Telomere Function and the G-Quadruplex Formation are Regulated by hnRNP U
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmid Constructs and Transfections
2.3. Recombinant Protein Expression and Purification
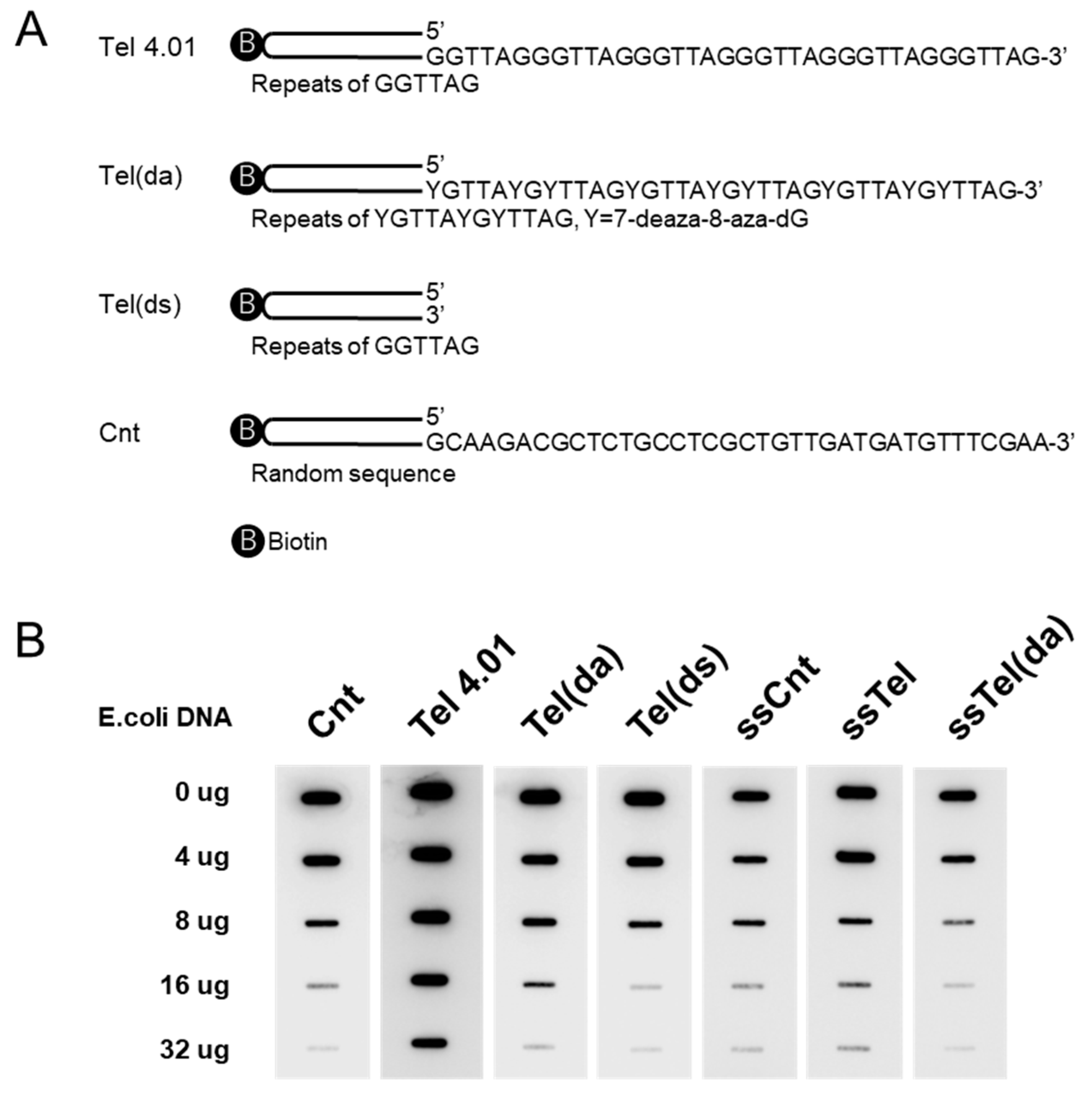
2.4. Competition Assay with E. coli DNA
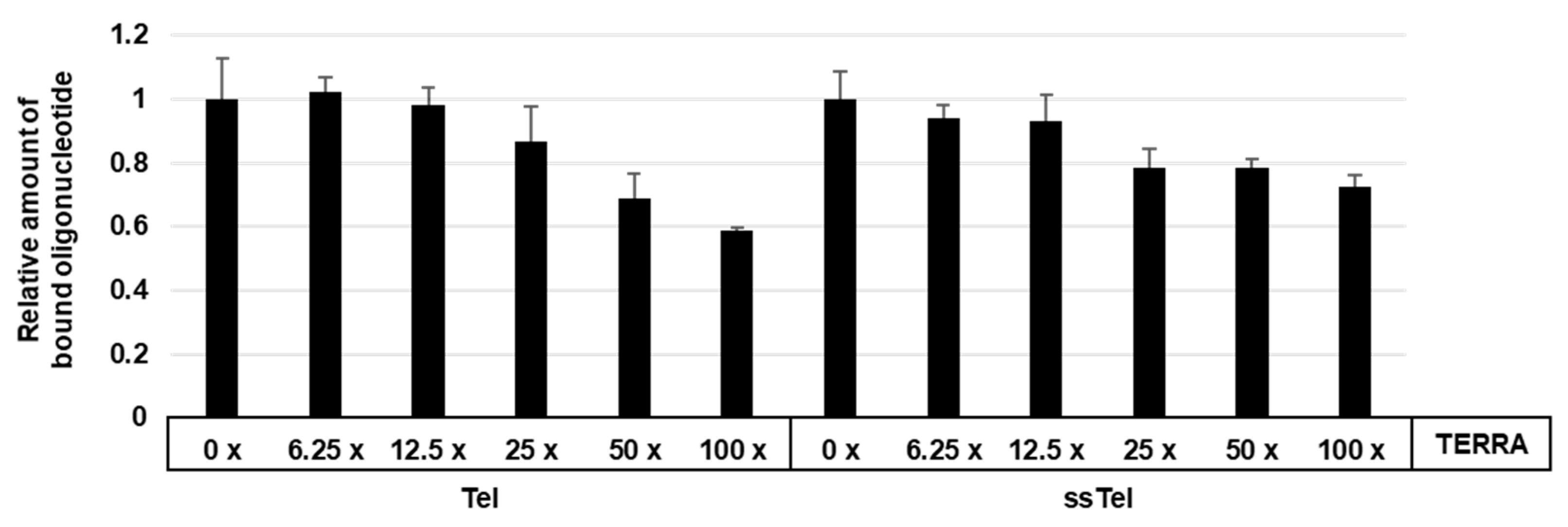
2.5. Effect of TERRA on Binding between hnRNP U 683C and Tel or Single-stranded(ss)Tel Oligonucleotide
2.6. Western Blotting
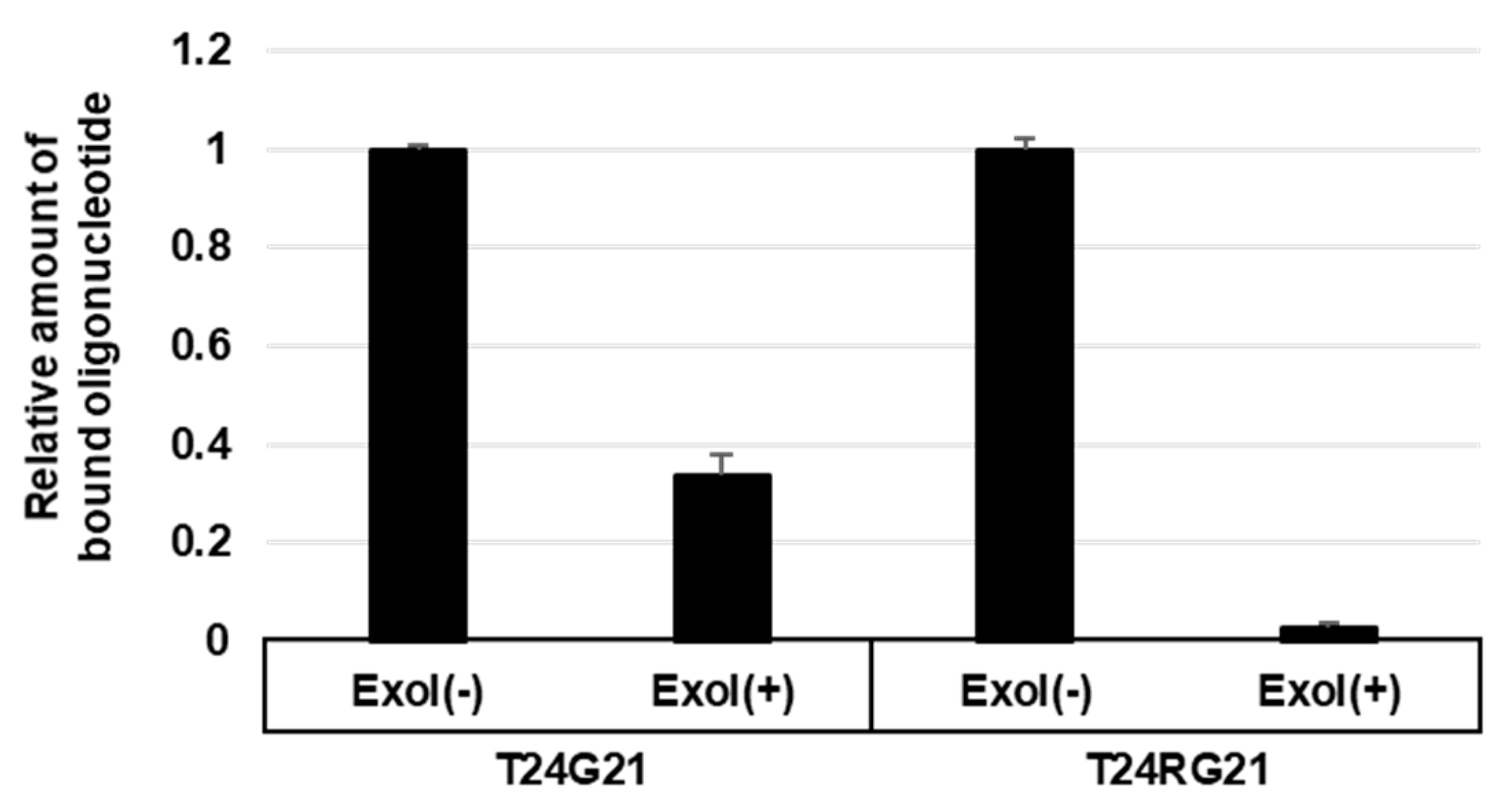
2.7. Exonuclease I Protection Assay
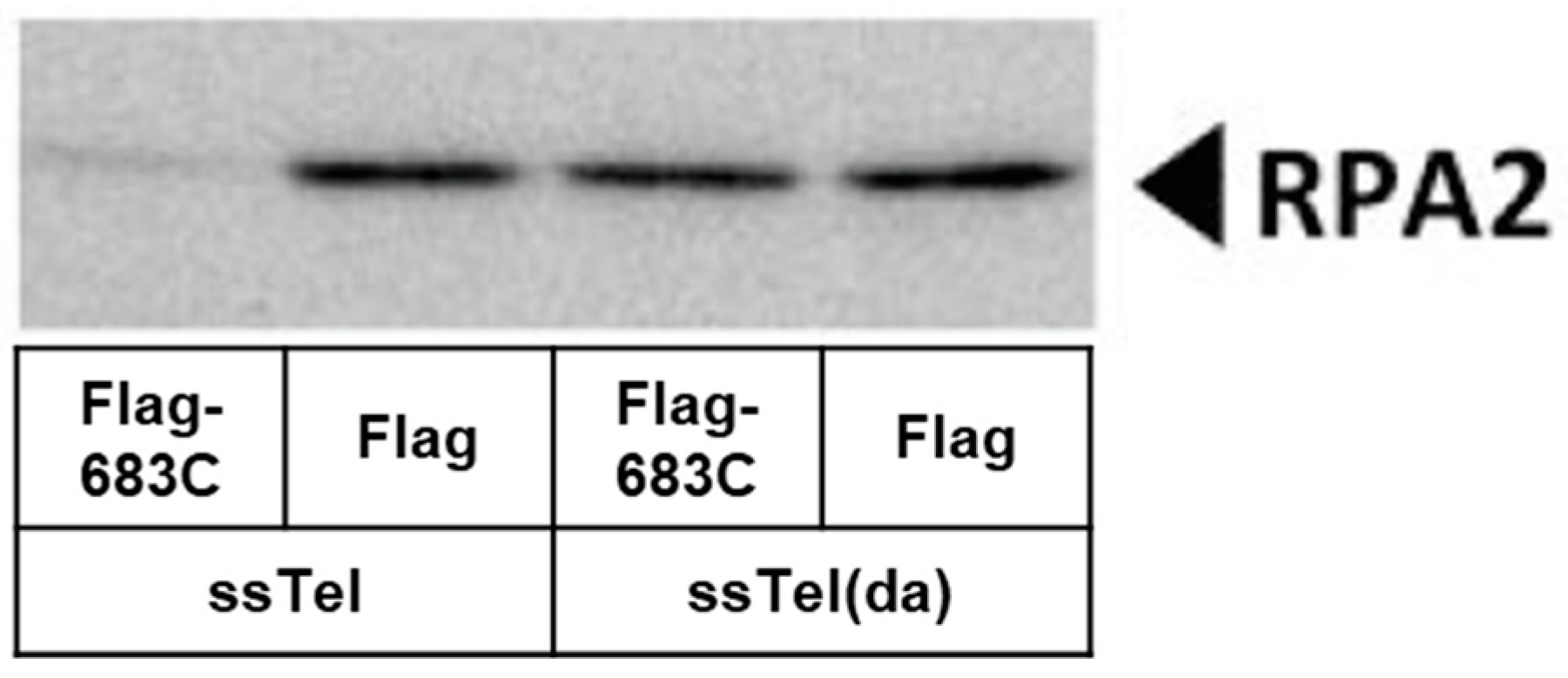
2.8. huRNP U Prevents RPA2 from Binding Telomeres
2.9. Oligonucleotides
- Tel 4.01: 5′—CTAACCCTAA CCCTAACCCT AACCCTAACC CTAACCCTAA CCCTAACCCT AAACTXCGGT TTAGGGTTAG GGTTAGGGTT AGGGTTAGGG TTAGGGTTAG GGTTAGGGTT AGGGTTAGGG TTAGGGTTAG GGTTAGGGTT AGGGTTAG—3′
- Tel(da): 5′—CTAACCCTAA CCCTAACCCT AACCCTAACC CTAACCCTAA CCCTAACCCT AAACTXCGYT TTAGYGTTAY GYTTAGYGTT AYGYTTAGYG TTAYGYTTAG YGTTAYGYTT AGYGTTAYGY TTAGYGTTAY GYTTAGYGTT AYGYTTAG—3′
- Tel(ds): 5′—CTAACCCTAA CCCTAACCCT AACCCTAACC CTAACCCTAA CCCTAACCCT AAACTXCGGT TTAGGGTTAG GGTTAGGGTTA GGGTTAGGGT TAGGGTTAGG GTTAGGGTTA G—3′
