Opposite Effects of Moderate and Extreme Cx43 Deficiency in Conditional Cx43-Deficient Mice on Angiotensin II-Induced Cardiac Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Experimental Protocol
2.2. Systolic Cardiac Function by Transthoracic Echocardiography
2.3. Collagen Content and Cardiomyocyte Size
2.4. Immunohistochemistry
2.5. Real Time-PCR
2.6. Total Myocardial Homogenates
2.7. Gelatin Zymography
2.8. Cardiac Fibroblasts Isolation
2.9. Western Blot Analysis
2.10. Statistics
3. Results
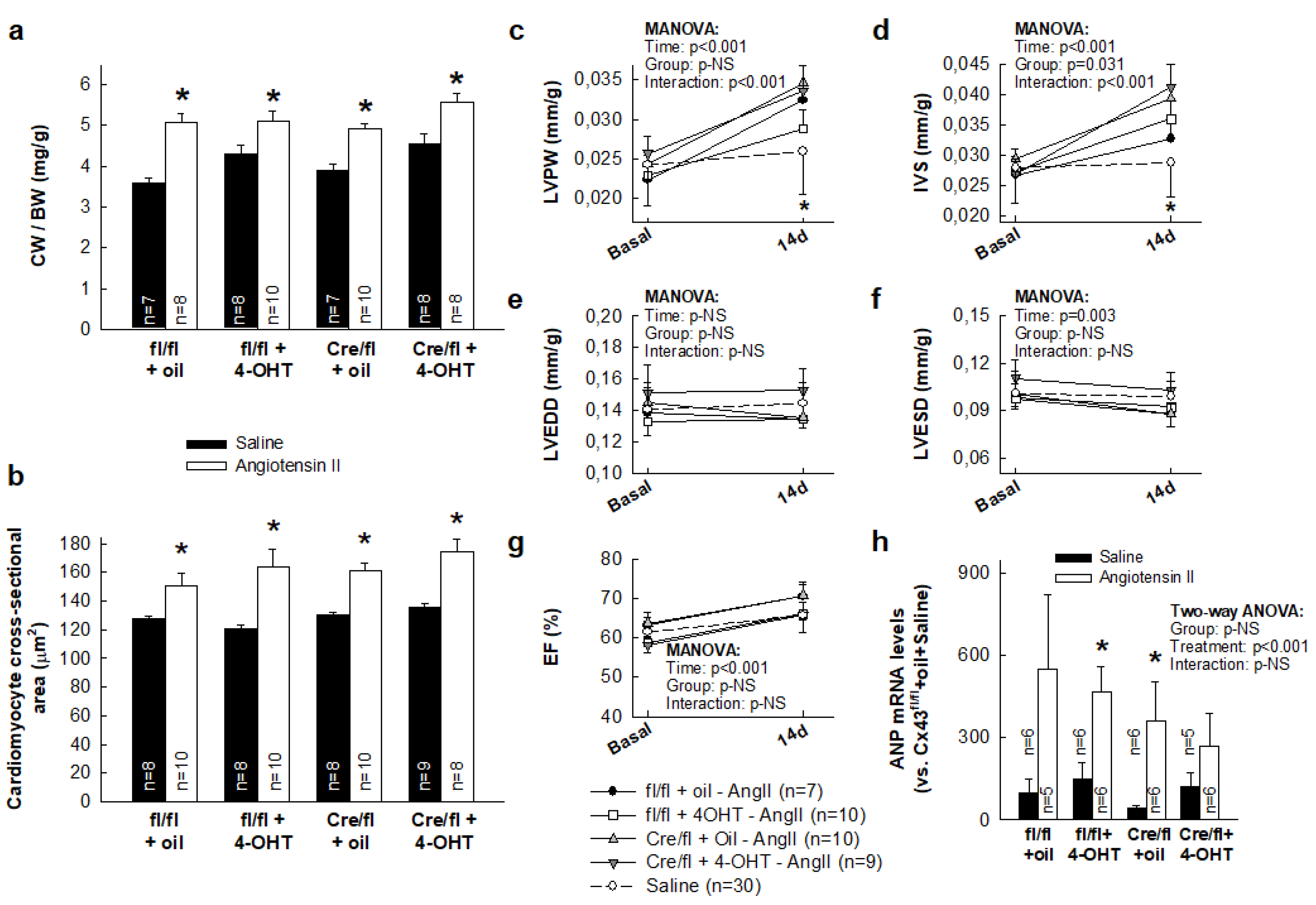
3.1. AngII Treatment Induces a Similar Hypertrophic Response in Hearts from Cx43fl/fl and Cx43Cre-ER(T)/fl Mice Independently of Cx43 Levels
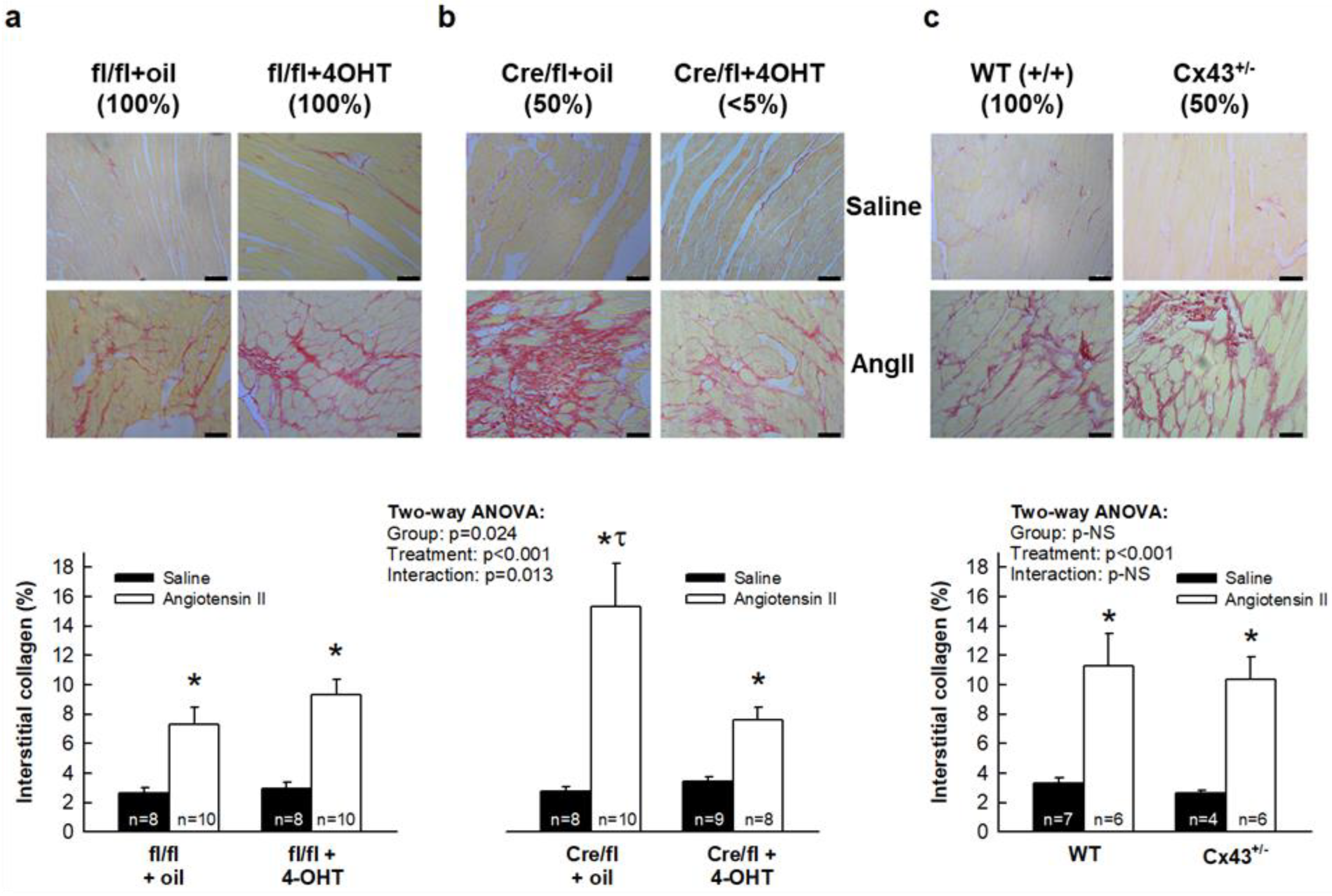
3.2. AngII Treatment Differentially Modulates Cardiac Fibrosis in Cx43Cre-ER(T)/fl Mice
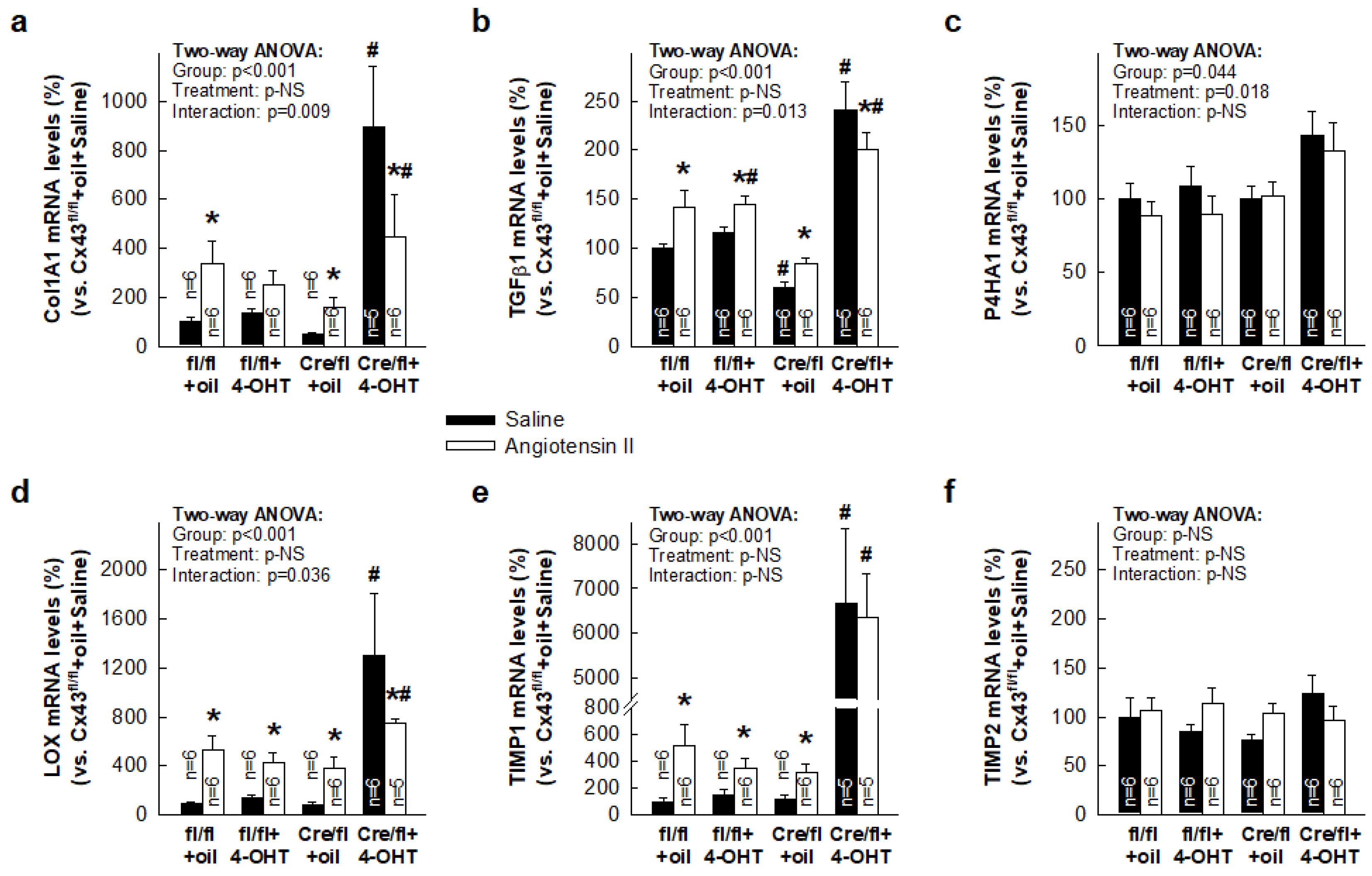
3.3. Paradoxical Overexpression of mRNAs Coding for Proteins Involved in Collagen Turnover in Cx43-Deficient Mice
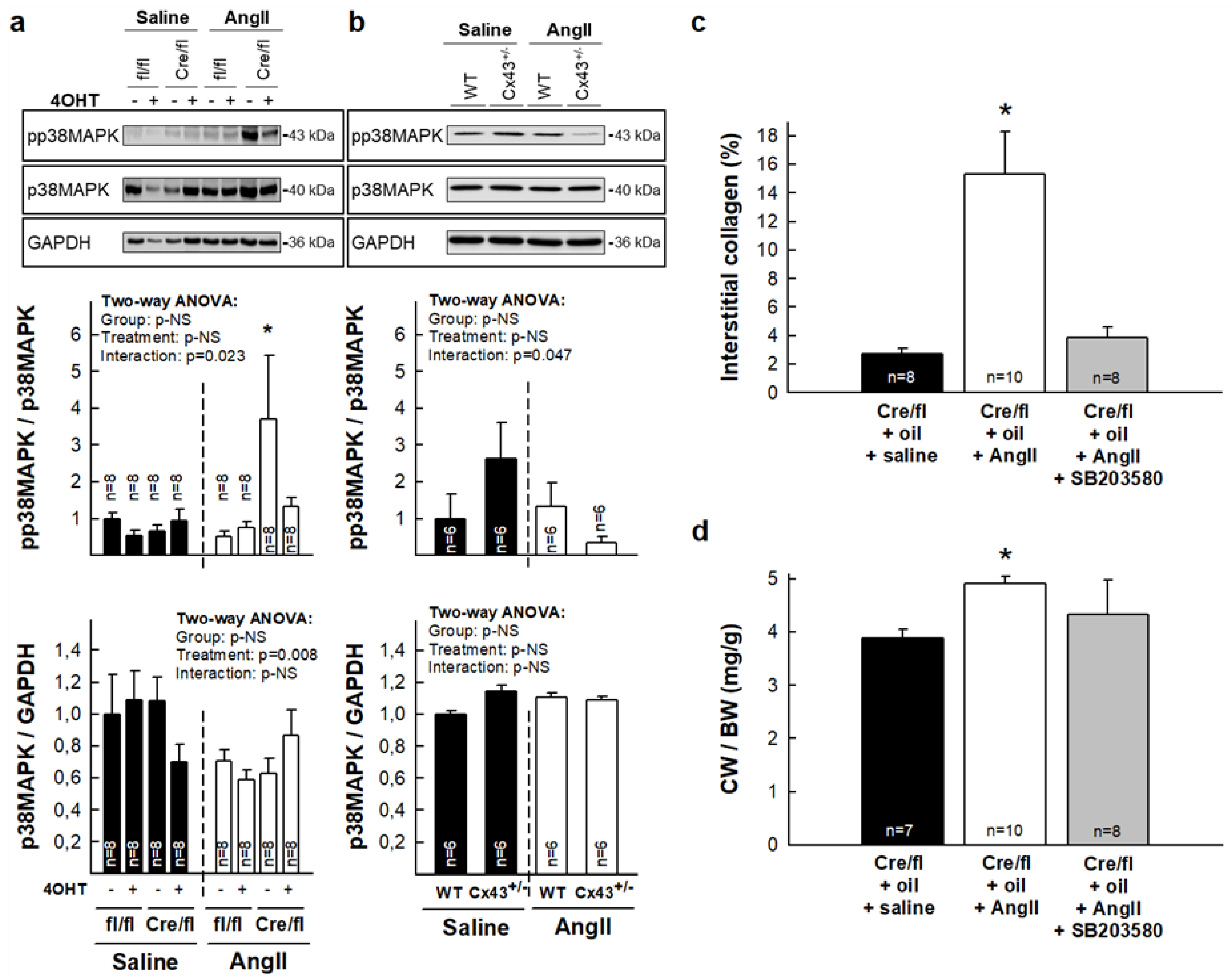
3.4. Enhanced Collagen Deposition in Response to AngII in Hearts from Partially Deficient Cx43Cre-ER(T)/fl Mice Correlates with Increased p38 MAPK Activation
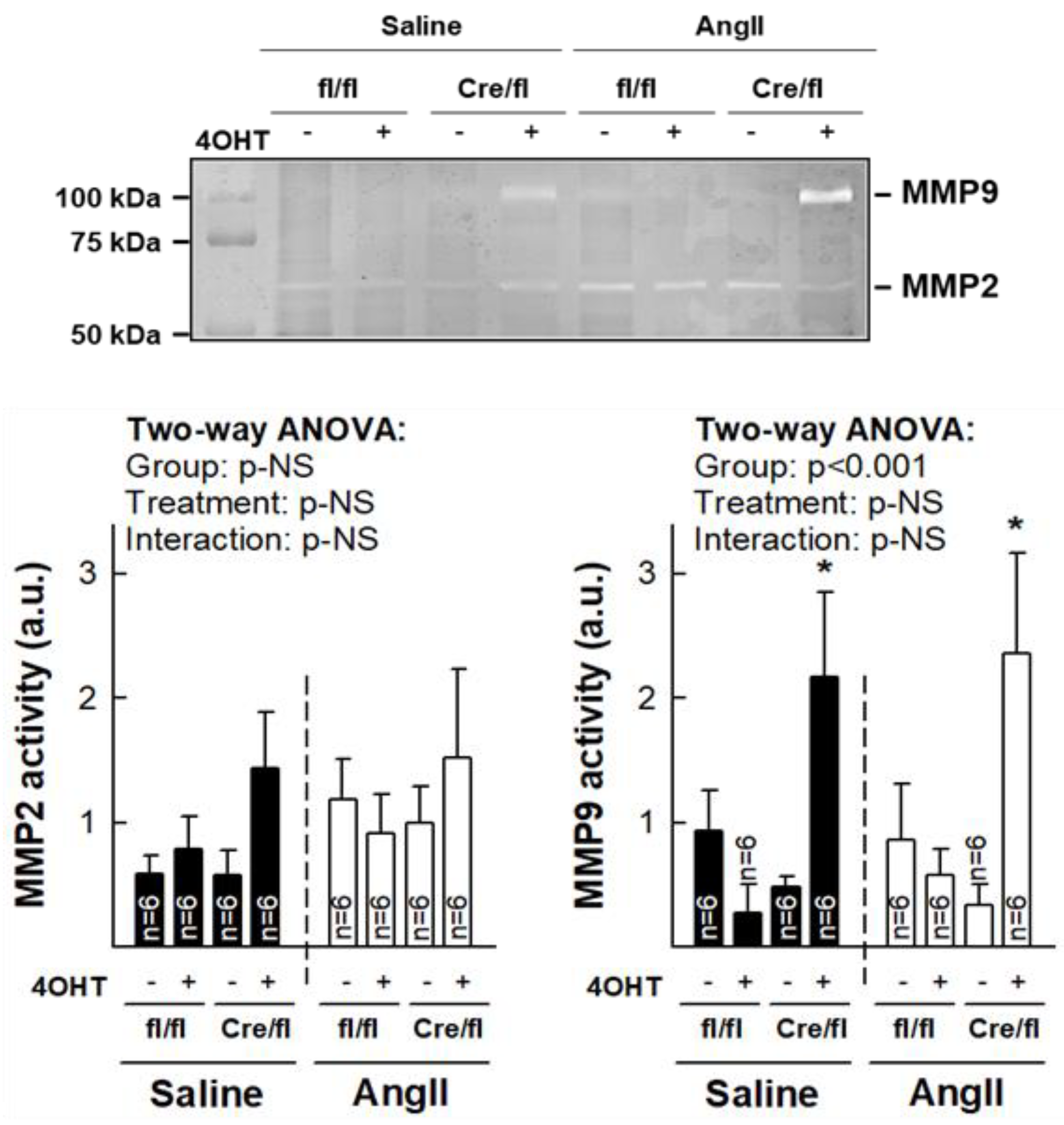
3.5. Normalization of Collagen Content in Hearts from Cx43Cre-ER(T)/fl Mice Injected with 4-OHT After AngII Treatment is Associated with Increased MMP-9 Activity
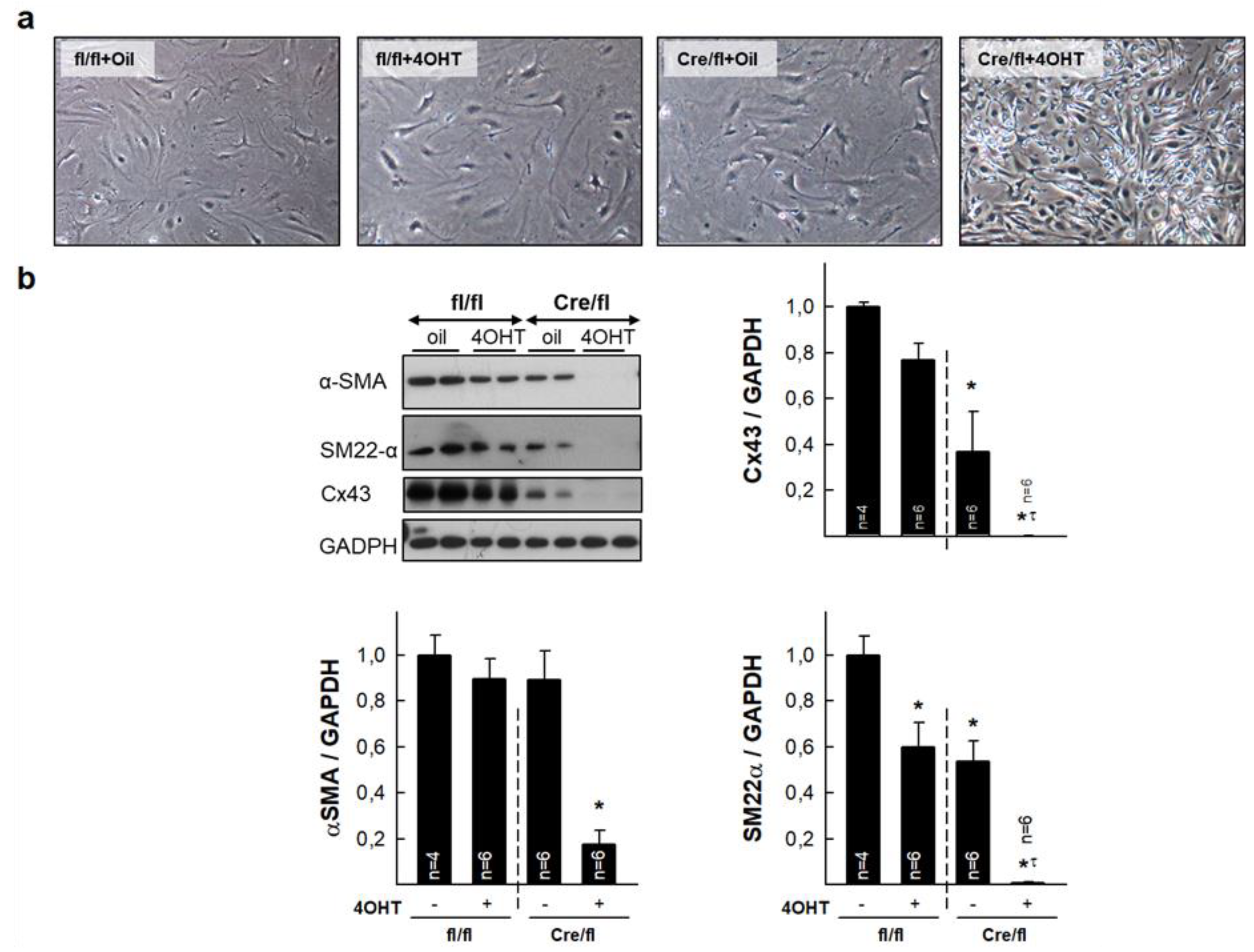
3.6. Isolated Cardiac Fibroblasts from Highly Deficient Cx43Cre-ER(T)/fl Mice Have Altered Phenotype and Differentiation Capacity
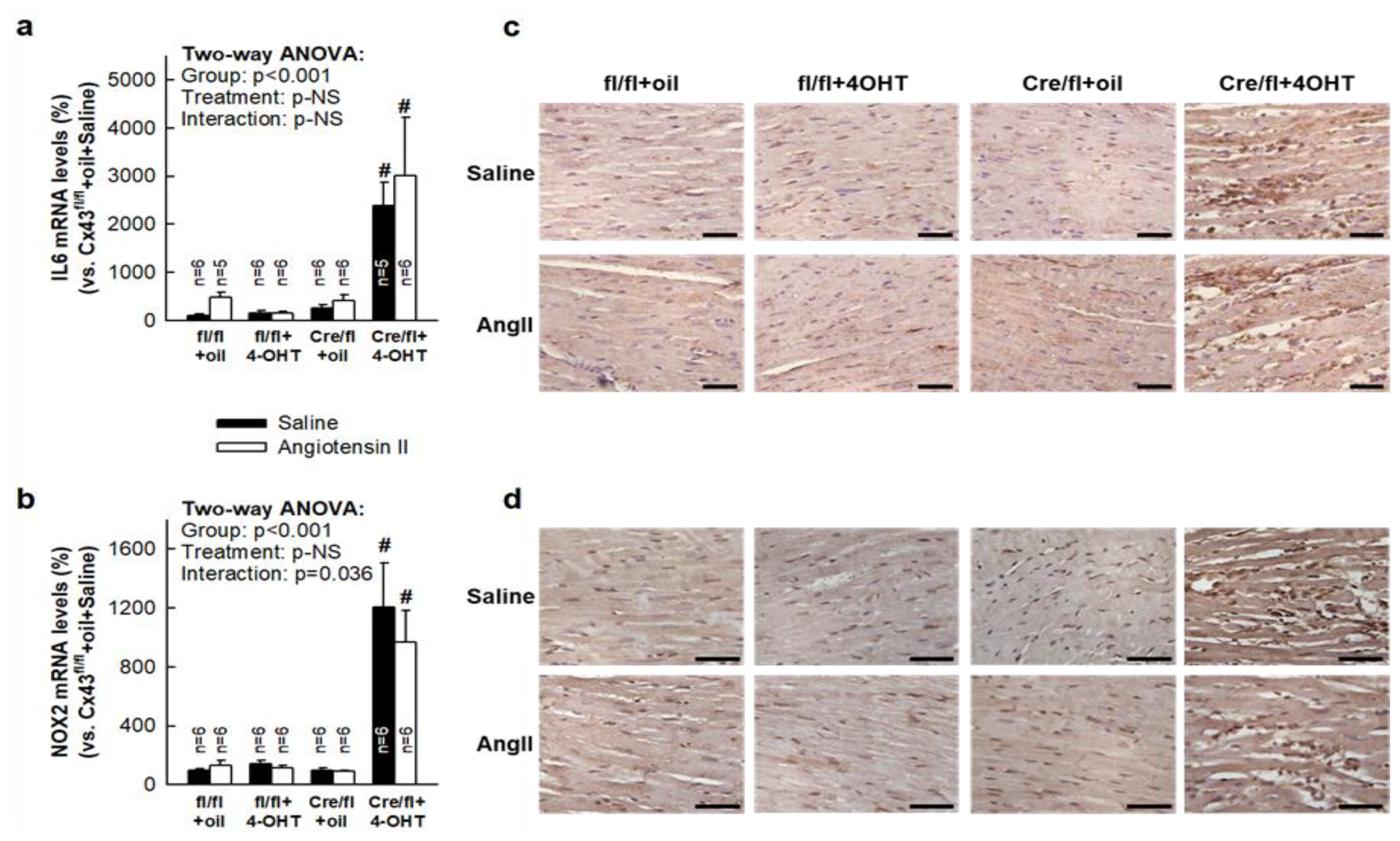
3.7. Deletion of Cx43 in Cx43Cre-ER(T)/fl Mice Injected with 4-OHT is Associated with Enhanced Cardiac Expression of Inflammatory Markers
4. Discussion
Ethical Statement
