Mast Cell Protease 7 Promotes Angiogenesis by Degradation of Integrin Subunits
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
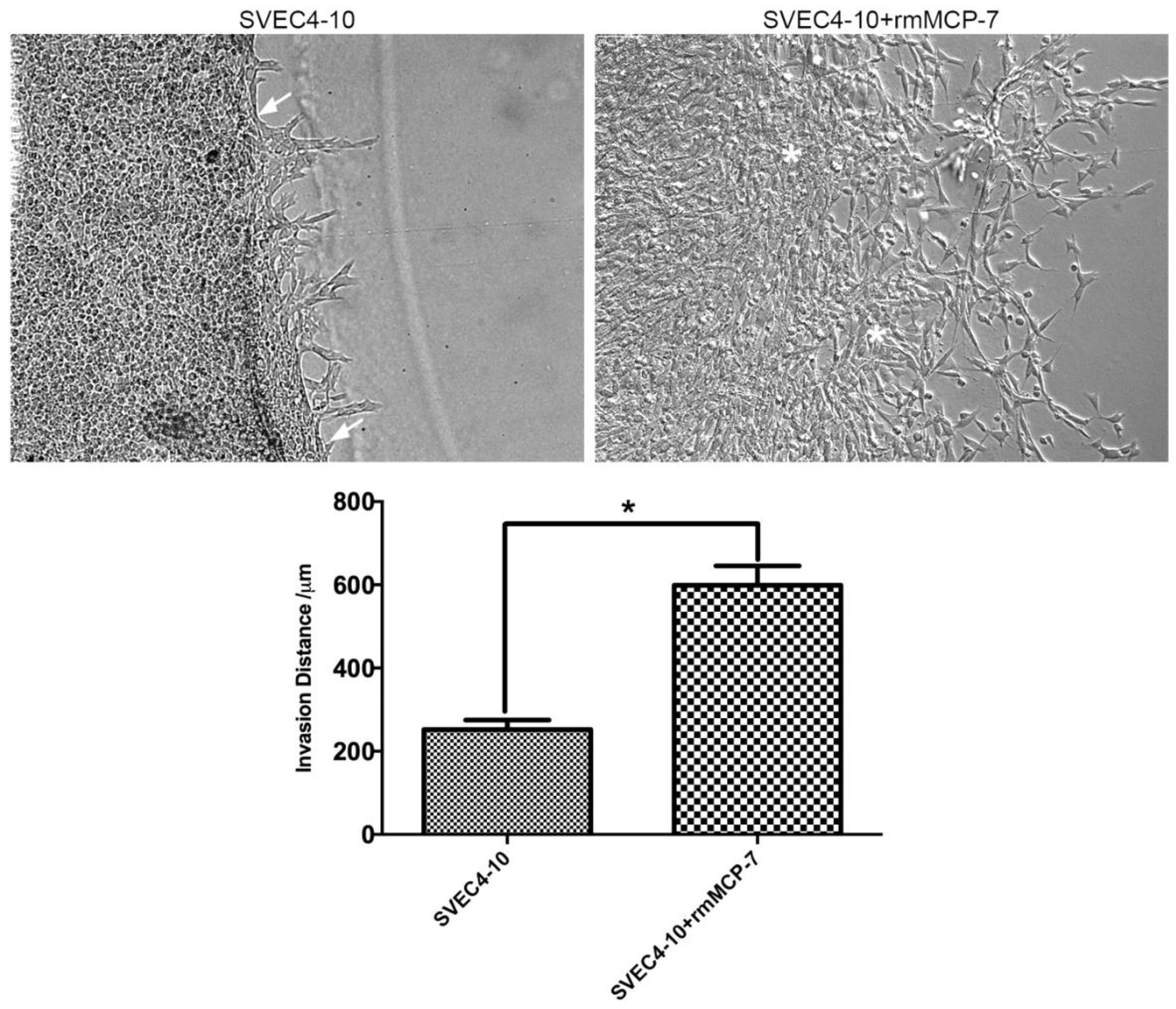
2.2. Cells Invasion Assay
2.3. Directed In Vivo Angiogenesis AssayTM (DIVAATM)
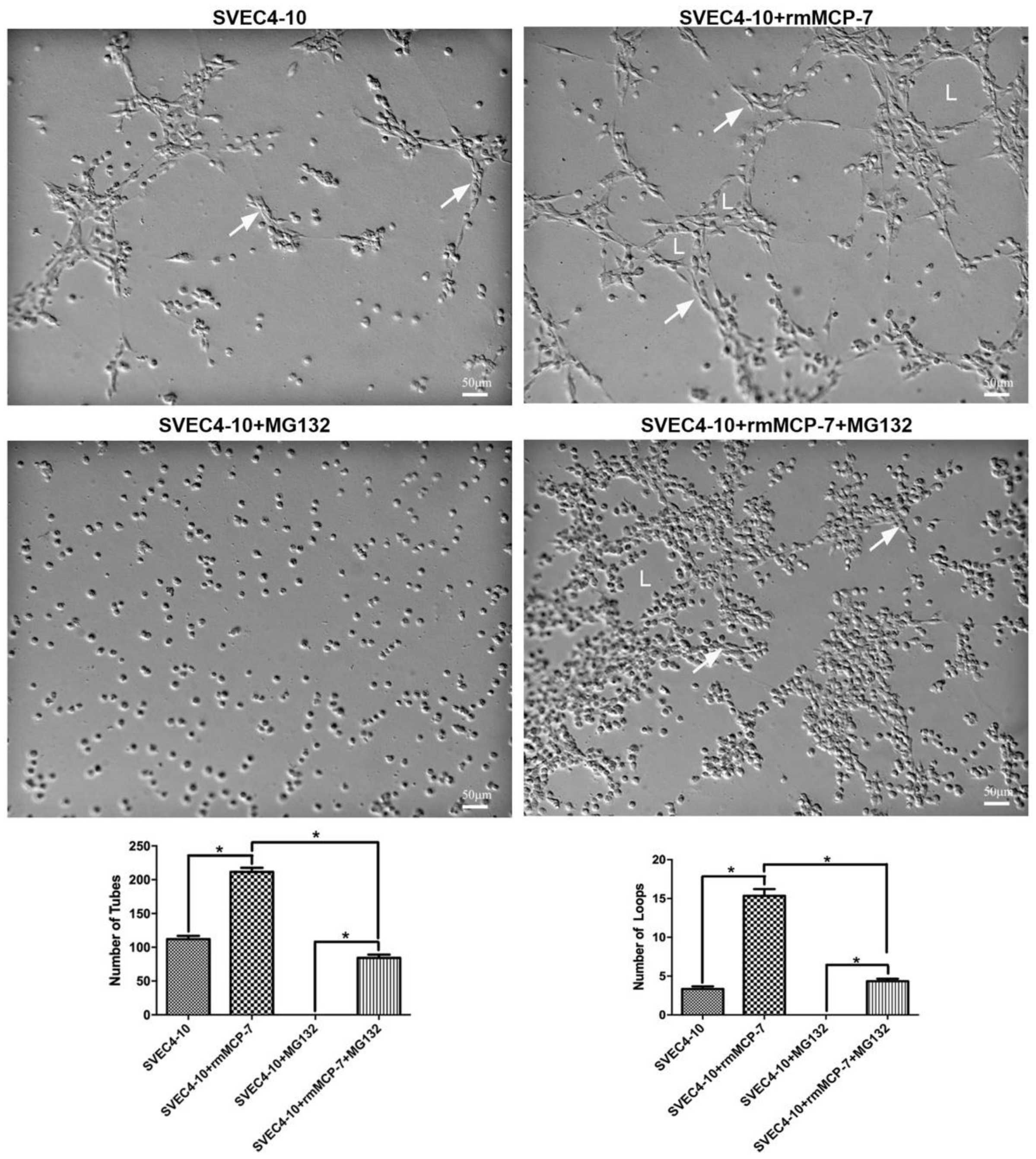
2.4. In Vitro Angiogenesis: Tube Formation Assay
2.5. Expression of Integrin Subunits
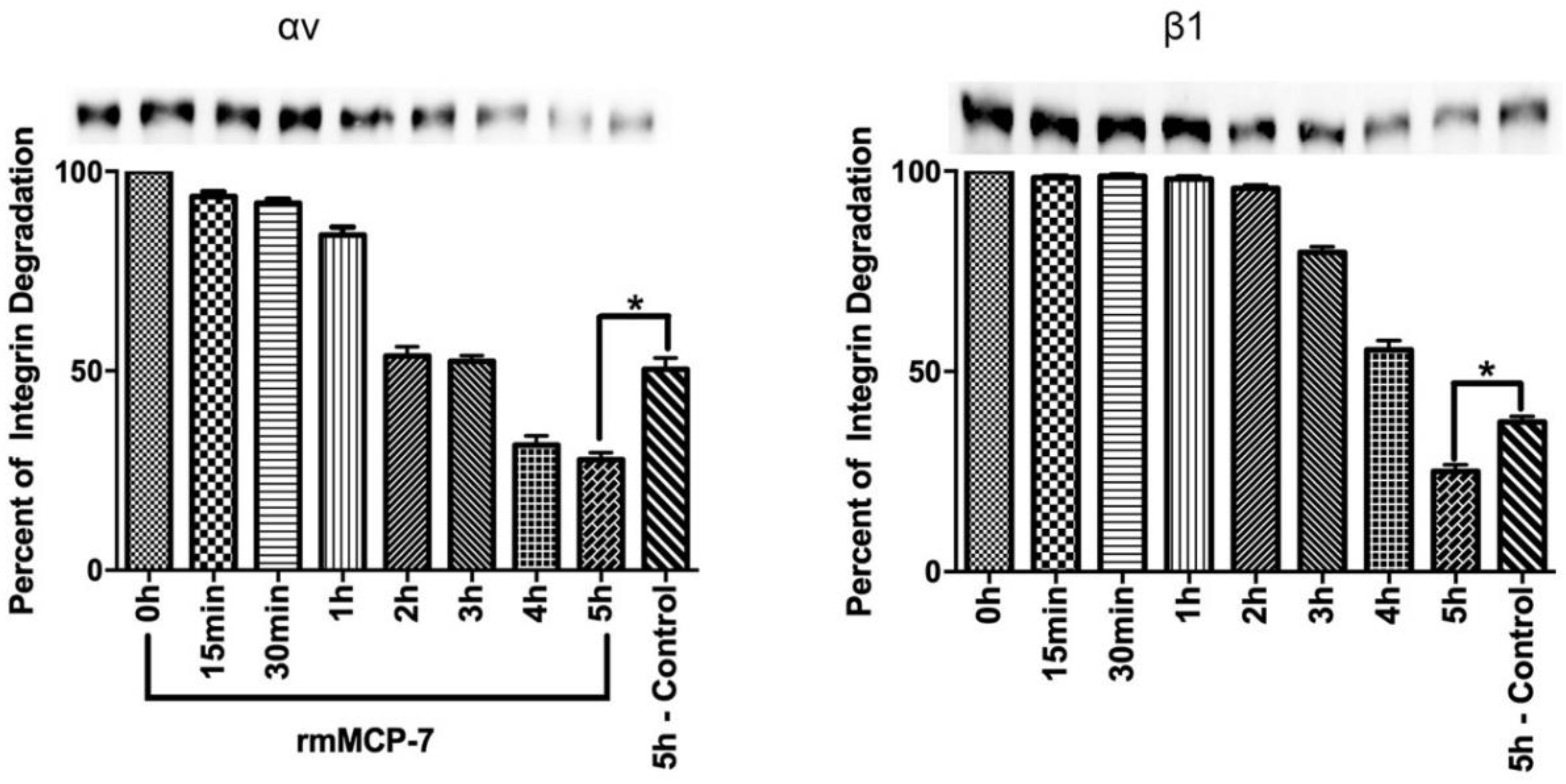
2.6. Integrin Degradation Assay
2.7. Proteasome Inhibition
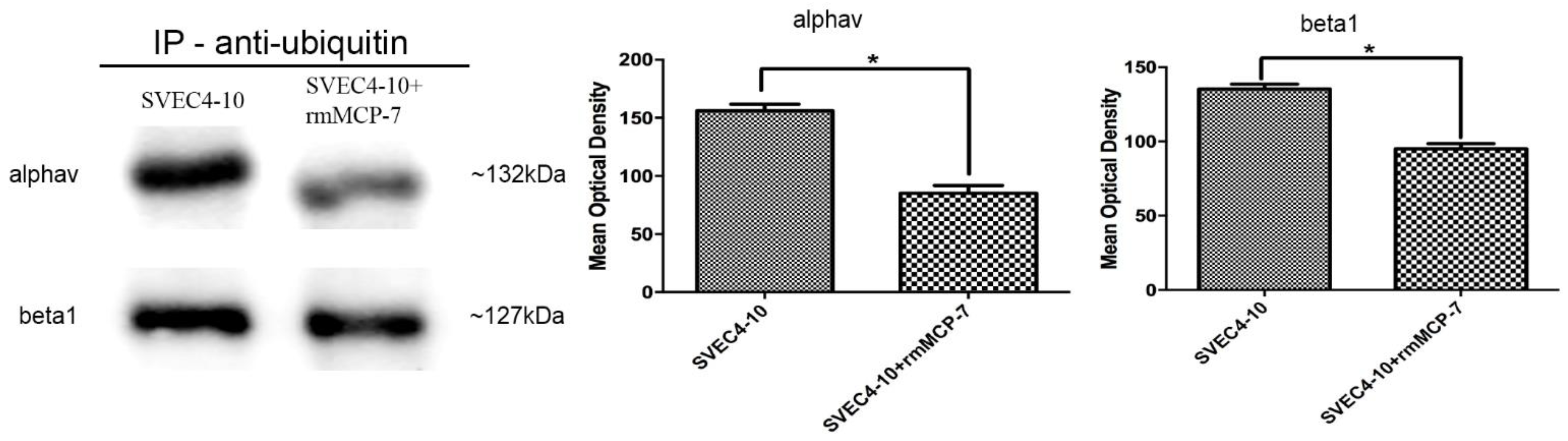
2.8. Immunoprecitions Assay Using Anti-Ubiquitin
2.9. Statistical Analysis
3. Results
3.1. rmMCP-7 Protease Induces Endothelial Cell Invasion
3.2. rmMCP-7 Induces In Vivo Angiogenesis
3.3. rmMCP-7 Induces Changes in Integrin Subunit Levels during Angiogenesis
3.4. rmMCP-7 Acts directly to Degrade Integrin Subunits
3.5. The Degradation of Integrin Subunits Occurs Indirectly via the Ubiquitin/Proteasome System
3.6. rmMCP-7 Induces Angiogenesis Independent of the Ubiquitin/Proteasome System
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kerbel, R.S. Tumor angiogenesis: Past, present and the near future. Carcinogenesis 2000, 21, 505–515. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Ausprunk, D.H.; Folkman, J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc. Res. 1977, 14, 53–65. [Google Scholar] [CrossRef]
- Gacche, R.N.; Meshram, R.J. Angiogenic factors as potential drug target: Efficacy and limitations of anti-angiogenic therapy. Biochim. Biophys. Acta 2014, 1846, 161–179. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Shan, S.; Huang, Q.; Braun, R.D.; Lanzen, J.; Hu, K.; Lin, P.; Dewhirst, M.W. Initial stages of tumor cell-induced angiogenesis: Evaluation via skin window chambers in rodent models. J. Natl. Cancer Inst. 2000, 92, 143–147. [Google Scholar] [CrossRef]
- Senger, D.R.; Davis, G.E. Angiogenesis. Cold Spring Harb Perspect. Biol. 2011, 3, a005090. [Google Scholar] [CrossRef]
- Tahergorabi, Z.; Khazaei, M. A review on angiogenesis and its assays. Iran. J. Basic Med. Sci. 2012, 15, 1110–1126. [Google Scholar] [PubMed]
- Yana, I.; Sagara, H.; Takaki, S.; Takatsu, K.; Nakamura, K.; Nakao, K.; Katsuki, M.; Taniguchi, S.; Aoki, T.; Sato, H.; et al. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J. Cell Sci. 2007, 120, 1607–1614. [Google Scholar] [CrossRef]
- Thurston, G.; Kitajewski, J. VEGF and Delta-Notch: Interacting signalling pathways in tumour angiogenesis. Br. J. Cancer 2008, 99, 1204–1209. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Ruhrberg, C.; Gerhardt, H.; Golding, M.; Watson, R.; Ioannidou, S.; Fujisawa, H.; Betsholtz, C.; Shima, D.T. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002, 16, 2684–2698. [Google Scholar] [CrossRef]
- Beets, K.; Huylebroeck, D.; Moya, I.M.; Umans, L.; Zwijsen, A. Robustness in angiogenesis: Notch and BMP shaping waves. Trends Genet. 2013, 29, 140–149. [Google Scholar] [CrossRef]