- Cnt: 5′—TGGCATATCA CGGTCGTGCG CGTAGAGATG TGATCGGAGA AAAGAGTCAT TACTXCGGTA ATGACTCTTT TCTCCGATCA CATCTCTACG CGCACGACCG TGATATGCC AGCAAGACGC TCTGCCTCGC TGTTGATGAT GTTTCGAA—3′
- ssTel: 5′—ZGGTTAGGGT TAGGGTTAGG GTTAGGGTTA GGGTTAG—3′
- ssTel(da): 5′—ZYGTTAYGYT TAGYGTTAYG YTTAGYGTTA YGYTTAG—3′
- ssCnt: 5′—ZCAAGACGCTC TGCCTCGCTG TTGATGATGT TTCGAA—3′
- TERRA: 5′—GGUUAGGGUU AGGGUUAGGG UUAGGGUUAG GGUUAG—3′
- dGGG(TTAGGG)3: 5′—ZGGGTTAGG GTTAGGGTTA GGG—3′
- TelC: 5′—CTAACCCTAA CCCTAACCCT AACCZ—3′
- T24G21: 5′—Biotin-T24(G3T2A)3G3—3′
- T24RG21: 5′—Biotin-T24GTGTGAGTGGAGGTGTGAGGT—3′
- Tel linker: 5′—GGGCTGGCAA GCCACGTTTG GTGTAAAACG ACGGCCAGTA GAAGGCACAG TCGAGGCCTC TGACACATGC AGCTCCCGGC TAACCCTAAC CCTAACCCT—3′
- T24G21 linker: 5′—GGGCTGGCAA GCCACGTTTG GTGTAAAACG ACGGCCAGTA GAAGGCACAG TCGAGGCCTC TGACACATGC AGCTCCCGGC CCTAACCCTA ACCCTAACCC—3′
- T24RG21 linker: 5′—GGGCTGGCAA GCCACGTTTG GTGTAAAACG ACGGCCAGTA GAAGGCACAG TCGAGGCCTC TGACACATGC AGCTCCCGGA CCTCACACCT CCACTCACAC—3′
- Linker primer 1: 5′—GGGCTGGCAA GCCACGTTTG GTG—3′
- Linker primer 2: 5′—CCGGGAGCTG CATGTGTCAG AGG—3′
2.10. Antibodies
3. Results
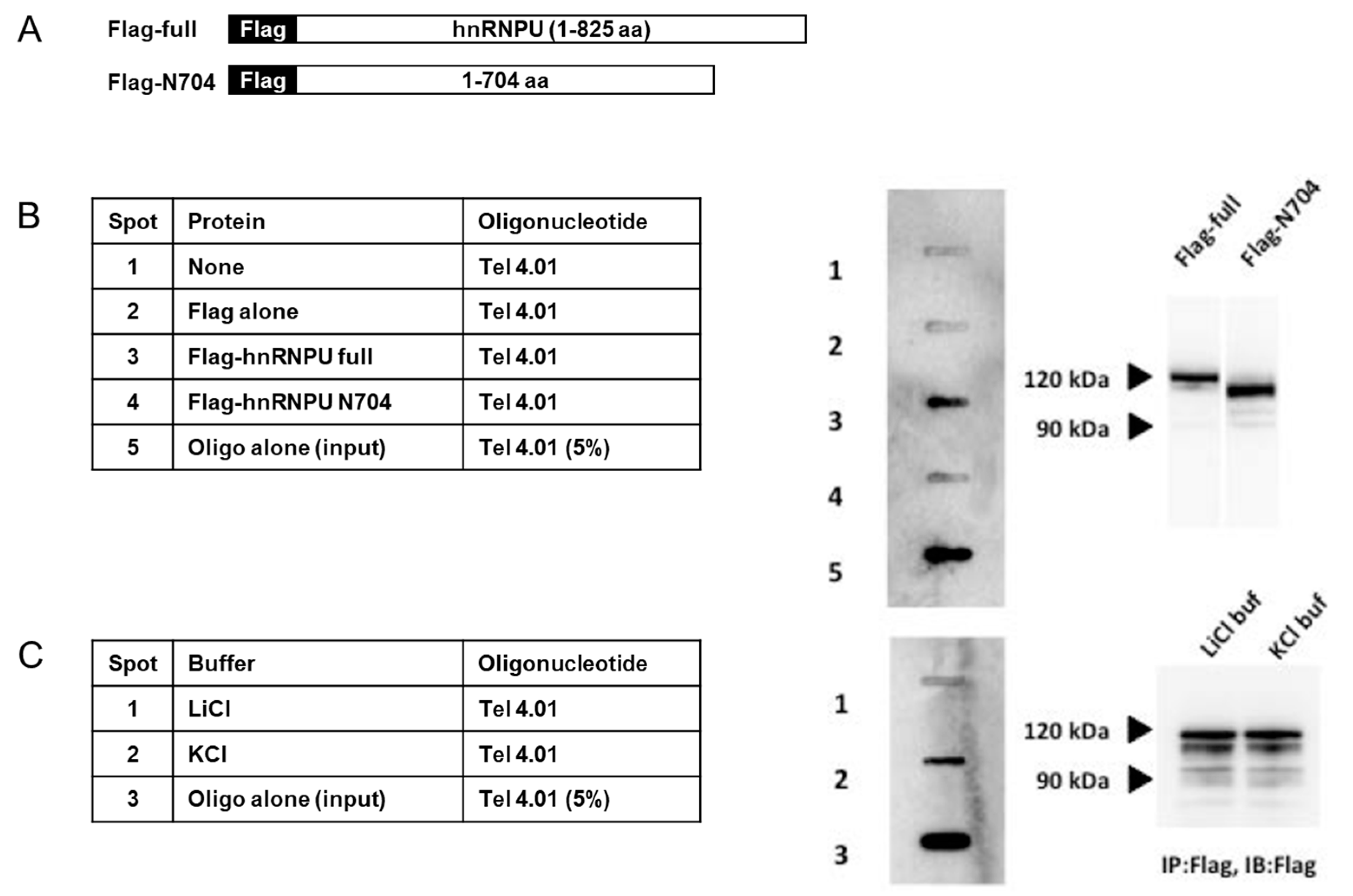
3.1. hnRNP U Associates with Telomeres