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sosinsky, G.E.; Nicholson, B.J. Structural organization of gap junction channels. Biochim. Biophys. Acta 2005, 1711, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Severs, N.J. The cardiac gap junction and intercalated disc. Int. J. Cardiol. 1990, 26, 137–173. [Google Scholar] [CrossRef]
- Kleber, A.G.; Saffitz, J.E. Role of the intercalated disc in cardiac propagation and arrhythmogenesis. Front. Physiol. 2014, 5, 404. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Nygaard, A.L.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N.H. Gap junctions. Compr. Physiol. 2012, 2, 1981–2035. [Google Scholar] [PubMed]
- Ongstad, E.; Kohl, P. Fibroblast-myocyte coupling in the heart: Potential relevance for therapeutic interventions. J. Mol. Cell. Cardiol. 2016, 91, 238–246. [Google Scholar] [CrossRef]
- Lambiase, P.D.; Tinker, A. Connexins in the heart. Cell Tissue Res. 2015, 360, 675–684. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Rodriguez-Sinovas, A.; Fernandez-Sanz, C.; Ruiz-Meana, M.; Garcia-Dorado, D. Effects of a reduction in the number of gap junction channels or in their conductance on ischemia-reperfusion arrhythmias in isolated mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2442–H2453. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Sinovas, A.; Cabestrero, A.; Lopez, D.; Torre, I.; Morente, M.; Abellan, A.; Miro, E.; Ruiz-Meana, M.; Garcia-Dorado, D. The modulatory effects of connexin 43 on cell death/survival beyond cell coupling. Prog. Biophys. Mol. Biol. 2007, 94, 219–232. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Rodriguez-Sinovas, A.; Ruiz-Meana, M. Gap junction-mediated spread of cell injury and death during myocardial ischemia-reperfusion. Cardiovasc. Res. 2004, 61, 386–401. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Inserte, J.; Ruiz-Meana, M.; Gonzalez, M.A.; Solares, J.; Julia, M.; Barrabes, J.A.; Soler-Soler, J. Gap junction uncoupler heptanol prevents cell-to-cell progression of hypercontracture and limits necrosis during myocardial reperfusion. Circulation 1997, 96, 3579–3586. [Google Scholar] [CrossRef]
- Rodriguez-Sinovas, A.; Sanchez, J.A.; Gonzalez-Loyola, A.; Barba, I.; Morente, M.; Aguilar, R.; Agullo, E.; Miro-Casas, E.; Esquerda, N.; Ruiz-Meana, M.; et al. Effects of substitution of Cx43 by Cx32 on myocardial energy metabolism, tolerance to ischemia and preconditioning protection. J. Physiol. 2010, 588, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.A.; Rodriguez-Sinovas, A.; Barba, I.; Miro-Casas, E.; Fernandez-Sanz, C.; Ruiz-Meana, M.; Alburquerque-Bejar, J.J.; Garcia-Dorado, D. Activation of RISK and SAFE pathways is not involved in the effects of Cx43 deficiency on tolerance to ischemia-reperfusion injury and preconditioning protection. Basic Res. Cardiol. 2013, 108, 351. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; De Vuyst, E.; Ponsaerts, R.; Boengler, K.; Palacios-Prado, N.; Wauman, J.; Lai, C.P.; De Bock, M.; Decrock, E.; Bol, M.; et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2013, 108, 309. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Coutinho, P.; Frank, S.; Franke, S.; Law, L.Y.; Martin, P.; Green, C.R.; Becker, D.L. Targeting connexin43 expression accelerates the rate of wound repair. Curr. Biol. 2003, 13, 1697–1703. [Google Scholar] [CrossRef]
- Elbadawy, H.M.; Mirabelli, P.; Xeroudaki, M.; Parekh, M.; Bertolin, M.; Breda, C.; Cagini, C.; Ponzin, D.; Lagali, N.; Ferrari, S. Effect of connexin 43 inhibition by the mimetic peptide Gap27 on corneal wound healing, inflammation and neovascularization. Br. J. Pharmacol. 2016, 173, 2880–2893. [Google Scholar] [CrossRef]
