Cholesterol Trafficking: An Emerging Therapeutic Target for Angiogenesis and Cancer
Abstract
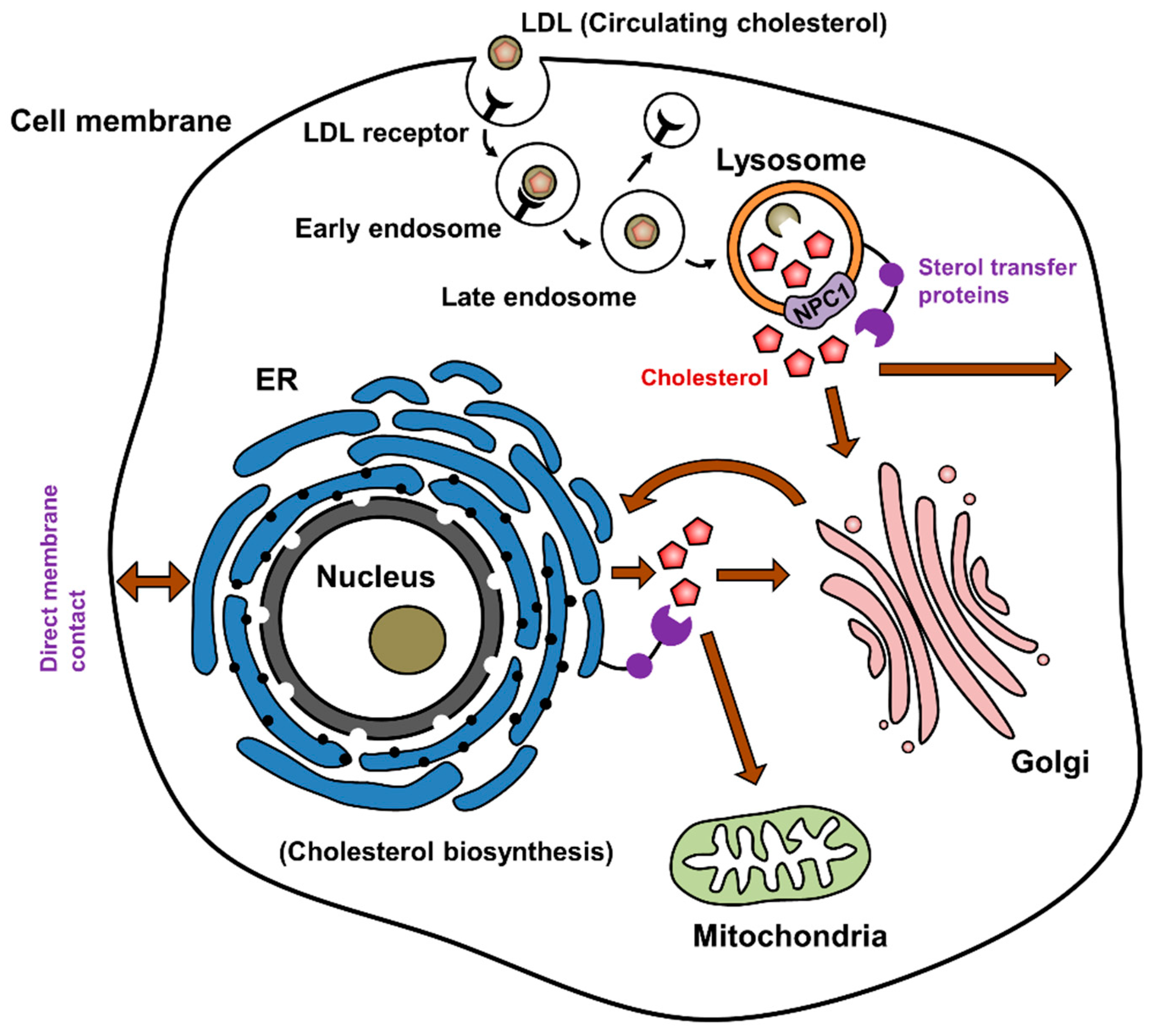
1. Cholesterol Synthesis and Distribution
2. Cholesterol and Angiogenic Signaling
2.1. Cholesterol Biosynthesis and Angiogenesis
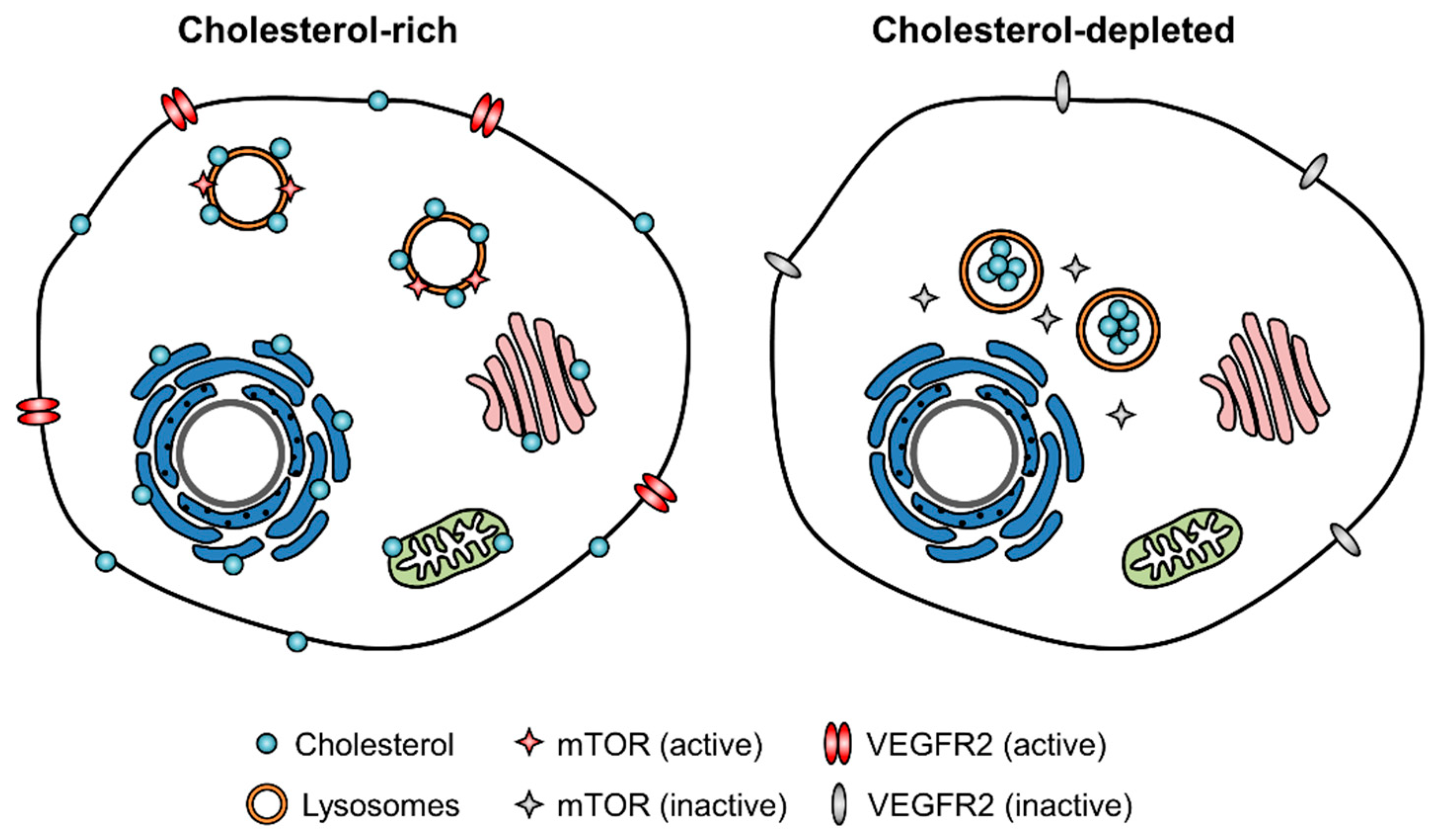
2.2. Endothelial Cell Cholesterol Level and Angiogenic Signaling
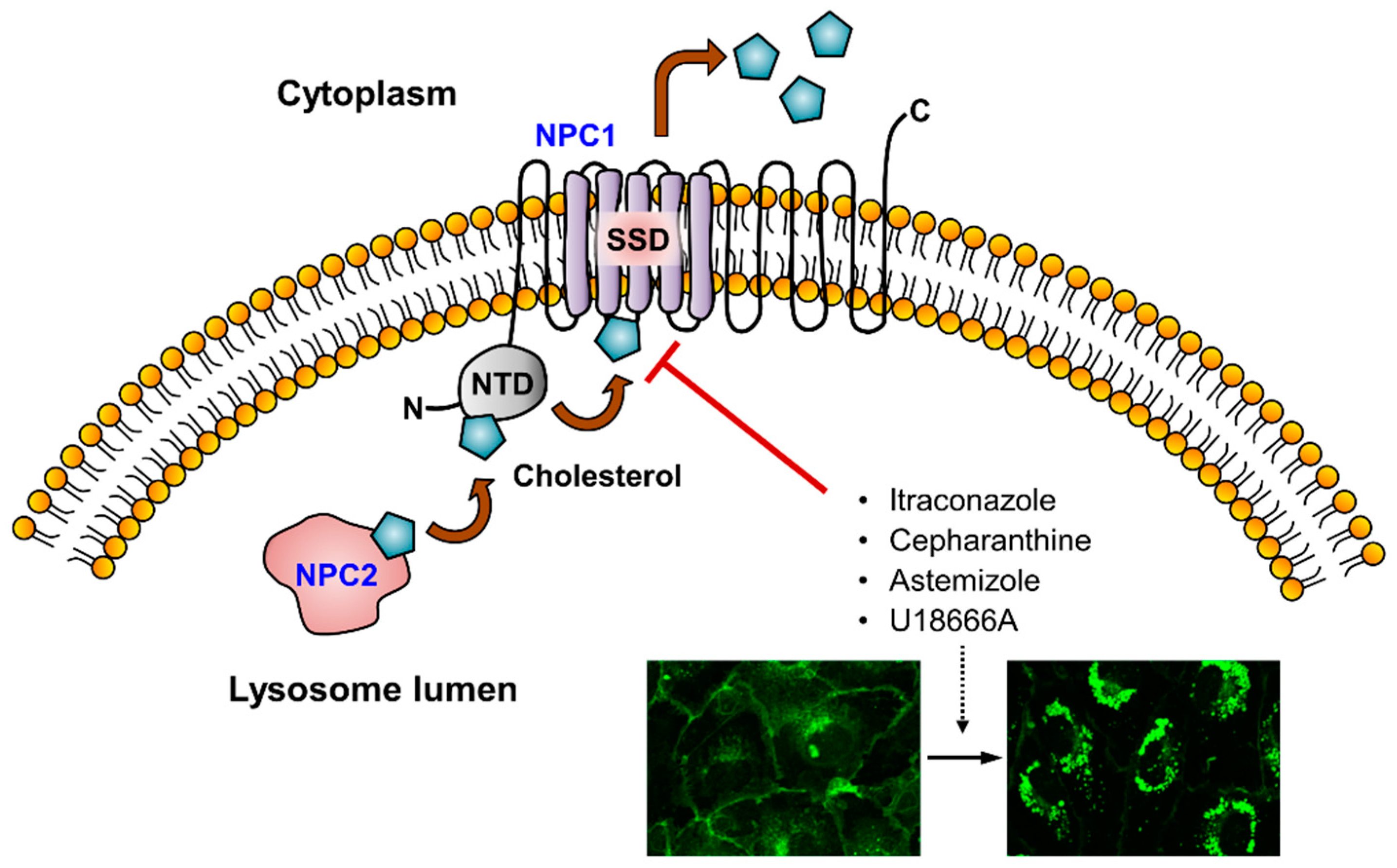
3. Cholesterol Trafficking Inhibitors as Anti-Angiogenic Agents
3.1. Itraconazole
3.2. Selective Estrogen Receptor Modulators (SERM)
3.3. Cepharanthine and Astemizole
4. Summary and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
| ABC | adenosine triphosphate-binding cassette |
| AIBP | apoA-I binding protein |
| ER | endoplasmic reticulum |
| HDL | high-density lipoprotein |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| HSMC | human vascular smooth muscle cell |
| HUVEC | human umbilical vein endothelial cells |
| LAL | lysosomal acid lipase |
| LDL | low-density lipoprotein |
| LXR | liver X receptor |
| NPC | Niemann-Pick type C |
| NPC1L1 | NPC1-like 1 |
| NTD | N-terminal domain |
| ORP | OSBP-related protein |
| OSBP | oxysterol binding protein |
| SCAP | SREBP cleavage-activating protein |
| SERM | selective estrogen receptor modulators |
| SREBP | sterol regulatory element-binding protein |
| SSD | sterol-sensing domain |
References
- Incardona, J.P.; Eaton, S. Cholesterol in signal transduction. Curr. Opin. Cell. Biol. 2000, 12, 193–203. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Tabas, I. Role of cholesterol and lipid organization in disease. Nature 2005, 438, 612–621. [Google Scholar] [CrossRef]
- Galan, C.; Woodard, G.E.; Dionisio, N.; Salido, G.M.; Rosado, J.A. Lipid rafts modulate the activation but not the maintenance of store-operated Ca(2+) entry. Biochim. Biophys. Acta 2010, 1803, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Feigenson, G.W. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 1999, 76, 2142–2157. [Google Scholar] [CrossRef]