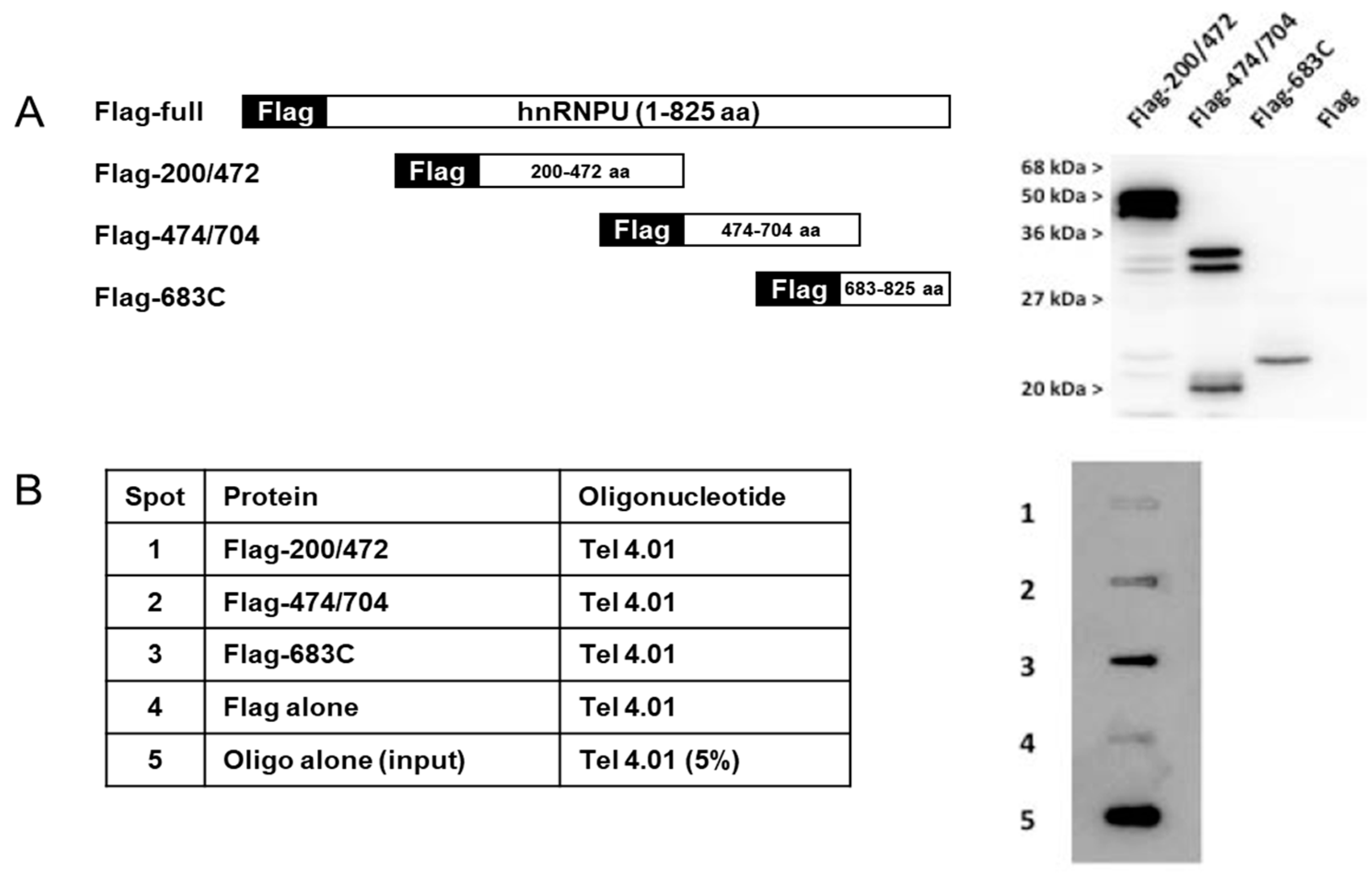
3.2. Binding Assays of Flag-hnRNP U and Several Telomere-Associated Oligonucleotides in Competition with E. coli DNA
3.3. Binding Assays of Flag-hnRNP U683C and Tel or ssTel. Competition with TERRA
3.4. Flag-hnRNP U 683 C Facilitates G-Quadruplex Formation and DNA Degradation
3.5. huRNP U Prevents RPA2 Accumulation on Telomeres
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shampay, J.; Szostak, J.W.; Blackburn, E.H. DNA sequences of telomeres maintained in yeast. Nature 1984, 310, 154–157. [Google Scholar] [CrossRef]
- Meyne, J.; Ratliff, R.L.; Moyzis, R.K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl. Acad. Sci. USA 1989, 86, 7049–7053. [Google Scholar] [CrossRef] [PubMed]
- Hemann, M.T.; Strong, M.A.; Hao, L.Y.; Greider, C.W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 2001, 107, 67–77. [Google Scholar] [CrossRef]
- Simonsson, T. G-quadruplex DNA structures--variations on a theme. Biol. Chem. 2001, 382, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, G.N.; Lee, M.P.; Neidle, S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature 2002, 417, 876–880. [Google Scholar] [CrossRef]
