Analysis of Genetic Alterations in Tunisian Patients with Lung Adenocarcinoma
Abstract
1. Introduction
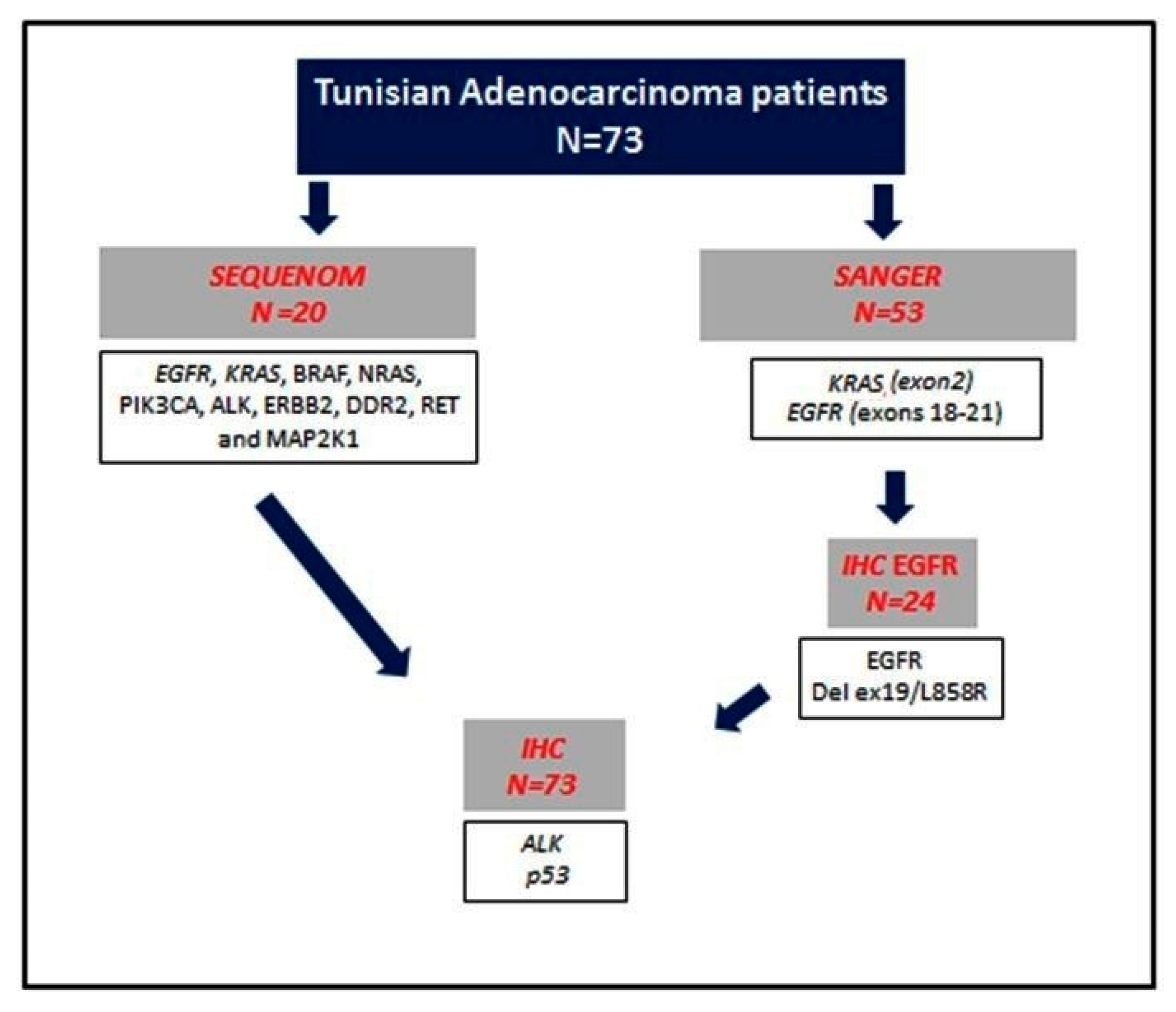
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Molecular Analysis
2.2.1. DNA Extraction
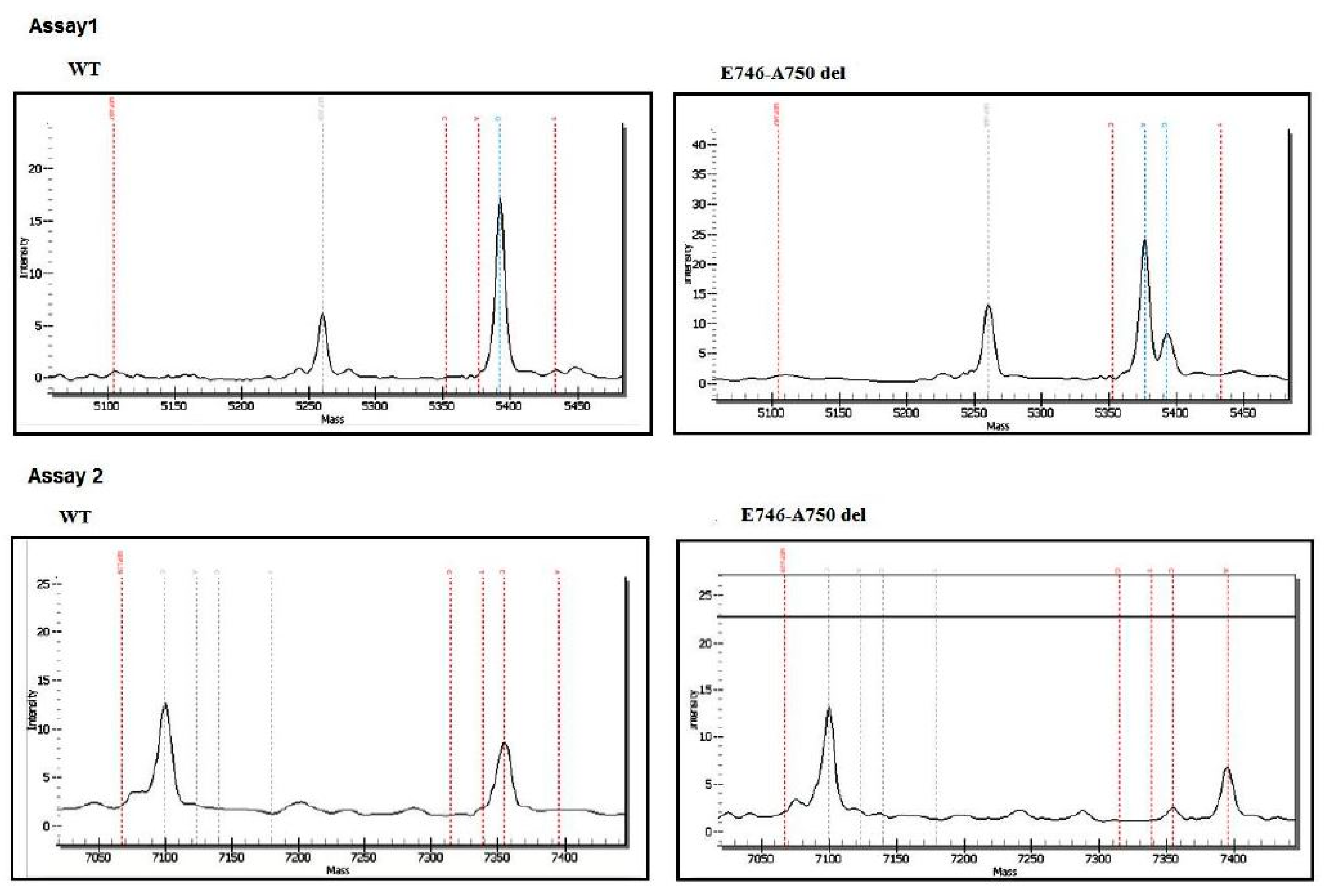
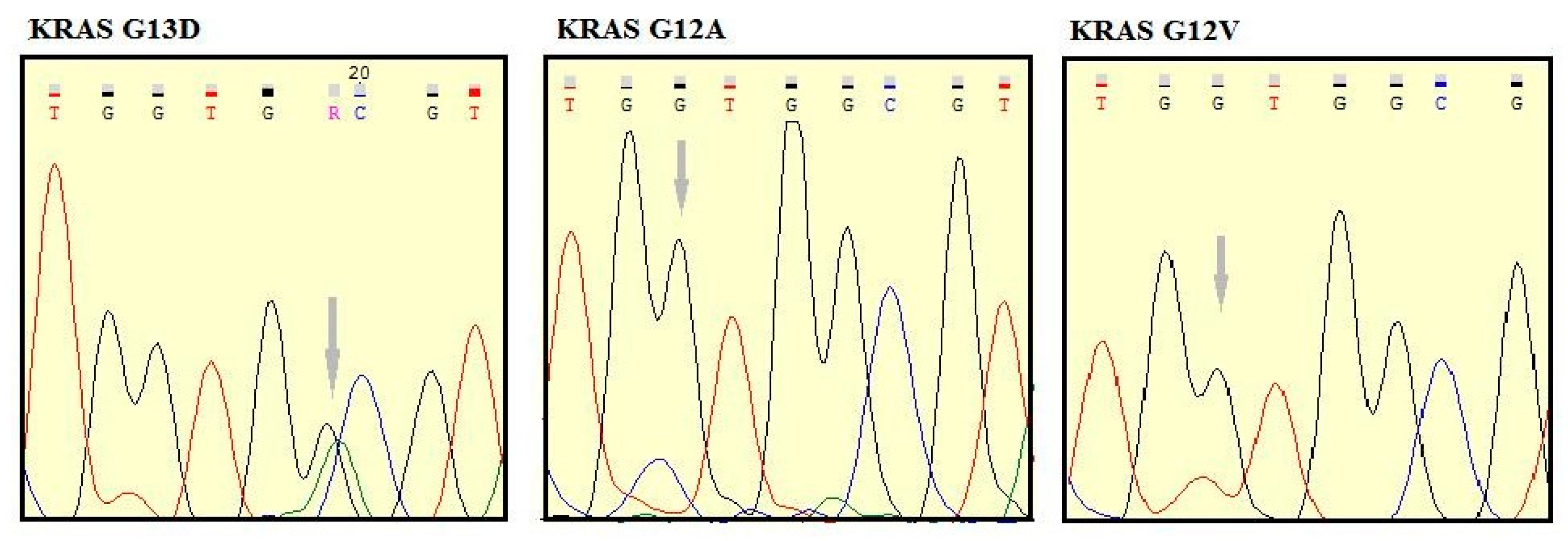
2.2.2. Mutation Analysis
2.3. Immunohistochemistry
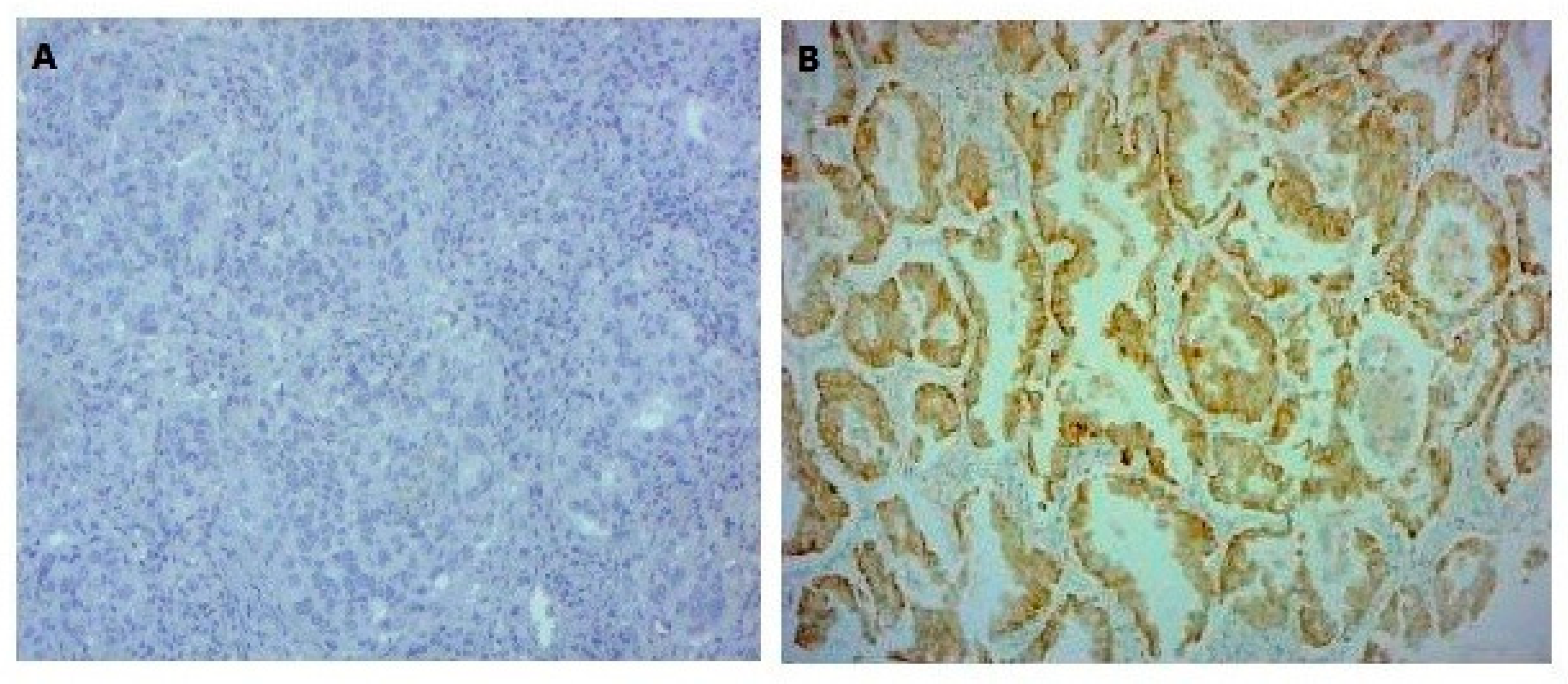
2.3.1. EGFR Mutation-Specific Immunohistochemistry
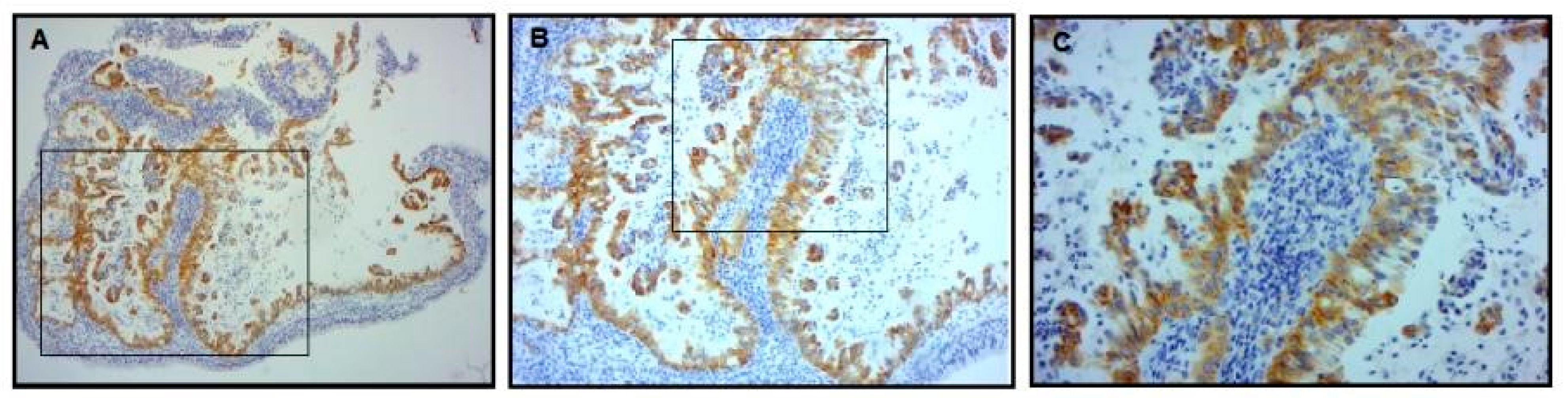
2.3.2. ALK Expression Analysis
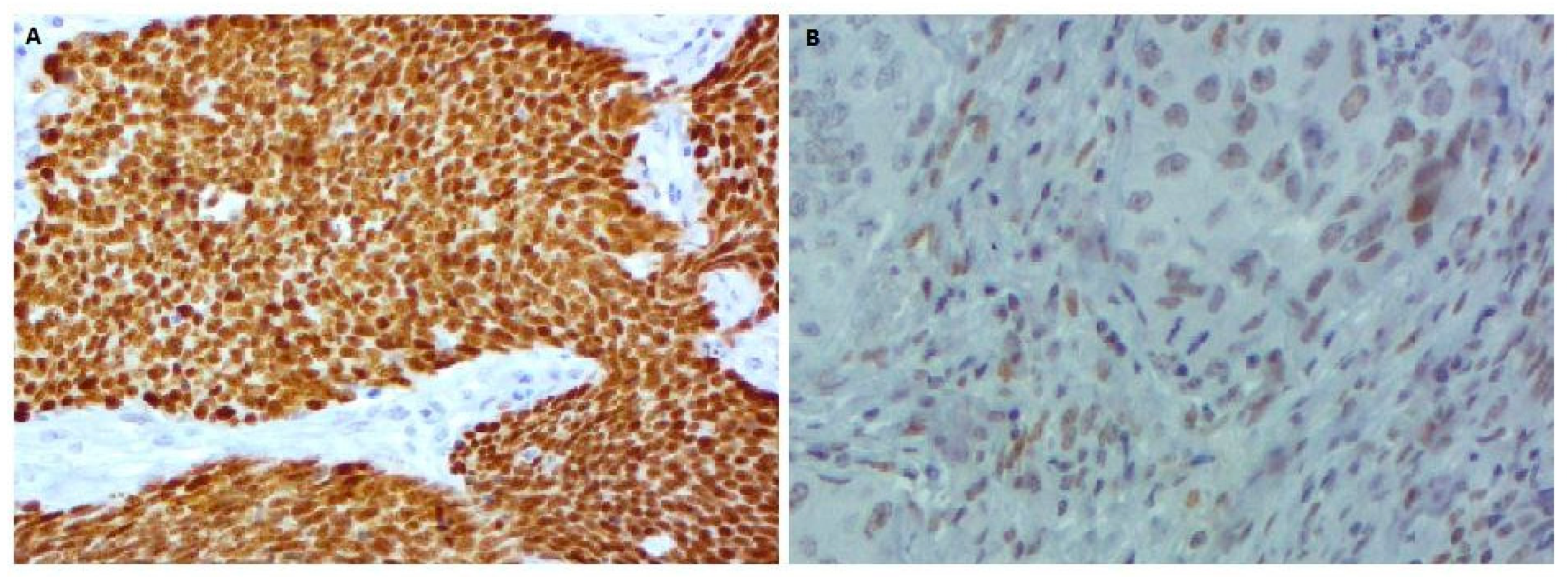
2.3.3. p53 Expression Analysis
2.4. Compliance with Ethical Standards and Informed Consent
3. Results
3.1. Patient Characteristics
3.2. EGFR and KRAS Analysis
3.3. ALK and p53 Protein Expression Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALK | anaplastic lymphoma kinase |
| EGFR | epidermal growth factor receptor |
| FFPE | paraffin-embedded |
| IHC | immunohistochemistry |
| KRAS | kirsten rat sarcoma |
| LAD | lung adenocarcinoma |
| NSCLC | non-small cell lung cancer |
| PCR | polymerase chain reaction |
| TKIs | tyrosine kinase inhibitors |
References
- Pao, W.; Girard, N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011, 12, 175–180. [Google Scholar] [CrossRef]
- Author Correction: Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 514, 262. [CrossRef]
- Marchetti, A.; Martella, C.; Felicioni, L.; Barassi, F.; Salvatore, S.; Chella, A.; Camplese, P.P.; Iarussi, T.; Mucilli, F.; Mezzetti, A.; et al. EGFR mutations in non-small-cell lung cancer: Analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 2005, 23, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Matsuoka, M.; Sutani, A.; Gemma, A.; Maemondo, M.; Inoue, A.; Okinaga, S.; Nagashima, M.; Oizumi, S.; Uematsu, K.; et al. Frequency of and variables associated with the EGFR mutation and its subtypes. Int. J. Cancer 2010, 126, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M. Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Choi, S.-J.; Ho Cho, J.; Joo Choi, H.; Lee, J.; Jung, K.; Irwin, D.; Liu, X.; Lira, M.E.; Mao, M.; et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget 2015, 6, 5465–5474. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Yang, J.C.H.; Wu, Y.L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Hu, C.P.; O’Byrne, K.; Feng, J.; et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.I.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Inamura, K.; Takeuchi, K.; Togashi, Y.; Nomura, K.; Ninomiya, H.; Okui, M.; Satoh, Y.; Okumura, S.; Nakagawa, K.; Soda, M.; et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J. Thorac. Oncol. 2008, 3, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Yang, X.; Yang, J.; Zhou, Q.; Yin, L.; An, S.; Lin, J.; Chen, S.; Xie, Z.; et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol. Cancer 2010, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; VanderLaan, P.A.; Folch, E.; Boucher, D.H.; Canepa, H.M.; Kent, M.S.; Gangadharan, S.P.; Majid, A.; Kocher, O.N.; Goldstein, M.A.; et al. Smoking status and self-reported race affect the frequency of clinically relevant oncogenic alterations in non-small-cell lung cancers at a United States-based academic medical practice. Lung Cancer 2013, 82, 31–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaw, A.T.; Kim, D.-W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.-J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus Chemotherapy in Advanced ALK -Positive Lung Cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.M.; Gandhi, L.; Riely, G.J.; Chiappori, A.A.; West, H.L.; Azada, M.C.; Morcos, P.N.; Lee, R.M.; Garcia, L.; Yu, L.; et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014, 15, 1119–1128. [Google Scholar] [CrossRef]
- Bauml, J.; Mick, R.; Zhang, Y.; Watt, C.D.; Vachani, A.; Aggarwal, C.; Evans, T.; Langer, C. Frequency of EGFR and KRAS mutations in patients with non small cell lung cancer by racial background: Do disparities exist? Lung Cancer 2013, 81, 347–353. [Google Scholar] [CrossRef]
- Rouquette, I.; Lauwers-Cances, V.; Allera, C.; Brouchet, L.; Milia, J.; Nicaise, Y.; Laurent, J.; Delisle, M.B.; Favre, G.; Didier, A.; et al. Characteristics of lung cancer in women: Importance of hormonal and growth factors. Lung Cancer 2012, 76, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Varghese, A.M.; Ou, S.H.I.; Kabraji, S.; Awad, M.M.; Katayama, R.; Pawlak, A.; Mino-Kenudson, M.; Yeap, B.Y.; Riely, G.J.; et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: An analysis of 1,683 patients with non-small cell lung cancer. Clin. Cancer Res. 2013, 19, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Won, J.K.; Keam, B.; Koh, J.; Cho, H.J.; Jeon, Y.K.; Kim, T.M.; Lee, S.H.; Lee, D.S.; Kim, D.W.; Chung, D.H. Concomitant ALK translocation and EGFR mutation in lung cancer: A comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann. Oncol. 2015, 26, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Baldi, L.; Barbieri, F.; Bertolini, F.; Tiseo, M. Concomitant EGFR and KRAS mutations in ALK-rearranged lung cancer. Ann. Oncol. 2015, 26, 1035–1036. [Google Scholar] [CrossRef]
- Levine, A.J.; Oren, M. The first 30 years of p53: Growing ever more complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Lain, S.; Verma, C.S.; Fersht, A.R.; Lane, D.P. Awakening guardian angels: Drugging the P53 pathway. Nat. Rev. Cancer 2009, 9, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Gannon, J.V.; Greavesl, R.; Iggo, R.; Lane, D.P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990, 9, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Husgafvel-Pursiainen, K.; Kannio, A. Cigarette smoking and p53 mutations in lung cancer and bladder cancer. Environ. Health Perspect. 1996, 104, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.; Chirieac, L.R.; D’Amico, T.A.; Decamp, M.M.; Dilling, T.J.; Dobelbower, M.; et al. Non-small cell lung cancer, version 5.2017: Clinical practice guidelines in oncology. JNCCN J. Natl. Compr. Cancer Netw. 2017, 15, 504–535. [Google Scholar] [CrossRef]
- Schaaij-Visser, T.B.M.; De Wit, M.; Lam, S.W.; Jiménez, C.R. The cancer secretome, current status and opportunities in the lung, breast and colorectal cancer context. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 2242–2258. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Callejo, D.; Romero, A.; Provencio, M.; Torrente, M. Liquid biopsy based biomarkers in non-small cell lung cancer for diagnosis and treatment monitoring. Transl. Lung Cancer Res. 2016, 5, 455–465. [Google Scholar] [CrossRef]
