Hepatitis C Virus Improves Human Tregs Suppressive Function and Promotes Their Recruitment to the Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Donors
2.2. Isolation of Immune Cells and Flow Cytometry
2.3. Isolation of Hepatic Cells
2.4. Culture Conditions and Viral Inoculation
2.5. Migration Assay
2.6. Gene Expression
2.7. Statistical Analysis
2.8. Supplementary Material and Methods
3. Results
3.1. Characterization of Freshly Isolated Natural Human Regulatory T Cells
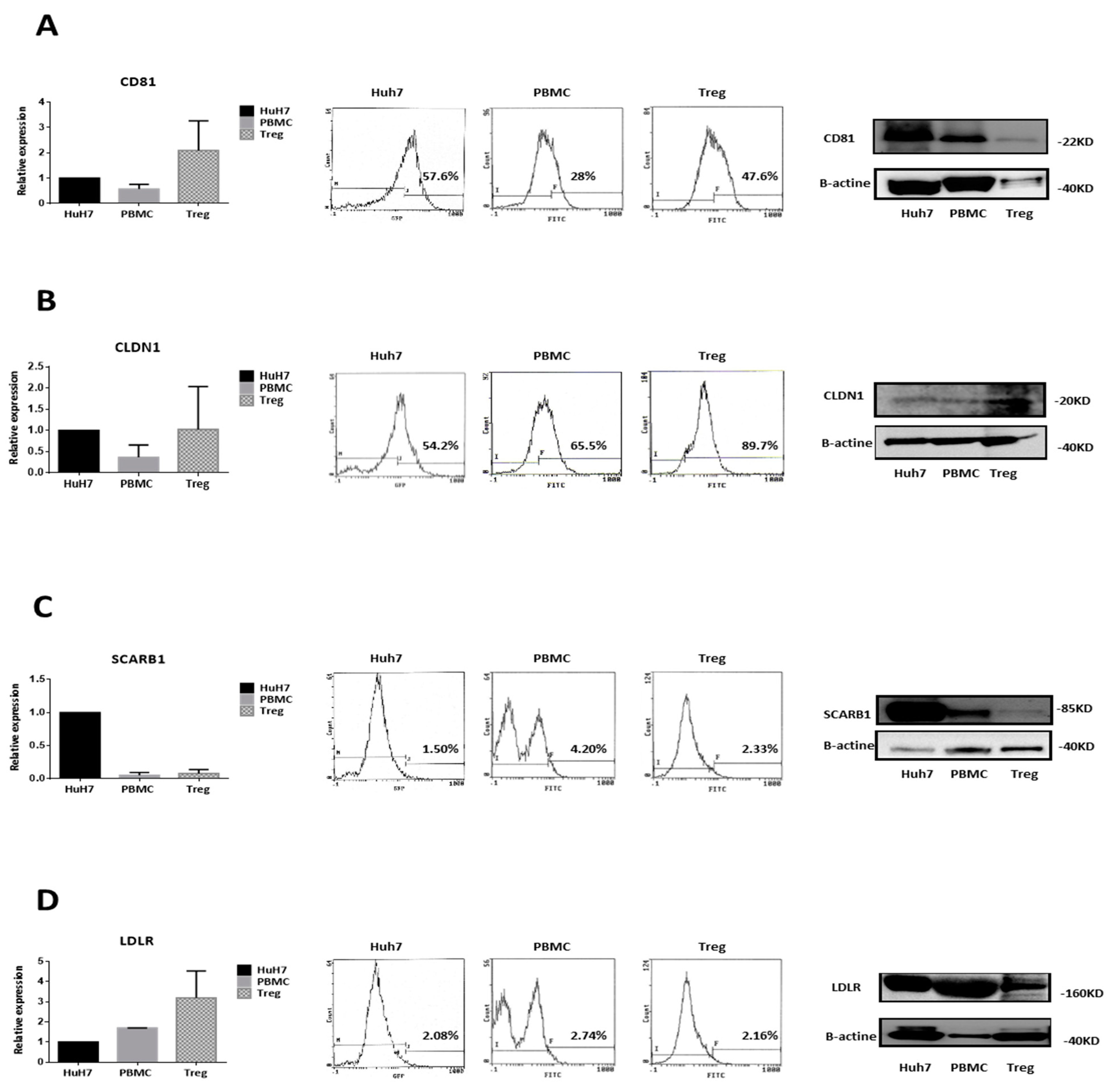
3.2. Circulating CD4+CD25+/highCD127−/low Tregs Possess the Classical HCV Entry Receptors
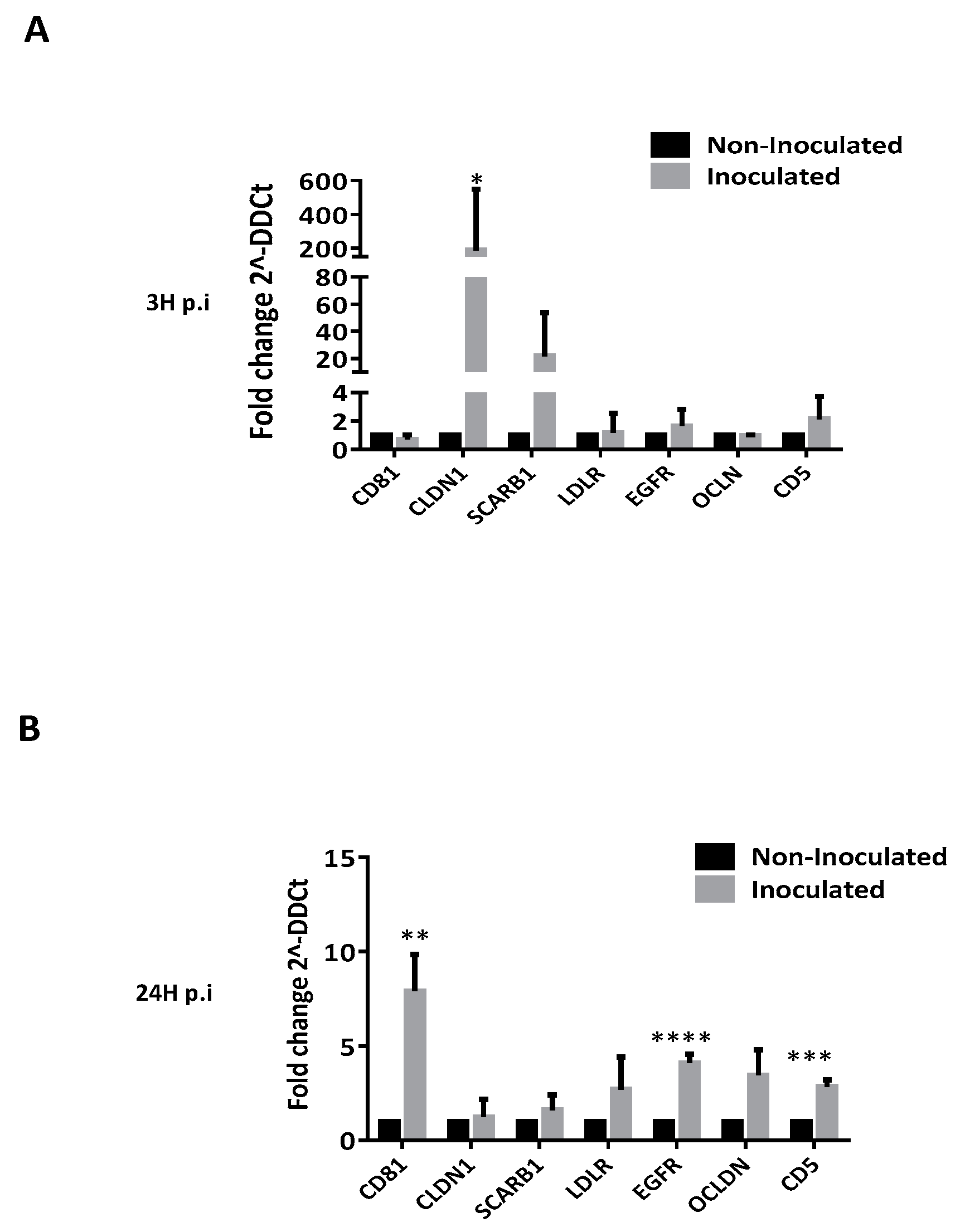
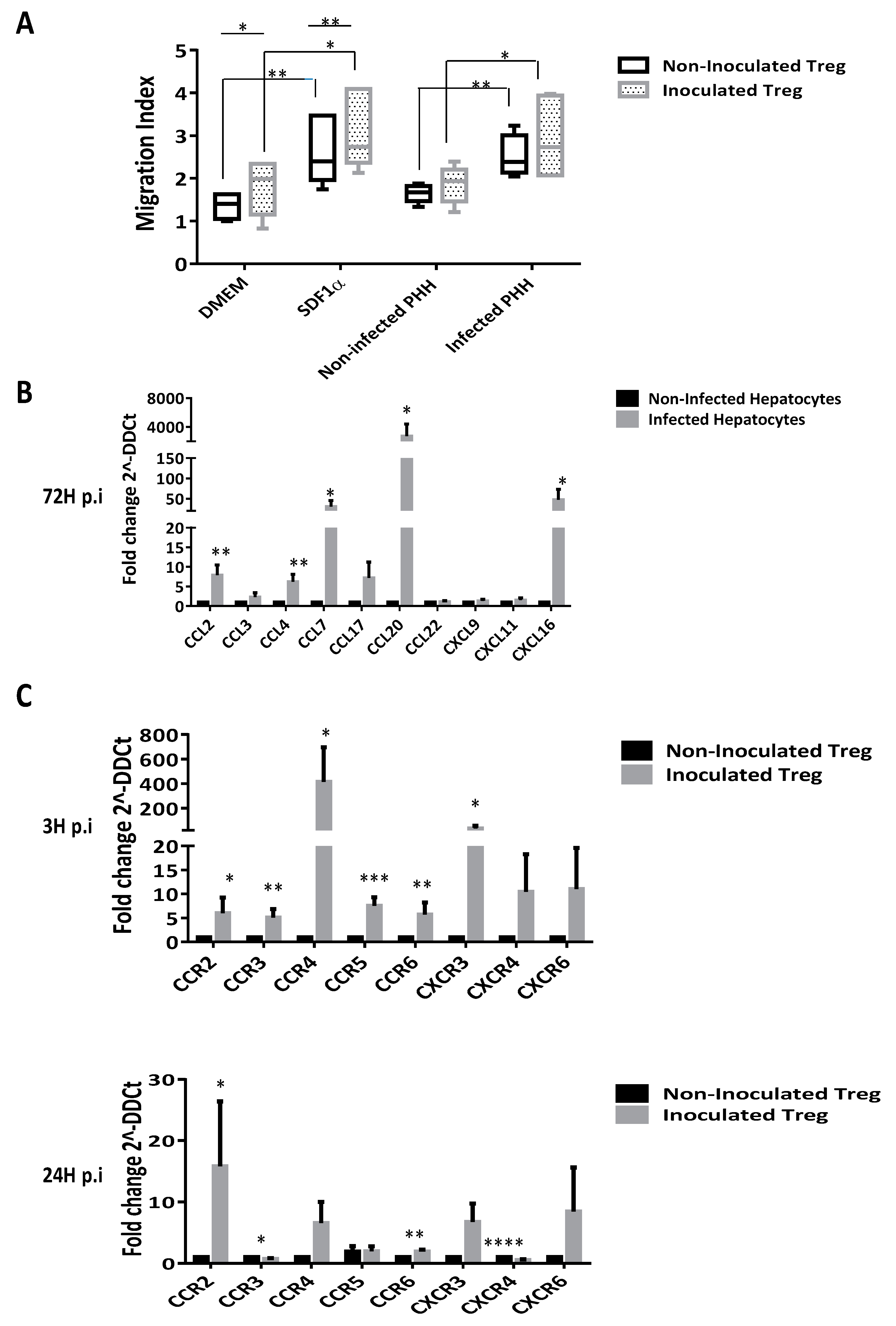
3.3. HCV Inoculation Increases the Expression of Its Receptors on Tregs
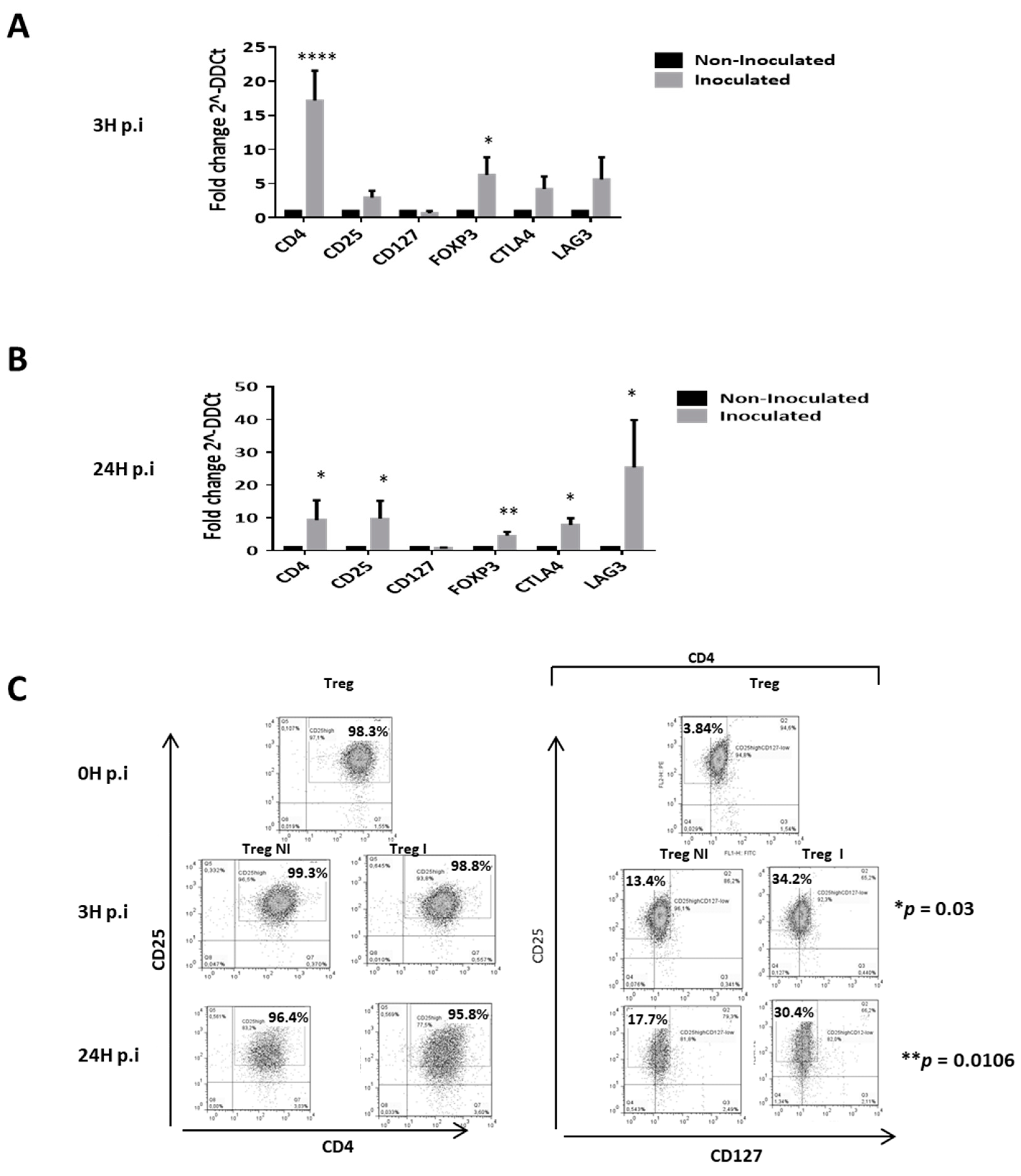
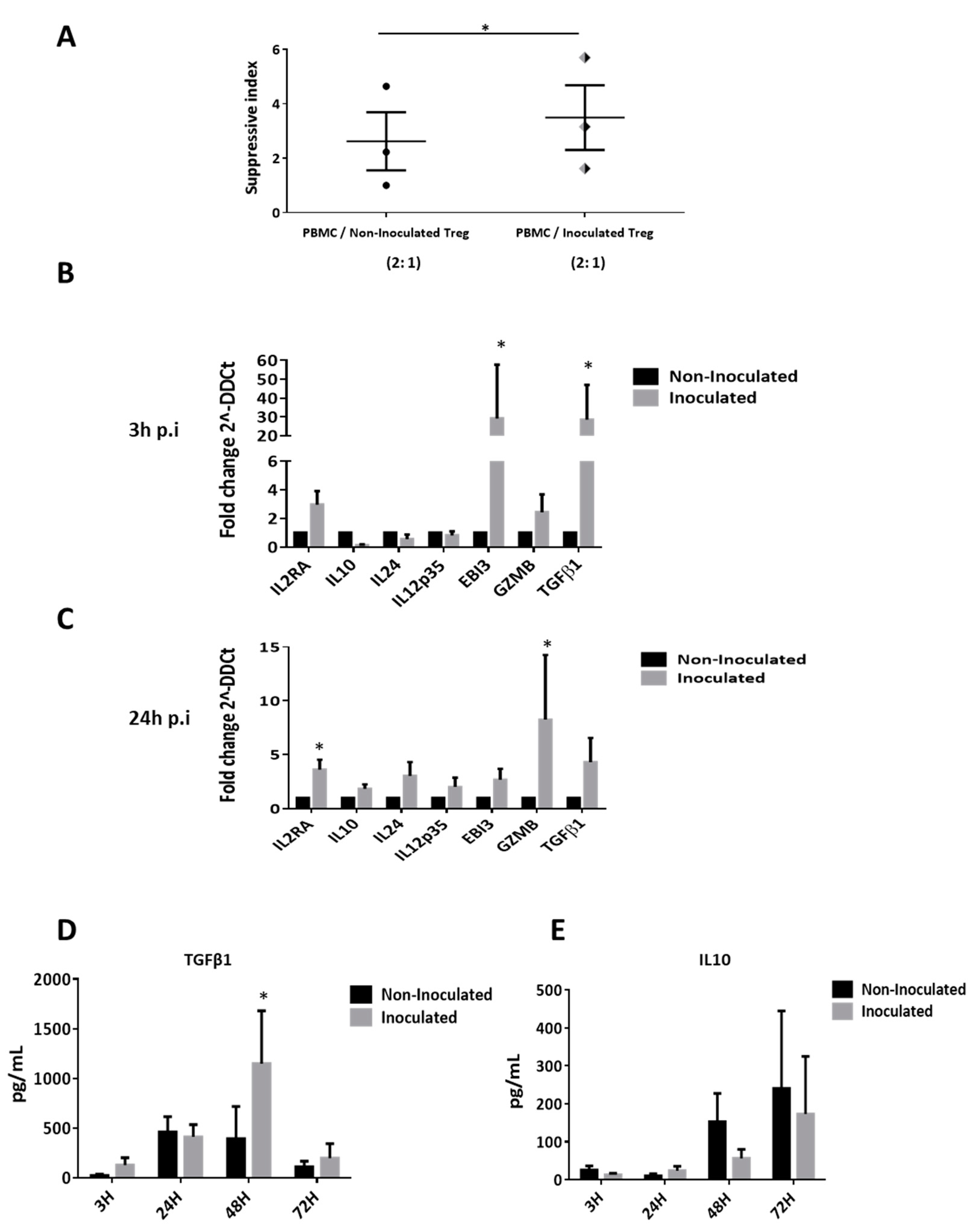
3.4. Supernantant Containing HCVcc Significantly Modifies the Suppressive Phenotype of Tregs
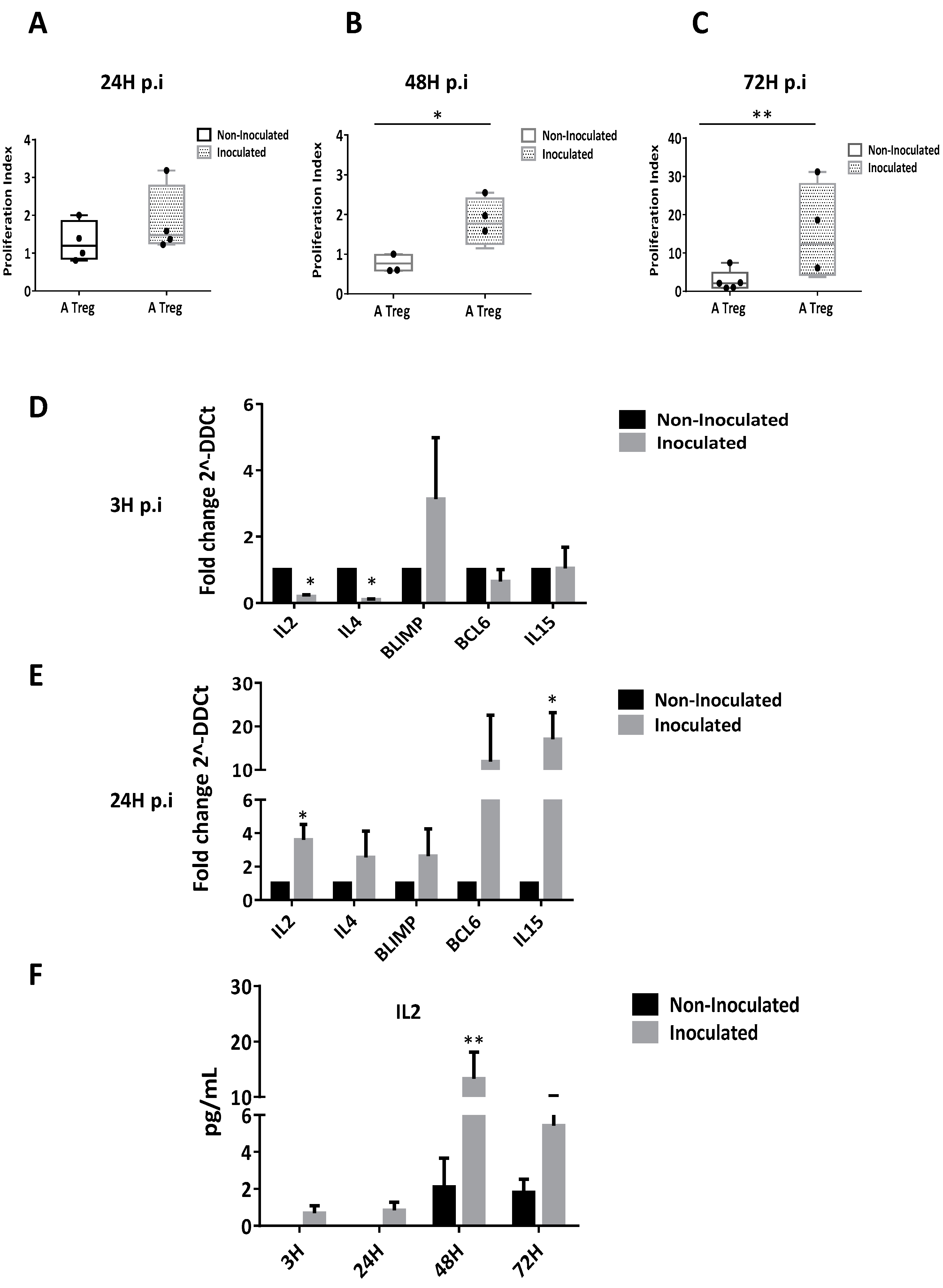
3.5. HCV Inoculation Induces Treg Proliferation
3.6. HCV Inoculation Increases the Suppressive Activity of Tregs
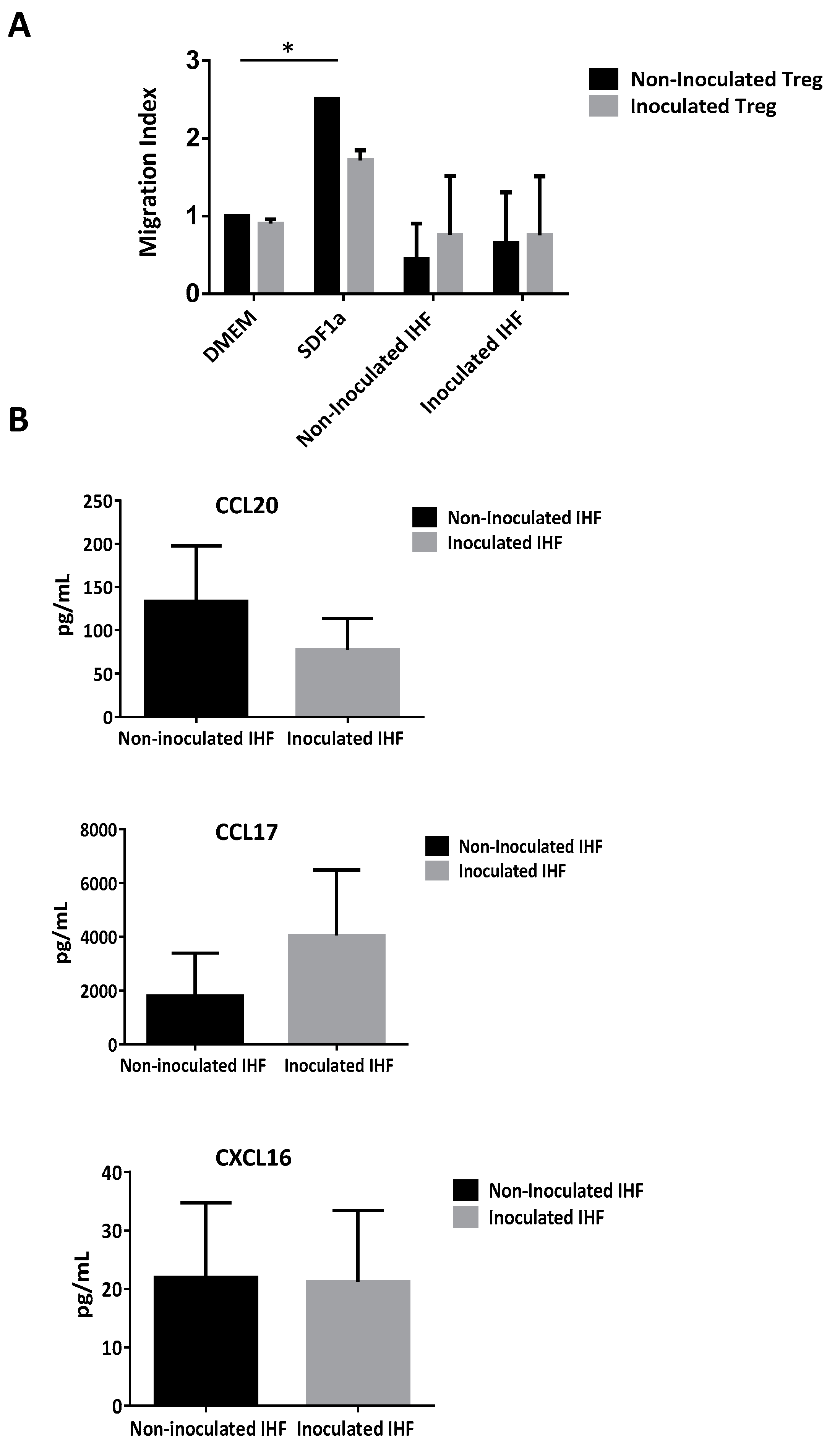
3.7. CD4+CD25highCD127− Tregs are Recruited by Infected Human Hepatic Cells Supernatants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CCL | chemokines (C-C motif) |
| CD | cluster of differentiation |
| CTLA4 | cytotoxic T-lymphocyte-associated protein 4; |
| CXCL | chemokine (C-X-C motif) |
| E1 | envelop glycoprotein 1 |
| EGFR | epidermal growth factor receptor |
| ELISA | enzyme-linked immunosorbant assay |
| FACS | fluorescence-activated cell sorting |
| Foxp3 | forkhead box protein 3 |
| GZMB | granzyme B |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| PHH | primary human hepatocyte |
| IHF | intra-hepatic fibroblast |
| LAG3 | lymphocytes activating gene 3 |
| IL-10 | interleukin 10 |
| mRNA | messenger ribonucleic acid |
| NS5A | Non-structural protein 5A |