- Bochman, M.L.; Paeschke, K.; Zakian, V.A. DNA secondary structures: Stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef]
- Biffi, G.; Tannahill, D.; McCafferty, J.; Balasubramanian, S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013, 5, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.; Wu, Y.; Huang, Y.C.; Chavez, E.A.; Platt, J.; Johnson, F.B.; Brosh, R.M., Jr.; Sen, D.; Lansdorp, P.M. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res. 2014, 42, 860–869. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Qi, H.; Liu, L.F. Protection of DNA ends by telomeric 3′ G-tail sequences. J. Biol. Chem. 2007, 282, 18786–18792. [Google Scholar] [CrossRef]
- Flynn, R.L.; Centore, R.C.; O’Sullivan, R.J.; Rai, R.; Tse, A.; Songyang, Z.; Chang, S.; Karlseder, J.; Zou, L. TERRA and hnRNPA1 orchestrate an RPA-to-POT1 switch on telomeric single-stranded DNA. Nature 2011, 471, 532–536. [Google Scholar] [CrossRef]
- Verdun, R.E.; Karlseder, J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell 2006, 127, 709–720. [Google Scholar] [CrossRef]
- Ray, S.; Bandaria, J.N.; Qureshi, M.H.; Yildiz, A.; Balci, H. G-quadruplex formation in telomeres enhances POT1/TPP1 protection against RPA binding. Proc. Natl. Acad. Sci. USA 2014, 111, 2990–2995. [Google Scholar] [CrossRef]