- Mlika, M.; Braham, E.; Laabidi, S.; Boussen, H.; El Mezni, F. About the Feasibility of Personalized Medicine in a Low Income Country? Open J. Soc. Sci. 2015, 3, 41–45. [Google Scholar] [CrossRef][Green Version]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Kato, Y.; Peled, N.; Wynes, M.W.; Yoshida, K.; Pardo, M.; Mascaux, C.; Ohira, T.; Tsuboi, M.; Matsubayashi, J.; Nagao, T.; et al. Novel epidermal growth factor receptor mutation-specific antibodies for non-small cell lung cancer: Immunohistochemistry as a possible screening method for epidermal growth factor receptor mutations. J. Thorac. Oncol. 2010, 5, 1551–1558. [Google Scholar] [CrossRef]
- Prabhakar, C.N. Epidermal growth factor receptor in non-small cell lung cancer. Transl. Lung Cancer 2015, 4, 110–118. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Xu, H.; Yu, B.; Shi, S.; Zhang, J.; Wang, X.; Wang, J.; Lu, Z.; Ma, H.; et al. Immunostaining with EGFR mutation-specific antibodies: A reliable screening method for lung adenocarcinomas harboring EGFR mutation in biopsy and resection samples. Hum. Pathol. 2013, 44, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, S.H.; Park, S.Y.; Yoo, J.; Kim, S.K.; Kim, H.K. Identification of EGFR mutations by immunohistochemistry with EGFR mutation-specific antibodies in biopsy and resection specimens from pulmonary adenocarcinoma. Cancer Res. Treat. 2015, 47, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Mraihi, Z.; Ben Amar, J.; Bouacha, H.; Rammeh, S.; Hila, L. EGFR mutation status in Tunisian nonsmall-cell lung cancer patients evaluated by mutation-specific immunohistochemistry. BMC Pulm. Med. 2018, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Moiseyenko, V.M.; Procenko, S.A.; Levchenko, E.V.; Barchuk, A.S.; Moiseyenko, F.V.; Iyevleva, A.G.; Mitiushkina, N.V.; Togo, A.V.; Semionov, I.I.; Ivantsov, A.O.; et al. High efficacy of first-line gefitinib in non-asian patients with EGFR-Mutated Lung adenocarcinoma. Onkologie 2010, 33, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Arfaoui Toumi, A.; Blel, A.; Aloui, R.; Zaibi, H.; Ksentinini, M.; Boudaya, M.S.; Znaidi, N.; Zidi, Y.; Aouina, H.; Rammeh Rommani, S. Assessment of