- Suchting, S.; Freitas, C.; le Noble, F.; Benedito, R.; Bréant, C.; Duarte, A.; Eichmann, A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA 2007, 104, 3225–3230. [Google Scholar] [CrossRef]
- Hellström, M.; Phng, L.K.; Hofmann, J.J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.; Nilsson, A.K.; Karlsson, L.; Gaiano, N.; et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007, 445, 776–780. [Google Scholar] [CrossRef]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lai, M.D.; Lei, H.Y.; Wing, L.Y. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology 2006, 147, 1278–1286. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Phagocytes & granulocytes. Angiogenic neutrophils: A novel subpopulation paradigm. Blood 2012, 120, 4455–4457. [Google Scholar] [CrossRef]
- Norton, K.A.; Popel, A.S. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci. Rep. 2016, 6, 36992. [Google Scholar] [CrossRef]
- Da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Espinosa, E.; Valitutti, S. New roles and controls of mast cells. Curr. Opin. Immunol. 2017, 50, 39–47. [Google Scholar] [CrossRef]
- Marichal, T.; Tsai, M.; Galli, S.J. Mast cells: Potential positive and negative roles in tumor biology. Cancer Immunol. Res. 2013, 1, 269–279. [Google Scholar] [CrossRef]
- Wedemeyer, J.; Galli, S.J. Mast cells and basophils in acquired immunity. Br. Med. Bull. 2000, 56, 936–955. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Soucie, E.; Brenet, F.; Dubreuil, P. Molecular basis of mast cell disease. Mol. Immunol. 2015, 63, 55–60. [Google Scholar] [CrossRef]
- Jamur, M.C.; Oliver, C. Origin, maturation and recruitment of mast cell precursors. Front. Biosci. (Sch. Ed.) 2011, 3, 1390–1406. [Google Scholar]
- Harvima, I.T.; Levi-Schaffer, F.; Draber, P.; Friedman, S.; Polakovicova, I.; Gibbs, B.F.; Blank, U.; Nilsson, G.; Maurer, M. Molecular targets on mast cells and basophils for novel therapies. J. Allergy Clin. Immunol. 2014, 134, 530–544. [Google Scholar] [CrossRef]
- Pejler, G.; Abrink, M.; Ringvall, M.; Wernersson, S. Mast cell proteases. Adv. Immunol. 2007, 95, 167–255. [Google Scholar] [CrossRef]
- McNeil, H.P.; Shin, K.; Campbell, I.K.; Wicks, I.P.; Adachi, R.; Lee, D.M.; Stevens, R.L. The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 2008, 58, 2338–2346. [Google Scholar] [CrossRef]
- Huang, C.; Wong, G.W.; Ghildyal, N.; Gurish, M.F.; Sali, A.; Matsumoto, R.; Qiu, W.T.; Stevens, R.L. The tryptase, mouse mast cell protease 7, exhibits anticoagulant activity in vivo and in vitro due to its ability to degrade fibrinogen in the presence of the diverse array of protease inhibitors in plasma. J. Biol. Chem. 1997, 272, 31885–31893. [Google Scholar] [CrossRef]
- Lundequist, A.; Pejler, G. Biological implications of preformed mast cell mediators. Cell. Mol. Life Sci. 2011, 68, 965–975. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Shubin, N.J.; Glukhova, V.A.; Clauson, M.; Truong, P.; Abrink, M.; Pejler, G.; White, N.J.; Deutsch, G.H.; Reeves, S.R.; Vaisar, T.; et al. Proteome analysis of mast cell releasates reveals a role for chymase in the regulation of coagulation factor XIIIA levels via proteolytic degradation. J. Allergy Clin. Immunol. 2016, 139, 323–334. [Google Scholar] [CrossRef]