- Fu, D.; Collins, K. Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol. Cell 2007, 28, 773–785. [Google Scholar] [CrossRef]
- Takahama, K.; Takada, A.; Tada, S.; Shimizu, M.; Sayama, K.; Kurokawa, R.; Oyoshi, T. Regulation of telomere length by G-quadruplex telomere DNA- and TERRA-binding protein TLS/FUS. Chem. Biol. 2013, 20, 341–350. [Google Scholar] [CrossRef]
- Romig, H.; Fackelmayer, F.O.; Renz, A.; Ramsperger, U.; Richter, A. Characterization of SAF-A, a novel nuclear DNA binding protein from HeLa cells with high affinity for nuclear matrix/scaffold attachment DNA elements. EMBO J. 1992, 11, 3431–3440. [Google Scholar] [CrossRef]
- Xiao, R.; Tang, P.; Yang, B.; Huang, J.; Zhou, Y.; Shao, C.; Li, H.; Sun, H.; Zhang, Y.; Fu, X.D. Nuclear matrix factor hnRNP U/SAF-A exerts a global control of alternative splicing by regulating U2 snRNP maturation. Mol. Cell 2012, 45, 656–668. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, M. RGG-box in hnRNPA1 specifically recognizes the telomere G-quadruplex DNA and enhances the G-quadruplex unfolding ability of UP1 domain. Nucleic Acids Res. 2018, 46, 10246–10261. [Google Scholar] [CrossRef]