| PBMC | peripheral blood mononuclear cells |
| p.i. | post-inoculation |
| Q-PCR | quantitative polymerase chain reaction |
| TGF-β1 | transforming growth factor βeta 1 |
| TLR | toll like receptor |
| Tregs | regulatory T cells |
| TR1 | regulatory T cell type 1 |
| WB | Western Blot |
References
- Hadigan, C.; Kottilil, S. Hepatitis C virus infection and coinfection with human immunodeficiency virus: Challenges and advancements in management. JAMA 2011, 306, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Baumert, T.F.; Ito, S.; Wong, D.T.; Liang, T.J. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J. Virol. 1998, 72, 3827–3836. [Google Scholar] [PubMed]
- Ding, Q.; von Schaewen, M.; Ploss, A. The impact of hepatitis C virus entry on viral tropism. Cell Host Microbe 2014, 16, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Lupberger, J.; Zeisel, M.B.; Xiao, F.; Thumann, C.; Fofana, I.; Zona, L.; Davis, C.; Mee, C.J.; Turek, M.; Gorke, S.; et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med. 2011, 17, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, M.A.; Chen, A.Y.; Michalak, T.I. Differential expression of candidate virus receptors in human T lymphocytes prone or resistant to infection with patient-derived hepatitis C virus. PLoS ONE 2013, 8, e62159. [Google Scholar] [CrossRef] [PubMed]
- Pape, G.R.; Gerlach, T.J.; Diepolder, H.M.; Gruner, N.; Jung, M.; Santantonio, T. Role of the specific T-cell response for clearance and control of hepatitis C virus. J. Viral. Hepat. 1999, 6, 36–40. [Google Scholar] [CrossRef]
- Houldsworth, A.; Metzner, M.; Hodgkinson, A.; Shaw, S.; Kaminski, E.; Demaine, A.G.; Cramp, M.E. Haplotype analysis finds linkage disequilibrium in the IL-12 gene in patients with HCV. J. Med. Virol. 2015, 87, 1207–1217. [Google Scholar] [CrossRef]
- Napoli, J.; Bishop, G.A.; McGuinness, P.H.; Painter, D.M.; McCaughan, G.W. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology 1996, 24, 759–765. [Google Scholar] [CrossRef]