- Pani, B.; Ong, H.L.; Liu, X.B.; Rauser, K.; Ambudkar, I.S.; Singh, B.B. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE). J. Biol. Chem. 2008, 283, 17333–17340. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K. The biological synthesis of cholesterol. Science 1965, 150, 19–28. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell. Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Fielding, C.J.; Fielding, P.E. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995, 36, 211–228. [Google Scholar]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Yang, T.; Espenshade, P.J.; Wright, M.E.; Yabe, D.; Gong, Y.; Aebersold, R.; Goldstein, J.L.; Brown, M.S. Crucial step in cholesterol homeostasis: Sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 2002, 110, 489–500. [Google Scholar] [CrossRef]
- Johnson, W.J.; Mahlberg, F.H.; Rothblat, G.H.; Phillips, M.C. Cholesterol Transport between Cells and High-Density-Lipoproteins. Biochim. Biophys. Acta 1991, 1085, 273–298. [Google Scholar] [CrossRef]
- Beaven, S.W.; Tontonoz, P. Nuclear receptors in lipid metabolism: Targeting the heart of dyslipidemia. Annu. Rev. Med. 2006, 57, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Abi-Mosleh, L.; Wang, M.L.; Deisenhofer, J.; Goldstein, J.L.; Brown, M.S.; Infante, R.E. Structure of N-Terminal Domain of NPC1 Reveals Distinct Subdomains for Binding and Transfer of Cholesterol. Cell 2009, 137, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Carstea, E.D.; Morris, J.A.; Coleman, K.G.; Loftus, S.K.; Zhang, D.; Cummings, C.; Gu, J.; Rosenfeld, M.A.; Pavan, W.J.; Krizman, D.B.; et al. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science 1997, 277, 228–231. [Google Scholar] [CrossRef]
- Davies, J.P.; Ioannou, Y.A. Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J. Biol. Chem. 2000, 275, 24367–24374. [Google Scholar]
- Sleat, D.E.; Wiseman, J.A.; El-Banna, M.; Price, S.M.; Verot, L.; Shen, M.M.; Tint, G.S.; Vanier, M.T.; Walkley, S.U.; Lobel, P. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl. Acad. Sci. USA 2004, 101, 5886–5891. [Google Scholar] [CrossRef]