- Crespo, Y.S.; da Silva, T.C.; Pereira, I.V.A.; Willebrords, J.; Maes, M.; Sayuri, N.M.; de Castro, I.A.; Leclercq, I.; Romualdo, G.R.; Barbisan, L.F.; et al. TAT-Gap19 and Carbenoxolone Alleviate Liver Fibrosis in Mice. Int. J. Mol. Sci. 2018, 19, 817. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Kovacs, A.; Kanter, E.M.; Yamada, K.A. Reduced expression of Cx43 attenuates ventricular remodeling after myocardial infarction via impaired TGF-beta signaling. Am. J. Physiol. Heart Circ Physiol. 2010, 298, H477–H487. [Google Scholar] [CrossRef]
- Kanno, S.; Kovacs, A.; Yamada, K.A.; Saffitz, J.E. Connexin43 as a determinant of myocardial infarct size following coronary occlusion in mice. J. Am. Coll. Cardiol. 2003, 41, 681–686. [Google Scholar] [CrossRef]
- O’Quinn, M.P.; Palatinus, J.A.; Harris, B.S.; Hewett, K.W.; Gourdie, R.G. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ. Res. 2011, 108, 704–715. [Google Scholar] [CrossRef]
- Ongstad, E.L.; O’Quinn, M.P.; Ghatnekar, G.S.; Yost, M.J.; Gourdie, R.G. A Connexin43 Mimetic Peptide Promotes Regenerative Healing and Improves Mechanical Properties in Skin and Heart. Adv. Wound Care 2013, 2, 55–62. [Google Scholar] [CrossRef]
- Jansen, J.A.; van Veen, T.A.; de Jong, S.; van der, N.R.; van Stuijvenberg, L.; Driessen, H.; Labzowski, R.; Oefner, C.M.; Bosch, A.A.; Nguyen, T.Q.; et al. Reduced cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circ. Arrhythm. Electrophysiol. 2012, 5, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, D.; Theis, M.; Degen, J.; Ott, T.; van Rijen, H.V.; Kirchhoff, S.; Kim, J.S.; de Bakker, J.M.; Willecke, K. Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J. Mol. Cell. Cardiol. 2004, 36, 101–110. [Google Scholar] [CrossRef] [PubMed]