- Ouaguia, L.; Mrizak, D.; Renaud, S.; Morales, O.; Delhem, N. Control of the inflammatory response mechanisms mediated by natural and induced regulatory T-cells in HCV-, HTLV-1-, and EBV-associated cancers. Mediat. Inflamm. 2014, 2014, 564296. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Wing, K.; Miyara, M. Regulatory T cells—A brief history and perspective. Eur. J. Immunol. 2007, 37, S116–S123. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [PubMed]
- Shevach, E.M.; Thornton, A.M. tTregs, pTregs, and iTregs: Similarities and differences. Immunol. Rev. 2014, 259, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Fabien, S.; Olivier, M.; Khaldoun, G.; Vivian, V.; Lynda, A.; Laurissa, O.; Gautier, G.; Yvon, C.; Nadira, D.; Filomena, C. CD49b, a major marker of regulatory T-cells type 1, predicts the response to antiviral therapy of recurrent hepatitis C after liver transplantation. BioMed Res. Int. 2013, 2014, 290878. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Su, W.; Zhang, Y.; Feng, Y.; Lu, Y. Relationship between CD4+CD25(High)CD127(low) regularly T cells in the peripheral blood and tumor regression after neoadjuvant therapy in patients with rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2015, 18, 361–364. [Google Scholar] [PubMed]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Delhem, N.; Cottrez, F.; Carpentier, A.; Miroux, C.; Morales, O.; Francois, V.; Groux, H.; Auriault, C.; Pancre, V. Role of the Regulatory T lymphocytes in hepatitis C fibrosis progression. Bull. Cancer 2008, 95, 1029–1038. [Google Scholar] [PubMed]
- Delhem, N.; Carpentier, A.; Morales, O.; Miroux, C.; Groux, H.; Auriault, C.; Pancre, V. Regulatory T-cells and hepatocellular carcinoma: Implication of the regulatory T lymphocytes in the control of the immune response. Bull. Cancer 2008, 95, 1219–1225. [Google Scholar]