- Lu, F.; Liang, Q.; Abi-Mosleh, L.; Das, A.; De Brabander, J.K.; Goldstein, J.L.; Brown, M.S. Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. eLife 2015, 4. [Google Scholar] [CrossRef]
- Van Hinsbergh, V.W.; Havekes, L.; Emeis, J.J.; van Corven, E.; Scheffer, M. Low density lipoprotein metabolism by endothelial cells from human umbilical cord arteries and veins. Arteriosclerosis 1983, 3, 547–559. [Google Scholar] [CrossRef]
- Negre-Aminou, P.; van Vliet, A.K.; van Erck, M.; van Thiel, G.C.; van Leeuwen, R.E.; Cohen, L.H. Inhibition of proliferation of human smooth muscle cells by various HMG-CoA reductase inhibitors; comparison with other human cell types. Biochim. Biophys. Acta 1997, 1345, 259–268. [Google Scholar] [CrossRef]
- Feleszko, W.; Balkowiec, E.Z.; Sieberth, E.; Marczak, M.; Dabrowska, A.; Giermasz, A.; Czajka, A.; Jakobisiak, M. Lovastatin and tumor necrosis factor-alpha exhibit potentiated antitumor effects against Ha-ras-transformed murine tumor via inhibition of tumor-induced angiogenesis. Int. J. Cancer 1999, 81, 560–567. [Google Scholar] [CrossRef]
- Vincent, L.; Chen, W.; Hong, L.; Mirshahi, F.; Mishal, Z.; Mirshahi-Khorassani, T.; Vannier, J.P.; Soria, J.; Soria, C. Inhibition of endothelial cell migration by cerivastatin, an HMG-CoA reductase inhibitor: Contribution to its anti-angiogenic effect. FEBS Lett. 2001, 495, 159–166. [Google Scholar] [CrossRef]
- Kureishi, Y.; Luo, Z.Y.; Shiojima, I.; Bialik, A.; Fulton, D.; Lefer, D.J.; Sessa, W.C.; Walsh, K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000, 6, 1004–1010. [Google Scholar] [CrossRef]
- Weis, M.; Heeschen, C.; Glassford, A.J.; Cooke, J.P. Statins have biphasic effects on angiogenesis. Circulation 2002, 105, 739–745. [Google Scholar] [CrossRef]
- Garcia-Calvo, M.; Lisnock, J.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R., Jr.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef]
