Hsp60 in Skeletal Muscle Fiber Biogenesis and Homeostasis: From Physical Exercise to Skeletal Muscle Pathology
Abstract
1. Hsp60 Function, Mechanisms and Interactions
2. Skeletal Muscle Fiber and Adaptations
3. Hsp60 in Skeletal Muscle Fibers of Human and Small Rodent: Meaning and Implications
4. Hsp60 and Skeletal Muscle Pathology
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bukau, B.; Horwich, A.L. The Hsp70 and Hsp60 chaperone machines. Cell 1998, 92, 351–366. [Google Scholar] [CrossRef]
- Christensen, J.H.; Nielsen, M.N.; Hansen, J.; Fuchtbauer, A.; Fuchtbauer, E.M.; West, M.; Corydon, T.J.; Gregersen, N.; Bross, P. Inactivation of the hereditary spastic paraplegia-associated Hspd1 gene encoding the Hsp60 chaperone results in early embryonic lethality in mice. Cell Stress Chaperones 2010, 15, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Rath, E.; Yuan, D.; Waldschmitt, N.; Khaloian, S.; Allgauer, M.; Staszewski, O.; Lobner, E.M.; Schottl, T.; Giesbertz, P.; et al. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nature Commun. 2016, 7, 13171. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, J.; Liu, X.; Wang, G.; Luo, M.; Deng, H. Down-regulation of HSP60 Suppresses the Proliferation of Glioblastoma Cells via the ROS/AMPK/mTOR Pathway. Sci. Rep. 2016, 6, 28388. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Patel, H.V.; Ridley, R.G.; Freeman, K.B.; Gupta, R.S. Mitochondrial import of the human chaperonin (hsp60) protein. Biochem. Biophys. Res. Commun. 1990, 169, 391–396. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Hartl, F.U.; Horwich, A.L. The mitochondrial chaperonin hsp60 is required for its own assembly. Nature 1990, 348, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Vilasi, S.; Bulone, D.; Caruso Bavisotto, C.; Campanella, C.; Marino Gammazza, A.; San Biagio, P.L.; Cappello, F.; Conway de Macario, E.; Macario, A.J.L. Chaperonin of Group I: Oligomeric Spectrum and Biochemical and Biological Implications. Front. Mol. Biosci. 2017, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Hohn, T.; Hohn, B.; Engel, A.; Wurtz, M.; Smith, P.R. Isolation and characterization of the host protein groE involved in bacteriophage lambda assembly. J. Mol. Biol. 1979, 129, 359–373. [Google Scholar] [CrossRef]
- Horwich, A.L. Protein folding in the cell: An inside story. Nat. Med. 2011, 17, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Fares, M.A.; Lund, P.A. Chaperonin 60: A paradoxical, evolutionarily conserved protein family with multiple moonlighting functions. Biol. Rev. Camb. Philos. Soc. 2013, 88, 955–987. [Google Scholar] [CrossRef] [PubMed]
- Viitanen, P.V.; Lorimer, G.H.; Seetharam, R.; Gupta, R.S.; Oppenheim, J.; Thomas, J.O.; Cowan, N.J. Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J. Biol. Chem. 1992, 267, 695–698. [Google Scholar] [PubMed]
- Vilasi, S.; Carrotta, R.; Mangione, M.R.; Campanella, C.; Librizzi, F.; Randazzo, L.; Martorana, V.; Marino Gammazza, A.; Ortore, M.G.; Vilasi, A.; et al. Human Hsp60 with its mitochondrial import signal occurs in solution as heptamers and tetradecamers remarkably stable over a wide range of concentrations. PLoS ONE 2014, 9, e97657. [Google Scholar] [CrossRef] [PubMed]
- Spinello, A.; Ortore, M.G.; Spinozzi, F.; Ricci, C.; Barone, G.; Gammazza, A.M.; Piccionello, A.P. Quaternary structures of GroEL and naïve-Hsp60 chaperonins in solution: A combined SAXS-MD study. RSC Adv. 2015, 5, 49871–49879. [Google Scholar] [CrossRef]
- Nielsen, K.L.; Cowan, N.J. A single ring is sufficient for productive chaperonin-mediated folding in vivo. Mol. Cell 1998, 2, 93–99. [Google Scholar] [CrossRef]
- Nisemblat, S.; Parnas, A.; Yaniv, O.; Azem, A.; Frolow, F. Crystallization and structure determination of a symmetrical ‘football’ complex of the mammalian mitochondrial Hsp60-Hsp10 chaperonins. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Marino Gammazza, A.; Palumbo Piccionello, A.; Campanella, C.; Pace, A.; Conway de Macario, E.; Macario, A.J. Hsp60 chaperonopathies and chaperonotherapy: Targets and agents. Expert Opin. Ther. Targets 2014, 18, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, S.R.; Gupta, S.; Knowlton, A.A. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation 2002, 105, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Pfister, G.; Stroh, C.M.; Perschinka, H.; Kind, M.; Knoflach, M.; Hinterdorfer, P.; Wick, G. Detection of HSP60 on the membrane surface of stressed human endothelial cells by atomic force and confocal microscopy. J. Cell Sci. 2005, 118, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.; D’Anneo, A.; Marino Gammazza, A.; Caruso Bavisotto, C.; Barone, R.; Emanuele, S.; Lo Cascio, F.; Mocciaro, E.; Fais, S.; Conway De Macario, E.; et al. The histone deacetylase inhibitor SAHA induces HSP60 nitration and its extracellular release by exosomal vesicles in human lung-derived carcinoma cells. Oncotarget 2016, 7, 28849–28867. [Google Scholar] [CrossRef] [PubMed]
