IL-33 and MRGPRX2-Triggered Activation of Human Skin Mast Cells—Elimination of Receptor Expression on Chronic Exposure, but Reinforced Degranulation on Acute Priming
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Human Skin MCs
2.2. MC Treatment
2.3. Accell®-Mediated RNA Interference
2.4. Flow Cytometry
2.5. Determination of Histamine Release
2.6. β-Hexosaminidase Release Assay
2.7. Reverse Transcription-Quantitative PCR (RT-qPCR)
2.8. Immunoblot Analysis
2.9. Statistics
3. Results
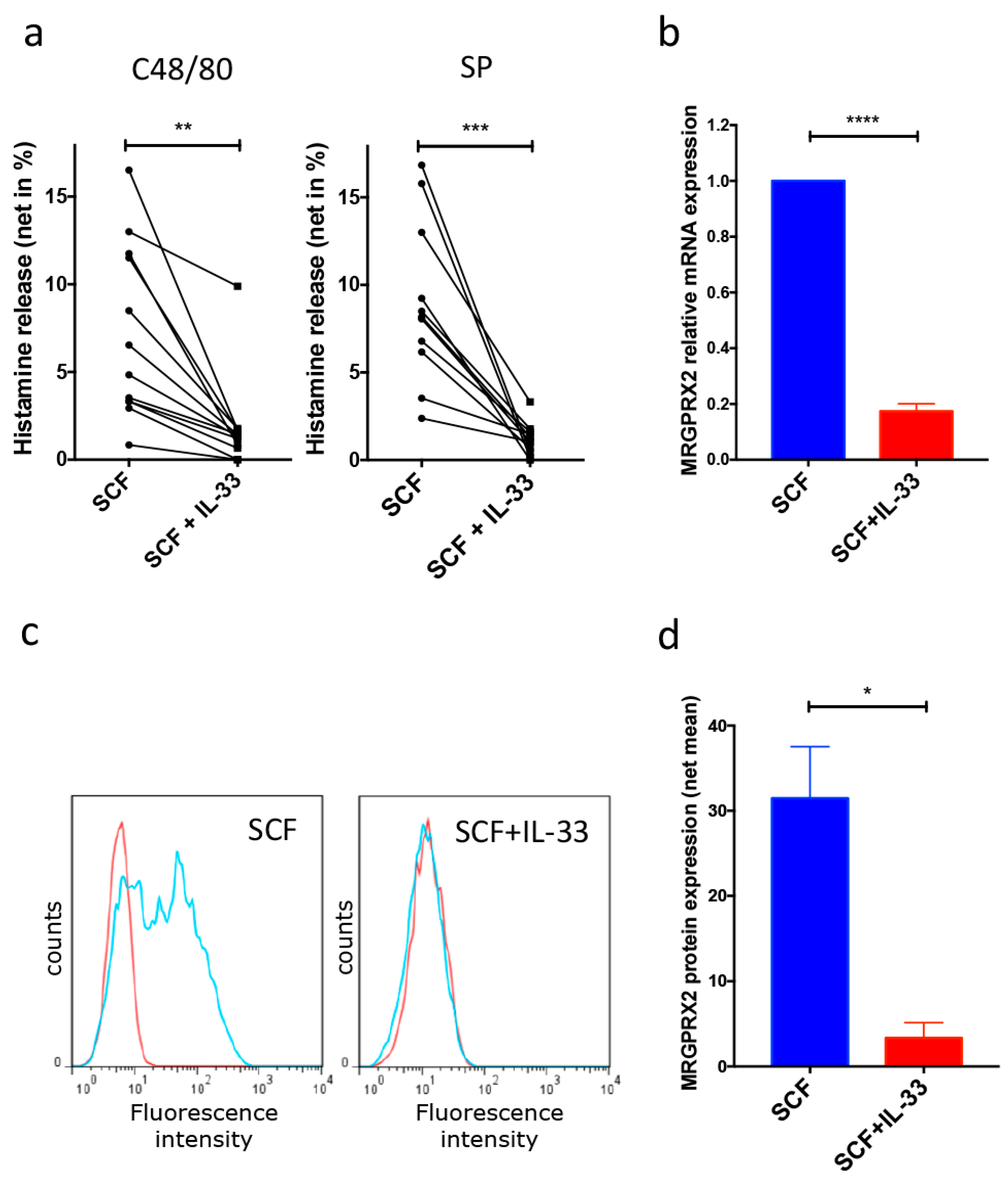
3.1. Skin MCs Lose Responsiveness to MRGPRX2 Ligands and Massively Downregulate MRGPRX2 Expression after Long-Term Exposure to IL-33
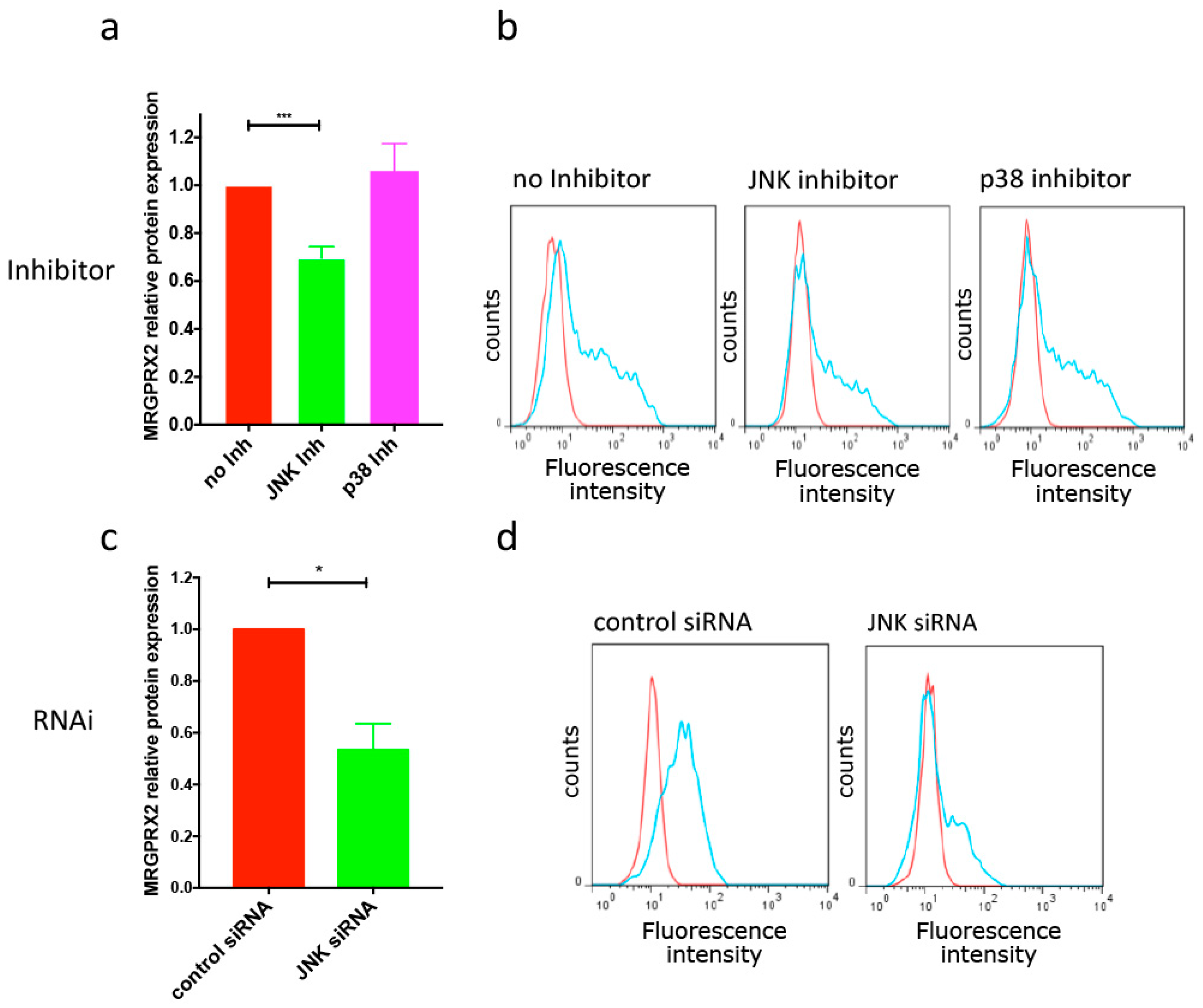
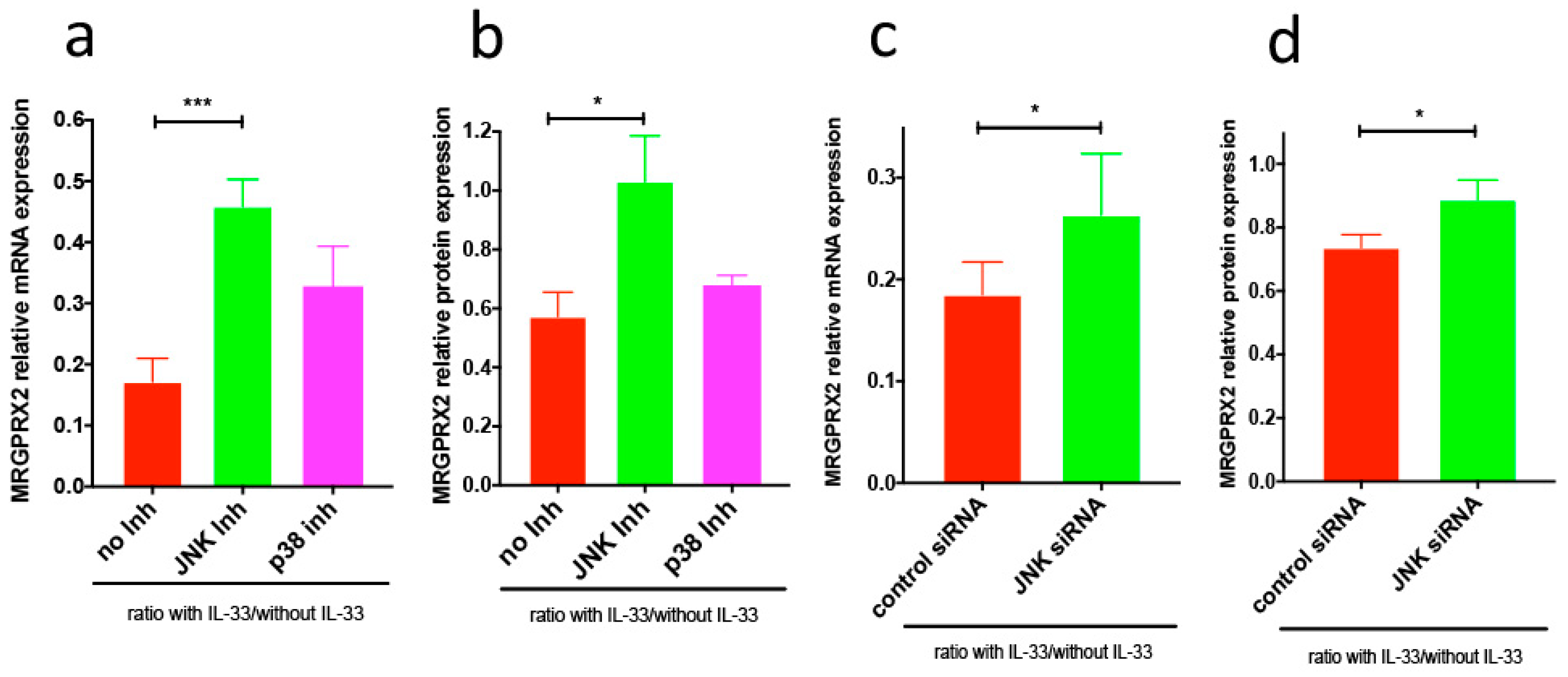
3.2. IL-33-Triggered Downregulation of MRGPRX2 Is Partially JNK-Dependent, although JNK Is A Positive Regulator of MRGPRX2 in the Absence of IL-33
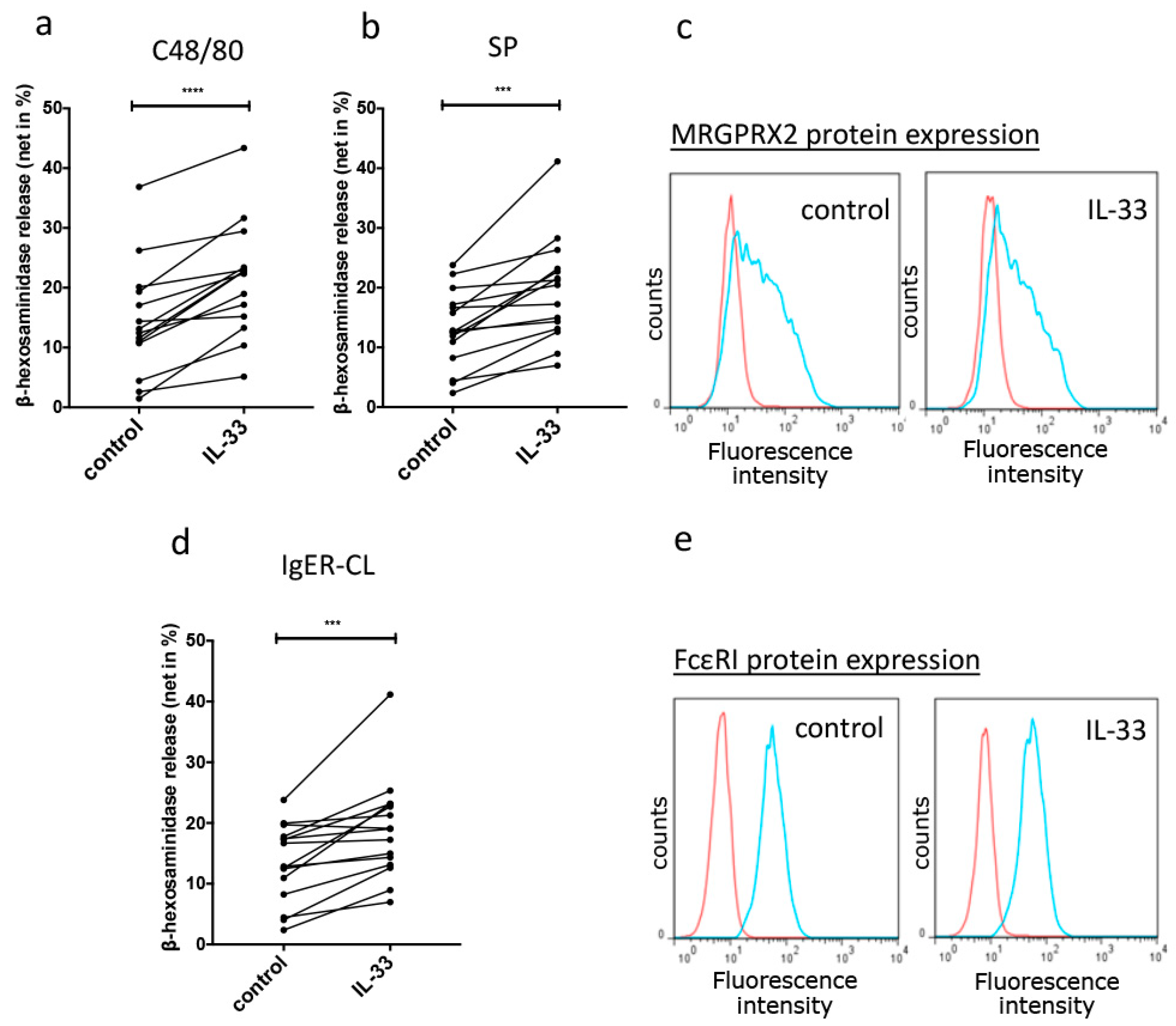
3.3. Short-Term Priming by IL-33 Fosters Skin MC Degranulation
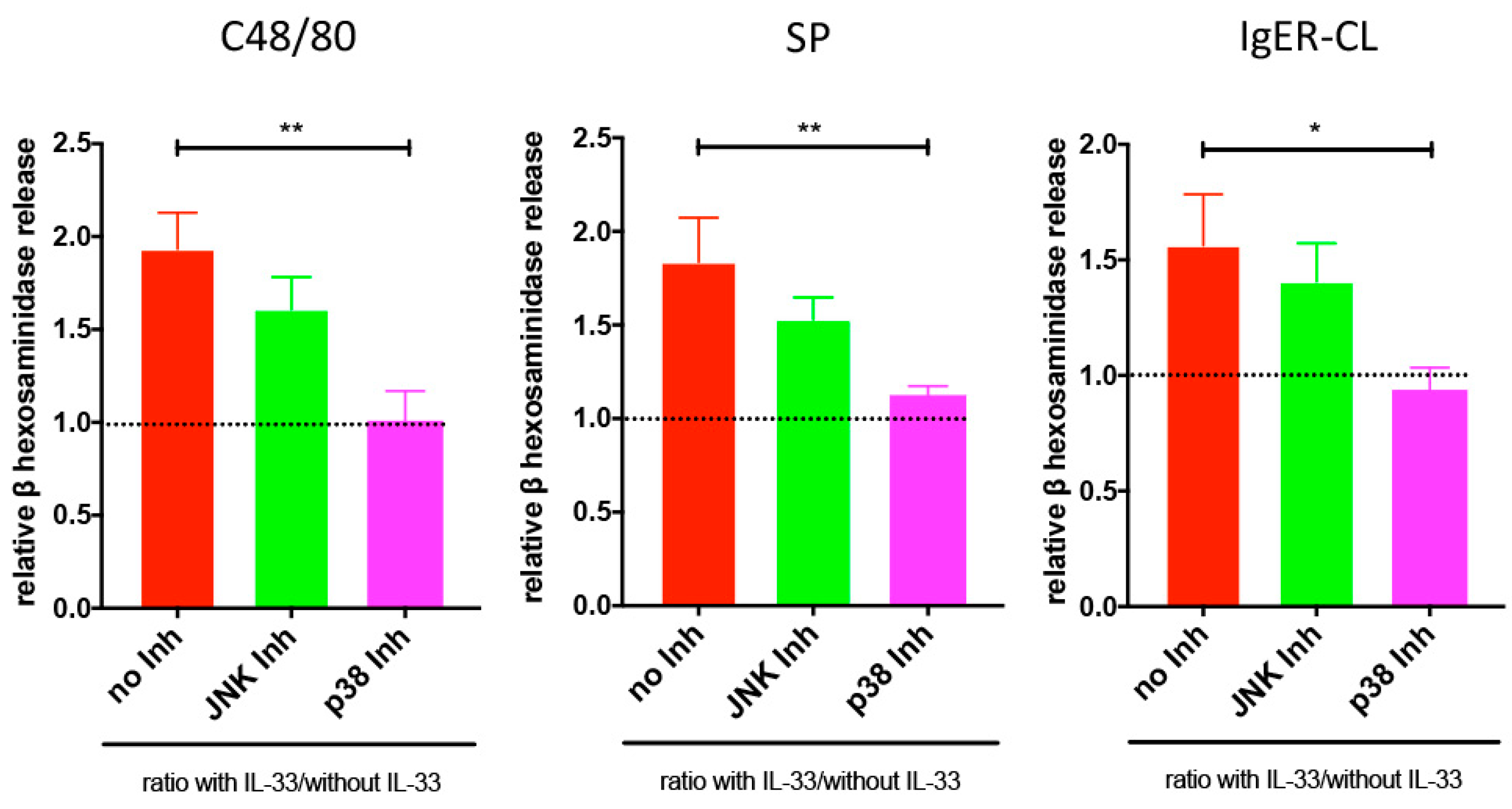
3.4. Reinforced Degranulation by IL-33 Depends on p38
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, H. Emerging Roles for MAS-Related G Protein-Coupled Receptor-X2 in Host Defense Peptide, Opioid, and Neuropeptide-Mediated Inflammatory Reactions. Adv. Immunol. 2017, 136, 123–162. [Google Scholar] [CrossRef] [PubMed]
- Castells, M. Drug Hypersensitivity and Anaphylaxis in Cancer and Chronic Inflammatory Diseases: The Role of Desensitizations. Front. Immunol. 2017, 8, 1472. [Google Scholar] [CrossRef] [PubMed]
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol. 2018, 9, 3027. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P.; Garvey, L.H. Mast Cells and Anaphylaxis. Curr. Allergy Asthma Rep. 2016, 16, 20. [Google Scholar] [CrossRef]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Tainsh, K.R.; Lau, H.Y.; Liu, W.L.; Pearce, F.L. The human skin mast cell: A comparison with the human lung cell and a novel mast cell type, the uterine mast cell. Agents Actions 1991, 33, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K.; Clough, G.F. Human skin mast cells: In vitro and in vivo studies. Ann Allergy Asthma Immunol. 1999, 83, 471–475. [Google Scholar] [CrossRef]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef]
