Differences between the Proliferative Effects of Human Platelet Lysate and Fetal Bovine Serum on Human Adipose-Derived Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Isolation of hASCs
2.3. Cell Proliferation Assay
2.4. BrdU Incorporation Assay
2.5. Cell Cycle Assay
2.6. Cell Surface Marker of hASCs
2.7. Immunofluorescence Confocal Microscopy
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. PLTMax Stimulated Proliferation of hASCs
3.2. PLTMax Promoted Cell Cycle Transition from G0/G1 to S Phase
3.3. Effect of PLTMax on the Number of Ki-67 Positive Cells
3.4. Effect of PLTMax on the Cell Surface Marker of hASCs
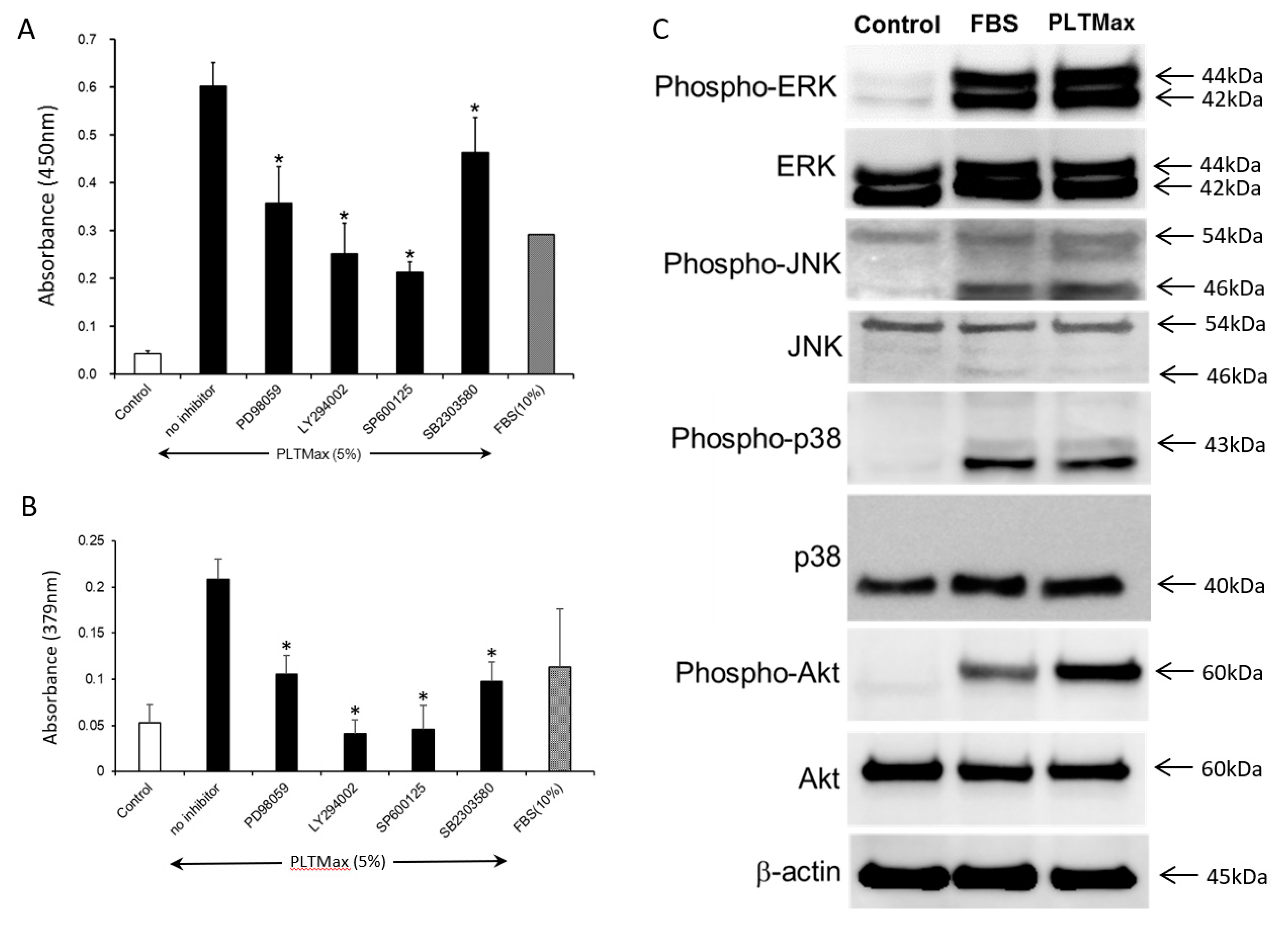
3.5. PLTMax Activated ERK1/2, AKT, and JNK Signaling Pathways
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Boil. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H. Adipose-derived stem and stromal cells for cell-based therapy: Current status of preclinical studies and clinical trials. Curr. Opin. Mol. Ther. 2010, 12, 442–449. [Google Scholar] [PubMed]
- Hara, Y.; Steiner, M.; Baldini, M.G. Platelets as a source of growth-promoting factor(s) for tumor cells. Cancer Res. 1980, 40, 1212–1216. [Google Scholar] [PubMed]
- Umeno, Y.; Okuda, A.; Kimura, G. Proliferative behaviour of fibroblasts in plasma-rich culture medium. J. Cell Sci. 1989, 94, 567–575. [Google Scholar] [PubMed]
- Doucet, C.; Ernou, I.; Zhang, Y.; Begot, L.; Holy, X.; Llense, J.-R.; Lataillade, J.-J.; Llense, J.; Lataillade, J. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J. Cell. Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, N.; Minakata, T.; Mitsui, T.; Kushida, S.; Notodihardjo, F.Z.; Kusumoto, K. Proliferation-Promoting Effect of Platelet-Rich Plasma on Human Adipose–Derived Stem Cells and Human Dermal Fibroblasts. Plast. Reconstr. Surg. 2008, 122, 1352–1360. [Google Scholar] [CrossRef]
- Trojahn Kolle, S.; Oliveri, R.S.; Glovinski, P.V.; Kirchhoff, M.; Mathiasen, A.B.; Elberg, J.J.; Andersen, P.S.; Drzewiecki, K.T.; Fischer-Nielsen, A. Pooled Human Platelet Lysate Versus Fetal Bovine Serum-Investigating the Proliferation Rate, Chromosome Stability and Angiogenic Potential of Human Adipose Tissue-Derived Stem Cells Intended for Clinical Use. Cytotherapy 2013, 15, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, V.; Scioli, M.G.; Gentile, P.; Doldo, E.; Bonanno, E.; Spagnoli, L.G.; Orlandi, A. Platelet-Rich Plasma Greatly Potentiates Insulin-Induced Adipogenic Differentiation of Human Adipose-Derived Stem Cells Through a Serine/Threonine Kinase Akt-Dependent Mechanism and Promotes Clinical Fat Graft Maintenance. Stem Cells Transl. Med. 2012, 1, 206–220. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Huang, C.-J.; Sun, Y.-C.; Christopher, K.; Pai, A.S.-I.; Lu, C.-J.; Hu, F.-R.; Lin, S.-Y.; Chen, W.-L. Comparison of corneal epitheliotrophic capacities among human platelet lysates and other blood derivatives. PLoS ONE 2017, 12, 0171008. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, N.; Kushida, S.; Suzuki, K.; Ogura, T.; Notodihardjo, P.V.; Hara, T.; Kusumoto, K. Effects of transforming growth factor-beta1 on cell motility, collagen gel contraction, myofibroblastic differentiation, and extracellular matrix expression of human adipose-derived stem cell. Hum. Cell 2012, 25, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, N.; Morimoto, N.; Ogawa, T.; Taketani, S.; Kusumoto, K. Hypoxia Enhances Proliferation of Human Adipose-Derived Stem Cells via HIF-1a Activation. PLoS ONE 2015, 10, e0139890. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Kakudo, N.; Morimoto, N.; Taketani, S.; Hara, T.; Ogawa, T.; Kusumoto, K. Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res. Ther. 2018, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef]
- Selvaggi, T.A.; Walker, R.E.; Fleisher, T.A. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood 1997, 89, 776–779. [Google Scholar]
- Mackensen, A.; Dräger, R.; Schlesier, M.; Mertelsmann, R.; Lindemann, A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol. Immunother. 2000, 49, 152–156. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Gordon, P.L.; Koo, W.K.K.; Marx, J.C.; Neel, M.D.; McNall, R.Y.; Muul, L.; Hofmann, T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA 2002, 99, 8932–8937. [Google Scholar] [CrossRef]
- Rauch, C. Alternatives to the use of fetal bovine serum: Human platelet lysates as a serum substitute in cell culture media. ALTEX 2011, 28, 305–316. [Google Scholar] [CrossRef]
- Schallmoser, K.; Strunk, D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J. Vis. Exp. 2009, 32, e1523. [Google Scholar] [CrossRef]
- Schallmoser, K.; Strunk, D. Generation of a pool of human platelet lysate and efficient use in cell culture. Methods Mol. Biol. 2013, 946, 349–362. [Google Scholar] [PubMed]
- Carrancio, S.; Lopez-Holgado, N.; Sanchez-Guijo, F.M.; Villaron, E.; Barbado, V.; Tabera, S.; Diez-Campelo, M.; Blanco, J.; Miguel, J.F.S.; Del Cañizo, M.C. Optimization of mesenchymal stem cell expansion procedures by cell separation and culture conditions modification. Exp. Hematol. 2008, 36, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Azouna, N.B.; Jenhani, F.; Regaya, Z.; Berraeis, L.; Othman, T.B.; Ducrocq, E.; Domenech, J. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: Comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res. Ther. 2012, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Huang, C.-J.; Tu, W.-H.; Lu, C.-J.; Sun, Y.-C.; Lin, S.-Y.; Chen, W.-L. The corneal epitheliotrophic abilities of lyophilized powder form human platelet lysates. PLoS ONE 2018, 13, e0194345. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-J.; Huang, S.-F.; Lai, J.-Y.; Ma, S.-C.; Chen, H.-C.; Wu, S.-E.; Wang, T.-K.; Sun, C.-C.; Ma, K.S.-K.; Chen, J.-K.; et al. Preservation of epithelial progenitor cells from collagenase-digested oral mucosa during ex vivo cultivation. Sci. Rep. 2016, 6, 36266. [Google Scholar] [CrossRef]
