Neurogenesis and Neuroinflammation in Dialogue: Mapping Gaps, Modulating Microglia, Rewiring Aging
Highlights
- Five mechanistic gaps were defined that shape the neurogenesis–neuroinflammation dialogue in aging.
- Translational strategies such as imaging, immunomodulation, and glial reprogramming offer testable intervention pathways.
- Tuning immune and epigenetic environments may preserve or even restore neurogenic potential.
- An integrated roadmap links mechanistic precision to clinical innovation, aiming to delay cognitive decline.
Abstract
1. Introduction

2. Neurogenesis and Neuroinflammation in the Aging Brain: An Overview
2.1. Adult Neurogenesis: Mechanisms and Age-Related Decline
2.2. Neuroinflammation in Aging: Microglia and Beyond
2.3. Microglia–Neural Stem Cell Crosstalk
3. Critical Gaps in Current Knowledge
3.1. Gap 1—Region-Specific Microglial Diversity in Aging
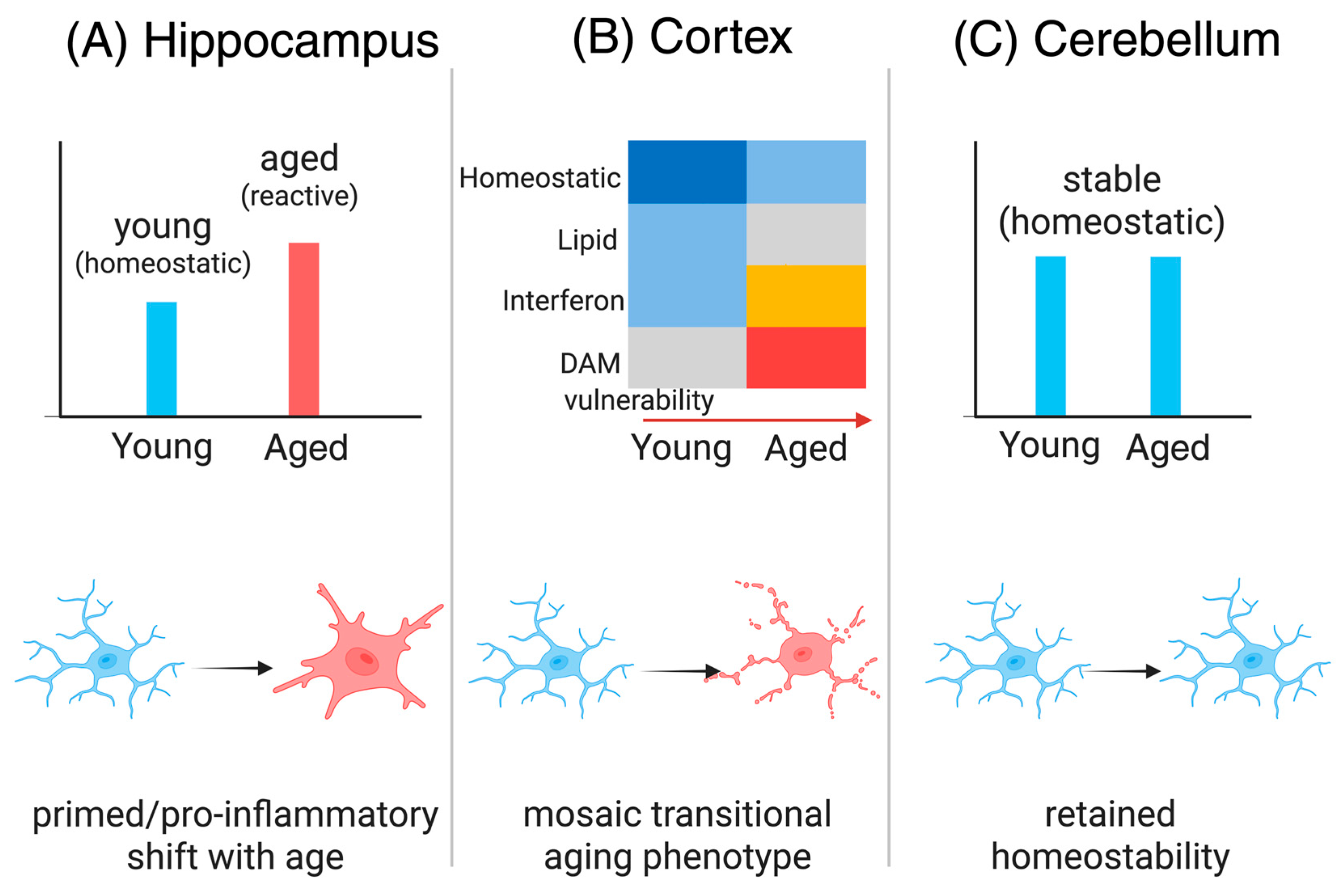
3.2. Gap 2—Inflammasome-Driven Epigenetic Alterations
3.3. Gap 3—Longitudinal Dynamics of Neuroimmune Interactions
3.4. Gap 4—Niche-Specific Immune Mechanisms
3.5. Gap 5—Translational and Cross-Species Disconnects
4. Strategies and Emerging Approaches to Bridge the Gaps

4.1. Longitudinal Neuroimmune Imaging
4.2. Niche-Focused Immunomodulation
4.3. Glial Subtype Reprogramming
4.4. Brain-Penetrant NLRP3 Inflammasome Inhibitors
4.5. CRISPR-Based Epigenetic Editing
5. Comparative Perspectives: Human vs. Animal Models

5.1. Adult Neurogenesis: Rodents vs. Humans
5.2. Microglial States Across Species
5.3. Inflammatory Pathways and Neuroimmune Crosstalk
5.4. Intervention Efficacy and Translational Readiness
5.5. Bridging the Gap: Models, Ethics, and Future Outlook
6. Integrating Mechanisms with Therapeutics: Toward Rewiring the Aging Brain
6.1. Mechanistic Gaps as Opportunities
6.2. Translational Roadmap
6.3. Ethical and Clinical Considerations
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AAV | adeno-associated virus |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| CNS | central nervous system |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| CSF | cerebrospinal fluid |
| CX3CL1 | C-X3-C motif chemokine ligand 1 |
| CX3CR1 | C-X3-C motif chemokine receptor 1 |
| DAM | disease-associated microglia |
| DCX | doublecortin |
| DG | dentate gyrus |
| EVs | extracellular vesicles |
| IFN-γ | interferon-gamma |
| IL-1β | interleukin-1 beta |
| IL-18 | interleukin-18 |
| iPSC | induced pluripotent stem cell |
| JAK/STAT1 | janus kinase/signal transducer and activator of transcription 1 |
| MCC950 | nlrp3 inflammasome inhibitor mcc950 |
| MRI | magnetic resonance imaging |
| NLRP3 | nod-like receptor protein 3 |
| NSAID | non-steroidal anti-inflammatory drug |
| NSPCs | neural stem and progenitor cells |
| PET | positron emission tomography |
| PSA-NCAM | polysialylated neural cell adhesion molecule |
| RNA-seq | RNA sequencing |
| SVZ | subventricular zone |
| TBI | traumatic brain injury |
| TNF-α | tumor necrosis factor-alpha |
| TSPO | translocator protein 18 kDa |
| Wnt | wingless-related integration site signaling pathway |
References
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599.e585. [Google Scholar] [CrossRef]
- Babcock, K.R.; Page, J.S.; Fallon, J.R.; Webb, A.E. Adult Hippocampal Neurogenesis in Aging and Alzheimer’s Disease. Stem Cell Rep. 2021, 16, 681–693. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15, 124. [Google Scholar] [CrossRef]
- Culig, L.; Chu, X.; Bohr, V.A. Neurogenesis in aging and age-related neurodegenerative diseases. Ageing Res. Rev. 2022, 78, 101636. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Valero, J.; Bernardino, L.; Cardoso, F.L.; Silva, A.P.; Fontes-Ribeiro, C.; Ambrósio, A.F.; Malva, J.O. Impact of Neuroinflammation on Hippocampal Neurogenesis: Relevance to Aging and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, S161–S168. [Google Scholar] [CrossRef] [PubMed]
- Amanollahi, M.; Jameie, M.; Heidari, A.; Rezaei, N. The Dialogue Between Neuroinflammation and Adult Neurogenesis: Mechanisms Involved and Alterations in Neurological Diseases. Mol. Neurobiol. 2023, 60, 923–959. [Google Scholar] [CrossRef]
- Mészáros, Á.; Molnár, K.; Nógrádi, B.; Hernádi, Z.; Nyúl-Tóth, Á.; Wilhelm, I.; Krizbai, I.A. Neurovascular Inflammaging in Health and Disease. Cells 2020, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, M.C.; Jurcau, A.; Cristian, A.; Hogea, V.O.; Diaconu, R.G.; Nunkoo, V.S. Inflammaging and Brain Aging. Int. J. Mol. Sci. 2024, 25, 10535. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of neuropathology-associated reactive astrocytes: A systematic review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef]
- Propson, N.E.; Roy, E.R.; Litvinchuk, A.; Köhl, J.; Zheng, H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J. Clin. Investig. 2021, 131, e140966. [Google Scholar] [CrossRef]
- Elahy, M.; Jackaman, C.; Mamo, J.C.; Lam, V.; Dhaliwal, S.S.; Giles, C.; Nelson, D.; Takechi, R. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun. Ageing 2015, 12, 2. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16, 2721. [Google Scholar] [CrossRef]
- Ramnauth, A.D.; Tippani, M.; Divecha, H.R.; Papariello, A.R.; Miller, R.A.; Nelson, E.D.; Thompson, J.R.; Pattie, E.A.; Kleinman, J.E.; Maynard, K.R.; et al. Spatiotemporal analysis of gene expression in the human dentate gyrus reveals age-associated changes in cellular maturation and neuroinflammation. Cell Rep. 2025, 44, 115300. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Korobeynyk, V.I.; Zamboni, M.; Waern, F.; Cole, J.D.; Mundt, S.; Greter, M.; Frisén, J.; Llorens-Bobadilla, E.; Jessberger, S. Multimodal transcriptomics reveal neurogenic aging trajectories and age-related regional inflammation in the dentate gyrus. Nat. Neurosci. 2025, 28, 415–430. [Google Scholar] [CrossRef]
- Mathews, K.J.; Allen, K.M.; Boerrigter, D.; Ball, H.; Shannon Weickert, C.; Double, K.L. Evidence for reduced neurogenesis in the aging human hippocampus despite stable stem cell markers. Aging Cell 2017, 16, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Houtman, J.; Eguiguren, J.S.; Ghassemzadeh, S.; Rund, N.; Novaresi, N.M.; Hu, L.; Parylak, S.L.; Denli, A.M.; Randolph-Moore, L.; et al. Lamin B1 decline underlies age-related loss of adult hippocampal neurogenesis. EMBO J. 2021, 40, e105819. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, T.; Nakajima, K. Inflammatory cytokines TNFα, IL-1β, and IL-6 are induced in endotoxin- stimulated microglia through different signaling cascades. Sci. Prog. 2021, 104, 368504211054985. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S. From Biomarkers to Behavior: Mapping the Neuroimmune Web of Pain, Mood, and Memory. Biomedicines 2025, 13, 2226. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Ikegaya, Y.; Koyama, R. The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur. J. Neurosci. 2021, 54, 5880–5901. [Google Scholar] [CrossRef]