- Marino Gammazza, A.; Colangeli, R.; Orban, G.; Pierucci, M.; Di Gennaro, G.; Lo Bello, M.; D’Aniello, A.; Bucchieri, F.; Pomara, C.; Valentino, M.; et al. Hsp60 response in experimental and human temporal lobe epilepsy. Sci. Rep. 2015, 5, 9434. [Google Scholar] [CrossRef] [PubMed]
- Marino Gammazza, A.; Campanella, C.; Barone, R.; Caruso Bavisotto, C.; Gorska, M.; Wozniak, M.; Carini, F.; Cappello, F.; D’Anneo, A.; Lauricella, M.; et al. Doxorubicin anti-tumor mechanisms include Hsp60 post-translational modifications leading to the Hsp60/p53 complex dissociation and instauration of replicative senescence. Cancer Lett. 2017, 385, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Voos, W.; Rottgers, K. Molecular chaperones as essential mediators of mitochondrial biogenesis. Biochim. Biophys. Acta 2002, 1592, 51–62. [Google Scholar] [CrossRef]
- Fenton, W.A.; Weissman, J.S.; Horwich, A.L. Putting a lid on protein folding: Structure and function of the co-chaperonin, GroES. Chem. Biol. 1996, 3, 157–161. [Google Scholar] [CrossRef]
- Martin, J.; Mayhew, M.; Langer, T.; Hartl, F.U. The reaction cycle of GroEL and GroES in chaperonin-assisted protein folding. Nature 1993, 366, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Voos, W. Chaperone-protease networks in mitochondrial protein homeostasis. Biochim. Biophys. Acta 2013, 1833, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Y.; Hartl, F.U.; Martin, J.; Pollock, R.A.; Kalousek, F.; Neupert, W.; Hallberg, E.M.; Hallberg, R.L.; Horwich, A.L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature 1989, 337, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Reading, D.S.; Hallberg, R.L.; Myers, A.M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature 1989, 337, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.A.; Newman, S.M.; Hallberg, R.L.; Slaughter, C.A.; Perlman, P.S.; Butow, R.A. In organello formaldehyde crosslinking of proteins to mtDNA: Identification of bifunctional proteins. Proc. Natl. Acad. Sci. USA 2000, 97, 7772–7777. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.A.; Kolesar, J.E.; Perlman, P.S.; Butow, R.A. A function for the mitochondrial chaperonin Hsp60 in the structure and transmission of mitochondrial DNA nucleoids in Saccharomyces cerevisiae. J. Cell Biol. 2003, 163, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Madan, D.; Rye, H.S. GroEL stimulates protein folding through forced unfolding. Nat. Struct. Mol. Biol. 2008, 15, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Marino Gammazza, A.; Bavisotto, C.C.; Barone, R.; de Macario, E.C.; Macario, A.J. Alzheimer’s Disease and Molecular Chaperones: Current Knowledge and the Future of Chaperonotherapy. Curr. Pharm. Des. 2016, 22, 4040–4049. [Google Scholar] [CrossRef] [PubMed]
- Deocaris, C.C.; Kaul, S.C.; Wadhwa, R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 2006, 11, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Sigal, L.H.; Williams, S.; Soltys, B.; Gupta, R. H9724, a monoclonal antibody to Borrelia burgdorferi’s flagellin, binds to heat shock protein 60 (HSP60) within live neuroblastoma cells: A potential role for HSP60 in peptide hormone signaling and in an autoimmune pathogenesis of the neuropathy of Lyme disease. Cell Mol. Neurobiol. 2001, 21, 477–495. [Google Scholar] [PubMed]
- Chandra, D.; Choy, G.; Tang, D.G. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: Evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J. Biol. Chem. 2007, 282, 31289–31301. [Google Scholar] [CrossRef] [PubMed]
- Chaiwatanasirikul, K.A.; Sala, A. The tumour-suppressive function of CLU is explained by its localisation and interaction with HSP60. Cell Death Dis. 2011, 2, e219. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.C.; Dohi, T.; Kang, B.H.; Altieri, D.C. Hsp60 regulation of tumor cell apoptosis. J. Biol. Chem. 2008, 283, 5188–5194. [Google Scholar] [CrossRef] [PubMed]