- Irani, A.M.; Bradford, T.R.; Kepley, C.L.; Schechter, N.M.; Schwartz, L.B. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J. Histochem. Cytochem. 1989, 37, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Irani, A.A.; Schechter, N.M.; Craig, S.S.; DeBlois, G.; Schwartz, L.B. Two types of human mast cells that have distinct neutral protease compositions. Proc. Natl. Acad. Sci. USA 1986, 83, 4464–4468. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood 2014, 123, e58–e67. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Subramanian, H.; Ali, H. Modulation of host defense peptide-mediated human mast cell activation by LPS. Innate Immun. 2016, 22, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Idahosa, C.; Roy, S.; Lee, D.; Subramanian, H.; Dhingra, A.; Boesze-Battaglia, K.; Korostoff, J.; Ali, H. Differential Regulation of Mas-Related G Protein-Coupled Receptor X2-Mediated Mast Cell Degranulation by Antimicrobial Host Defense Peptides and Porphyromonas gingivalis Lipopolysaccharide. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. Allergic FcepsilonRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy 2018, 73, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Gaudenzio, N.; Sibilano, R.; Marichal, T.; Starkl, P.; Reber, L.L.; Cenac, N.; McNeil, B.D.; Dong, X.; Hernandez, J.D.; Sagi-Eisenberg, R.; et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Investig. 2016, 126, 3981–3998. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef]
- Babina, M.; Wang, Z.; Artuc, M.; Guhl, S.; Zuberbier, T. MRGPRX2 is negatively targeted by SCF and IL-4 to diminish pseudo-allergic stimulation of skin mast cells in culture. Exp. Dermatol. 2018, 27, 1298–1303. [Google Scholar] [CrossRef]
- Babina, M.; Artuc, M.; Guhl, S.; Zuberbier, T. Retinoic Acid Negatively Impacts Proliferation and MCTC Specific Attributes of Human Skin Derived Mast Cells, but Reinforces Allergic Stimulability. Int. J. Mol. Sci. 2017, 18, 525. [Google Scholar] [CrossRef] [PubMed]
- Lunderius-Andersson, C.; Enoksson, M.; Nilsson, G. Mast Cells Respond to Cell Injury through the Recognition of IL-33. Front. Immunol. 2012, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Ketelaar, M.E.; Hawro, T.; Church, M.K.; Maurer, M.; Nawijn, M.C. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol. Immunol. 2015, 63, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef]