- Nelson, L.H.; Peketi, P.; Lenz, K.M. Microglia Regulate Cell Genesis in a Sex-dependent Manner in the Neonatal Hippocampus. Neuroscience 2021, 453, 237–255. [Google Scholar] [CrossRef]
- Mecca, C.; Giambanco, I.; Donato, R.; Arcuri, C. Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int. J. Mol. Sci. 2018, 19, 318. [Google Scholar] [CrossRef]
- Jurgens, H.A.; Johnson, R.W. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp. Neurol. 2012, 233, 40–48. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Qiao, Y.; Zhou, T.; He, H.; Yi, S.; Zhang, L.; Mo, L.; Li, Y.; Jiang, W.; et al. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia 2020, 68, 2674–2692. [Google Scholar] [CrossRef]
- Ekdahl, C.T. Microglial activation—Tuning and pruning adult neurogenesis. Front. Pharmacol. 2012, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Früholz, I.; Meyer-Luehmann, M. The intricate interplay between microglia and adult neurogenesis in Alzheimer’s disease. Front. Cell Neurosci. 2024, 18, 1456253. [Google Scholar] [CrossRef] [PubMed]
- Al-Onaizi, M.; Al-Khalifah, A.; Qasem, D.; ElAli, A. Role of Microglia in Modulating Adult Neurogenesis in Health and Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 6875. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Sanchez-Molina, P.; Almolda, B.; Giménez-Llort, L.; González, B.; Castellano, B. Chronic IL-10 overproduction disrupts microglia-neuron dialogue similar to aging, resulting in impaired hippocampal neurogenesis and spatial memory. Brain Behav. Immun. 2022, 101, 231–245. [Google Scholar] [CrossRef]
- De Lucia, C.; Rinchon, A.; Olmos-Alonso, A.; Riecken, K.; Fehse, B.; Boche, D.; Perry, V.H.; Gomez-Nicola, D. Microglia regulate hippocampal neurogenesis during chronic neurodegeneration. Brain Behav. Immun. 2016, 55, 179–190. [Google Scholar] [CrossRef]
- Domínguez-Rivas, E.; Ávila-Muñoz, E.; Schwarzacher, S.W.; Zepeda, A. Adult hippocampal neurogenesis in the context of lipopolysaccharide-induced neuroinflammation: A molecular, cellular and behavioral review. Brain Behav. Immun. 2021, 97, 286–302. [Google Scholar] [CrossRef]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016, 58, 1–8. [Google Scholar] [CrossRef]
- Rusznák, K.; Horváth Á, I.; Pohli-Tóth, K.; Futácsi, A.; Kemény, Á.; Kiss, G.; Helyes, Z.; Czéh, B. Experimental Arthritis Inhibits Adult Hippocampal Neurogenesis in Mice. Cells 2022, 11, 791. [Google Scholar] [CrossRef]
- Zonis, S.; Pechnick, R.N.; Ljubimov, V.A.; Mahgerefteh, M.; Wawrowsky, K.; Michelsen, K.S.; Chesnokova, V. Chronic intestinal inflammation alters hippocampal neurogenesis. J. Neuroinflamm. 2015, 12, 65. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Xiao, C.; Su, D.; Li, L.; Yang, C.; Zhao, Z.; Jiang, W.; You, Z.; Zhou, T. Akebia saponin D protects hippocampal neurogenesis from microglia-mediated inflammation and ameliorates depressive-like behaviors and cognitive impairment in mice through the PI3K-Akt pathway. Front. Pharmacol. 2022, 13, 927419. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, C.T.; Claasen, J.H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Golia, M.T.; Poggini, S.; Alboni, S.; Garofalo, S.; Ciano Albanese, N.; Viglione, A.; Ajmone-Cat, M.A.; St-Pierre, A.; Brunello, N.; Limatola, C.; et al. Interplay between inflammation and neural plasticity: Both immune activation and suppression impair LTP and BDNF expression. Brain Behav. Immun. 2019, 81, 484–494. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Pang, Y. Role of neuroinflammation in the establishment of the neurogenic microenvironment in brain diseases. Curr. Tissue Microenviron. Rep. 2021, 2, 17–28. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Jin, Y.; Zhang, Y.; Wu, J.; Xu, Z.; Huang, Y.; Cai, L.; Gao, S.; Liu, T.; et al. Transcriptional and epigenetic decoding of the microglial aging process. Nat. Aging 2023, 3, 1288–1311. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Yang, M.; Zeng, B.; Qiu, W.; Ma, Q.; Jing, X.; Zhang, Q.; Wang, B.; Yin, C.; et al. Transcriptome dynamics of hippocampal neurogenesis in macaques across the lifespan and aged humans. Cell Res. 2022, 32, 729–743. [Google Scholar] [CrossRef]
- Grabert, K.; Michoel, T.; Karavolos, M.H.; Clohisey, S.; Baillie, J.K.; Stevens, M.P.; Freeman, T.C.; Summers, K.M.; McColl, B.W. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef]
- Tan, Y.L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef]
- Yu, H.; Chang, Q.; Sun, T.; He, X.; Wen, L.; An, J.; Feng, J.; Zhao, Y. Metabolic reprogramming and polarization of microglia in Parkinson’s disease: Role of inflammasome and iron. Ageing Res. Rev. 2023, 90, 102032. [Google Scholar] [CrossRef] [PubMed]
- Petralla, S.; De Chirico, F.; Miti, A.; Tartagni, O.; Massenzio, F.; Poeta, E.; Virgili, M.; Zuccheri, G.; Monti, B. Epigenetics and Communication Mechanisms in Microglia Activation with a View on Technological Approaches. Biomolecules 2021, 11, 306. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Chen, H.; Jin, C.; Jin, Z.; Lu, J.; Xu, L.; Lu, Y.; Zhang, J.; Shi, L. Aged microglia promote peripheral T cell infiltration by reprogramming the microenvironment of neurogenic niches. Immun. Ageing 2022, 19, 34. [Google Scholar] [CrossRef]
- Bisht, K.; Okojie, K.A.; Sharma, K.; Lentferink, D.H.; Sun, Y.Y.; Chen, H.R.; Uweru, J.O.; Amancherla, S.; Calcuttawala, Z.; Campos-Salazar, A.B.; et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat. Commun. 2021, 12, 5289. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, D.K.; Lauffenburger, D.A. Translating preclinical models to humans. Science 2020, 367, 742–743. [Google Scholar] [CrossRef] [PubMed]
- Gault, N.; Szele, F.G. Immunohistochemical evidence for adult human neurogenesis in health and disease. WIREs Mech. Dis. 2021, 13, e1526. [Google Scholar] [CrossRef]
- Cutler, R.R.; Kokovay, E. Rejuvenating subventricular zone neurogenesis in the aging brain. Curr. Opin. Pharmacol. 2020, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular rejuvenation: Molecular mechanisms and potential therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 116. [Google Scholar] [CrossRef]
- Gillotin, S.; Sahni, V.; Lepko, T.; Hanspal, M.A.; Swartz, J.E.; Alexopoulou, Z.; Marshall, F.H. Targeting impaired adult hippocampal neurogenesis in ageing by leveraging intrinsic mechanisms regulating Neural Stem Cell activity. Ageing Res. Rev. 2021, 71, 101447. [Google Scholar] [CrossRef]
- Niklison-Chirou, M.V.; Agostini, M.; Amelio, I.; Melino, G. Regulation of Adult Neurogenesis in Mammalian Brain. Int. J. Mol. Sci. 2020, 21, 4869. [Google Scholar] [CrossRef]