- Wong, G.W.; Li, L.; Madhusudhan, M.S.; Krilis, S.A.; Gurish, M.F.; Rothenberg, M.E.; Sali, A.; Stevens, R.L. Tryptase 4, a new member of the chromosome 17 family of mouse serine proteases. J. Biol. Chem. 2001, 276, 20648–20658. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell proteases as pharmacological targets. Eur. J. Pharmacol. 2016, 778, 44–55. [Google Scholar] [CrossRef]
- Reynolds, D.S.; Stevens, R.L.; Lane, W.S.; Carr, M.H.; Austen, K.F.; Serafin, W.E. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc. Natl. Acad. Sci. USA 1990, 87, 3230–3234. [Google Scholar] [CrossRef]
- Stevens, R.L.; Friend, D.S.; McNeil, H.P.; Schiller, V.; Ghildyal, N.; Austen, K.F. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc. Natl. Acad. Sci. USA 1994, 91, 128–132. [Google Scholar] [CrossRef]
- Gurish, M.F.; Pear, W.S.; Stevens, R.L.; Scott, M.L.; Sokol, K.; Ghildyal, N.; Webster, M.J.; Hu, X.; Austen, K.F.; Baltimore, D. Tissue-regulated differentiation and maturation of a v-abl-immortalized mast cell-committed progenitor. Immunity 1995, 3, 175–186. [Google Scholar] [CrossRef]
- Vangansewinkel, T.; Geurts, N.; Quanten, K.; Nelissen, S.; Lemmens, S.; Geboes, L.; Dooley, D.; Vidal, P.M.; Pejler, G.; Hendrix, S. Mast cells promote scar remodeling and functional recovery after spinal cord injury via mouse mast cell protease 6. FASEB J. 2016, 30, 2040–2057. [Google Scholar] [CrossRef]
- Reynolds, D.S.; Gurley, D.S.; Austen, K.F.; Serafin, W.E. Cloning of the cDNA and gene of mouse mast cell protease-6. Transcription by progenitor mast cells and mast cells of the connective tissue subclass. J. Biol. Chem. 1991, 266, 3847–3853. [Google Scholar]
- McNeil, H.P.; Reynolds, D.S.; Schiller, V.; Ghildyal, N.; Gurley, D.S.; Austen, K.F.; Stevens, R.L. Isolation, characterization, and transcription of the gene encoding mouse mast cell protease 7. Proc. Natl. Acad. Sci. USA 1992, 89, 11174–11178. [Google Scholar] [CrossRef]
- Morii, E.; Tsujimura, T.; Jippo, T.; Hashimoto, K.; Takebayashi, K.; Tsujino, K.; Nomura, S.; Yamamoto, M.; Kitamura, Y. Regulation of mouse mast cell protease 6 gene expression by transcription factor encoded by the mi locus. Blood 1996, 88, 2488–2494. [Google Scholar]
- Hiromatsu, Y.; Toda, S. Mast cells and angiogenesis. Microsc. Res. Tech. 2003, 60, 64–69. [Google Scholar] [CrossRef]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef]
- Paolino, G.; Corsetti, P.; Moliterni, E.; Corsetti, S.; Didona, D.; Albanesi, M.; Mattozzi, C.; Lido, P.; Calvieri, S. Mast cells and cancer: A review of literature. Giornale Italiano di Dermatologia e Venereologia 2017. [Google Scholar] [CrossRef]
- Norrby, K. Mast cells and angiogenesis. APMIS 2002, 110, 355–371. [Google Scholar] [CrossRef]