- Babina, M.; Wang, Z.; Franke, K.; Guhl, S.; Artuc, M.; Zuberbier, T. Yin-yang of IL-33 in human skin mast cells: Reduced degranulation, but augmented histamine synthesis through p38 activation. J. Investig. Dermatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cop, N.; Ebo, D.G.; Bridts, C.H.; Elst, J.; Hagendorens, M.M.; Mertens, C.; Faber, M.A.; De Clerck, L.S.; Sabato, V. Influence of IL-6, IL-33, and TNF-alpha on human mast cell activation: Lessons from single cell analysis by flow cytometry. Cytometry B Clin. Cytom. 2018, 94, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Joulia, R.; L’Faqihi, F.E.; Valitutti, S.; Espinosa, E. IL-33 fine tunes mast cell degranulation and chemokine production at the single-cell level. J. Allergy Clin. Immunol. 2017, 140, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Fux, M.; Pecaric-Petkovic, T.; Odermatt, A.; Hausmann, O.V.; Lorentz, A.; Bischoff, S.C.; Virchow, J.C.; Dahinden, C.A. IL-33 is a mediator rather than a trigger of the acute allergic response in humans. Allergy 2014, 69, 216–222. [Google Scholar] [CrossRef]
- Galand, C.; Leyva-Castillo, J.M.; Yoon, J.; Han, A.; Lee, M.S.; McKenzie, A.N.J.; Stassen, M.; Oyoshi, M.K.; Finkelman, F.D.; Geha, R.S. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J. Allergy Clin. Immunol. 2016, 138, 1356–1366. [Google Scholar] [CrossRef]
- Jung, M.Y.; Smrz, D.; Desai, A.; Bandara, G.; Ito, T.; Iwaki, S.; Kang, J.H.; Andrade, M.V.; Hilderbrand, S.C.; Brown, J.M.; et al. IL-33 induces a hyporesponsive phenotype in human and mouse mast cells. J. Immunol. 2013, 190, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Guhl, S.; Artuc, M.; Trivedi, N.N.; Zuberbier, T. Phenotypic variability in human skin mast cells. Exp. Dermatol. 2016, 25, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. Skin mast cell phenotypes between two highly divergent cohorts—More pronounced variability within than between groups. Exp. Dermatol. 2017, 26, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Guhl, S.; Starke, A.; Kirchhof, L.; Zuberbier, T.; Henz, B.M. Comparative cytokine profile of human skin mast cells from two compartments--strong resemblance with monocytes at baseline but induction of IL-5 by IL-4 priming. J. Leukoc. Biol. 2004, 75, 244–252. [Google Scholar] [CrossRef]