- Samali, A.; Cai, J.; Zhivotovsky, B.; Jones, D.P.; Orrenius, S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999, 18, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G.; Multhoff, G. Cell stress proteins in extracellular fluids: Friend or foe? Novartis Found. Symp. 2008, 291, 86–95. [Google Scholar] [PubMed]
- Quintana, F.J.; Cohen, I.R. HSP60 speaks to the immune system in many voices. Novartis Found. Symp. 2008, 291, 101–111. [Google Scholar] [PubMed]
- De Kleer, I.; Vercoulen, Y.; Klein, M.; Meerding, J.; Albani, S.; van der Zee, R.; Sawitzki, B.; Hamann, A.; Kuis, W.; Prakken, B. CD30 discriminates heat shock protein 60-induced FOXP3+ CD4+ T cells with a regulatory phenotype. J. Immunol. 2010, 185, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Aalberse, J.A.; Kapitein, B.; de Roock, S.; Klein, M.R.; de Jager, W.; van der Zee, R.; Hoekstra, M.O.; van Wijk, F.; Prakken, B.J. Cord blood CD4+ T cells respond to self heat shock protein 60 (HSP60). PLoS ONE 2011, 6, e24119. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.K.; Wang, H.; Yim, A.M.; Le Naour, F.; Brichory, F.; Jang, J.H.; Zhao, R.; Puravs, E.; Tra, J.; Michael, C.W.; et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J. Biol. Chem. 2003, 278, 7607–7616. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G.; Muthana, M.; Calderwood, S.K. The dual immunoregulatory roles of stress proteins. Trends Biochem. Sci. 2008, 33, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Sfady, M.; Nussbaum, G.; Pevsner-Fischer, M.; Mor, F.; Carmi, P.; Zanin-Zhorov, A.; Lider, O.; Cohen, I.R. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J. Immunol. 2005, 175, 3594–3602. [Google Scholar] [CrossRef] [PubMed]
- Kol, A.; Lichtman, A.H.; Finberg, R.W.; Libby, P.; Kurt-Jones, E.A. Cutting edge: Heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 2000, 164, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Van Eden, W.; van der Zee, R.; Prakken, B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat. Rev. Immunol. 2005, 5, 318–330. [Google Scholar] [CrossRef] [PubMed]
- De Kleer, I.M.; Kamphuis, S.M.; Rijkers, G.T.; Scholtens, L.; Gordon, G.; De Jager, W.; Hafner, R.; van de Zee, R.; van Eden, W.; Kuis, W.; et al. The spontaneous remission of juvenile idiopathic arthritis is characterized by CD30+ T cells directed to human heat-shock protein 60 capable of producing the regulatory cytokine interleukin-10. Arthritis Rheum. 2003, 48, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Shamaei-Tousi, A.; Steptoe, A.; O’Donnell, K.; Palmen, J.; Stephens, J.W.; Hurel, S.J.; Marmot, M.; Homer, K.; D’Aiuto, F.; Coates, A.R.; et al. Plasma heat shock protein 60 and cardiovascular disease risk: The role of psychosocial, genetic, and biological factors. Cell Stress Chaperones 2007, 12, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Rappa, F.; Macaluso, F.; Caruso Bavisotto, C.; Sangiorgi, C.; Di Paola, G.; Tomasello, G.; Di Felice, V.; Marciano, V.; Farina, F.; et al. Alcoholic Liver Disease: A Mouse Model Reveals Protection by Lactobacillus fermentum. Clin. Transl. Gastroenterol. 2016, 7, e138. [Google Scholar] [CrossRef] [PubMed]
- Hayoun, D.; Kapp, T.; Edri-Brami, M.; Ventura, T.; Cohen, M.; Avidan, A.; Lichtenstein, R.G. HSP60 is transported through the secretory pathway of 3-MCA-induced fibrosarcoma tumour cells and undergoes N-glycosylation. FEBS J. 2012, 279, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Tian, E.; Liu, C.; Wang, Q.; Deng, H. Oxidative stress induces monocyte necrosis with enrichment of cell-bound albumin and overexpression of endoplasmic reticulum and mitochondrial chaperones. PLoS ONE 2013, 8, e59610. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Rodolico, V.; Zerilli, M.; Martorana, A.; Bucchieri, F.; Pitruzzella, A.; Marino Gammazza, A.; David, S.; Rappa, F.; Zummo, G.; et al. Changes in immunohistochemical levels and subcellular localization after therapy and correlation and colocalization with CD68 suggest a pathogenetic role of Hsp60 in ulcerative colitis. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, G.; Dardiotis, E.; Liaskos, C.; Marou, E.; Tsimourtou, V.; Scheper, T.; Meyer, W.; Daponte, A.; Sakkas, L.I.; Hadjigeorgiou, G.; et al. Anti-hsp60 antibody responses based on Helicobacter pylori in patients with multiple sclerosis: (ir)Relevance to disease pathogenesis. J. Neuroimmunol. 2016, 298, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, H.; Zheng, C.; Gao, J.; Fu, Q.; Hu, N.; Shao, X.; Zhou, Y.; Xiong, J.; Nie, K.; et al. Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth. Cell Death Dis. 2018, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Chang, H.; Huang, X.; Guo, X.; Du, X.; Tian, S.; Wang, L.; Lyv, Y.; Yuan, P.; et al. Hsp60 exerts a tumor suppressor function by inducing cell differentiation and inhibiting invasion in hepatocellular carcinoma. Oncotarget 2016, 7, 68976–68989. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, J.; Chen, H.; Adam, A.; Tang, S.; Kemper, N.; Hartung, J.; Bao, E. Expression and location of HSP60 and HSP10 in the heart tissue of heat-stressed rats. Exp. Ther. Med. 2016, 12, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Agababaoglu, I.; Onen, A.; Demir, A.B.; Aktas, S.; Altun, Z.; Ersoz, H.; Sanl, A.; Ozdemir, N.; Akkoclu, A. Chaperonin (HSP60) and annexin-2 are candidate biomarkers for non-small cell lung carcinoma. Medicine 2017, 96, e5903. [Google Scholar] [CrossRef] [PubMed]
- Cechetto, J.D.; Soltys, B.J.; Gupta, R.S. Localization of mitochondrial 60-kD heat shock chaperonin protein (Hsp60) in pituitary growth hormone secretory granules and pancreatic zymogen granules. J. Histochem. Cytochem. 2000, 48, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Spangenburg, E.E.; Booth, F.W. Molecular regulation of individual skeletal muscle fibre types. Acta Physiol. Scand. 2003, 178, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Zierath, J.R.; Hawley, J.A. Skeletal muscle fiber type: Influence on contractile and metabolic properties. PLoS Biol. 2004, 2, e348. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Fluck, M.; Hoppeler, H. Molecular basis of skeletal muscle plasticity-from gene to form and function. Rev. Physiol. Biochem. Pharmacol. 2003, 146, 159–216. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H. Exercise-induced ultrastructural changes in skeletal muscle. Int. J. Sports. Med. 1986, 7, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.; Puntschart, A.; Howald, H.; Mueller, B.; Mannhart, C.; Gfeller-Tuescher, L.; Mullis, P.; Hoppeler, H. Effects of dietary fat on muscle substrates, metabolism, and performance in athletes. Med. Sci. Sports. Exerc. 2003, 35, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.; Barone, R.; Catanese, P.; Carini, F.; Rizzuto, L.; Farina, F.; Di Felice, V. Do fat supplements increase physical performance? Nutrients 2013, 5, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.; Isaacs, A.W.; Di Felice, V.; Myburgh, K.H. Acute change of titin at mid-sarcomere remains despite 8 wk of plyometric training. J. Appl. Physiol. 2014, 116, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.; Myburgh, K.H. Current evidence that exercise can increase the number of adult stem cells. J. Muscle Res. Cell Motil. 2012, 33, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.; Brooks, N.E.; Niesler, C.U.; Myburgh, K.H. Satellite cell pool expansion is affected by skeletal muscle characteristics. Muscle Nerve 2013, 48, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Libardi, C.A.; Ugrinowitsch, C.; Vechin, F.C.; Lixandrao, M.E.; Snijders, T.; Nederveen, J.P.; Bacurau, A.V.; Brum, P.; Tricoli, V.; et al. Early- and later-phases satellite cell responses and myonuclear content with resistance training in young men. PLoS ONE 2018, 13, e0191039. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.; Brooks, N.E.; van de Vyver, M.; Van Tubbergh, K.; Niesler, C.U.; Myburgh, K.H. Satellite cell count, VO(2max), and p38 MAPK in inactive to moderately active young men. Scand. J. Med. Sci. Sports. 2012, 22, e38–e44. [Google Scholar] [CrossRef] [PubMed]
- Beiter, T.; Hoene, M.; Prenzler, F.; Mooren, F.C.; Steinacker, J.M.; Weigert, C.; Niess, A.M.; Munz, B. Exercise, skeletal muscle and inflammation: ARE-binding proteins as key regulators in inflammatory and adaptive networks. Exerc. Immunol. Rev. 2015, 21, 42–57. [Google Scholar] [PubMed]
- Smith, C.; Kruger, M.J.; Smith, R.M.; Myburgh, K.H. The inflammatory response to skeletal muscle injury: Illuminating complexities. Sports Med. 2008, 38, 947–969. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Bilan, P.J.; Fink, L.N.; Klip, A. Cross-talk between skeletal muscle and immune cells: Muscle-derived mediators and metabolic implications. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E453–E465. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.A.; Apponi, L.H.; Long, K.K.; Pavlath, G.K. Chemokine expression and control of muscle cell migration during myogenesis. J. Cell Sci. 2010, 123, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, J.; Pedersen, B.K.; Kratchmarova, I. Quantitative analysis of the secretion of the MCP family of chemokines by muscle cells. Mol. Biosyst. 2011, 7, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Scheler, M.; Irmler, M.; Lehr, S.; Hartwig, S.; Staiger, H.; Al-Hasani, H.; Beckers, J.; de Angelis, M.H.; Haring, H.U.; Weigert, C. Cytokine response of primary human myotubes in an in vitro exercise model. Am. J. Physiol. Cell Physiol. 2013, 305, C877–C886. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, M.A.; Leggate, M.; Viana, J.L.; King, J.A. The effect of physical activity on mediators of inflammation. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 51–60. [Google Scholar] [CrossRef]