- Solomon, K.R.; Pelton, K.; Boucher, K.; Joo, J.; Tully, C.; Zurakowski, D.; Schaffner, C.P.; Kim, J.; Freeman, M.R. Ezetimibe Is an Inhibitor of Tumor Angiogenesis. Am. J. Pathol. 2009, 174, 1017–1026. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid rafts as major platforms for signaling regulation in cancer. Adv. Biol. Regul. 2015, 57, 130–146. [Google Scholar] [CrossRef]
- Kabouridis, P.S.; Janzen, J.; Magee, A.L.; Ley, S.C. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur. J. Immunol. 2000, 30, 954–963. [Google Scholar] [CrossRef]
- Fang, L.H.; Choi, S.H.; Baek, J.S.; Liu, C.; Almazan, F.; Ulrich, F.; Wiesner, P.; Taleb, A.; Deer, E.; Pattison, J.; et al. Control of angiogenesis by AIBP-mediated cholesterol efflux. Nature 2013, 498, 118–122. [Google Scholar] [CrossRef]
- Noghero, A.; Perino, A.; Seano, G.; Saglio, E.; Lo Sasso, G.; Veglio, F.; Primo, L.; Hirsch, E.; Bussolino, F.; Morello, F. Liver X Receptor Activation Reduces Angiogenesis by Impairing Lipid Raft Localization and Signaling of Vascular Endothelial Growth Factor Receptor-2. Arterioscl. Throm. Vas. 2012, 32, 2280–2288. [Google Scholar] [CrossRef]
- Zelcer, N.; Tontonoz, P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 2006, 116, 607–614. [Google Scholar] [CrossRef]
- Chong, C.R.; Xu, J.; Lu, J.; Bhat, S.; Sullivan, D.J.; Liu, J.O. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem. Biol. 2007, 2, 263–270. [Google Scholar] [CrossRef]
- Aftab, B.T.; Dobromilskaya, I.; Liu, J.O.; Rudin, C.M. Itraconazole Inhibits Angiogenesis and Tumor Growth in Non-Small Cell Lung Cancer. Cancer Res. 2011, 71, 6764–6772. [Google Scholar] [CrossRef]
- Shim, J.S.; Li, R.J.; Bumpus, N.N.; Head, S.A.; Pasunooti, K.K.; Yang, E.J.; Lv, J.; Shi, W.; Liu, J.O. Divergence of Antiangiogenic Activity and Hepatotoxicity of Different Stereoisomers of Itraconazole. Clin. Cancer Res. 2016, 22, 2709–2720. [Google Scholar] [CrossRef]