- Abbott, L.C.; Nigussie, F. Adult neurogenesis in the mammalian dentate gyrus. Anat. Histol. Embryol. 2020, 49, 3–16. [Google Scholar] [CrossRef]
- Nishiyama, R.; Nakagomi, T.; Nakano-Doi, A.; Kuramoto, Y.; Tsuji, M.; Yoshimura, S. Neonatal Brains Exhibit Higher Neural Reparative Activities than Adult Brains in a Mouse Model of Ischemic Stroke. Cells 2024, 13, 519. [Google Scholar] [CrossRef]
- Merkle, F.T.; Tramontin, A.D.; García-Verdugo, J.M.; Alvarez-Buylla, A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. USA 2004, 101, 17528–17532. [Google Scholar] [CrossRef]
- Zaritsky, R.; Kumari, E.; Velloso, F.J.; Lemenze, A.; Husain, S.; Levison, S.W. Transcriptional Profiling Defines Unique Subtypes of Transit Amplifying Neural Progenitors Within the Neonatal Mouse Subventricular Zone. Biomolecules 2025, 15, 1438. [Google Scholar] [CrossRef]
- Nicaise, A.M.; Willis, C.M.; Crocker, S.J.; Pluchino, S. Stem Cells of the Aging Brain. Front. Aging Neurosci. 2020, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Whitney, N.; Wu, Y.; Tian, C.; Dou, H.; Zhou, Y.; Zheng, J. HIV-1-infected and/or immune-activated macrophage-secreted TNF-alpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia 2008, 56, 903–916. [Google Scholar] [CrossRef]
- Vidal, P.M.; Lemmens, E.; Dooley, D.; Hendrix, S. The role of “anti-inflammatory” cytokines in axon regeneration. Cytokine Growth Factor. Rev. 2013, 24, 1–12. [Google Scholar] [CrossRef]
- Liang, Z.; Jin, N.; Guo, W. Neural stem cell heterogeneity in adult hippocampus. Cell Regen. 2025, 14, 6. [Google Scholar] [CrossRef]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. From Microbial Switches to Metabolic Sensors: Rewiring the Gut-Brain Kynurenine Circuit. Biomedicines 2025, 13, 2020. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals 2025, 18, 133. [Google Scholar] [CrossRef]
- Duan, X.; Kang, E.; Liu, C.Y.; Ming, G.L.; Song, H. Development of neural stem cell in the adult brain. Curr. Opin. Neurobiol. 2008, 18, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef]
- Kempermann, G.; Song, H.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Cold Spring Harb. Perspect. Biol. 2015, 7, a018812. [Google Scholar] [CrossRef] [PubMed]
- Cope, E.C.; Gould, E. Adult Neurogenesis, Glia, and the Extracellular Matrix. Cell Stem Cell 2019, 24, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Kageyama, R.; Imayoshi, I. The functional significance of newly born neurons integrated into olfactory bulb circuits. Front. Neurosci. 2014, 8, 121. [Google Scholar] [CrossRef]
- Deshpande, A.; Bergami, M.; Ghanem, A.; Conzelmann, K.K.; Lepier, A.; Götz, M.; Berninger, B. Retrograde monosynaptic tracing reveals the temporal evolution of inputs onto new neurons in the adult dentate gyrus and olfactory bulb. Proc. Natl. Acad. Sci. USA 2013, 110, E1152–E1161. [Google Scholar] [CrossRef]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Z.; Wang, Z.; Shao, G.; Li, X. Epigenetic regulation in adult neural stem cells. Front. Cell Dev. Biol. 2024, 12, 1331074. [Google Scholar] [CrossRef]
- Horgusluoglu, E.; Nudelman, K.; Nho, K.; Saykin, A.J. Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Bátiz, L.F.; Castro, M.A.; Burgos, P.V.; Velásquez, Z.D.; Muñoz, R.I.; Lafourcade, C.A.; Troncoso-Escudero, P.; Wyneken, U. Exosomes as Novel Regulators of Adult Neurogenic Niches. Front. Cell Neurosci. 2015, 9, 501. [Google Scholar] [CrossRef]
- Li, Y.; Guo, W. Neural Stem Cell Niche and Adult Neurogenesis. Neuroscientist 2021, 27, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, S.; Istiaq, A.; Jono, H.; Cacci, E.; Ohta, K.; Lupo, G. Assessing the Role of Ependymal and Vascular Cells as Sources of Extracellular Cues Regulating the Mouse Ventricular-Subventricular Zone Neurogenic Niche. Front. Cell Dev. Biol. 2022, 10, 845567. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Clemenson, G.D.; Gage, F.H. New neurons in an aged brain. Behav. Brain Res. 2012, 227, 497–507. [Google Scholar] [CrossRef]
- Bin Imtiaz, M.K.; Jaeger, B.N.; Bottes, S.; Machado, R.A.C.; Vidmar, M.; Moore, D.L.; Jessberger, S. Declining lamin B1 expression mediates age-dependent decreases of hippocampal stem cell activity. Cell Stem Cell 2021, 28, 967–977.e968. [Google Scholar] [CrossRef]
- Bast, L.; Calzolari, F.; Strasser, M.K.; Hasenauer, J.; Theis, F.J.; Ninkovic, J.; Marr, C. Increasing Neural Stem Cell Division Asymmetry and Quiescence Are Predicted to Contribute to the Age-Related Decline in Neurogenesis. Cell Rep. 2018, 25, 3231–3240.e3238. [Google Scholar] [CrossRef]
- Pineda, J.R.; Daynac, M.; Chicheportiche, A.; Cebrian-Silla, A.; Sii Felice, K.; Garcia-Verdugo, J.M.; Boussin, F.D.; Mouthon, M.A. Vascular-derived TGF-β increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol. Med. 2013, 5, 548–562. [Google Scholar] [CrossRef]
- Buckwalter, M.S.; Yamane, M.; Coleman, B.S.; Ormerod, B.K.; Chin, J.T.; Palmer, T.; Wyss-Coray, T. Chronically increased transforming growth factor-beta1 strongly inhibits hippocampal neurogenesis in aged mice. Am. J. Pathol. 2006, 169, 154–164. [Google Scholar] [CrossRef]
- DeCarolis, N.A.; Kirby, E.D.; Wyss-Coray, T.; Palmer, T.D. The Role of the Microenvironmental Niche in Declining Stem-Cell Functions Associated with Biological Aging. Cold Spring Harb. Perspect. Med. 2015, 5, a025874. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. A Decade of Dedication: Pioneering Perspectives on Neurological Diseases and Mental Illnesses. Biomedicines 2024, 12, 1083. [Google Scholar] [CrossRef]
- Jiménez Peinado, P.; Urbach, A. From Youthful Vigor to Aging Decline: Unravelling the Intrinsic and Extrinsic Determinants of Hippocampal Neural Stem Cell Aging. Cells 2023, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef]
- Flor-García, M.; Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Ávila, J.; Rábano, A.; Llorens-Martín, M. Unraveling human adult hippocampal neurogenesis. Nat. Protoc. 2020, 15, 668–693. [Google Scholar] [CrossRef]
- Terreros-Roncal, J.; Flor-García, M.; Moreno-Jiménez, E.P.; Rodríguez-Moreno, C.B.; Márquez-Valadez, B.; Gallardo-Caballero, M.; Rábano, A.; Llorens-Martín, M. Methods to study adult hippocampal neurogenesis in humans and across the phylogeny. Hippocampus 2023, 33, 271–306. [Google Scholar] [CrossRef]
- Seki, T. Understanding the Real State of Human Adult Hippocampal Neurogenesis From Studies of Rodents and Non-human Primates. Front. Neurosci. 2020, 14, 839. [Google Scholar] [CrossRef]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef]
- Costa, J.; Martins, S.; Ferreira, P.A.; Cardoso, A.M.S.; Guedes, J.R.; Peça, J.; Cardoso, A.L. The old guard: Age-related changes in microglia and their consequences. Mech. Ageing Dev. 2021, 197, 111512. [Google Scholar] [CrossRef] [PubMed]