- Carpentier, A.; Conti, F.; Stenard, F.; Aoudjehane, L.; Miroux, C.; Podevin, P.; Morales, O.; Chouzenoux, S.; Scatton, O.; Groux, H.; et al. Increased expression of regulatory Tr1 cells in recurrent hepatitis C after liver transplantation. Am. J. Transpl. 2009, 9, 2102–2112. [Google Scholar] [CrossRef]
- Langhans, B.; Braunschweiger, I.; Arndt, S.; Schulte, W.; Satoguina, J.; Layland, L.E.; Vidovic, N.; Hoerauf, A.; Oldenburg, J.; Sauerbruch, T.; et al. Core-specific adaptive regulatory T-cells in different outcomes of hepatitis C. Clin. Sci. 2010, 119, 97–109. [Google Scholar] [CrossRef]
- Miroux, C.; Vausselin, T.; Delhem, N. Regulatory T cells in HBV and HCV liver diseases: Implication of regulatory T lymphocytes in the control of immune response. Expert Opin. Biol. Ther. 2010, 10, 1563–1572. [Google Scholar] [CrossRef]
- Ouaguia, L.; Morales, O.; Mrizak, D.; Ghazal, K.; Boleslawski, E.; Auriault, C.; Pancre, V.; de Launoit, Y.; Conti, F.; Delhem, N. Overexpression of Regulatory T Cells Type 1 (Tr1) Specific Markers in a Patient with HCV-Induced Hepatocellular Carcinoma. ISRN Hepatol. 2013, 2013, 928485. [Google Scholar] [CrossRef] [PubMed]
- Riezu-Boj, J.I.; Larrea, E.; Aldabe, R.; Guembe, L.; Casares, N.; Galeano, E.; Echeverria, I.; Sarobe, P.; Herrero, I.; Sangro, B.; et al. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J. Hepatol. 2011, 54, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Podevin, P.; Carpentier, A.; Pene, V.; Aoudjehane, L.; Carriere, M.; Zaidi, S.; Hernandez, C.; Calle, V.; Meritet, J.F.; Scatton, O.; et al. Production of infectious hepatitis C virus in primary cultures of human adult hepatocytes. Gastroenterology 2010, 139, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Aoudjehane, L.; Bisch, G.; Scatton, O.; Granier, C.; Gaston, J.; Housset, C.; Roingeard, P.; Cosset, F.L.; Perdigao, F.; Balladur, P.; et al. Infection of Human Liver Myofibroblasts by Hepatitis C Virus: A Direct Mechanism of Liver Fibrosis in Hepatitis C. PLoS ONE 2015, 10, e0134141. [Google Scholar] [CrossRef]