- Choi, C.H.; Ryu, J.Y.; Cho, Y.J.; Jeon, H.K.; Choi, J.J.; Ylaya, K.; Lee, Y.Y.; Kim, T.J.; Chung, J.Y.; Hewitt, S.M.; et al. The anti-cancer effects of itraconazole in epithelial ovarian cancer. Sci. Rep. 2017, 7, 6552. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.S.; Yang, K.; Kong, C.; Liu, C.; Chen, H.J.; Huang, J.F.; Qian, F. Tumor Progression of Non-Small Cell Lung Cancer Controlled by Albumin and Micellar Nanoparticles of Itraconazole, a Multitarget Angiogenesis Inhibitor. Mol. Pharmaceut. 2017, 14, 4705–4713. [Google Scholar] [CrossRef]
- Xu, J.; Dang, Y.; Ren, Y.R.; Liu, J.O. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4764–4769. [Google Scholar] [CrossRef]
- Head, S.A.; Shi, W.Q.; Yang, E.J.; Nacev, B.A.; Hong, S.Y.; Pasunooti, K.K.; Li, R.J.; Shim, J.S.; Liu, J.O. Simultaneous Targeting of NPC1 and VDAC1 by Itraconazole Leads to Synergistic Inhibition of mTOR Signaling and Angiogenesis. ACS Chem. Biol. 2017, 12, 174–182. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Heath, E.I.; Smith, D.C.; Rathkopf, D.; Blackford, A.L.; Danila, D.C.; King, S.; Frost, A.; Ajiboye, A.S.; Zhao, M.; et al. Repurposing itraconazole as a treatment for advanced prostate cancer: A noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist 2013, 18, 163–173. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, J.; Spaunhurst, K.; Montoya, J.; Khodosh, R.; Chandra, K.; Fu, T.; Gilliam, A.; Molgo, M.; Beachy, P.A.; et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J. Clin. Oncol. 2014, 32, 745–751. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brahmer, J.R.; Juergens, R.A.; Hann, C.L.; Ettinger, D.S.; Sebree, R.; Smith, R.; Aftab, B.T.; Huang, P.; Liu, J.O. Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 619–623. [Google Scholar] [CrossRef]
- Tsubamoto, H.; Sonoda, T.; Ikuta, S.; Tani, S.; Inoue, K.; Yamanaka, N. Combination Chemotherapy with Itraconazole for Treating Metastatic Pancreatic Cancer in the Second-line or Additional Setting. Anticancer Res. 2015, 35, 4191–4196. [Google Scholar]
- Jordan, V.C. Long-term adjuvant tamoxifen therapy for breast cancer. Breast Cancer Res. Treat. 1990, 15, 125–136. [Google Scholar] [CrossRef]
- Jordan, V.C.; Murphy, C.S. Endocrine pharmacology of antiestrogens as antitumor agents. Endocr. Rev. 1990, 11, 578–610. [Google Scholar] [CrossRef]