- Koellhoffer, E.C.; McCullough, L.D.; Ritzel, R.M. Old Maids: Aging and Its Impact on Microglia Function. Int. J. Mol. Sci. 2017, 18, 769. [Google Scholar] [CrossRef]
- Ana, B. Aged-Related Changes in Microglia and Neurodegenerative Diseases: Exploring the Connection. Biomedicines 2024, 12, 1737. [Google Scholar] [CrossRef]
- Škandík, M.; Friess, L.; Vázquez-Cabrera, G.; Keane, L.; Grabert, K.; Cruz De Los Santos, M.; Posada-Pérez, M.; Baleviciute, A.; Cheray, M.; Joseph, B. Age-associated microglial transcriptome leads to diminished immunogenicity and dysregulation of MCT4 and P2RY12/P2RY13 related functions. Cell Death Discov. 2025, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Edler, M.K.; Mhatre-Winters, I.; Richardson, J.R. Microglia in Aging and Alzheimer’s Disease: A Comparative Species Review. Cells 2021, 10, 1138. [Google Scholar] [CrossRef]
- O’Neil, S.M.; Hans, E.E.; Jiang, S.; Wangler, L.M.; Godbout, J.P. Astrocyte immunosenescence and deficits in interleukin 10 signaling in the aged brain disrupt the regulation of microglia following innate immune activation. Glia 2022, 70, 913–934. [Google Scholar] [CrossRef] [PubMed]
- Tamatta, R.; Pai, V.; Jaiswal, C.; Singh, I.; Singh, A.K. Neuroinflammaging and the Immune Landscape: The Role of Autophagy and Senescence in Aging Brain. Biogerontology 2025, 26, 52. [Google Scholar] [CrossRef] [PubMed]
- Lutshumba, J.; Nikolajczyk, B.S.; Bachstetter, A.D. Dysregulation of Systemic Immunity in Aging and Dementia. Front. Cell Neurosci. 2021, 15, 652111. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef]
- Sobral, A.F.; Costa, I.; Teixeira, V.; Silva, R.; Barbosa, D.J. Molecular Motors in Blood-Brain Barrier Maintenance by Astrocytes. Brain Sci. 2025, 15, 279. [Google Scholar] [CrossRef]
- Mohammad, Z.B.; Yudin, S.C.Y.; Goldberg, B.J.; Serra, K.L.; Klegeris, A. Exploring neuroglial signaling: Diversity of molecules implicated in microglia-to-astrocyte neuroimmune communication. Rev. Neurosci. 2025, 36, 91–117. [Google Scholar] [CrossRef]
- Wang, S.; Pan, Y.; Zhang, C.; Zhao, Y.; Wang, H.; Ma, H.; Sun, J.; Zhang, S.; Yao, J.; Xie, D.; et al. Transcriptome Analysis Reveals Dynamic Microglial-Induced A1 Astrocyte Reactivity via C3/C3aR/NF-κB Signaling After Ischemic Stroke. Mol. Neurobiol. 2024, 61, 10246–10270. [Google Scholar] [CrossRef]
- Bhusal, A.; Afridi, R.; Lee, W.H.; Suk, K. Bidirectional Communication Between Microglia and Astrocytes in Neuroinflammation. Curr. Neuropharmacol. 2023, 21, 2020–2029. [Google Scholar] [CrossRef]
- Norden, D.M.; Godbout, J.P. Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013, 39, 19–34. [Google Scholar] [CrossRef]
- Elmore, M.R.P.; Hohsfield, L.A.; Kramár, E.A.; Soreq, L.; Lee, R.J.; Pham, S.T.; Najafi, A.R.; Spangenberg, E.E.; Wood, M.A.; West, B.L.; et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 2018, 17, e12832. [Google Scholar] [CrossRef] [PubMed]
- Niraula, A.; Sheridan, J.F.; Godbout, J.P. Microglia Priming with Aging and Stress. Neuropsychopharmacology 2017, 42, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Muccigrosso, M.M.; Godbout, J.P. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 2015, 96, 29–41. [Google Scholar] [CrossRef]
- Neher, J.J.; Cunningham, C. Priming Microglia for Innate Immune Memory in the Brain. Trends Immunol. 2019, 40, 358–374. [Google Scholar] [CrossRef]
- Hoeijmakers, L.; Heinen, Y.; van Dam, A.M.; Lucassen, P.J.; Korosi, A. Microglial Priming and Alzheimer’s Disease: A Possible Role for (Early) Immune Challenges and Epigenetics? Front. Hum. Neurosci. 2016, 10, 398. [Google Scholar] [CrossRef]
- O’Neil, S.M.; Witcher, K.G.; McKim, D.B.; Godbout, J.P. Forced turnover of aged microglia induces an intermediate phenotype but does not rebalance CNS environmental cues driving priming to immune challenge. Acta Neuropathol. Commun. 2018, 6, 129. [Google Scholar] [CrossRef]
- Wahl, D.; Risen, S.J.; Osburn, S.C.; Emge, T.; Sharma, S.; Gilberto, V.S.; Chatterjee, A.; Nagpal, P.; Moreno, J.A.; LaRocca, T.J. Nanoligomers targeting NF-κB and NLRP3 reduce neuroinflammation and improve cognitive function with aging and tauopathy. J. Neuroinflamm. 2024, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, Y.; Yin, S.; Wan, F.; Hu, J.; Kou, L.; Sun, Y.; Wu, J.; Zhou, Q.; Huang, J.; et al. Targeting Microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 Inflammasome Axis in Parkinson’s Disease. Front. Immunol. 2021, 12, 719807. [Google Scholar] [CrossRef]
- Lei, L.Y.; Wang, R.C.; Pan, Y.L.; Yue, Z.G.; Zhou, R.; Xie, P.; Tang, Z.S. Mangiferin inhibited neuroinflammation through regulating microglial polarization and suppressing NF-κB, NLRP3 pathway. Chin. J. Nat. Med. 2021, 19, 112–119. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Lu, Y.; Chi, L. NTN-1 attenuates amyloid-β-mediated microglial neuroinflammation and memory impairment via the NF-κB pathway and NLRP3 inflammasome in a rat model of Alzheimer’s disease. Front. Aging Neurosci. 2025, 17, 1516399. [Google Scholar] [CrossRef] [PubMed]
- Fornari Laurindo, L.; Aparecido Dias, J.; Cressoni Araújo, A.; Torres Pomini, K.; Machado Galhardi, C.; Rucco Penteado Detregiachi, C.; Santos de Argollo Haber, L.; Donizeti Roque, D.; Dib Bechara, M.; Vialogo Marques de Castro, M.; et al. Immunological dimensions of neuroinflammation and microglial activation: Exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front. Immunol. 2023, 14, 1305933. [Google Scholar] [CrossRef]
- Borsini, A.; Zunszain, P.A.; Thuret, S.; Pariante, C.M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015, 38, 145–157. [Google Scholar] [CrossRef]
- Kong, X.; Gong, Z.; Zhang, L.; Sun, X.; Ou, Z.; Xu, B.; Huang, J.; Long, D.; He, X.; Lin, X.; et al. JAK2/STAT3 signaling mediates IL-6-inhibited neurogenesis of neural stem cells through DNA demethylation/methylation. Brain Behav. Immun. 2019, 79, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Kauppinen, A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: Impact on the aging process and age-related diseases. Cell Mol. Life Sci. 2012, 69, 2999–3013. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Redefining Roles: A Paradigm Shift in Tryptophan-Kynurenine Metabolism for Innovative Clinical Applications. Int. J. Mol. Sci. 2024, 25, 12767. [Google Scholar] [CrossRef]
- Juhász, L.; Spisák, K.; Szolnoki, B.Z.; Nászai, A.; Szabó, Á.; Rutai, A.; Tallósy, S.P.; Szabó, A.; Toldi, J.; Tanaka, M.; et al. The Power Struggle: Kynurenine Pathway Enzyme Knockouts and Brain Mitochondrial Respiration. J. Neurochem. 2025, 169, e70075. [Google Scholar] [CrossRef]