- Guhl, S.; Neou, A.; Artuc, M.; Zuberbier, T.; Babina, M. Skin mast cells develop non-synchronized changes in typical lineage characteristics upon culture. Exp. Dermatol. 2014, 23, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Guhl, S.; Artuc, M.; Neou, A.; Babina, M.; Zuberbier, T. Long-term cultured human skin mast cells are suitable for pharmacological studies of anti-allergic drugs due to high responsiveness to FcepsilonRI cross-linking. Biosci. Biotechnol. Biochem. 2011, 75, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Hazzan, T.; Eberle, J.; Worm, M.; Babina, M. Apoptotic resistance of human skin mast cells is mediated by Mcl-1. Cell Death Discov. 2017, 3, 17048. [Google Scholar] [CrossRef]
- Hazzan, T.; Guhl, S.; Artuc, M.; Franke, K.; Worm, M.; Zuberbier, T.; Babina, M. An efficient method for gene knock-down by RNA interference in human skin mast cells. Exp. Dermatol. 2017, 26, 1136–1139. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef]
- Liew, F.Y.; Pitman, N.I.; McInnes, I.B. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat. Rev. Immunol. 2010, 10, 103–110. [Google Scholar] [CrossRef]
- Halim, T.Y.; Steer, C.A.; Matha, L.; Gold, M.J.; Martinez-Gonzalez, I.; McNagny, K.M.; McKenzie, A.N.; Takei, F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef]
- Enoksson, M.; Moller-Westerberg, C.; Wicher, G.; Fallon, P.G.; Forsberg-Nilsson, K.; Lunderius-Andersson, C.; Nilsson, G. Intraperitoneal influx of neutrophils in response to IL-33 is mast cell-dependent. Blood 2013, 121, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Klonowska, J.; Glen, J.; Nowicki, R.J.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis-New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.; et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.H.; Lee, E.; Lee, B.C.; Choi, M.J.; Kwon, T.R.; Hong, J.; Mun, S.K.; Lee, K.; Kim, S.; Na, J.; et al. Therapeutic potential of topically administered gamma-AlOOH on 2,4-dinitrochlorobenzene-induced atopic dermatitis-like lesions in Balb/c mice. Exp. Dermatol. 2018. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, J. Serum levels of IL-31, IL-33 and ST2 in allergic rhinitis of children in China. Cell Mol. Biol. (Noisy-le-grand) 2018, 64, 52–55. [Google Scholar] [CrossRef]
