, H.J.; Wilmeth, L.J.; Haas, L.L.; Peterson, T.A.; Johnson, D.L.; Shuster, C.B.; Lyons, B.A. The cell migration protein Grb7 associates with transcriptional regulator FHL2 in a Grb7 phosphorylation-dependent manner. J. Mol. Recognit. 2009, 22, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, P.; Shrestha, S.; Madanayake, T.; Shuster, C.B.; Rohrschneider, L.R.; Rowland, A.; Lyons, B.A. Grb7 and Filamin-a associate and are colocalized to cell membrane ruffles upon EGF stimulation. J. Mol. Recognit. 2013, 26, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Ferguson, K.M.; Abrams, C.S. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 2002, 513, 71–76. [Google Scholar] [CrossRef]
- Shen, T.L.; Han, D.C.; Guan, J.L. Association of Grb7 with phosphoinositides and its role in the regulation of cell migration. J. Biol. Chem. 2002, 277, 29069–29077. [Google Scholar] [CrossRef]
- Li, H.; Sanchez-Torres, J.; del Carpio, A.F.; Nogales-Gonzalez, A.; Molina-Ortiz, P.; Moreno, M.J.; Torok, K.; Villalobo, A. The adaptor Grb7 is a novel calmodulin-binding protein: Functional implications of the interaction of calmodulin with Grb7. Oncogene 2005, 24, 4206–4219. [Google Scholar] [CrossRef][Green Version]
- Kasus-Jacobi, A.; Bereziat, V.; Perdereau, D.; Girard, J.; Burnol, A.F. Evidence for an interaction between the insulin receptor and Grb7. A role for two of its binding domains, PIR and SH2. Oncogene 2000, 19, 2052–2059. [Google Scholar] [CrossRef]
- He, W.; Rose, D.W.; Olefsky, J.M.; Gustafson, T.A. Grb10 interacts differentially with the insulin receptor, insulin-like growth factor I receptor, and epidermal growth factor receptor via the Grb10 Src homology 2 (SH2) domain and a second novel domain located between the pleckstrin homology and SH2 domains. J. Biol. Chem. 1998, 273, 6860–6867. [Google Scholar]
- Kasus-Jacobi, A.; Perdereau, D.; Auzan, C.; Clauser, E.; Van Obberghen, E.; Mauvais-Jarvis, F.; Girard, J.; Burnol, A.F. Identification of the rat adapter Grb14 as an inhibitor of insulin actions. J. Biol. Chem. 1998, 273, 26026–26035. [Google Scholar] [CrossRef]
- Stein, E.G.; Gustafson, T.A.; Hubbard, S.R. The BPS domain of Grb10 inhibits the catalytic activity of the insulin and IGF1 receptors. FEBS Lett. 2001, 493, 106–111. [Google Scholar] [CrossRef]
- Depetris, R.S.; Hu, J.; Gimpelevich, I.; Holt, L.J.; Daly, R.J.; Hubbard, S.R. Structural basis for inhibition of the insulin receptor by the adaptor protein Grb14. Mol. Cell 2005, 20, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Janes, P.W.; Lackmann, M.; Church, W.B.; Sanderson, G.M.; Sutherland, R.L.; Daly, R.J. Structural determinants of the interaction between the erbB2 receptor and the Src homology 2 domain of Grb7. J. Biol. Chem. 1997, 272, 8490–8497. [Google Scholar] [CrossRef] [PubMed]
- Fiddes, R.J.; Campbell, D.H.; Janes, P.W.; Sivertsen, S.P.; Sasaki, H.; Wallasch, C.; Daly, R.J. Analysis of Grb7 recruitment by heregulin-activated erbB receptors reveals a novel target selectivity for erbB3. J. Biol. Chem. 1998, 273, 7717–7724. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Liu, X.; Dixon, J.E.; Di Fiore, P.P.; Dixit, V.M. Direct association between the Ret receptor tyrosine kinase and the Src homology 2-containing adapter protein Grb7. J. Biol. Chem. 1996, 271, 10607–10610. [Google Scholar] [CrossRef]