- Sarkar, S.; Malovic, E.; Harishchandra, D.S.; Ghaisas, S.; Panicker, N.; Charli, A.; Palanisamy, B.N.; Rokad, D.; Jin, H.; Anantharam, V.; et al. Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. NPJ Park. Dis. 2017, 3, 30. [Google Scholar] [CrossRef]
- Hansen, C.E.; Vacondio, D.; van der Molen, L.; Jüttner, A.A.; Fung, W.K.; Karsten, M.; van Het Hof, B.; Fontijn, R.D.; Kooij, G.; Witte, M.E.; et al. Endothelial-Ercc1 DNA repair deficiency provokes blood-brain barrier dysfunction. Cell Death Dis. 2025, 16, 1. [Google Scholar] [CrossRef]
- Dulken, B.W.; Buckley, M.T.; Navarro Negredo, P.; Saligrama, N.; Cayrol, R.; Leeman, D.S.; George, B.M.; Boutet, S.C.; Hebestreit, K.; Pluvinage, J.V.; et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 2019, 571, 205–210. [Google Scholar] [CrossRef]
- Moreno-Valladares, M.; Moreno-Cugnon, L.; Silva, T.M.; Garcés, J.P.; Saenz-Antoñanzas, A.; Álvarez-Satta, M.; Matheu, A. CD8(+) T cells are increased in the subventricular zone with physiological and pathological aging. Aging Cell 2020, 19, e13198. [Google Scholar] [CrossRef]
- Solano Fonseca, R.; Mahesula, S.; Apple, D.M.; Raghunathan, R.; Dugan, A.; Cardona, A.; O’Connor, J.; Kokovay, E. Neurogenic Niche Microglia Undergo Positional Remodeling and Progressive Activation Contributing to Age-Associated Reductions in Neurogenesis. Stem Cells Dev. 2016, 25, 542–555. [Google Scholar] [CrossRef]
- Fonken, L.K.; Gaudet, A.D. Neuroimmunology of healthy brain aging. Curr. Opin. Neurobiol. 2022, 77, 102649. [Google Scholar] [CrossRef]
- Chintamen, S.; Imessadouene, F.; Kernie, S.G. Immune Regulation of Adult Neurogenic Niches in Health and Disease. Front. Cell Neurosci. 2020, 14, 571071. [Google Scholar] [CrossRef]
- Diaz-Aparicio, I.; Paris, I.; Sierra-Torre, V.; Plaza-Zabala, A.; Rodríguez-Iglesias, N.; Márquez-Ropero, M.; Beccari, S.; Huguet, P.; Abiega, O.; Alberdi, E.; et al. Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. J. Neurosci. 2020, 40, 1453–1482. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Kurematsu, C.; Sawada, M.; Ohmuraya, M.; Tanaka, M.; Kuboyama, K.; Ogino, T.; Matsumoto, M.; Oishi, H.; Inada, H.; Ishido, Y.; et al. Synaptic pruning of murine adult-born neurons by microglia depends on phosphatidylserine. J. Exp. Med. 2022, 219, e20202304. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yi, S.; Liu, Q.; Zhang, J. The secretome of microglia induced by IL-4 of IFN-γ differently regulate proliferation, differentiation and survival of adult neural stem/progenitor cell by targeting the PI3K-Akt pathway. Cytotechnology 2022, 74, 407–420. [Google Scholar] [CrossRef]
- Matsui, T.K.; Mori, E. Microglia support neural stem cell maintenance and growth. Biochem. Biophys. Res. Commun. 2018, 503, 1880–1884. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, A.; Holtman, I.R.; Krueger, M.; Yogev, N.; Bruttger, J.; Khorooshi, R.; Benmamar-Badel, A.; de Boer-Bergsma, J.J.; Martin, N.A.; Karram, K.; et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. Embo J 2017, 36, 3292–3308. [Google Scholar] [CrossRef] [PubMed]
- Mallard, C.; Tremblay, M.E.; Vexler, Z.S. Microglia and Neonatal Brain Injury. Neuroscience 2019, 405, 68–76. [Google Scholar] [CrossRef]
- Harley, S.B.R.; Willis, E.F.; Shaikh, S.N.; Blackmore, D.G.; Sah, P.; Ruitenberg, M.J.; Bartlett, P.F.; Vukovic, J. Selective Ablation of BDNF from Microglia Reveals Novel Roles in Self-Renewal and Hippocampal Neurogenesis. J. Neurosci. 2021, 41, 4172–4186. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gelyana, E.; Rajsombath, M.; Yang, T.; Li, S.; Selkoe, D. Environmental Enrichment Potently Prevents Microglia-Mediated Neuroinflammation by Human Amyloid β-Protein Oligomers. J. Neurosci. 2016, 36, 9041–9056. [Google Scholar] [CrossRef]
- Mee-Inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, J.Y.; Kim, J.Y.; Park, J.; Lee, W.T.; Lee, J.E. M2 Phenotype Microglia-derived Cytokine Stimulates Proliferation and Neuronal Differentiation of Endogenous Stem Cells in Ischemic Brain. Exp. Neurobiol. 2017, 26, 33–41. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Vay, S.U.; Flitsch, L.J.; Rabenstein, M.; Rogall, R.; Blaschke, S.; Kleinhaus, J.; Reinert, N.; Bach, A.; Fink, G.R.; Schroeter, M.; et al. The plasticity of primary microglia and their multifaceted effects on endogenous neural stem cells in vitro and in vivo. J. Neuroinflamm. 2018, 15, 226. [Google Scholar] [CrossRef]
- Carrier, M.; Šimončičová, E.; St-Pierre, M.K.; McKee, C.; Tremblay, M. Psychological Stress as a Risk Factor for Accelerated Cellular Aging and Cognitive Decline: The Involvement of Microglia-Neuron Crosstalk. Front. Mol. Neurosci. 2021, 14, 749737. [Google Scholar] [CrossRef]
- Afridi, R.; Lee, W.H.; Suk, K. Microglia Gone Awry: Linking Immunometabolism to Neurodegeneration. Front. Cell Neurosci. 2020, 14, 246. [Google Scholar] [CrossRef]
- Pawelec, P.; Ziemka-Nalecz, M.; Sypecka, J.; Zalewska, T. The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders. Cells 2020, 9, 2277. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, J.; Colditz, M.J.; Blackmore, D.G.; Ruitenberg, M.J.; Bartlett, P.F. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J. Neurosci. 2012, 32, 6435–6443. [Google Scholar] [CrossRef]
- Bolós, M.; Perea, J.R.; Terreros-Roncal, J.; Pallas-Bazarra, N.; Jurado-Arjona, J.; Ávila, J.; Llorens-Martín, M. Absence of microglial CX3CR1 impairs the synaptic integration of adult-born hippocampal granule neurons. Brain Behav. Immun. 2018, 68, 76–89. [Google Scholar] [CrossRef]
- Morton, M.C.; Neckles, V.N.; Seluzicki, C.M.; Holmberg, J.C.; Feliciano, D.M. Neonatal Subventricular Zone Neural Stem Cells Release Extracellular Vesicles that Act as a Microglial Morphogen. Cell Rep. 2018, 23, 78–89. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, M.; Li, H.; Wang, Y.; Shen, H.; Li, X.; Zhang, Y.; Wu, J.; Yu, Z.; Chen, G. CX3CL1/CX3CR1 axis attenuates early brain injury via promoting the delivery of exosomal microRNA-124 from neuron to microglia after subarachnoid hemorrhage. J. Neuroinflamm. 2020, 17, 209. [Google Scholar] [CrossRef]
- Qian, H.D.; Song, X.Y.; He, G.W.; Peng, X.N.; Chen, Y.; Huang, P.; Zhang, J.; Lin, X.Y.; Gao, Q.; Zhu, S.M.; et al. Müller Glial-Derived Small Extracellular Vesicles Mitigate RGC Degeneration by Suppressing Microglial Activation via Cx3cl1-Cx3cr1 Signaling. Adv. Healthc. Mater. 2025, 14, e2404306. [Google Scholar] [CrossRef]
- Fritze, J.; Muralidharan, C.; Stamp, E.; Ahlenius, H. Microglia undergo disease-associated transcriptional activation and CX3C motif chemokine receptor 1 expression regulates neurogenesis in the aged brain. Dev. Neurobiol. 2024, 84, 128–141. [Google Scholar] [CrossRef]