- van Rijen, H.V.; Eckardt, D.; Degen, J.; Theis, M.; Ott, T.; Willecke, K.; Jongsma, H.J.; Opthof, T.; de Bakker, J.M. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 2004, 109, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Galan, M.; Varona, S.; Guadall, A.; Orriols, M.; Navas, M.; Aguilo, S.; de Diego, A.; Navarro, M.A.; Garcia-Dorado, D.; Rodriguez-Sinovas, A.; et al. Lysyl oxidase overexpression accelerates cardiac remodeling and aggravates angiotensin II-induced hypertrophy. FASEB J. 2017, 31, 3787–3799. [Google Scholar] [CrossRef]
- Poncelas, M.; Inserte, J.; Aluja, D.; Hernando, V.; Vilardosa, U.; Garcia-Dorado, D. Delayed, oral pharmacological inhibition of calpains attenuates adverse post-infarction remodelling. Cardiovasc. Res. 2017, 113, 950–961. [Google Scholar] [CrossRef]
- Rodriguez-Calvo, R.; Ferran, B.; Alonso, J.; Marti-Pamies, I.; Aguilo, S.; Calvayrac, O.; Rodriguez, C.; Martinez-Gonzalez, J. NR4A receptors up-regulate the antiproteinase alpha-2 macroglobulin (A2M) and modulate MMP-2 and MMP-9 in vascular smooth muscle cells. Thromb. Haemost. 2015, 113, 1323–1334. [Google Scholar] [CrossRef]
- Boengler, K.; Dodoni, G.; Rodriguez-Sinovas, A.; Cabestrero, A.; Ruiz-Meana, M.; Gres, P.; Konietzka, I.; Lopez-Iglesias, C.; Garcia-Dorado, D.; Di Lisa, F.; et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc. Res. 2005, 67, 234–244. [Google Scholar] [CrossRef]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef]
- Touyz, R.M.; Schiffrin, E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000, 52, 639–672. [Google Scholar]
- Kim, S.; Iwao, H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 2000, 52, 11–34. [Google Scholar]