- Yokote, K.; Margolis, B.; Heldin, C.H.; Claesson-Welsh, L. Grb7 is a downstream signaling component of platelet-derived growth factor alpha- and beta-receptors. J. Biol. Chem. 1996, 271, 30942–30949. [Google Scholar] [CrossRef]
- Han, D.C.; Shen, T.L.; Miao, H.; Wang, B.; Guan, J.L. EphB1 associates with Grb7 and regulates cell migration. J. Biol. Chem. 2002, 277, 45655–45661. [Google Scholar] [CrossRef]
- Thommes, K.; Lennartsson, J.; Carlberg, M.; Ronnstrand, L. Identification of Tyr-703 and Tyr-936 as the primary association sites for Grb2 and Grb7 in the c-Kit/stem cell factor receptor. Biochem. J. 1999, 341 Pt 1, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Han, D.C.; Guan, J.L. Association of focal adhesion kinase with Grb7 and its role in cell migration. J. Biol. Chem. 1999, 274, 24425–24430. [Google Scholar] [CrossRef]
- Jones, N.; Master, Z.; Jones, J.; Bouchard, D.; Gunji, Y.; Sasaki, H.; Daly, R.; Alitalo, K.; Dumont, D.J. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J. Biol. Chem. 1999, 274, 30896–30905. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.; Cooper, J.A. Use of the two-hybrid system to detect the association of the protein-tyrosine-phosphatase, SHPTP2, with another SH2-containing protein, Grb7. Oncogene 1996, 12, 1537–1544. [Google Scholar] [PubMed]
- Tanaka, S.; Mori, M.; Akiyoshi, T.; Tanaka, Y.; Mafune, K.; Wands, J.R.; Sugimachi, K. A novel variant of human Grb7 is associated with invasive esophageal carcinoma. J. Clin. Invest 1998, 102, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chan, D.W.; Liu, V.W.; Chiu, P.; Ngan, H.Y. Differential functions of growth factor receptor-bound protein 7 (GRB7) and its variant GRB7v in ovarian carcinogenesis. Clin. Cancer Res. 2010, 16, 2529–2539. [Google Scholar] [CrossRef]
- Tanaka, S.; Sugimachi, K.; Kawaguchi, H.; Saeki, H.; Ohno, S.; Wands, J.R. Grb7 signal transduction protein mediates metastatic progression of esophageal carcinoma. J. Cell Physiol. 2000, 183, 411–415. [Google Scholar] [CrossRef]
- Han, D.C.; Shen, T.L.; Guan, J.L. Role of Grb7 targeting to focal contacts and its phosphorylation by focal adhesion kinase in regulation of cell migration. J. Biol. Chem. 2000, 275, 28911–28917. [Google Scholar] [CrossRef]
- Chu, P.Y.; Huang, L.Y.; Hsu, C.H.; Liang, C.C.; Guan, J.L.; Hung, T.H.; Shen, T.L. Tyrosine phosphorylation of growth factor receptor-bound protein-7 by focal adhesion kinase in the regulation of cell migration, proliferation, and tumorigenesis. J. Biol. Chem. 2009, 284, 20215–20226. [Google Scholar] [CrossRef]
- Siamakpour-Reihani, S.; Peterson, T.A.; Bradford, A.M.; Argiros, H.J.; Haas, L.L.; Lor, S.N.; Haulsee, Z.M.; Spuches, A.M.; Johnson, D.L.; Rohrschneider, L.R.; et al. Grb7 binds to Hax-1 and undergoes an intramolecular domain association that offers a model for Grb7 regulation. J. Mol. Recognit. 2011, 24, 314–321. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. BioMed Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef]
- Tai, Y.L.; Tung, L.H.; Lin, Y.C.; Lu, P.J.; Chu, P.Y.; Wang, M.Y.; Huang, W.P.; Chen, K.C.; Lee, H.; Shen, T.L. Grb7 Protein Stability Modulated by Pin1 in Association with Cell Cycle Progression. PLoS ONE 2016, 11, e0163617. [Google Scholar] [CrossRef]
- Tsai, N.P.; Ho, P.C.; Wei, L.N. Regulation of stress granule dynamics by Grb7 and FAK signalling pathway. EMBO J. 2008, 27, 715–726. [Google Scholar] [CrossRef]