EGFR mutation status in Tunisian patients with pulmonary adenocarcinoma. Curr. Res. Transl. Med. 2018, 66, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Mosier, S.L.; Thiess, M.; Beierl, K.F.; Debeljak, M.; Tseng, L.H.; Chen, G.; Yegnasubramanian, S.; Ho, H.; Cope, L.; et al. Clinical validation of KRAS, BRAF, and EGFR mutation detection using next-generation sequencing. Am. J. Clin. Pathol. 2014, 141, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; An, J.; Jiang, Q.Q.; Li, M.; Tan, J.; Hu, C.P. Analysis of EGFR, EML4-ALK, KRAS, and c-MET mutations in Chinese lung adenocarcinoma patients. Exp. Lung Res. 2013, 39, 328–335. [Google Scholar] [CrossRef]
- Gao, B.; Sun, Y.; Zhang, J.; Ren, Y.; Fang, R.; Han, X.; Shen, L.; Liu, X.Y.; Pao, W.; Chen, H.; et al. Spectrum of LKB1, EGFR, and KRAS mutations in Chinese lung adenocarcinomas. J. Thorac. Oncol. 2010, 5, 1130–1135. [Google Scholar] [CrossRef]
- Takamochi, K.; Oh, S.; Suzuki, K. Differences in EGFR and KRAS mutation spectra in lung adenocarcinoma of never and heavy smokers. Oncol. Lett. 2013, 6, 1207–1212. [Google Scholar] [CrossRef]
- Arrieta, O.; Cardona, A.F.; Federico Bramuglia, G.; Gallo, A.; Campos-Parra, A.D.; Serrano, S.; Castro, M.; Avilés, A.; Amorin, E.; Kirchuk, R.; et al. Genotyping non-small cell lung cancer (NSCLC) in latin America. J. Thorac. Oncol. 2011, 6, 1955–1959. [Google Scholar] [CrossRef]
- McQuitty, E.; Zhang, W.; Hendrickson, H.; Tio, F.O.; Jagirdar, J.; Olsen, R.; Cagle, P.T. Lung adenocarcinoma biomarker incidence in hispanic versus non-hispanic white patients. Arch. Pathol. Lab. Med. 2014, 38, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.Y.; Niu, F.Y.; Wu, Y.L.; Zhong, W.Z. Differences in driver genes between smoking-related and non-smoking-related lung cancer in the Chinese population. Cancer 2015, 17, 3069–3079. [Google Scholar] [CrossRef] [PubMed]