- Raschke, S.; Eckel, J. Adipo-myokines: Two sides of the same coin--mediators of inflammation and mediators of exercise. Mediat. Inflamm. 2013, 2013, 320724. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, J.V.; Jones, K.M.; Basson, M.A.; Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 2012, 490, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Boppart, M.D. Influence of exercise and aging on extracellular matrix composition in the skeletal muscle stem cell niche. J. Appl. Physiol. 2016, 121, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Tarnopolsky, M.A.; Beckman, K.; Felkey, K.; Hubbard, A. Resistance exercise reverses aging in human skeletal muscle. PLoS ONE 2007, 2, e465. [Google Scholar] [CrossRef] [PubMed]
- Ornatsky, O.I.; Connor, M.K.; Hood, D.A. Expression of stress proteins and mitochondrial chaperonins in chronically stimulated skeletal muscle. Biochem. J. 1995, 311 Pt 1, 119–123. [Google Scholar] [CrossRef]
- Bornman, L.; Polla, B.S.; Lotz, B.P.; Gericke, G.S. Expression of heat-shock/stress proteins in Duchenne muscular dystrophy. Muscle Nerve 1995, 18, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.P.; Maclaren, D.P.; Cable, N.T.; Campbell, I.T.; Evans, L.; Kayani, A.C.; McArdle, A.; Drust, B. Trained men display increased basal heat shock protein content of skeletal muscle. Med. Sci. Sports. Exerc. 2008, 40, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, M.; Mackey, A.L.; Langberg, H.; Oskarsson, E.; Piehl-Aulin, K.; Henriksson, J.; Kadi, F. The expression of heat shock protein in human skeletal muscle: Effects of muscle fibre phenotype and training background. Acta Physiol. 2013, 209, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Takahashi, M.; Connor, M.K.; Freyssenet, D. Assembly of the cellular powerhouse: Current issues in muscle mitochondrial biogenesis. Exerc. Sport Sci. Rev. 2000, 28, 68–73. [Google Scholar] [PubMed]
- Mattson, J.P.; Ross, C.R.; Kilgore, J.L.; Musch, T.I. Induction of mitochondrial stress proteins following treadmill running. Med. Sci. Sports. Exerc. 2000, 32, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Samelman, T.R. Heat shock protein expression is increased in cardiac and skeletal muscles of Fischer 344 rats after endurance training. Exp. Physiol. 2000, 85, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Oishi, Y.; Higashida, K.; Higuchi, M.; Muraoka, I. Prolonged exercise training induces long-term enhancement of HSP70 expression in rat plantaris muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1557–R1563. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.S.; Lollo, P.C.; Morato, P.N.; Nisishima, L.H.; Carneiro, E.M.; Amaya-Farfan, J. Whey protein hydrolysate enhances HSP90 but does not alter HSP60 and HSP25 in skeletal muscle of rats. PLoS ONE 2014, 9, e83437. [Google Scholar] [CrossRef]
- Barone, R.; Macaluso, F.; Sangiorgi, C.; Campanella, C.; Marino Gammazza, A.; Moresi, V.; Coletti, D.; Conway de Macario, E.; Macario, A.J.; Cappello, F.; et al. Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 alpha1 expression. Sci. Rep. 2016, 6, 19781. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Sangiorgi, C.; Marino Gammazza, A.; D’Amico, D.; Salerno, M.; Cappello, F.; Pomara, C.; Zummo, G.; Farina, F.; Di Felice, V.; et al. Effects of Conjugated Linoleic Acid Associated With Endurance Exercise on Muscle Fibres and Peroxisome Proliferator-Activated Receptor gamma Coactivator 1 alpha Isoforms. J. Cell. Physiol. 2017, 232, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.A.; Cerutti, R.; Beninca, C.; Brea-Calvo, G.; Jacobs, H.T.; Zeviani, M.; Szibor, M.; Viscomi, C. Perturbed Redox Signaling Exacerbates a Mitochondrial Myopathy. Cell Metab. 2018, 28, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Ramadasan-Nair, R.; Gayathri, N.; Mishra, S.; Sunitha, B.; Mythri, R.B.; Nalini, A.; Subbannayya, Y.; Harsha, H.C.; Kolthur-Seetharam, U.; Srinivas Bharath, M.M. Mitochondrial alterations and oxidative stress in an acute transient mouse model of muscle degeneration: Implications for muscular dystrophy and related muscle pathologies. J. Biol. Chem. 2014, 289, 485–509. [Google Scholar] [CrossRef] [PubMed]