- Balato, A.; Lembo, S.; Mattii, M.; Schiattarella, M.; Marino, R.; De Paulis, A.; Balato, N.; Ayala, F. IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp. Dermatol. 2012, 21, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimaki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Zhang, B.; Kempuraj, D.; Tagen, M.; Vasiadi, M.; Angelidou, A.; Alysandratos, K.D.; Kalogeromitros, D.; Asadi, S.; Stavrianeas, N.; et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. USA 2010, 107, 4448–4453. [Google Scholar] [CrossRef]
- Afferni, C.; Buccione, C.; Andreone, S.; Galdiero, M.R.; Varricchi, G.; Marone, G.; Mattei, F.; Schiavoni, G. The Pleiotropic Immunomodulatory Functions of IL-33 and Its Implications in Tumor Immunity. Front. Immunol. 2018, 9, 2601. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Irani, A.M.; Schwartz, L.B. Human mast cell heterogeneity. Allergy Proc. 1994, 15, 303–308. [Google Scholar] [CrossRef]
- Azimi, E.; Reddy, V.B.; Shade, K.C.; Anthony, R.M.; Talbot, S.; Pereira, P.J.S.; Lerner, E.A. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight 2016, 1, e89362. [Google Scholar] [CrossRef]
- Azimi, E.; Reddy, V.B.; Lerner, E.A. Brief communication: MRGPRX2, atopic dermatitis and red man syndrome. Itch 2017, 2. [Google Scholar] [CrossRef]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019, 101, 412–420.e13. [Google Scholar] [CrossRef]
- Muto, Y.; Wang, Z.; Vanderberghe, M.; Two, A.; Gallo, R.L.; Di Nardo, A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J. Investig. Dermatol. 2014, 134, 2728–2736. [Google Scholar] [CrossRef]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Yamada, K.; Sato, H.; Sakamaki, K.; Kamada, M.; Okuno, Y.; Fukuishi, N.; Furuta, K.; Tanaka, S. Suppression of IgE-Independent Degranulation of Murine Connective Tissue-Type Mast Cells by Dexamethasone. Cells 2019, 8, 112. [Google Scholar] [CrossRef]
- Kaur, D.; Gomez, E.; Doe, C.; Berair, R.; Woodman, L.; Saunders, R.; Hollins, F.; Rose, F.R.; Amrani, Y.; May, R.; et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: Airway smooth muscle crosstalk. Allergy 2015, 70, 556–567. [Google Scholar] [CrossRef]