- Knabbe, C.; Zugmaier, G.; Schmahl, M.; Dietel, M.; Lippman, M.E.; Dickson, R.B. Induction of transforming growth factor beta by the antiestrogens droloxifene, tamoxifen, and toremifene in MCF-7 cells. Am. J. Clin. Oncol. 1991, 14, S15–20. [Google Scholar] [CrossRef]
- Gagliardi, A.; Collins, D.C. Inhibition of angiogenesis by antiestrogens. Cancer Res. 1993, 53, 533–535. [Google Scholar]
- Blackwell, K.L.; Haroon, Z.A.; Shan, S.; Saito, W.; Broadwater, G.; Greenberg, C.S.; Dewhirst, M.W. Tamoxifen inhibits angiogenesis in estrogen receptor-negative animal models. Clin. Cancer Res. 2000, 6, 4359–4364. [Google Scholar]
- Haran, E.F.; Maretzek, A.F.; Goldberg, I.; Horowitz, A.; Degani, H. Tamoxifen enhances cell death in implanted MCF7 breast cancer by inhibiting endothelium growth. Cancer Res. 1994, 54, 5511–5514. [Google Scholar]
- Shim, J.S.; Li, R.J.; Lv, J.; Head, S.A.; Yang, E.J.; Liu, J.O. Inhibition of angiogenesis by selective estrogen receptor modulators through blockade of cholesterol trafficking rather than estrogen receptor antagonism. Cancer Lett. 2015, 362, 106–115. [Google Scholar] [CrossRef]
- Altan, N.; Chen, Y.; Schindler, M.; Simon, S.M. Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 4432–4437. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Reigada, D.; Nguyen, J.; Laties, A.M.; Mitchell, C.H. Restoration of lysosomal pH in RPE cells from cultured human and ABCA4(-/-) mice: Pharmacologic approaches and functional recovery. Invest. Ophthalmol. Vis. Sci. 2008, 49, 772–780. [Google Scholar] [CrossRef]
- Saito, R.; Tsuchiya, S.; Ishizuka, T.; Fueki, N.; Ezawa, K.; Minato, K.; Nakano, H.; Takise, A.; Kurihara, M.; Fueki, R. Clinical effects of cepharanthin (Ceph.) on leukopenia by chemotherapy in lung cancer patients. Nihon Gan. Chiryo Gakkai Shi 1989, 24, 2587–2593. [Google Scholar]
- Kato, T.; Suzumura, Y. Potentiation of antitumor activity of vincristine by the biscoclaurine alkaloid cepharanthine. J. Natl. Cancer. Inst. 1987, 79, 527–532. [Google Scholar]