- Mrizak, D.; Martin, N.; Barjon, C.; Jimenez-Pailhes, A.S.; Mustapha, R.; Niki, T.; Guigay, J.; Pancre, V.; de Launoit, Y.; Busson, P.; et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J. Natl. Cancer Inst. 2015, 107, 363. [Google Scholar] [CrossRef]
- Aoudjehane, L.; Pissaia, A., Jr.; Scatton, O.; Podevin, P.; Massault, P.P.; Chouzenoux, S.; Soubrane, O.; Calmus, Y.; Conti, F. Interleukin-4 induces the activation and collagen production of cultured human intrahepatic fibroblasts via the STAT-6 pathway. Lab. Investig. 2008, 88, 973–985. [Google Scholar] [CrossRef]
- Roque-Cuellar, M.C.; Sanchez, B.; Garcia-Lozano, J.R.; Garrido-Serrano, A.; Sayago, M.; Praena-Fernandez, J.M.; Nunez-Roldan, A.; Aguilar-Reina, J. Expression of CD81, SR-BI and LDLR in lymphocytes and monocytes from patients with classic and occult hepatitis C virus infection. J. Med. Virol. 2012, 84, 1727–1736. [Google Scholar] [CrossRef]
- Chen, G.; Gharib, T.G.; Huang, C.C.; Taylor, J.M.; Misek, D.E.; Kardia, S.L.; Giordano, T.J.; Iannettoni, M.D.; Orringer, M.B.; Hanash, S.M.; et al. Discordant protein and mRNA expression in lung adenocarcinomas. Mol. Cell Proteom. 2002, 1, 304–313. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Lahtvee, P.J.; Sanchez, B.J.; Smialowska, A.; Kasvandik, S.; Elsemman, I.E.; Gatto, F.; Nielsen, J. Absolute Quantification of Protein and mRNA Abundances Demonstrate Variability in Gene-Specific Translation Efficiency in Yeast. Cell Syst. 2017, 4, 495–504. [Google Scholar] [CrossRef]
- Kumar, D.; Bansal, G.; Narang, A.; Basak, T.; Abbas, T.; Dash, D. Integrating transcriptome and proteome profiling: Strategies and applications. Proteomics 2016, 16, 2533–2544. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Neyen, C.; Gordon, S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology 2012, 217, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmy, A.R.; Malathi, J.; Madhavan, H.N. Hepatitis C Virus NS3 Mediated Microglial Inflammation via TLR2/TLR6 MyD88/NF-kappaB Pathway and Toll Like Receptor Ligand Treatment Furnished Immune Tolerance. PLoS ONE 2015, 10, e0125419. [Google Scholar] [CrossRef]