- Silver, M.R.; Margulis, A.; Wood, N.; Goldman, S.J.; Kasaian, M.; Chaudhary, D. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm. Res. 2010, 59, 207–218. [Google Scholar] [CrossRef]
- Rivellese, F.; Suurmond, J.; de Paulis, A.; Marone, G.; Huizinga, T.W.; Toes, R.E. IgE and IL-33-mediated triggering of human basophils inhibits TLR4-induced monocyte activation. Eur. J. Immunol. 2014, 44, 3045–3055. [Google Scholar] [CrossRef] [PubMed]
- Rivellese, F.; Suurmond, J.; Habets, K.; Dorjee, A.L.; Ramamoorthi, N.; Townsend, M.J.; de Paulis, A.; Marone, G.; Huizinga, T.W.; Pitzalis, C.; et al. Ability of Interleukin-33- and Immune Complex-Triggered Activation of Human Mast Cells to Down-Regulate Monocyte-Mediated Immune Responses. Arthritis Rheumatol. 2015, 67, 2343–2353. [Google Scholar] [CrossRef]
- Ishizuka, T.; Okajima, F.; Ishiwara, M.; Iizuka, K.; Ichimonji, I.; Kawata, T.; Tsukagoshi, H.; Dobashi, K.; Nakazawa, T.; Mori, M. Sensitized mast cells migrate toward the antigen: A response regulated by p38 mitogen-activated protein kinase and Rho-associated coiled-coil-forming protein kinase. J. Immunol. 2001, 167, 2298–2304. [Google Scholar] [CrossRef]
- Craig, A.W.; Greer, P.A. Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells. Mol. Cell Biol. 2002, 22, 6363–6374. [Google Scholar] [CrossRef]
- Sundstrom, M.; Alfredsson, J.; Olsson, N.; Nilsson, G. Stem cell factor-induced migration of mast cells requires p38 mitogen-activated protein kinase activity. Exp. Cell. Res. 2001, 267, 144–151. [Google Scholar] [CrossRef]
- Teshima, R.; Onose, J.; Okunuki, H.; Sawada, J. Effect of Ca(2+) ATPase inhibitors on MCP-1 release from bone marrow-derived mast cells and the involvement of p38 MAP kinase activation. Int. Arch Allergy Immunol. 2000, 121, 34–43. [Google Scholar] [CrossRef]
- Koranteng, R.D.; Swindle, E.J.; Davis, B.J.; Dearman, R.J.; Kimber, I.; Flanagan, B.F.; Coleman, J.W. Differential regulation of mast cell cytokines by both dexamethasone and the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580. Clin. Exp. Immunol. 2004, 137, 81–87. [Google Scholar] [CrossRef]