- Garcia-Palmero, I.; Villalobo, A. Calmodulin regulates the translocation of Grb7 into the nucleus. FEBS Lett. 2012, 586, 1533–1539. [Google Scholar] [CrossRef]
- Tsai, N.P.; Lin, Y.L.; Tsui, Y.C.; Wei, L.N. Dual action of epidermal growth factor: Extracellular signal-stimulated nuclear-cytoplasmic export and coordinated translation of selected messenger RNA. J. Cell Biol. 2010, 188, 325–333. [Google Scholar] [CrossRef]
- Porter, C.J.; Wilce, M.C.; Mackay, J.P.; Leedman, P.; Wilce, J.A. Grb7-SH2 domain dimerisation is affected by a single point mutation. Eur. Biophys. J. 2005, 34, 454–460. [Google Scholar] [CrossRef]
- Porter, C.J.; Matthews, J.M.; Mackay, J.P.; Pursglove, S.E.; Schmidberger, J.W.; Leedman, P.J.; Pero, S.C.; Krag, D.N.; Wilce, M.C.; Wilce, J.A. Grb7 SH2 domain structure and interactions with a cyclic peptide inhibitor of cancer cell migration and proliferation. BMC Struct. Biol. 2007, 7, 58. [Google Scholar] [CrossRef]
- Peterson, T.A.; Benallie, R.L.; Bradford, A.M.; Pias, S.C.; Yazzie, J.; Lor, S.N.; Haulsee, Z.M.; Park, C.K.; Johnson, D.L.; Rohrschneider, L.R.; et al. Dimerization in the Grb7 protein. J. Mol. Recognit. 2012, 25, 427–434. [Google Scholar] [CrossRef]
- Zhao, H.B.; Zhang, X.F.; Jia, X.L.; Wang, H.B. Grb7 is over-expressed in cervical cancer and facilitate invasion and inhibit apoptosis in cervical cancer cells. Pathol. Res. Pract. 2017, 213, 1180–1184. [Google Scholar] [CrossRef]
- Giricz, O.; Calvo, V.; Pero, S.C.; Krag, D.N.; Sparano, J.A.; Kenny, P.A. GRB7 is required for triple-negative breast cancer cell invasion and survival. Breast Cancer Res. Treat. 2012, 133, 607–615. [Google Scholar] [CrossRef]
- Krisenko, M.O.; Higgins, R.L.; Ghosh, S.; Zhou, Q.; Trybula, J.S.; Wang, W.H.; Geahlen, R.L. Syk Is Recruited to Stress Granules and Promotes Their Clearance through Autophagy. J. Biol. Chem. 2015, 290, 27803–27815. [Google Scholar] [CrossRef]
- Qian, L.; Bradford, A.M.; Cooke, P.H.; Lyons, B.A. Grb7 and Hax1 may colocalize partially to mitochondria in EGF-treated SKBR3 cells and their interaction can affect Caspase3 cleavage of Hax1. J. Mol. Recognit. 2016, 29, 318–333. [Google Scholar] [CrossRef]
- Sahlberg, K.K.; Hongisto, V.; Edgren, H.; Makela, R.; Hellstrom, K.; Due, E.U.; Moen Vollan, H.K.; Sahlberg, N.; Wolf, M.; Borresen-Dale, A.L.; et al. The HER2 amplicon includes several genes required for the growth and survival of HER2 positive breast cancer cells. Mol. Oncol. 2013, 7, 392–401. [Google Scholar] [CrossRef]
- Pradip, D.; Bouzyk, M.; Dey, N.; Leyland-Jones, B. Dissecting GRB7-mediated signals for proliferation and migration in HER2 overexpressing breast tumor cells: GTP-ase rules. Am. J. Cancer Res. 2013, 3, 173–195. [Google Scholar]
- Lim, R.C.; Price, J.T.; Wilce, J.A. Context-dependent role of Grb7 in HER2+ve and triple-negative breast cancer cell lines. Breast Cancer Res. Treat. 2014, 143, 593–603. [Google Scholar] [CrossRef]
- Bai, T.; Luoh, S.W. GRB-7 facilitates HER-2/Neu-mediated signal transduction and tumor formation. Carcinogenesis 2008, 29, 473–479. [Google Scholar] [CrossRef][Green Version]
- Kao, J.; Pollack, J.R. RNA interference-based functional dissection of the 17q12 amplicon in breast cancer reveals contribution of coamplified genes. Genes Chromosomes Cancer 2006, 45, 761–769. [Google Scholar] [CrossRef]