- Quintana, F.J.; Cohen, I.R. The HSP60 immune system network. Trends Immunol. 2011, 32, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E.; Zembron-Lacny, A.; Kasperska, A.; Antosiewicz, J.; Grzywacz, T.; Garsztka, T.; Laskowski, R. Exercise training-induced changes in inflammatory mediators and heat shock proteins in young tennis players. J. Sports Sci. Med. 2013, 12, 282–289. [Google Scholar] [PubMed]
- Molanouri Shamsi, M.; Mahdavi, M.; Quinn, L.S.; Gharakhanlou, R.; Isanegad, A. Effect of resistance exercise training on expression of Hsp70 and inflammatory cytokines in skeletal muscle and adipose tissue of STZ-induced diabetic rats. Cell Stress Chaperones 2016, 21, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Khadir, A.; Kavalakatt, S.; Cherian, P.; Warsame, S.; Abubaker, J.A.; Dehbi, M.; Tiss, A. Physical Exercise Enhanced Heat Shock Protein 60 Expression and Attenuated Inflammation in the Adipose Tissue of Human Diabetic Obese. Front. Endocrinol. 2018, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Elst, E.F.; Klein, M.; de Jager, W.; Kamphuis, S.; Wedderburn, L.R.; van der Zee, R.; Albani, S.; Kuis, W.; Prakken, B.J. Hsp60 in inflamed muscle tissue is the target of regulatory autoreactive T cells in patients with juvenile dermatomyositis. Arthritis Rheum. 2008, 58, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pickrell, A.M.; Rossi, S.G.; Pinto, M.; Dillon, L.M.; Hida, A.; Rotundo, R.L.; Moraes, C.T. Transient systemic mtDNA damage leads to muscle wasting by reducing the satellite cell pool. Hum. Mol. Genet. 2013, 22, 3976–3986. [Google Scholar] [CrossRef] [PubMed]
- Kinawi, A.; Onken, A.; Rozyczka, B.; Konig, W. Studies on the reactability of chlordiazepoxide to its N-nitrosos derivative in physiological conditions (author’s translation). Arzneimittel-Forschung 1977, 27, 363–367. [Google Scholar] [PubMed]
- Fellows, I.W. Hereditary (primary) haemochromatosis. BMJ 1990, 301, 350. [Google Scholar] [CrossRef]
- Theilen, N.T.; Kunkel, G.H.; Tyagi, S.C. The Role of Exercise and TFAM in Preventing Skeletal Muscle Atrophy. J. Cell. Physiol. 2017, 232, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, G.H.; Chaturvedi, P.; Tyagi, S.C. Mitochondrial pathways to cardiac recovery: TFAM. Heart Fail. Rev. 2016, 21, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Morici, G.; Frinchi, M.; Pitruzzella, A.; Di Liberto, V.; Barone, R.; Pace, A.; Di Felice, V.; Belluardo, N.; Cappello, F.; Mudo, G.; et al. Mild Aerobic Exercise Training Hardly Affects the Diaphragm of mdx Mice. J. Cell. Physiol. 2017, 232, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, K.; Une, S.; Komatsu, M.; Yamaji, R.; Akiyama, J. Heat stress prevents the decrease in succinate dehydrogenase activity in the extensor digitorum longus of streptozotocin-induced diabetic rats. Physiol. Res. 2018, 67, 117–126. [Google Scholar] [PubMed]
- Huckriede, A.; Heikema, A.; Sjollema, K.; Briones, P.; Agsteribbe, E. Morphology of the mitochondria in heat shock protein 60 deficient fibroblasts from mitochondrial myopathy patients. Effects of stress conditions. Virchows Arch. 1995, 427, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Gammazza, A.M.; Bucchieri, F.; Grimaldi, L.M.; Benigno, A.; de Macario, E.C.; Macario, A.J.; Zummo, G.; Cappello, F. The molecular anatomy of human Hsp60 and its similarity with that of bacterial orthologs and acetylcholine receptor reveal a potential pathogenetic role of anti-chaperonin immunity in myasthenia gravis. Cell. Mol. Neurobiol. 2012, 32, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.J.; Durr, A.; Cournu-Rebeix, I.; Georgopoulos, C.; Ang, D.; Nielsen, M.N.; Davoine, C.S.; Brice, A.; Fontaine, B.; Gregersen, N.; et al. Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am. J. Hum. Genet. 2002, 70, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Parnas, A.; Nadler, M.; Nisemblat, S.; Horovitz, A.; Mandel, H.; Azem, A. The MitCHAP-60 disease is due to entropic destabilization of the human mitochondrial Hsp60 oligomer. J. Biol. Chem. 2009, 284, 28198–28203. [Google Scholar] [CrossRef] [PubMed]
- SWISS-MODEL Website. Available online: https://swissmodel.expasy.org (accessed on 30 October 2018).