- Lensch, M.; Muise, A.; White, L.; Badowski, M.; Harris, D. Comparison of Synthetic Media Designed for Expansion of Adipose-Derived Mesenchymal Stromal Cells. Biomedicines 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Shang, H.; Khurgel, M.; Katz, A. Low serum and serum-free culture of multipotential human adipose stem cells. Cytotherapy 2007, 9, 637–646. [Google Scholar] [CrossRef]
- Mizuno, N.; Shiba, H.; Ozeki, Y.; Mouri, Y.; Niitani, M.; Inui, T.; Hayashi, H.; Suzuki, K.; Tanaka, S.; Kawaguchi, H. Human autologous serum obtained using a completely closed bag system as a substitute for foetal calf serum in human mesenchymal stem cell cultures. Cell Boil. Int. 2006, 30, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Nimura, A.; Muneta, T.; Koga, H.; Mochizuki, T.; Suzuki, K.; Makino, H.; Umezawa, A.; Sekiya, I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: Comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008, 58, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Shahdadfar, A.; Frønsdal, K.; Haug, T.; Reinholt, F.P.; Brinchmann, J.E. In Vitro Expansion of Human Mesenchymal Stem Cells: Choice of Serum Is a Determinant of Cell Proliferation, Differentiation, Gene Expression, and Transcriptome Stability. Stem Cells 2005, 23, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Hemeda, H.; Giebel, B.; Wagner, W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 2014, 16, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Haack-Sorensen, M.; Friis, T.; Bindslev, L.; Mortensen, S.; Johnsen, H.E.; Kastrup, J. Comparison of different culture conditions for human mesenchymal stromal cells for clinical stem cell therapy. Scand. J. Clin. Lab. Investig. 2008, 68, 192–203. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakudo, N.; Morimoto, N.; Ma, Y.; Kusumoto, K. Differences between the Proliferative Effects of Human Platelet Lysate and Fetal Bovine Serum on Human Adipose-Derived Stem Cells. Cells 2019, 8, 1218. https://doi.org/10.3390/cells8101218
Kakudo N, Morimoto N, Ma Y, Kusumoto K. Differences between the Proliferative Effects of Human Platelet Lysate and Fetal Bovine Serum on Human Adipose-Derived Stem Cells. Cells. 2019; 8(10):1218. https://doi.org/10.3390/cells8101218
Chicago/Turabian StyleKakudo, Natsuko, Naoki Morimoto, Yuanyuan Ma, and Kenji Kusumoto. 2019. "Differences between the Proliferative Effects of Human Platelet Lysate and Fetal Bovine Serum on Human Adipose-Derived Stem Cells" Cells 8, no. 10: 1218. https://doi.org/10.3390/cells8101218
APA StyleKakudo, N., Morimoto, N., Ma, Y., & Kusumoto, K. (2019). Differences between the Proliferative Effects of Human Platelet Lysate and Fetal Bovine Serum on Human Adipose-Derived Stem Cells. Cells, 8(10), 1218. https://doi.org/10.3390/cells8101218