- Han, B.; Izumi, H.; Yasuniwa, Y.; Akiyama, M.; Yamaguchi, T.; Fujimoto, N.; Matsumoto, T.; Wu, B.; Tanimoto, A.; Sasaguri, Y.; et al. Human mitochondrial transcription factor A functions in both nuclei and mitochondria and regulates cancer cell growth. Biochem. Biophys. Res. Commun. 2011, 408, 45–51. [Google Scholar] [CrossRef]
- Sfeir, A.; Kosiyatrakul, S.T.; Hockemeyer, D.; MacRae, S.L.; Karlseder, J.; Schildkraut, C.L.; de Lange, T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 2009, 138, 90–103. [Google Scholar] [CrossRef]
- Mohaghegh, P.; Karow, J.K.; Brosh, R.M., Jr.; Bohr, V.A.; Hickson, I.D. The Bloom’s and Werner’s syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 2001, 29, 2843–2849. [Google Scholar] [CrossRef]
- Paeschke, K.; Bochman, M.L.; Garcia, P.D.; Cejka, P.; Friedman, K.L.; Kowalczykowski, S.C.; Zakian, V.A. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature 2013, 497, 458–462. [Google Scholar] [CrossRef]
- Davis, M.; Hatzubai, A.; Andersen, J.S.; Ben-Shushan, E.; Fisher, G.Z.; Yaron, A.; Bauskin, A.; Mercurio, F.; Mann, M.; Ben-Neriah, Y. Pseudosubstrate regulation of the SCF(beta-TrCP) ubiquitin ligase by hnRNP-U. Genes Dev. 2002, 16, 439–451. [Google Scholar] [CrossRef]