- Vacca, A.; Ria, R.; Reale, A.; Ribatti, D. Angiogenesis in multiple myeloma. Chem. Immunol. Allergy 2014, 99, 180–196. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Vacca, A. Mast Cells and Angiogenesis in Human Plasma Cell Malignancies. Int. J. Mol. Sci. 2019, 20, 481. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta 2012, 1822, 2–8. [Google Scholar] [CrossRef]
- Crivellato, E.; Nico, B.; Ribatti, D. Mast cells and tumour angiogenesis: New insight from experimental carcinogenesis. Cancer Lett. 2008, 269, 1–6. [Google Scholar] [CrossRef]
- Ribatti, D.; Ranieri, G.; Nico, B.; Benagiano, V.; Crivellato, E. Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int. J. Dev. Biol. 2011, 55, 99–102. [Google Scholar] [CrossRef]
- de Souza, D.A.; Toso, V.D.; Campos, M.R.; Lara, V.S.; Oliver, C.; Jamur, M.C. Expression of mast cell proteases correlates with mast cell maturation and angiogenesis during tumor progression. PLoS ONE 2012, 7, e40790. [Google Scholar] [CrossRef]
- Gaber, M.A.; Seliet, I.A.; Ehsan, N.A.; Megahed, M.A. Mast cells and angiogenesis in wound healing. Anal. Quant. Cytopathol. Histpathol. 2014, 36, 32–40. [Google Scholar]
- Sawatsubashi, M.; Yamada, T.; Fukushima, N.; Mizokami, H.; Tokunaga, O.; Shin, T. Association of vascular endothelial growth factor and mast cells with angiogenesis in laryngeal squamous cell carcinoma. Virchows Arch. 2000, 436, 243–248. [Google Scholar] [CrossRef]
- De Souza, D.A.; Borges, A.C.; Santana, A.C.; Oliver, C.; Jamur, M.C. Mast Cell Proteases 6 and 7 Stimulate Angiogenesis by Inducing Endothelial Cells to Release Angiogenic Factors. PLoS ONE 2015, 10, e0144081. [Google Scholar] [CrossRef]
- de Souza Junior, D.A.; Mazucato, V.M.; Santana, A.C.; Oliver, C.; Jamur, M.C. Mast Cells Interact with Endothelial Cells to Accelerate In Vitro Angiogenesis. Int. J. Mol. Sci. 2017, 18, 2674. [Google Scholar] [CrossRef]
- Bayless, K.J.; Kwak, H.I.; Su, S.C. Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat. Protoc. 2009, 4, 1888–1898. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lee, W.S.; Park, Y.L.; Kim, N.; Oh, H.H.; Son, D.J.; Kim, M.Y.; Oak, C.Y.; Chung, C.Y.; Park, H.C.; Kim, J.S.; et al. Myeloid cell leukemia-1 is associated with tumor progression by inhibiting apoptosis and enhancing angiogenesis in colorectal cancer. Am. J. Cancer Res. 2015, 5, 101–113. [Google Scholar]
- Askou, A.L.; Aagaard, L.; Kostic, C.; Arsenijevic, Y.; Hollensen, A.K.; Bek, T.; Jensen, T.G.; Mikkelsen, J.G.; Corydon, T.J. Multigenic lentiviral vectors for combined and tissue-specific expression of miRNA- and protein-based antiangiogenic factors. Mol. Ther. Methods Clin. Dev. 2015, 2, 14064. [Google Scholar] [CrossRef]
- Khoo, C.P.; Micklem, K.; Watt, S.M. A comparison of methods for quantifying angiogenesis in the Matrigel assay in vitro. Tissue Eng. Part C Methods 2011, 17, 895–906. [Google Scholar] [CrossRef]
- Subramanian, M.; Rao, S.R.; Thacker, P.; Chatterjee, S.; Karunagaran, D. MiR-29b downregulates canonical Wnt signaling by suppressing coactivators of β-catenin in human colorectal cancer cells. J. Cell. Biochem. 2014, 115, 1974–1984. [Google Scholar] [CrossRef]