- Gemma, C.; Bachstetter, A.D.; Bickford, P.C. Neuron-Microglia Dialogue and Hippocampal Neurogenesis in the Aged Brain. Aging Dis. 2010, 1, 232–244. [Google Scholar] [PubMed]
- Lonnemann, N.; Hosseini, S.; Marchetti, C.; Skouras, D.B.; Stefanoni, D.; D’Alessandro, A.; Dinarello, C.A.; Korte, M. The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 32145–32154. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Madhu, L.N.; Attaluri, S.; Gitaí, D.L.G.; Pinson, M.R.; Kodali, M.; Shetty, G.; Zanirati, G.; Kumar, S.; Shuai, B.; et al. Extracellular vesicles from human iPSC-derived neural stem cells: miRNA and protein signatures, and anti-inflammatory and neurogenic properties. J. Extracell. Vesicles 2020, 9, 1809064. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.; Kong, C.; Su, X.; Zhang, X.; Bai, W.; He, X. MiR-124 Enriched Exosomes Promoted the M2 Polarization of Microglia and Enhanced Hippocampus Neurogenesis After Traumatic Brain Injury by Inhibiting TLR4 Pathway. Neurochem. Res. 2019, 44, 811–828. [Google Scholar] [CrossRef] [PubMed]
- Giacomoni, J.; Åkerblom, M.; Habekost, M.; Fiorenzano, A.; Kajtez, J.; Davidsson, M.; Parmar, M.; Björklund, T. Identification and validation of novel engineered AAV capsid variants targeting human glia. Front. Neurosci. 2024, 18, 1435212. [Google Scholar] [CrossRef]
- Barko, K.; Shelton, M.; Xue, X.; Afriyie-Agyemang, Y.; Puig, S.; Freyberg, Z.; Tseng, G.C.; Logan, R.W.; Seney, M.L. Brain region- and sex-specific transcriptional profiles of microglia. Front. Psychiatry 2022, 13, 945548. [Google Scholar] [CrossRef]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e256. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.P.; Henze, D.E.; Ramirez Lopez, E.; Haberberger, J.; Dong, C.; Lu, N.; Atkins, M.; Costa, E.K.; Farinas, A.; Oh, H.S.-H. Spatial and molecular insights into microglial roles in cerebellar aging. bioRxiv 2025. [Google Scholar] [CrossRef]
- La Sala, G.; Farini, D. Glial Cells and Aging: From the CNS to the Cerebellum. Int. J. Mol. Sci. 2025, 26, 7553. [Google Scholar] [CrossRef]
- Spencer, S.J.; Basri, B.; Sominsky, L.; Soch, A.; Ayala, M.T.; Reineck, P.; Gibson, B.C.; Barrientos, R.M. High-fat diet worsens the impact of aging on microglial function and morphology in a region-specific manner. Neurobiol. Aging 2019, 74, 121–134. [Google Scholar] [CrossRef]
- Ribeiro Xavier, A.L.; Kress, B.T.; Goldman, S.A.; Lacerda de Menezes, J.R.; Nedergaard, M. A Distinct Population of Microglia Supports Adult Neurogenesis in the Subventricular Zone. J. Neurosci. 2015, 35, 11848–11861. [Google Scholar] [CrossRef]
- Smith, L.K.; White, C.W., 3rd; Villeda, S.A. The systemic environment: At the interface of aging and adult neurogenesis. Cell Tissue Res. 2018, 371, 105–113. [Google Scholar] [CrossRef]
- Lana, D.; Magni, G.; Landucci, E.; Wenk, G.L.; Pellegrini-Giampietro, D.E.; Giovannini, M.G. Phenomic Microglia Diversity as a Druggable Target in the Hippocampus in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 13668. [Google Scholar] [CrossRef]
- Sato, K. Effects of Microglia on Neurogenesis. Glia 2015, 63, 1394–1405. [Google Scholar] [CrossRef]
- Chintamen, S.; Gaur, P.; Vo, N.; Bradshaw, E.M.; Menon, V.; Kernie, S.G. Distinct microglial transcriptomic signatures within the hippocampus. PLoS ONE 2024, 19, e0296280. [Google Scholar] [CrossRef]
- McKee, C.G.; Hoffos, M.; Vecchiarelli, H.A.; Tremblay, M. Microglia: A pharmacological target for the treatment of age-related cognitive decline and Alzheimer’s disease. Front. Pharmacol. 2023, 14, 1125982. [Google Scholar] [CrossRef]
- McGroarty, J.; Salinas, S.; Evans, H.; Jimenez, B.; Tran, V.; Kadavakollu, S.; Vashist, A.; Atluri, V. Inflammasome-Mediated Neuroinflammation: A Key Driver in Alzheimer’s Disease Pathogenesis. Biomolecules 2025, 15, 676. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, Y.; Zhou, R. NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell Mol. Immunol. 2025, 22, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Khilazheva, E.D.; Mosiagina, A.I.; Panina, Y.A.; Belozor, O.S.; Komleva, Y.K. Impact of NLRP3 Depletion on Aging-Related Metaflammation, Cognitive Function, and Social Behavior in Mice. Int. J. Mol. Sci. 2023, 24, 16580. [Google Scholar] [CrossRef]
- Zhang, X.; Kracht, L.; Lerario, A.M.; Dubbelaar, M.L.; Brouwer, N.; Wesseling, E.M.; Boddeke, E.; Eggen, B.J.L.; Kooistra, S.M. Epigenetic regulation of innate immune memory in microglia. J. Neuroinflamm. 2022, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Day, K.; Guo, W.; Haus, D.L.; Nguyen, H.X.; Scarfone, V.M.; Booher, K.; Jia, X.Y.; Cummings, B.J.; Anderson, A.J. Injured inflammatory environment overrides the TET2 shaped epigenetic landscape of pluripotent stem cell derived human neural stem cells. Sci. Rep. 2024, 14, 25186. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, N.; Oshima, M.; Koide, S.; Takayama, N.; Kuribayashi, W.; Nakajima-Takagi, Y.; Aoyama, K.; Yamazaki, S.; Yamaguchi, K.; Furukawa, Y.; et al. Epigenetic traits inscribed in chromatin accessibility in aged hematopoietic stem cells. Nat. Commun. 2022, 13, 2691. [Google Scholar] [CrossRef]
- Zocher, S.; Toda, T. Epigenetic aging in adult neurogenesis. Hippocampus 2023, 33, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Waseem, A.; Siddiqui, A.J.; Naime, M.; Khan, M.A.; Robertson, A.A.; Boltze, J.; Raza, S.S. MCC950 mitigates SIRT3-NLRP3-driven inflammation and rescues post-stroke neurogenesis. Biomed. Pharmacother. 2025, 183, 117861. [Google Scholar] [CrossRef]
- Kodi, T.; Sankhe, R.; Gopinathan, A.; Nandakumar, K.; Kishore, A. New Insights on NLRP3 Inflammasome: Mechanisms of Activation, Inhibition, and Epigenetic Regulation. J. Neuroimmune Pharmacol. 2024, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, T. Unlocking the potential of regionally activated injury/ischemia-induced stem cells for neural regeneration. Stem Cells 2025, 43, sxaf015. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, T.; Nakano-Doi, A.; Kubo, S.; Sawano, T.; Kuramoto, Y.; Yamahara, K.; Matsuyama, T.; Takagi, T.; Doe, N.; Yoshimura, S. Transplantation of Human Brain-Derived Ischemia-Induced Multipotent Stem Cells Ameliorates Neurological Dysfunction in Mice After Stroke. Stem Cells Transl. Med. 2023, 12, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Holleman, J.; Daniilidou, M.; Kåreholt, I.; Aspö, M.; Hagman, G.; Udeh-Momoh, C.T.; Spulber, G.; Kivipelto, M.; Solomon, A.; Matton, A.; et al. Diurnal cortisol, neuroinflammation, and neuroimaging visual rating scales in memory clinic patients. Brain Behav. Immun. 2024, 118, 499–509. [Google Scholar] [CrossRef]
- Dutta, P.; Quax, R.; Crielaard, L.; Badiali, L.; Sloot, P.M.A. Inferring temporal dynamics from cross-sectional data using Langevin dynamics. R. Soc. Open Sci. 2021, 8, 211374. [Google Scholar] [CrossRef]