- Ye, J.; Beetz, N.; O’Keeffe, S.; Tapia, J.C.; Macpherson, L.; Chen, W.V.; Bassel-Duby, R.; Olson, E.N.; Maniatis, T. hnRNP U protein is required for normal pre-mRNA splicing and postnatal heart development and function. Proc. Natl. Acad. Sci. USA 2015, 112, E3020–E3029. [Google Scholar] [CrossRef]
- Smith, J.S.; Chen, Q.; Yatsunyk, L.A.; Nicoludis, J.M.; Garcia, M.S.; Kranaster, R.; Balasubramanian, S.; Monchaud, D.; Teulade-Fichou, M.P.; Abramowitz, L.; et al. Rudimentary G-quadruplex-based telomere capping in Saccharomyces cerevisiae. Nat. Struct. Mol. Biol. 2011, 18, 478–485. [Google Scholar] [CrossRef]
- Vannier, J.B.; Pavicic-Kaltenbrunner, V.; Petalcorin, M.I.; Ding, H.; Boulton, S.J. RTEL1 dismantles T loops and counteracts telomeric G4-DNA to maintain telomere integrity. Cell 2012, 149, 795–806. [Google Scholar] [CrossRef]
- Takahama, K.; Arai, S.; Kurokawa, R.; Oyoshi, T. Identification of DNA binding specificity for TLS. Nucleic Acids Symp. Ser. (Oxf) 2009, 53, 247–248. [Google Scholar] [CrossRef][Green