- Waern, I.; Jonasson, S.; Hjoberg, J.; Bucht, A.; Abrink, M.; Pejler, G.; Wernersson, S. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J. Immunol. 2009, 183, 6369–6376. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Crivellato, E. The dual role of mast cells in tumor fate. Cancer Lett. 2018, 433, 252–258. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Crivellato, E. Cross talk between natural killer cells and mast cells in tumor angiogenesis. Inflamm. Res. 2019, 68, 19–23. [Google Scholar] [CrossRef]
- Kritas, S.K.; Gallenga, C.E.; D Ovidio, C.; Ronconi, G.; Caraffa, A.; Toniato, E.; Lauritano, D.; Conti, P. Impact of mold on mast cell-cytokine immune response. J. Biol. Regul. Homeost. Agents 2018, 32, 763–768. [Google Scholar]
- Qian, N.; Li, X.; Wang, X.; Wu, C.; Yin, L.; Zhi, X. Tryptase promotes breast cancer angiogenesis through PAR-2 mediated endothelial progenitor cell activation. Oncol. Lett. 2018, 16, 1513–1520. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Vescio, G.; Zuccalà, V.; Luposella, M.; Patruno, R.; Zizzo, N.; Gadaleta, C.; Marech, I.; Ruggieri, R.; et al. Tryptase mast cell density, protease-activated receptor-2 microvascular density, and classical microvascular density evaluation in gastric cancer patients undergoing surgery: Possible translational relevance. Therap. Adv. Gastroenterol. 2017, 10, 353–360. [Google Scholar] [CrossRef]
- Kielty, C.M.; Lees, M.; Shuttleworth, C.A.; Woolley, D. Catabolism of intact type VI collagen microfibrils: Susceptibility to degradation by serine proteinases. Biochem. Biophys. Res. Commun. 1993, 191, 1230–1236. [Google Scholar] [CrossRef]
- Ribatti, D.; Ranieri, G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef]
- Stack, M.S.; Johnson, D.A. Human mast cell tryptase activates single-chain urinary-type plasminogen activator (pro-urokinase). J. Biol. Chem. 1994, 269, 9416–9419. [Google Scholar]
- Dudeck, A.; Köberle, M.; Goldmann, O.; Meyer, N.; Dudeck, J.; Lemmens, S.; Rohde, M.; Roldán, N.G.; Dietze-Schwonberg, K.; Orinska, Z.; et al. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2018. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Shao, C.; Zeng, X.; Sun, L.; Kong, H.; Xie, W.; Wang, H. New dynamic viewing of mast cells in pulmonary arterial hypertension (PAH): Contributors or outsiders to cardiovascular remodeling. J. Thorac. Dis. 2018, 10, 3016–3026. [Google Scholar] [CrossRef]
- Schwartz, L.B.; Bradford, T.R.; Irani, A.M.; Deblois, G.; Craig, S.S. The major enzymes of human mast cell secretory granules. Am. Rev. Respir. Dis. 1987, 135, 1186–1189. [Google Scholar]
- Gruber, B.L.; Marchese, M.J.; Suzuki, K.; Schwartz, L.B.; Okada, Y.; Nagase, H.; Ramamurthy, N.S. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J. Clin. Investig. 1989, 84, 1657–1662. [Google Scholar] [CrossRef]
- Fang, K.C.; Wolters, P.J.; Steinhoff, M.; Bidgol, A.; Blount, J.L.; Caughey, G.H. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J. Immunol. 1999, 162, 5528–5535. [Google Scholar]