- Haran, M.; Chebatco, S.; Flaishon, L.; Lantner, F.; Harpaz, N.; Valinsky, L.; Berrebi, A.; Shachar, I. Grb7 expression and cellular migration in chronic lymphocytic leukemia: A comparative study of early and advanced stage disease. Leukemia 2004, 18, 1948–1950. [Google Scholar] [CrossRef]
- Chan, D.W.; Hui, W.W.; Cai, P.C.; Liu, M.X.; Yung, M.M.; Mak, C.S.; Leung, T.H.; Chan, K.K.; Ngan, H.Y. Targeting GRB7/ERK/FOXM1 signaling pathway impairs aggressiveness of ovarian cancer cells. PLoS ONE 2012, 7, e52578. [Google Scholar] [CrossRef]
- Lee, H.; Volonte, D.; Galbiati, F.; Iyengar, P.; Lublin, D.M.; Bregman, D.B.; Wilson, M.T.; Campos-Gonzalez, R.; Bouzahzah, B.; Pestell, R.G.; et al. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: Identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol. Endocrinol. 2000, 14, 1750–1775. [Google Scholar] [CrossRef]
- Tanaka, S.; Mori, M.; Akiyoshi, T.; Tanaka, Y.; Mafune, K.; Wands, J.R.; Sugimachi, K. Coexpression of Grb7 with epidermal growth factor receptor or Her2/erbB2 in human advanced esophageal carcinoma. Cancer Res. 1997, 57, 28–31. [Google Scholar]
- Priedigkeit, N.; Hartmaier, R.J.; Chen, Y.; Vareslija, D.; Basudan, A.; Watters, R.J.; Thomas, R.; Leone, J.P.; Lucas, P.C.; Bhargava, R.; et al. Intrinsic Subtype Switching and Acquired ERBB2/HER2 Amplifications and Mutations in Breast Cancer Brain Metastases. JAMA Oncol. 2017, 3, 666–671. [Google Scholar] [CrossRef]
- Nadler, Y.; Gonzalez, A.M.; Camp, R.L.; Rimm, D.L.; Kluger, H.M.; Kluger, Y. Growth factor receptor-bound protein-7 (Grb7) as a prognostic marker and therapeutic target in breast cancer. Ann. Oncol. 2010, 21, 466–473. [Google Scholar] [CrossRef]
- Walch, A.; Specht, K.; Braselmann, H.; Stein, H.; Siewert, J.R.; Hopt, U.; Hofler, H.; Werner, M. Coamplification and coexpression of GRB7 and ERBB2 is found in high grade intraepithelial neoplasia and in invasive Barrett’s carcinoma. Int. J. Cancer 2004, 112, 747–753. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, R.N.; Song, K.; Jeon, S.; Jeong, H.M.; Kim, J.S.; Han, J.; Hong, S.; Oh, E.; Choi, J.S.; et al. Genes co-amplified with ERBB2 or MET as novel potential cancer-promoting genes in gastric cancer. Oncotarget 2017, 8, 92209–92226. [Google Scholar] [CrossRef]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Perez, E.A.; Olson, J.A., Jr.; et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2015, 373, 2005–2014. [Google Scholar] [CrossRef]
- Ramsey, B.; Bai, T.; Hanlon Newell, A.; Troxell, M.; Park, B.; Olson, S.; Keenan, E.; Luoh, S.W. GRB7 protein over-expression and clinical outcome in breast cancer. Breast Cancer Res. Treat. 2011, 127, 659–669. [Google Scholar] [CrossRef]
- Sparano, J.A.; Goldstein, L.J.; Childs, B.H.; Shak, S.; Brassard, D.; Badve, S.; Baehner, F.L.; Bugarini, R.; Rowley, S.; Perez, E.A.; et al. Relationship between quantitative GRB7 RNA expression and recurrence after adjuvant anthracycline chemotherapy in triple-negative breast cancer. Clin. Cancer Res. 2011, 17, 7194–7203. [Google Scholar] [CrossRef]
- Saito, M.; Kato, Y.; Ito, E.; Fujimoto, J.; Ishikawa, K.; Doi, A.; Kumazawa, K.; Matsui, A.; Takebe, S.; Ishida, T.; et al. Expression screening of 17q12-21 amplicon reveals GRB7 as an ERBB2-dependent oncogene. FEBS Lett. 2012, 586, 1708–1714. [Google Scholar] [CrossRef]
- Paik, S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. Oncologist 2007, 12, 631–635. [Google Scholar] [CrossRef]