Version]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Bode, J.; Benham, C.; Knopp, A.; Mielke, C. Transcriptional augmentation: Modulation of gene expression by scaffold/matrix-attached regions (S/MAR elements). Crit. Rev. Eukaryot. Gene Expr. 2000, 10, 73–90. [Google Scholar] [CrossRef]
- Kwon, C.; Chung, I.K. Interaction of an Arabidopsis RNA-binding protein with plant single-stranded telomeric DNA modulates telomerase activity. J. Biol. Chem. 2004, 279, 12812–12818. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumi, H.; Funa, K. Telomere Function and the G-Quadruplex Formation are Regulated by hnRNP U. Cells 2019, 8, 390. https://doi.org/10.3390/cells8050390
Izumi H, Funa K. Telomere Function and the G-Quadruplex Formation are Regulated by hnRNP U. Cells. 2019; 8(5):390. https://doi.org/10.3390/cells8050390
Chicago/Turabian StyleIzumi, Hiroto, and Keiko Funa. 2019. "Telomere Function and the G-Quadruplex Formation are Regulated by hnRNP U" Cells 8, no. 5: 390. https://doi.org/10.3390/cells8050390
APA StyleIzumi, H., & Funa, K. (2019). Telomere Function and the G-Quadruplex Formation are Regulated by hnRNP U. Cells, 8(5), 390. https://doi.org/10.3390/cells8050390