- Sarhan, M.A.; Pham, T.N.; Chen, A.Y.; Michalak, T.I. Hepatitis C virus infection of human T lymphocytes is mediated by CD5. J. Virol. 2012, 86, 3723–3735. [Google Scholar] [CrossRef] [PubMed]
- Serti, E.; Doumba, P.P.; Thyphronitis, G.; Tsitoura, P.; Katsarou, K.; Foka, P.; Konstandoulakis, M.M.; Koskinas, J.; Mavromara, P.; Georgopoulou, U. Modulation of IL-2 expression after uptake of hepatitis C virus non-enveloped capsid-like particles: The role of p38 kinase. Cell Mol. Life Sci. 2012, 68, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Hougardy, J.M.; Verscheure, V.; Locht, C.; Mascart, F. In vitro expansion of CD4+CD25highFOXP3+CD127low/-regulatory T cells from peripheral blood lymphocytes of healthy Mycobacterium tuberculosis-infected humans. Microbes Infect. 2007, 9, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Egen, J.G.; Allison, J.P. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 2002, 16, 23–35. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef]
- Klein, S.; Kretz, C.C.; Krammer, P.H.; Kuhn, A. CD127(low/-) and FoxP3(+) expression levels characterize different regulatory T-cell populations in human peripheral blood. J. Investig. Dermatol. 2010, 130, 492–499. [Google Scholar] [CrossRef]
- Jin, X.; Lu, Y.; Zhao, Y.; Yi, S. Large-scale in vitro expansion of human regulatory T cells with potent xenoantigen-specific suppression. Cytotechnology 2015, 68, 935–945. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Vignali, D.A.; Rudensky, A.Y.; Niec, R.E.; Waldmann, H. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 2013, 13, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Lu, C.H.; Yan, D.C.; Lee, P.T.; Hsiao, H.S.; Kuo, M.L. Expansion of regulatory T cells from umbilical cord blood and adult peripheral blood CD4(+)CD25 (+) T cells. Immunol. Res. 2014, 60, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, H.C.; Jeffery, L.E.; Lutz, P.; Corrigan, M.; Webb, G.J.; Hirschfield, G.M.; Adams, D.H.; Oo, Y.H. Low-dose interleukin-2 promotes STAT-5 phosphorylation, Treg survival and CTLA-4-dependent function in autoimmune liver diseases. Clin. Exp. Immunol. 2017, 188, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Hwang, Y.S.; Chen, Y.Y.; Liu, C.L.; Shen, C.N.; Hong, W.H.; Lo, S.M.; Shen, C.R. Interleukin-4 Supports the Suppressive Immune Responses Elicited by Regulatory T Cells. Front. Immunol. 2017, 8, 1508. [Google Scholar] [CrossRef]
- Foster, R.G.; Golden-Mason, L.; Rutebemberwa, A.; Rosen, H.R. Interleukin (IL)-17/IL-22-producing T cells enriched within the liver of patients with chronic hepatitis C viral (HCV) infection. Dig. Dis. Sci. 2012, 57, 381–389. [Google Scholar] [CrossRef]
- Duhen, T.; Duhen, R.; Lanzavecchia, A.; Sallusto, F.; Campbell, D.J. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012, 119, 4430–4440. [Google Scholar] [CrossRef]
- Bansal, S.S.; Ismahil, M.A.; Goel, M.; Zhou, G.; Rokosh, G.; Hamid, T.; Prabhu, S.D. Dysfunctional and Proinflammatory Regulatory T-Lymphocytes Are Essential for Adverse Cardiac Remodeling in Ischemic Cardiomyopathy. Circulation 2019, 139, 206–221. [Google Scholar] [CrossRef]
- Beriou, G.; Costantino, C.M.; Ashley, C.W.; Yang, L.; Kuchroo, V.K.; Baecher-Allan, C.; Hafler, D.A. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood 2009, 113, 4240–4249. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, N.; Vasanthakumar, A.; Zhang, Y.; Chopin, M.; Nutt, S.L.; Kallies, A.; Lew, A.M. CCR2 enhances CD25 expression by FoxP3(+) regulatory T cells and regulates their abundance independently of chemotaxis and CCR2(+) myeloid cells. Cell Mol. Immunol. 2018. [Google Scholar] [CrossRef]