- Mroczek, M.; Desouky, A.; Sirry, W. Imaging Transcriptomics in Neurodegenerative Diseases. J. Neuroimaging 2021, 31, 244–250. [Google Scholar] [CrossRef]
- Lee, N.; Choi, J.Y.; Ryu, Y.H. The development status of PET radiotracers for evaluating neuroinflammation. Nucl. Med. Mol. Imaging 2024, 58, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Chaney, A.M.; Carlson, M.L.; Jackson, I.M.; Rao, A.; James, M.L. Neuroinflammation PET Imaging: Current Opinion and Future Directions. J. Nucl. Med. 2020, 61, 1107–1112. [Google Scholar] [CrossRef]
- Narayanaswami, V.; Dahl, K.; Bernard-Gauthier, V.; Josephson, L.; Cumming, P.; Vasdev, N. Emerging PET Radiotracers and Targets for Imaging of Neuroinflammation in Neurodegenerative Diseases: Outlook Beyond TSPO. Mol. Imaging 2018, 17, 1536012118792317. [Google Scholar] [CrossRef]
- Chen, Z.; Haider, A.; Chen, J.; Xiao, Z.; Gobbi, L.; Honer, M.; Grether, U.; Arnold, S.E.; Josephson, L.; Liang, S.H. The Repertoire of Small-Molecule PET Probes for Neuroinflammation Imaging: Challenges and Opportunities beyond TSPO. J. Med. Chem. 2021, 64, 17656–17689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, H.S.; Wang, X.; Dumont, A.S.; Liu, Q. Cellular senescence, DNA damage, and neuroinflammation in the aging brain. Trends Neurosci. 2024, 47, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Chan, A.K.Y.; Wu, J.; Lee, T.M.C. Relationships between Inflammation and Age-Related Neurocognitive Changes. Int. J. Mol. Sci. 2022, 23, 12573. [Google Scholar] [CrossRef]
- Gonzalez-Perez, O.; Gutierrez-Fernandez, F.; Lopez-Virgen, V.; Collas-Aguilar, J.; Quinones-Hinojosa, A.; Garcia-Verdugo, J.M. Immunological regulation of neurogenic niches in the adult brain. Neuroscience 2012, 226, 270–281. [Google Scholar] [CrossRef]
- Das, S.; Basu, A. Inflammation: A new candidate in modulating adult neurogenesis. J. Neurosci. Res. 2008, 86, 1199–1208. [Google Scholar] [CrossRef]
- Galea, I. The blood-brain barrier in systemic infection and inflammation. Cell Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Han, J.; Harris, R.A.; Zhang, X.M. An updated assessment of microglia depletion: Current concepts and future directions. Mol. Brain 2017, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shen, Q.; Chang, H.; Li, J.; Xing, D. Promoted CD4(+) T cell-derived IFN-γ/IL-10 by photobiomodulation therapy modulates neurogenesis to ameliorate cognitive deficits in APP/PS1 and 3xTg-AD mice. J. Neuroinflamm. 2022, 19, 253. [Google Scholar] [CrossRef]
- Brunet, A.; Goodell, M.A.; Rando, T.A. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell Biol. 2023, 24, 45–62. [Google Scholar] [CrossRef]
- Parkitny, L.; Maletic-Savatic, M. Glial PAMPering and DAMPening of Adult Hippocampal Neurogenesis. Brain Sci. 2021, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.E.; Blosser, T.R.; Sullivan, Z.A.; Dulac, C.; Zhuang, X. Molecular and spatial signatures of mouse brain aging at single-cell resolution. Cell 2023, 186, 194–208.e118. [Google Scholar] [CrossRef]
- Velikic, G.; Maric, D.M.; Maric, D.L.; Supic, G.; Puletic, M.; Dulic, O.; Vojvodic, D. Harnessing the Stem Cell Niche in Regenerative Medicine: Innovative Avenue to Combat Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 993. [Google Scholar] [CrossRef]
- Duque, A.; Arellano, J.I.; Rakic, P. An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases. Mol. Psychiatry 2022, 27, 377–382. [Google Scholar] [CrossRef]
- Geirsdottir, L.; David, E.; Keren-Shaul, H.; Weiner, A.; Bohlen, S.C.; Neuber, J.; Balic, A.; Giladi, A.; Sheban, F.; Dutertre, C.A.; et al. Cross-Species Single-Cell Analysis Reveals Divergence of the Primate Microglia Program. Cell 2019, 179, 1609–1622.e1616. [Google Scholar] [CrossRef]
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef] [PubMed]
- Denoth-Lippuner, A.; Jessberger, S. Formation and integration of new neurons in the adult hippocampus. Nat. Rev. Neurosci. 2021, 22, 223–236. [Google Scholar] [CrossRef]
- Tosoni, G.; Ayyildiz, D.; Bryois, J.; Macnair, W.; Fitzsimons, C.P.; Lucassen, P.J.; Salta, E. Mapping human adult hippocampal neurogenesis with single-cell transcriptomics: Reconciling controversy or fueling the debate? Neuron 2023, 111, 1714–1731.e1713. [Google Scholar] [CrossRef] [PubMed]
- Nutma, E.; Fancy, N.; Weinert, M.; Tsartsalis, S.; Marzin, M.C.; Muirhead, R.C.J.; Falk, I.; Breur, M.; de Bruin, J.; Hollaus, D.; et al. Translocator protein is a marker of activated microglia in rodent models but not human neurodegenerative diseases. Nat. Commun. 2023, 14, 5247. [Google Scholar] [CrossRef]
- Pediaditakis, I.; Kodella, K.R.; Manatakis, D.V.; Le, C.Y.; Barthakur, S.; Sorets, A.; Gravanis, A.; Ewart, L.; Rubin, L.L.; Manolakos, E.S.; et al. A microengineered Brain-Chip to model neuroinflammation in humans. iScience 2022, 25, 104813. [Google Scholar] [CrossRef]
- Tian, A.; Bhattacharya, A.; Muffat, J.; Li, Y. Expanding the neuroimmune research toolkit with in vivo brain organoid technologies. Dis. Model. Mech. 2025, 18, dmm052200. [Google Scholar] [CrossRef]
- Balestri, W.; Sharma, R.; da Silva, V.A.; Bobotis, B.C.; Curle, A.J.; Kothakota, V.; Kalantarnia, F.; Hangad, M.V.; Hoorfar, M.; Jones, J.L.; et al. Modeling the neuroimmune system in Alzheimer’s and Parkinson’s diseases. J. Neuroinflamm. 2024, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Parkinson’s Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation. Cells 2025, 14, 1161. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S. Dualistic Dynamics in Neuropsychiatry: From Monoaminergic Modulators to Multiscale Biomarker Maps. Biomedicines 2025, 13, 1456. [Google Scholar] [CrossRef]
- Tanaka, M. From Monoamines to Systems Psychiatry: Rewiring Depression Science and Care (1960s–2025). Biomedicines 2025, 2026, 35. [Google Scholar] [CrossRef]
- Tamura, Y.; Takahashi, K.; Takata, K.; Eguchi, A.; Yamato, M.; Kume, S.; Nakano, M.; Watanabe, Y.; Kataoka, Y. Noninvasive Evaluation of Cellular Proliferative Activity in Brain Neurogenic Regions in Rats under Depression and Treatment by Enhanced [18F]FLT-PET Imaging. J. Neurosci. 2016, 36, 8123–8131. [Google Scholar] [CrossRef]
- Chauveau, F.; Winkeler, A.; Chalon, S.; Boutin, H.; Becker, G. PET imaging of neuroinflammation: Any credible alternatives to TSPO yet? Mol. Psychiatry 2025, 30, 213–228. [Google Scholar] [CrossRef]
- Beaino, W.; Janssen, B.; Vugts, D.J.; de Vries, H.E.; Windhorst, A.D. Towards PET imaging of the dynamic phenotypes of microglia. Clin. Exp. Immunol. 2021, 206, 282–300. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, L.; Wu, Z.; Chen, Y.; Wang, F.; Chen, G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2014, 14, 188–202. [Google Scholar] [CrossRef]