- Tsushima, K.; Osawa, T.; Yanai, H.; Nakajima, A.; Takaoka, A.; Manabe, I.; Ohba, Y.; Imai, Y.; Taniguchi, T.; Nagai, R. IRF3 regulates cardiac fibrosis but not hypertrophy in mice during angiotensin II-induced hypertension. FASEB J. 2011, 25, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Olson, E.N. Cardiac hypertrophy: The good, the bad, and the ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef] [PubMed]
- Ghatnekar, G.S.; O’Quinn, M.P.; Jourdan, L.J.; Gurjarpadhye, A.A.; Draughn, R.L.; Gourdie, R.G. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Regen. Med. 2009, 4, 205–223. [Google Scholar] [CrossRef]
- Gilmartin, D.J.; Soon, A.; Thrasivoulou, C.; Phillips, A.R.; Jayasinghe, S.N.; Becker, D.L. Sustained Release of Cx43 Antisense Oligodeoxynucleotides from Coated Collagen Scaffolds Promotes Wound Healing. Adv. Healthc. Mater. 2016, 5, 1786–1799. [Google Scholar] [CrossRef]
- Montgomery, J.; Ghatnekar, G.S.; Grek, C.L.; Moyer, K.E.; Gourdie, R.G. Connexin 43-Based Therapeutics for Dermal Wound Healing. Int. J. Mol. Sci. 2018, 19, 1778. [Google Scholar] [CrossRef]
- Cogliati, B.; Vinken, M.; Silva, T.C.; Araujo, C.M.; Aloia, T.P.; Chaible, L.M.; Mori, C.M.; Dagli, M.L. Connexin 43 deficiency accelerates skin wound healing and extracellular matrix remodeling in mice. J. Dermatol. Sci. 2015, 79, 50–56. [Google Scholar] [CrossRef]
- Ghatnekar, G.S.; Grek, C.L.; Armstrong, D.G.; Desai, S.C.; Gourdie, R.G. The effect of a connexin43-based Peptide on the healing of chronic venous leg ulcers: A multicenter, randomized trial. J. Investig. Dermatol. 2015, 135, 289–298. [Google Scholar] [CrossRef]