- Nomoto, S.; Imada, H.; Ohguri, T.; Yahara, K.; Kato, F.; Morioka, T.; Korogi, Y. Effect of Cepharanthin in preventing radiation induced normal tissue damage in prostate cancer. Gan To Kagaku Ryoho 2004, 31, 1063–1066. [Google Scholar]
- Shimazu, R.; Tanaka, G.; Tomiyama, R.; Kuratomi, Y.; Inokuchi, A. Cepharanthin effect on radiation-induced xerostomia and taste disorder in patients with head and neck cancer. Nihon Jibiinkoka Gakkai Kaiho 2009, 112, 648–655. [Google Scholar] [CrossRef][Green Version]
- Lyu, J.; Yang, E.J.; Head, S.A.; Ai, N.; Zhang, B.; Wu, C.; Li, R.J.; Liu, Y.; Yang, C.; Dang, Y.; et al. Pharmacological blockade of cholesterol trafficking by cepharanthine in endothelial cells suppresses angiogenesis and tumor growth. Cancer Lett. 2017, 409, 91–103. [Google Scholar] [CrossRef]
- Lyu, J.; Yang, E.J.; Head, S.A.; Ai, N.; Zhang, B.; Wu, C.; Li, R.J.; Liu, Y.; Chakravarty, H.; Zhang, S.; et al. Astemizole Inhibits mTOR Signaling and Angiogenesis by Blocking Cholesterol Trafficking. Int J. Biol. Sci. 2018, 14, 1175–1185. [Google Scholar] [CrossRef]
- Gill, S.; Stevenson, J.; Kristiana, I.; Brown, A.J. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab. 2011, 13, 260–273. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, J.; Yang, E.J.; Shim, J.S. Cholesterol Trafficking: An Emerging Therapeutic Target for Angiogenesis and Cancer. Cells 2019, 8, 389. https://doi.org/10.3390/cells8050389
Lyu J, Yang EJ, Shim JS. Cholesterol Trafficking: An Emerging Therapeutic Target for Angiogenesis and Cancer. Cells. 2019; 8(5):389. https://doi.org/10.3390/cells8050389
Chicago/Turabian StyleLyu, Junfang, Eun Ju Yang, and Joong Sup Shim. 2019. "Cholesterol Trafficking: An Emerging Therapeutic Target for Angiogenesis and Cancer" Cells 8, no. 5: 389. https://doi.org/10.3390/cells8050389
APA StyleLyu, J., Yang, E. J., & Shim, J. S. (2019). Cholesterol Trafficking: An Emerging Therapeutic Target for Angiogenesis and Cancer. Cells, 8(5), 389. https://doi.org/10.3390/cells8050389