- Sendo, T.; Sumimura, T.; Itoh, Y.; Goromaru, T.; Aki, K.; Yano, T.; Oike, M.; Ito, Y.; Mori, S.; Nishibori, M.; et al. Involvement of proteinase-activated receptor-2 in mast cell tryptase-induced barrier dysfunction in bovine aortic endothelial cells. Cell Signal. 2003, 15, 773–781. [Google Scholar] [CrossRef]
- Itoh, Y.; Sendo, T.; Hirakawa, T.; Goromaru, T.; Takasaki, S.; Yahata, H.; Nakano, H.; Oishi, R. Role of sensory nerve peptides rather than mast cell histamine in paclitaxel hypersensitivity. Am. J. Respir. Crit. Care Med. 2004, 169, 113–119. [Google Scholar] [CrossRef]
- Ranieri, G.; Passantino, L.; Patruno, R.; Passantino, G.; Jirillo, F.; Catino, A.; Mattioli, V.; Gadaleta, C.; Ribatti, D. The dog mast cell tumour as a model to study the relationship between angiogenesis, mast cell density and tumour malignancy. Oncol. Rep. 2003, 10, 1189–1193. [Google Scholar] [CrossRef]
- Ranieri, G.; Labriola, A.; Achille, G.; Florio, G.; Zito, A.F.; Grammatica, L.; Paradiso, A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int. J. Oncol. 2002, 21, 1317–1323. [Google Scholar] [CrossRef]
- Gulubova, M.; Vlaykova, T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J. Gastroenterol. Hepatol. 2009, 24, 1265–1275. [Google Scholar] [CrossRef]
- Nakayama, T.; Yao, L.; Tosato, G. Mast cell-derived angiopoietin-1 plays a critical role in the growth of plasma cell tumors. J. Clin. Investig. 2004, 114, 1317–1325. [Google Scholar] [CrossRef]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim. Biophys. Acta 2009, 1796, 19–26. [Google Scholar] [CrossRef]
- Talreja, J.; Kabir, M.H.; B Filla, M.; Stechschulte, D.J.; Dileepan, K.N. Histamine induces Toll-like receptor 2 and 4 expression in endothelial cells and enhances sensitivity to Gram-positive and Gram-negative bacterial cell wall components. Immunology 2004, 113, 224–233. [Google Scholar] [CrossRef]
- Hall, C.L.; Dubyk, C.W.; Riesenberger, T.A.; Shein, D.; Keller, E.T.; van Golen, K.L. Type I collagen receptor (alpha2beta1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia 2008, 10, 797–803. [Google Scholar] [CrossRef]
- Chen, Q.; Manning, C.D.; Millar, H.; McCabe, F.L.; Ferrante, C.; Sharp, C.; Shahied-Arruda, L.; Doshi, P.; Nakada, M.T.; Anderson, G.M. CNTO 95, a fully human anti alphav integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clin. Exp. Metastasis 2008, 25, 139–148. [Google Scholar] [CrossRef]
- Ginsberg, M.H. Integrin activation. BMB Rep. 2014, 47, 655–659. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Zhan, J.; Chen, Y.; Fang, W.; Zhang, L.; Zhang, H. Smurf1 inhibits integrin activation by controlling Kindlin-2 ubiquitination and degradation. J. Cell Biol. 2017, 216, 1455–1471. [Google Scholar] [CrossRef]
- Matsuo, Y.; Sawai, H.; Ochi, N.; Yasuda, A.; Sakamoto, M.; Takahashi, H.; Funahashi, H.; Takeyama, H.; Guha, S. Proteasome inhibitor MG132 inhibits angiogenesis in pancreatic cancer by blocking NF-kappaB activity. Dig. Dis. Sci. 2010, 55, 1167–1176. [Google Scholar] [CrossRef]