- Wong, C.K.; Tsang, C.M.; Ip, W.K.; Lam, C.W. Molecular mechanisms for the release of chemokines from human leukemic mast cell line (HMC)-1 cells activated by SCF and TNF-alpha: Roles of ERK, p38 MAPK, and NF-kappaB. Allergy 2006, 61, 289–297. [Google Scholar] [CrossRef]
- Moon, T.C.; Befus, A.D. Exogenous nitric oxide regulates cyclooxygenase-2 expression and prostaglandin D(2) generation through p38 MAPK in mouse bone marrow-derived mast cells. Free Radic. Biol. Med. 2008, 45, 780–788. [Google Scholar] [CrossRef]
- Saluja, R.; Hawro, T.; Eberle, J.; Church, M.K.; Maurer, M. Interleukin-33 promotes the proliferation of mouse mast cells through ST2/MyD88 and p38 MAPK-dependent and Kit-independent pathways. J. Biol. Regul. Homeost. Agents 2014, 28, 575–585. [Google Scholar]
- McCarthy, P.C.; Phair, I.R.; Greger, C.; Pardali, K.; McGuire, V.A.; Clark, A.R.; Gaestel, M.; Arthur, J.S.C. IL-33 regulates cytokine production and neutrophil recruitment via the p38 MAPK-activated kinases MK2/3. Immunol. Cell Biol. 2019, 97, 54–71. [Google Scholar] [CrossRef]
- Zhang, C.; Baumgartner, R.A.; Yamada, K.; Beaven, M.A. Mitogen-activated protein (MAP) kinase regulates production of tumor necrosis factor-alpha and release of arachidonic acid in mast cells. Indications of communication between p38 and p42 MAP kinases. J. Biol. Chem. 1997, 272, 13397–13402. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Thorpe, M.; Alemayehu, R.; Roy, A.; Kervinen, J.; de Garavilla, L.; Abrink, M.; Hellman, L. Highly Selective Cleavage of Cytokines and Chemokines by the Human Mast Cell Chymase and Neutrophil Cathepsin G. J. Immunol. 2017, 198, 1474–1483. [Google Scholar] [CrossRef]
- Roy, A.; Ganesh, G.; Sippola, H.; Bolin, S.; Sawesi, O.; Dagalv, A.; Schlenner, S.M.; Feyerabend, T.; Rodewald, H.R.; Kjellen, L.; et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J. Biol. Chem. 2014, 289, 237–250. [Google Scholar] [CrossRef]
- Waern, I.; Lundequist, A.; Pejler, G.; Wernersson, S. Mast cell chymase modulates IL-33 levels and controls allergic sensitization in dust-mite induced airway inflammation. Mucosal. Immunol. 