- Tantraworasin, A.; Lertprasertsuke, N.; Kongkarnka, S.; Euathrongchit, J.; Wannasopha, Y.; Saeteng, S. Retrospective study of ALK rearrangement and clinicopathological implications in completely resected non- small cell lung cancer patients in Northern Thailand: Role of screening with D5F3 antibodies. Asian Pac. J. Cancer Prev. 2014, 15, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Minca, E.C.; Lanigan, C.P.; Reynolds, J.P.; Wang, Z.; Ma, P.C.; Cicenia, J.; Almeida, F.A.; Pennell, N.A.; Tubbs, R.R. ALK status testing in non-small-cell lung carcinoma by FISH on ThinPrep slides with cytology material. J. Thorac. Oncol. 2014, 9, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Cabillic, F.; Gros, A.; Dugay, F.; Begueret, H.; Mesturoux, L.; Chiforeanu, D.C.; Dufrenot, L.; Jauffret, V.; Dachary, D.; Corre, R.; et al. Parallel FISH and immunohistochemical studies of ALK status in 3244 non-small-cell lung cancers reveal major discordances. J. Thorac. Oncol. 2014, 9, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, J.S.M.; Brunnström, H.; Jabs, V.; Edlund, K.; Jirström, K.; Mindus, S.; la Fleur, L.; Pontén, F.; Karlsson, M.G.; Karlsson, C.; et al. Inconsistent results in the analysis of ALK rearrangements in non-small cell lung cancer. BMC Cancer 2016, 16, 603. [Google Scholar] [CrossRef]
- Wang, J.; Cai, Y.; Dong, Y.; Nong, J.; Zhou, L.; Liu, G.; Su, D.; Li, X.; Wu, S.; Chen, X.; et al. Clinical characteristics and outcomes of patients with primary lung adenocarcinoma harboring ALK rearrangements detected by FISH, IHC, and RT-PCR. PLoS ONE 2014, 9, e101551. [Google Scholar] [CrossRef]
- Mezni, F.; Mlika, M.; Boussen, H.; Ghedira, H.; Fenniche, S.; Faten, T.; Loriot, M.A. About molecular profile of lung cancer in Tunisian patients. J. Immunoass. Immunochem. 2018, 35, 256–268. [Google Scholar] [CrossRef]
- Fan, L.; Feng, Y.; Wan, H.; Shi, G.; Niu, W. Clinicopathological and demographical characteristics of non-small cell lung cancer patients with ALK rearrangements: A systematic review and meta-analysis. PLoS ONE 2014, 9, e100866. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, A.R.; Silwal-Pandit, L.; Meza-Zepeda, L.A.; Vodak, D.; Vu, P.; Sagerup, C.; Hovig, E.; Myklebost, O.; Børresen-Dale, A.L.; Brustugun, O.T.; et al. TP53 mutation spectrum in smokers and never smoking lung cancer patients. Front. Genet. 2016, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, D.L.; Byers, L.A.; Kurie, J.M. Smoking, p53 Mutation, and Lung Cancer. Mol. Cancer Res. 2014, 12, 3–13. [Google Scholar] [CrossRef] [PubMed]