- Bivi, N.; Nelson, M.T.; Faillace, M.E.; Li, J.; Miller, L.M.; Plotkin, L.I. Deletion of Cx43 from osteocytes results in defective bone material properties but does not decrease extrinsic strength in cortical bone. Calcif. Tissue Int. 2012, 91, 215–224. [Google Scholar] [CrossRef]
- Santiago, J.J.; Dangerfield, A.L.; Rattan, S.G.; Bathe, K.L.; Cunnington, R.H.; Raizman, J.E.; Bedosky, K.M.; Freed, D.H.; Kardami, E.; Dixon, I.M. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: Expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev. Dyn. 2010, 239, 1573–1584. [Google Scholar] [CrossRef]
- Fatigati, V.; Murphy, R.A. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J. Biol. Chem. 1984, 259, 14383–14388. [Google Scholar] [PubMed]
- Spinale, F.G. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circ. Res. 2002, 90, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Metalloproteinase production from macrophages—A perfect storm leading to atherosclerotic plaque rupture and myocardial infarction. Exp. Physiol. 2016, 101, 1327–1337. [Google Scholar] [CrossRef]
- Doble, B.W.; Dang, X.; Ping, P.; Fandrich, R.R.; Nickel, B.E.; Jin, Y.; Cattini, P.A.; Kardami, E. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J. Cell Sci. 2004, 117, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Doble, B.W.; Kardami, E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol. Cell. Biochem. 2003, 242, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Salat-Canela, C.; Sese, M.; Peula, C.; Cajal, S.; Aasen, T. Internal translation of the connexin 43 transcript. Cell Commun. Signal. 2014, 12, 31. [Google Scholar] [CrossRef]
- Smyth, J.W.; Shaw, R.M. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep. 2013, 5, 611–618. [Google Scholar] [CrossRef]
- Kardami, E.; Dang, X.; Iacobas, D.A.; Nickel, B.E.; Jeyaraman, M.; Srisakuldee, W.; Makazan, J.; Tanguy, S.; Spray, D.C. The role of connexins in controlling cell growth and gene expression. Prog. Biophys. Mol. Biol. 2007, 94, 245–264. [Google Scholar] [CrossRef]