- Watson, G.M.; Lucas, W.A.H.; Gunzburg, M.J.; Wilce, J.A. Insight into the Selectivity of the G7-18NATE Inhibitor Peptide for the Grb7-SH2 Domain Target. Front. Mol. Biosci. 2017, 4, 64. [Google Scholar] [CrossRef]
- Pero, S.C.; Shukla, G.S.; Cookson, M.M.; Flemer, S., Jr.; Krag, D.N. Combination treatment with Grb7 peptide and Doxorubicin or Trastuzumab (Herceptin) results in cooperative cell growth inhibition in breast cancer cells. Br. J. Cancer 2007, 96, 1520–1525. [Google Scholar] [CrossRef]
- Gunzburg, M.J.; Ambaye, N.D.; Del Borgo, M.P.; Perlmutter, P.; Wilce, J.A. Design and Testing of Bicyclic Inhibitors of Grb7-Are Two Cycles Better Than One? Biopolymers 2013, 100, 543–549. [Google Scholar] [CrossRef]
- Gunzburg, M.J.; Kulkarni, K.; Watson, G.M.; Ambaye, N.D.; Del Borgo, M.P.; Brandt, R.; Pero, S.C.; Perlmutter, P.; Wilce, M.C.; Wilce, J.A. Unexpected involvement of staple leads to redesign of selective bicyclic peptide inhibitor of Grb7. Sci. Rep. 2016, 6, 27060. [Google Scholar] [CrossRef]
- Watson, G.M.; Kulkarni, K.; Sang, J.; Ma, X.; Gunzburg, M.J.; Perlmutter, P.; Wilce, M.C.J.; Wilce, J.A. Discovery, Development, and Cellular Delivery of Potent and Selective Bicyclic Peptide Inhibitors of Grb7 Cancer Target. J. Med. Chem. 2017, 60, 9349–9359. [Google Scholar] [CrossRef]
- Watson, G.M.; Kulkarni, K.; Brandt, R.; Del Borgo, M.P.; Aguilar, M.I.; Wilce, J.A. Shortened Penetratin Cell-Penetrating Peptide Is Insufficient for Cytosolic Delivery of a Grb7 Targeting Peptide. ACS Omega 2017, 2, 670–677. [Google Scholar] [CrossRef]
- Ambaye, N.D.; Lim, R.C.C.; Clayton, D.J.; Gunzburg, M.J.; Price, J.T.; Pero, S.C.; Krag, D.N.; Wilce, M.C.J.; Aguilar, M.I.; Perlmutter, P.; et al. Uptake of a Cell Permeable G7-18NATE Construct Into Cells and Binding With the Grb7-SH2 Domain. Biopolymers 2011, 96, 181–188. [Google Scholar] [CrossRef]
- Chen, K.; Liu, M.X.; Mak, C.S.; Yung, M.M.; Leung, T.H.; Xu, D.; Ngu, S.F.; Chan, K.K.; Yang, H.; Ngan, H.Y.; et al. Methylation-associated silencing of miR-193a-3p promotes ovarian cancer aggressiveness by targeting GRB7 and MAPK/ERK pathways. Theranostics 2018, 8, 423–436. [Google Scholar] [CrossRef]
- Luoh, S.W.; Wagoner, W.; Wang, X.; Hu, Z.; Lai, X.; Chin, K.; Sears, R.; Ramsey, E. GRB7 dependent proliferation of basal-like, HER-2 positive human breast cancer cell lines is mediated in part by HER-1 signaling. Mol. Carcinog. 2019. [Google Scholar] [CrossRef]
- Rexer, B.N.; Arteaga, C.L. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: Mechanisms and clinical implications. Crit. Rev. Oncog. 2012, 17, 1–16. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, P.-Y.; Tai, Y.-L.; Shen, T.-L. Grb7, a Critical Mediator of EGFR/ErbB Signaling, in Cancer Development and as a Potential Therapeutic Target. Cells 2019, 8, 435. https://doi.org/10.3390/cells8050435
Chu P-Y, Tai Y-L, Shen T-L. Grb7, a Critical Mediator of EGFR/ErbB Signaling, in Cancer Development and as a Potential Therapeutic Target. Cells. 2019; 8(5):435. https://doi.org/10.3390/cells8050435
Chicago/Turabian StyleChu, Pei-Yu, Yu-Ling Tai, and Tang-Long Shen. 2019. "Grb7, a Critical Mediator of EGFR/ErbB Signaling, in Cancer Development and as a Potential Therapeutic Target" Cells 8, no. 5: 435. https://doi.org/10.3390/cells8050435
APA StyleChu, P.-Y., Tai, Y.-L., & Shen, T.-L. (2019). Grb7, a Critical Mediator of EGFR/ErbB Signaling, in Cancer Development and as a Potential Therapeutic Target. Cells, 8(5), 435. https://doi.org/10.3390/cells8050435