| No. (%) | |
|---|---|
| All patients | 73 (100) |
| Age, years | 73 |
| ≤61 | 36 (49.31) |
| >61 | 37 (50.68) |
| Gender | 73 |
| Male | 61 (83.56) |
| Female | 12 (16.43) |
| Tumor stage | 53 |
| I–II | 20 (37.73) |
| III–IV | 33 (62.26) |
| Tumor size | 44 |
| T1–T2 | 21 (47.72) |
| T3–T4 | 23 (52.27) |
| Lymph node (N) involvement | 44 |
| N0–N1 | 27 (61.36) |
| N2–N3 | 17 (38.63) |
| Metastasis | 52 |
| M0 | 33 (63.46) |
| M1 | 19 (36.53) |
| Smoking status | 59 |
| Non-smoker | 14 (23.72) |
| Smokers | 45 (76.27) |
| Forward Primer | Reverse Primer | Annealing Temperature (°C) | Product Size (bp) | |
|---|---|---|---|---|
| KRAS exon 2 | GACTGAATATAAACTTGTGGTAGTTGG | TTGGATCATATTCGTCCACAA | 60 | 101 |
| EGFR exon 18 | TCCAAATGAGCTGGCAAGTG | TCCCAAACACTCAGTGAAACAAA | 60 | 397 |
| EGFR exon 19 | CGTCTTCCTTCTCTCTCTGTC | GACATGAGAAAAGGTGGGC | 60 | 190 |
| EGFR exon 20 | CATTCATGCGTCTTCACCTG | CATATCCCCATGGCAAACTC | 60 | 377 |
| EGFR exon 21 | GCTCAGAGCCTGGCATGAA | CATCCTCCCCTGCATGTGT | 60 | 348 |
| Staining Intensity | No Staining 0 | Weak 1 | Moderate 2 | Strong 3 | Very Strong 4 |
|---|---|---|---|---|---|
| Proportion of positive tumor cells | 0% to 100% | ||||
| Immunostaining score = positive cell proportion score multiplied by staining intensity score H = 1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+) + 4 × (% cells 4+) | |||||
| 0–200 | 200–400 | ||||
| Degree of immunostaining | Negative or low | High | |||
| Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Staining intensity | No staining | Light yellow | Yellowish brown | Brown | Dark brown |
| Proportion of positive tumor cells | 0% | <10% | 11% to 50% | 51% to 80% | >80% |
| Immunostaining score = positive cell proportion score multiplied by staining intensity score | |||||
| Degree of immunostaining | 0 | 1–4 | >4 | ||
| Negative | Low | High | |||
| Patient No. | Gender | Age, Years | Smoking Data | Specimens | pTNM/Stage Grouping | Histological Subtype | Genetic Alteration | Method |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | Yes | Biopsy | Acinar | EGFR: EX-19 pE746-A750del | Sequenom | |