- Geng, L.; Chaudhuri, A.; Talmon, G.; Wisecarver, J.L.; Wang, J. TGF-Beta suppresses VEGFA-mediated angiogenesis in colon cancer metastasis. PLoS ONE 2013, 8, e59918. [Google Scholar] [CrossRef]
- Jridi, I.; Catacchio, I.; Majdoub, H.; Shahbazzadeh, D.; El Ayeb, M.; Frassanito, M.A.; Solimando, A.; Ribatti, D.; Vacca, A.; Borchani, L. The small subunit of Hemilipin2, a new heterodimeric phospholipase A2 from Hemiscorpius lepturus scorpion venom, mediates the antiangiogenic effect of the whole protein. Toxicon 2017, 126, 38–46. [Google Scholar] [CrossRef]
- Lobert, V.H.; Brech, A.; Pedersen, N.M.; Wesche, J.; Oppelt, A.; Malerød, L.; Stenmark, H. Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev. Cell 2010, 19, 148–159. [Google Scholar] [CrossRef]
- Hsia, H.C.; Nair, M.R.; Corbett, S.A. The fate of internalized α5 integrin is regulated by matrix-capable fibronectin. J. Surg. Res. 2014, 191, 268–279. [Google Scholar] [CrossRef]
- Teckchandani, A.; Cooper, J.A. The ubiquitin-proteasome system regulates focal adhesions at the leading edge of migrating cells. Elife 2016, 5, e17440. [Google Scholar] [CrossRef]
- Kleiger, G.; Mayor, T. Perilous journey: A tour of the ubiquitin-proteasome system. Trends Cell Biol. 2014, 24, 352–359. [Google Scholar] [CrossRef]
- Homrich, M. Not Available. Dtsch. Med. Wochenschr. 2016, 141, 438–439. [Google Scholar]
- Kim, S.; Harris, M.; Varner, J.A. Regulation of integrin alpha vbeta 3-mediated endothelial cell migration and angiogenesis by integrin alpha5beta1 and protein kinase A. J. Biol. Chem. 2000, 275, 33920–33928. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. αV integrins in angiogenesis and cancer. Cold Spring Harb Perspect. Med. 2011, 1, a006478. [Google Scholar] [CrossRef]
- Ivanov, A.N.; Norkin, I.A.; Puchin’ian, D.M.; Shirokov, V.I.U.; Zhdanova, O.I.U. Endothelial cell adhesion molecules. Uspekhi Fiziologicheskikh Nauk 2014, 45, 34–49. [Google Scholar]
- Arosio, D.; Manzoni, L.; Corno, C.; Perego, P. Integrin-Targeted Peptide- and Peptidomimetic-Drug Conjugates for the Treatment of Tumors. Recent Pat. Anticancer Drug Discov. 2017, 12, 148–168. [Google Scholar] [CrossRef]
- Walsh, N.; Clynes, M.; Crown, J.; O’Donovan, N. Alterations in integrin expression modulates invasion of pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2009, 28, 140. [Google Scholar] [CrossRef]
- Weis, S.M.; Lindquist, J.N.; Barnes, L.A.; Lutu-Fuga, K.M.; Cui, J.; Wood, M.R.; Cheresh, D.A. Cooperation between VEGF and beta3 integrin during cardiac vascular development. Blood 2007, 109, 1962–1970. [Google Scholar] [CrossRef]
- Reynolds, L.E.; Wyder, L.; Lively, J.C.; Taverna, D.; Robinson, S.D.; Huang, X.; Sheppard, D.; Hynes, R.O.; Hodivala-Dilke, K.M. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat. Med. 2002, 8, 27–34. [Google Scholar] [CrossRef]