2013, 6, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Arae, K.; Unno, H.; Miyauchi, K.; Toyama, S.; Nambu, A.; Oboki, K.; Ohno, T.; Motomura, K.; Matsuda, A.; et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity 2015, 43, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Salamon, P.; Shefler, I.; Moshkovits, I.; Munitz, A.; Horwitz Klotzman, D.; Mekori, Y.A.; Hershko, A.Y. IL-33 and IgE stimulate mast cell production of IL-2 and regulatory T cell expansion in allergic dermatitis. Clin. Exp. Allergy 2017, 47, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Zoltowska Nilsson, A.M.; Lei, Y.; Adner, M.; Nilsson, G.P. Mast cell-dependent IL-33/ST2 signaling is protective against the development of airway hyperresponsiveness in a house dust mite mouse model of asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L484–L492. [Google Scholar] [CrossRef]
- He, Z.; Song, J.; Hua, J.; Yang, M.; Ma, Y.; Yu, T.; Feng, J.; Liu, B.; Wang, X.; Li, Y.; et al. Mast cells are essential intermediaries in regulating IL-33/ST2 signaling for an immune network favorable to mucosal healing in experimentally inflamed colons. Cell Death Dis. 2018, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Guhl, S.; Franke, K.; Artuc, M.; Zuberbier, T.; Babina, M. IL-33 and MRGPRX2-Triggered Activation of Human Skin Mast Cells—Elimination of Receptor Expression on Chronic Exposure, but Reinforced Degranulation on Acute Priming. Cells 2019, 8, 341. https://doi.org/10.3390/cells8040341
Wang Z, Guhl S, Franke K, Artuc M, Zuberbier T, Babina M. IL-33 and MRGPRX2-Triggered Activation of Human Skin Mast Cells—Elimination of Receptor Expression on Chronic Exposure, but Reinforced Degranulation on Acute Priming. Cells. 2019; 8(4):341. https://doi.org/10.3390/cells8040341
Chicago/Turabian StyleWang, Zhao, Sven Guhl, Kristin Franke, Metin Artuc, Torsten Zuberbier, and Magda Babina. 2019. "IL-33 and MRGPRX2-Triggered Activation of Human Skin Mast Cells—Elimination of Receptor Expression on Chronic Exposure, but Reinforced Degranulation on Acute Priming" Cells 8, no. 4: 341. https://doi.org/10.3390/cells8040341
APA StyleWang, Z., Guhl, S., Franke, K., Artuc, M., Zuberbier, T., & Babina, M. (2019). IL-33 and MRGPRX2-Triggered Activation of Human Skin Mast Cells—Elimination of Receptor Expression on Chronic Exposure, but Reinforced Degranulation on Acute Priming. Cells, 8(4), 341. https://doi.org/10.3390/cells8040341