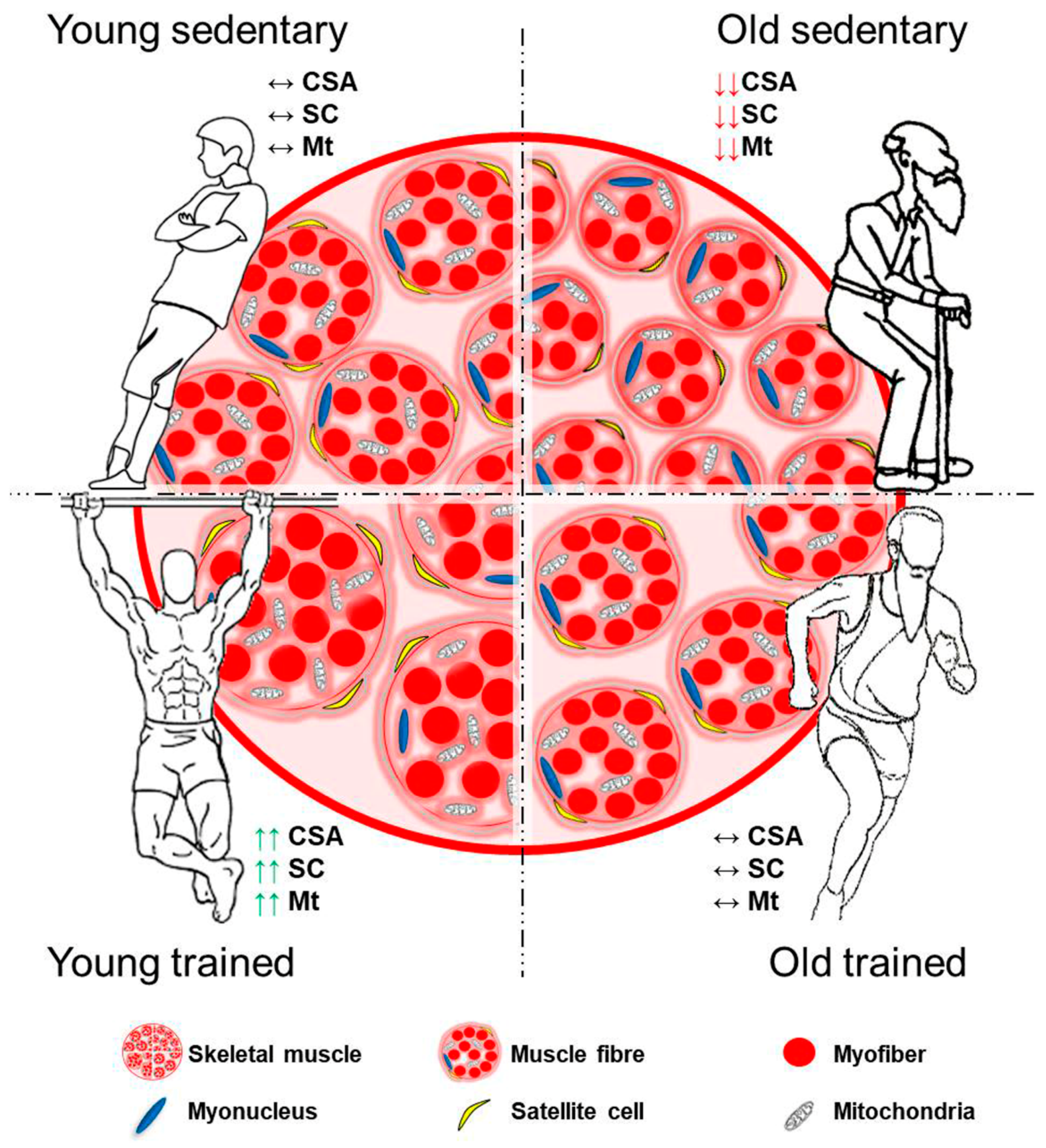

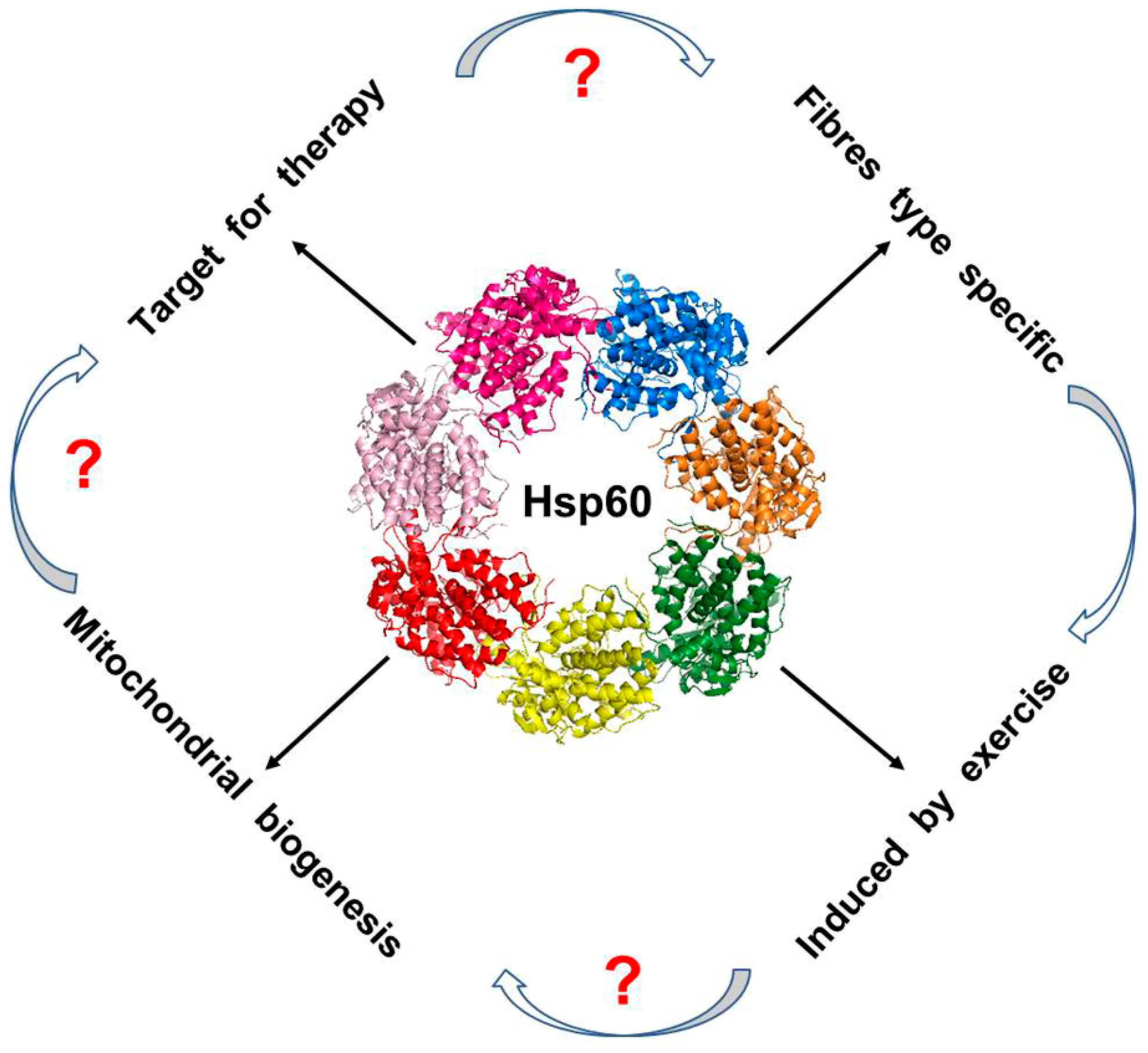
| Pathologic Condition | Main Mediators | References |
|---|---|---|
| Dystrophic-trained mice | No mediators, Hsp60 decrease in the diaphragm muscle | [106] |
| Diabetic rats | No mediators, Hsp60 decrease in the extensor digitorum longus muscle | [107] |
| Mitochondrial myopathy | No mediators, Hsp60 deficient mitochondria in fibroblast | [108] |
| Dermatomyositis | T cell | [100] |
| Myasthenia gravis | Shared epitopes between human, Chlamydia trachomatis and Chlamydia pneumoniae Hsp60 sequence with AChRα1 | [109] |
| Multiple sclerosis | Anti-Hsp60 Helicobacter pylori antibody reactivity | [53] |
| Hereditary spastic paraplegia | SPG13 associated with V72I substitution | [110] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino Gammazza, A.; Macaluso, F.; Di Felice, V.; Cappello, F.; Barone, R. Hsp60 in Skeletal Muscle Fiber Biogenesis and Homeostasis: From Physical Exercise to Skeletal Muscle Pathology. Cells 2018, 7, 224. https://doi.org/10.3390/cells7120224
Marino Gammazza A, Macaluso F, Di Felice V, Cappello F, Barone R. Hsp60 in Skeletal Muscle Fiber Biogenesis and Homeostasis: From Physical Exercise to Skeletal Muscle Pathology. Cells. 2018; 7(12):224. https://doi.org/10.3390/cells7120224
Chicago/Turabian StyleMarino Gammazza, Antonella, Filippo Macaluso, Valentina Di Felice, Francesco Cappello, and Rosario Barone. 2018. "Hsp60 in Skeletal Muscle Fiber Biogenesis and Homeostasis: From Physical Exercise to Skeletal Muscle Pathology" Cells 7, no. 12: 224. https://doi.org/10.3390/cells7120224
APA StyleMarino Gammazza, A., Macaluso, F., Di Felice, V., Cappello, F., & Barone, R. (2018). Hsp60 in Skeletal Muscle Fiber Biogenesis and Homeostasis: From Physical Exercise to Skeletal Muscle Pathology. Cells, 7(12), 224. https://doi.org/10.3390/cells7120224