- Kotini, M.; Barriga, E.H.; Leslie, J.; Gentzel, M.; Rauschenberger, V.; Schambon, A.; Mayor, R. Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat. Commun. 2018, 9, 3846. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valls-Lacalle, L.; Negre-Pujol, C.; Rodríguez, C.; Varona, S.; Valera-Cañellas, A.; Consegal, M.; Martínez-González, J.; Rodríguez-Sinovas, A. Opposite Effects of Moderate and Extreme Cx43 Deficiency in Conditional Cx43-Deficient Mice on Angiotensin II-Induced Cardiac Fibrosis. Cells 2019, 8, 1299. https://doi.org/10.3390/cells8101299
Valls-Lacalle L, Negre-Pujol C, Rodríguez C, Varona S, Valera-Cañellas A, Consegal M, Martínez-González J, Rodríguez-Sinovas A. Opposite Effects of Moderate and Extreme Cx43 Deficiency in Conditional Cx43-Deficient Mice on Angiotensin II-Induced Cardiac Fibrosis. Cells. 2019; 8(10):1299. https://doi.org/10.3390/cells8101299
Chicago/Turabian StyleValls-Lacalle, Laura, Corall Negre-Pujol, Cristina Rodríguez, Saray Varona, Antoni Valera-Cañellas, Marta Consegal, Jose Martínez-González, and Antonio Rodríguez-Sinovas. 2019. "Opposite Effects of Moderate and Extreme Cx43 Deficiency in Conditional Cx43-Deficient Mice on Angiotensin II-Induced Cardiac Fibrosis" Cells 8, no. 10: 1299. https://doi.org/10.3390/cells8101299
APA StyleValls-Lacalle, L., Negre-Pujol, C., Rodríguez, C., Varona, S., Valera-Cañellas, A., Consegal, M., Martínez-González, J., & Rodríguez-Sinovas, A. (2019). Opposite Effects of Moderate and Extreme Cx43 Deficiency in Conditional Cx43-Deficient Mice on Angiotensin II-Induced Cardiac Fibrosis. Cells, 8(10), 1299. https://doi.org/10.3390/cells8101299