- Senger, D.R.; Claffey, K.P.; Benes, J.E.; Perruzzi, C.A.; Sergiou, A.P.; Detmar, M. Angiogenesis promoted by vascular endothelial growth factor: Regulation through alpha1beta1 and alpha2beta1 integrins. Proc. Natl. Acad. Sci. USA 1997, 94, 13612–13617. [Google Scholar] [CrossRef]
- Pozzi, A.; Moberg, P.E.; Miles, L.A.; Wagner, S.; Soloway, P.; Gardner, H.A. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 2202–2207. [Google Scholar] [CrossRef]
- Zhang, Z.; Ramirez, N.E.; Yankeelov, T.E.; Li, Z.; Ford, L.E.; Qi, Y.; Pozzi, A.; Zutter, M.M. alpha2beta1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood 2008, 111, 1980–1988. [Google Scholar] [CrossRef]
- San Antonio, J.D.; Zoeller, J.J.; Habursky, K.; Turner, K.; Pimtong, W.; Burrows, M.; Choi, S.; Basra, S.; Bennett, J.S.; DeGrado, W.F.; et al. A key role for the integrin alpha2beta1 in experimental and developmental angiogenesis. Am. J. Pathol. 2009, 175, 1338–1347. [Google Scholar] [CrossRef]
- Ramirez, N.E.; Zhang, Z.; Madamanchi, A.; Boyd, K.L.; O’Rear, L.D.; Nashabi, A.; Li, Z.; Dupont, W.D.; Zijlstra, A.; Zutter, M.M. The α2β1 integrin is a metastasis suppressor in mouse models and human cancer. J. Clin. Investig. 2011, 121, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, D.; Huang, Y.; Jovin, I.; Shai, S.Y.; Kyriakides, T.; Ross, R.S.; Giordano, F.J. Endothelial expression of beta1 integrin is required for embryonic vascular patterning and postnatal vascular remodeling. Mol. Cell. Biol. 2008, 28, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Hakanpaa, L.; Kiss, E.A.; Jacquemet, G.; Miinalainen, I.; Lerche, M.; Guzmán, C.; Mervaala, E.; Eklund, L.; Ivaska, J.; Saharinen, P. Targeting β1-integrin inhibits vascular leakage in endotoxemia. Proc. Natl. Acad. Sci. USA 2018, 115, E6467–E6476. [Google Scholar] [CrossRef] [PubMed]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Primo, L.; Seano, G.; Roca, C.; Maione, F.; Gagliardi, P.A.; Sessa, R.; Martinelli, M.; Giraudo, E.; di Blasio, L.; Bussolino, F. Increased expression of alpha6 integrin in endothelial cells unveils a proangiogenic role for basement membrane. Cancer Res. 2010, 70, 5759–5769. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Junior, D.A.; Santana, C.; Vieira, G.V.; Oliver, C.; Jamur, M.C. Mast Cell Protease 7 Promotes Angiogenesis by Degradation of Integrin Subunits. Cells 2019, 8, 349. https://doi.org/10.3390/cells8040349
de Souza Junior DA, Santana C, Vieira GV, Oliver C, Jamur MC. Mast Cell Protease 7 Promotes Angiogenesis by Degradation of Integrin Subunits. Cells. 2019; 8(4):349. https://doi.org/10.3390/cells8040349
Chicago/Turabian Stylede Souza Junior, Devandir A., Carolina Santana, Gabriel V. Vieira, Constance Oliver, and Maria Celia Jamur. 2019. "Mast Cell Protease 7 Promotes Angiogenesis by Degradation of Integrin Subunits" Cells 8, no. 4: 349. https://doi.org/10.3390/cells8040349
APA Stylede Souza Junior, D. A., Santana, C., Vieira, G. V., Oliver, C., & Jamur, M. C. (2019). Mast Cell Protease 7 Promotes Angiogenesis by Degradation of Integrin Subunits. Cells, 8(4), 349. https://doi.org/10.3390/cells8040349