- Zhuang, H.; Cao, G.; Kou, C.; Liu, T. CCL2/CCR2 axis induces hepatocellular carcinoma invasion and epithelial-mesenchymal transition in vitro through activation of the Hedgehog pathway. Oncol. Rep. 2018, 39, 21–30. [Google Scholar] [CrossRef]
- Campbell, D.J.; Koch, M.A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011, 11, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Yamanouchi, K.; Ohashi, K.; Koike, M.; Utoh, R.; Hasegawa, H.; Muraoka, I.; Suematsu, T.; Soyama, A.; Hidaka, M.; et al. Vascularized subcutaneous human liver tissue from engineered hepatocyte/fibroblast sheets in mice. Biomaterials 2015, 65, 66–75. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouaguia, L.; Moralès, O.; Aoudjehane, L.; Wychowski, C.; Kumar, A.; Dubuisson, J.; Calmus, Y.; Conti, F.; Delhem, N. Hepatitis C Virus Improves Human Tregs Suppressive Function and Promotes Their Recruitment to the Liver. Cells 2019, 8, 1296. https://doi.org/10.3390/cells8101296
Ouaguia L, Moralès O, Aoudjehane L, Wychowski C, Kumar A, Dubuisson J, Calmus Y, Conti F, Delhem N. Hepatitis C Virus Improves Human Tregs Suppressive Function and Promotes Their Recruitment to the Liver. Cells. 2019; 8(10):1296. https://doi.org/10.3390/cells8101296
Chicago/Turabian StyleOuaguia, Laurissa, Olivier Moralès, Lynda Aoudjehane, Czeslaw Wychowski, Abhishek Kumar, Jean Dubuisson, Yvon Calmus, Filomena Conti, and Nadira Delhem. 2019. "Hepatitis C Virus Improves Human Tregs Suppressive Function and Promotes Their Recruitment to the Liver" Cells 8, no. 10: 1296. https://doi.org/10.3390/cells8101296
APA StyleOuaguia, L., Moralès, O., Aoudjehane, L., Wychowski, C., Kumar, A., Dubuisson, J., Calmus, Y., Conti, F., & Delhem, N. (2019). Hepatitis C Virus Improves Human Tregs Suppressive Function and Promotes Their Recruitment to the Liver. Cells, 8(10), 1296. https://doi.org/10.3390/cells8101296