| 2 | M | 53 | Yes | Biopsy | Acinar | KRAS G13D | Sequenom | |
| 3 | F | 61 | No | Surgical | pT2bN1M0/II | Mucinar | KRAS G12D | Sequenom |
| 4 | M | 74 | Yes | Biopsy | Lepidic | KRAS G12D | Sequenom | |
| 5 | F | 53 | No | Biopsy | Solid | EGFR: EX19 pE746-A750del | Sequenom | |
| 7 | M | 70 | No | Biopsy | Acinar | EGFR: EX19 pE746-A750del | IHC | |
| 8 | M | 71 | No | Surgical | pT1N1M0/II | - | EGFR: EX19 pE746-A750del | Sequenom |
| 9 | M | 61 | Yes | Biopsy | Lepedic | KRAS G12A | Sanger | |
| 10 | M | 66 | Yes | Biopsy | - | KRAS G13D | Sanger | |
| 11 | M | 58 | Yes | Biopsy | Solid | KRAS G12V | Sanger | |
| 12 | M | 62 | Yes | Biopsy | Acinar | KRAS G13D | Sanger | |
| 13 | M | 77 | Yes | Biopsy | - | ALK + | IHC |
| EGFR+ | KRAS+ | ALK+ | Abnormal p53 Expression | |
|---|---|---|---|---|
| Gender | ||||
| Male | 3 | 6 | 1 | 37 |
| Female | 1 | 1 | 0 | 4 |
| Smoking history | ||||
| Non-smoker | 3 | 1 | 0 | 5 |
| Smoker | 1 | 6 | 1 | 23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhieb, D.; Belguith, I.; Capelli, L.; Chiadini, E.; Canale, M.; Bravaccini, S.; Yangui, I.; Boudawara, O.; Jlidi, R.; Boudawara, T.; et al. Analysis of Genetic Alterations in Tunisian Patients with Lung Adenocarcinoma. Cells 2019, 8, 514. https://doi.org/10.3390/cells8060514
Dhieb D, Belguith I, Capelli L, Chiadini E, Canale M, Bravaccini S, Yangui I, Boudawara O, Jlidi R, Boudawara T, et al. Analysis of Genetic Alterations in Tunisian Patients with Lung Adenocarcinoma. Cells. 2019; 8(6):514. https://doi.org/10.3390/cells8060514
Chicago/Turabian StyleDhieb, Dhoha, Imen Belguith, Laura Capelli, Elisa Chiadini, Matteo Canale, Sara Bravaccini, Ilhem Yangui, Ons Boudawara, Rachid Jlidi, Tahya Boudawara, and et al. 2019. "Analysis of Genetic Alterations in Tunisian Patients with Lung Adenocarcinoma" Cells 8, no. 6: 514. https://doi.org/10.3390/cells8060514
APA StyleDhieb, D., Belguith, I., Capelli, L., Chiadini, E., Canale, M., Bravaccini, S., Yangui, I., Boudawara, O., Jlidi, R., Boudawara, T., Calistri, D., Ammar Keskes, L., & Ulivi, P. (2019). Analysis of Genetic Alterations in Tunisian Patients with Lung Adenocarcinoma. Cells, 8(6), 514. https://doi.org/10.3390/cells8060514