- Xiang, Z.; He, S.; Chen, R.; Liu, S.; Liu, M.; Xu, L.; Zheng, J.; Jiang, Z.; Ma, L.; Sun, Y.; et al. Two-photon live imaging of direct glia-to-neuron conversion in the mouse cortex. Neural Regen. Res. 2024, 19, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.; Billinton, A.; Bock, M.G.; Doedens, J.R.; Gabel, C.A.; Holloway, M.K.; Porter, R.A.; Reader, V.; Scanlon, J.; Schooley, K.; et al. Discovery of Clinical Candidate NT-0796, a Brain-Penetrant and Highly Potent NLRP3 Inflammasome Inhibitor for Neuroinflammatory Disorders. J. Med. Chem. 2023, 66, 14897–14911. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; Li, W.; Abdul, Y.; Jackson, L.; Dong, G.; Jamil, S.; Filosa, J.; Fagan, S.C.; Ergul, A. NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol. Res. 2019, 142, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Albornoz, E.A.; Christie, D.C.; Langley, M.R.; Kumar, V.; Mantovani, S.; Robertson, A.A.B.; Butler, M.S.; Rowe, D.B.; O’Neill, L.A.; et al. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018, 10, eaah4066. [Google Scholar] [CrossRef]
- Dempsey, C.; Rubio Araiz, A.; Bryson, K.J.; Finucane, O.; Larkin, C.; Mills, E.L.; Robertson, A.A.B.; Cooper, M.A.; O’Neill, L.A.J.; Lynch, M.A. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav. Immun. 2017, 61, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.; Velcicky, J.; Gommermann, N.; Mattes, H.; Janser, P.; Wright, M.; Dubois, C.; Brenneisen, S.; Ilic, S.; Vangrevelinghe, E.; et al. Discovery of NP3-253, a Potent Brain Penetrant Inhibitor of the NLRP3 Inflammasome. J. Med. Chem. 2024, 67, 20780–20798. [Google Scholar] [CrossRef]
- Mammoliti, O.; Carbajo, R.; Perez-Benito, L.; Yu, X.; Prieri, M.L.C.; Bontempi, L.; Embrechts, S.; Paesmans, I.; Bassi, M.; Bhattacharya, A.; et al. Discovery of Potent and Brain-Penetrant Bicyclic NLRP3 Inhibitors with Peripheral and Central In Vivo Activity. J. Med. Chem. 2025, 68, 4848–4887. [Google Scholar] [CrossRef]
- Cai, R.; Lv, R.; Shi, X.; Yang, G.; Jin, J. CRISPR/dCas9 Tools: Epigenetic Mechanism and Application in Gene Transcriptional Regulation. Int. J. Mol. Sci. 2023, 24, 14865. [Google Scholar] [CrossRef]
- Liu, X.S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; Czauderna, S.; Shu, J.; Dadon, D.; Young, R.A.; Jaenisch, R. Editing DNA Methylation in the Mammalian Genome. Cell 2016, 167, 233–247.e217. [Google Scholar] [CrossRef]
- O’Geen, H.; Ren, C.; Nicolet, C.M.; Perez, A.A.; Halmai, J.; Le, V.M.; Mackay, J.P.; Farnham, P.J.; Segal, D.J. dCas9-based epigenome editing suggests acquisition of histone methylation is not sufficient for target gene repression. Nucleic Acids Res. 2017, 45, 9901–9916. [Google Scholar] [CrossRef]
- Vojta, A.; Dobrinić, P.; Tadić, V.; Bočkor, L.; Korać, P.; Julg, B.; Klasić, M.; Zoldoš, V. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 2016, 44, 5615–5628. [Google Scholar] [CrossRef]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef]
- Nuñez, J.K.; Chen, J.; Pommier, G.C.; Cogan, J.Z.; Replogle, J.M.; Adriaens, C.; Ramadoss, G.N.; Shi, Q.; Hung, K.L.; Samelson, A.J.; et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 2021, 184, 2503–2519.e2517. [Google Scholar] [CrossRef]
- Takkinen, J.S.; López-Picón, F.R.; Al Majidi, R.; Eskola, O.; Krzyczmonik, A.; Keller, T.; Löyttyniemi, E.; Solin, O.; Rinne, J.O.; Haaparanta-Solin, M. Brain energy metabolism and neuroinflammation in ageing APP/PS1-21 mice using longitudinal (18)F-FDG and (18)F-DPA-714 PET imaging. J. Cereb. Blood Flow. Metab. 2017, 37, 2870–2882. [Google Scholar] [CrossRef]
- Wu, Y.; Bottes, S.; Fisch, R.; Zehnder, C.; Cole, J.D.; Pilz, G.A.; Helmchen, F.; Simons, B.D.; Jessberger, S. Chronic in vivo imaging defines age-dependent alterations of neurogenesis in the mouse hippocampus. Nat. Aging 2023, 3, 380–390. [Google Scholar] [CrossRef]
- Mathys, H.; Adaikkan, C.; Gao, F.; Young, J.Z.; Manet, E.; Hemberg, M.; De Jager, P.L.; Ransohoff, R.M.; Regev, A.; Tsai, L.H. Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell Rep. 2017, 21, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Kreisl, W.C.; Kim, M.J.; Coughlin, J.M.; Henter, I.D.; Owen, D.R.; Innis, R.B. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol. 2020, 19, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13, 167. [Google Scholar] [CrossRef]
- Kamei, R.; Urata, S.; Maruoka, H.; Okabe, S. In vivo Chronic Two-Photon Imaging of Microglia in the Mouse Hippocampus. J. Vis. Exp. 2022, 185, e64104. [Google Scholar] [CrossRef] [PubMed]
- Padmashri, R.; Tyner, K.; Dunaevsky, A. Implantation of a Cranial Window for Repeated In Vivo Imaging in Awake Mice. J. Vis. Exp. 2021. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Tang, F.; Guo, Y.; Xu, R.; Lei, P. Neural circuit changes in neurological disorders: Evidence from in vivo two-photon imaging. Ageing Res. Rev. 2023, 87, 101933. [Google Scholar] [CrossRef]
- Ulivi, A.F.; Castello-Waldow, T.P.; Weston, G.; Yan, L.; Yasuda, R.; Chen, A.; Attardo, A. Longitudinal Two-Photon Imaging of Dorsal Hippocampal CA1 in Live Mice. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Ren, W.; Ji, B.; Guan, Y.; Cao, L.; Ni, R. Recent Technical Advances in Accelerating the Clinical Translation of Small Animal Brain Imaging: Hybrid Imaging, Deep Learning, and Transcriptomics. Front. Med. 2022, 9, 771982. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, C.; Levi, J.; Huynh, T.L.; Tiret, B.; Blecha, J.; Tang, R.; VanBrocklin, H.; Chaumeil, M.M. Longitudinal Imaging of T Cells and Inflammatory Demyelination in a Preclinical Model of Multiple Sclerosis Using (18)F-FAraG PET and MRI. J. Nucl. Med. 2022, 63, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Best, L.; Ghadery, C.; Pavese, N.; Tai, Y.F.; Strafella, A.P. New and Old TSPO PET Radioligands for Imaging Brain Microglial Activation in Neurodegenerative Disease. Curr. Neurol. Neurosci. Rep. 2019, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.G. Uses of Human MR and PET Imaging in Research of Neurodegenerative Brain Diseases. Neurotherapeutics 2021, 18, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Mannheim, J.G.; Schmid, A.M.; Schwenck, J.; Katiyar, P.; Herfert, K.; Pichler, B.J.; Disselhorst, J.A. PET/MRI Hybrid Systems. Semin. Nucl. Med. 2018, 48, 332–347. [Google Scholar] [CrossRef]
- Tanaka, M.; He, Z.; Han, S.; Battaglia, S. Editorial: Noninvasive brain stimulation: A promising approach to study and improve emotion regulation. Front. Behav. Neurosci. 2025, 19, 1633936. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S.; Liloia, D. Navigating Neurodegeneration: Integrating Biomarkers, Neuroinflammation, and Imaging in Parkinson’s, Alzheimer’s, and Motor Neuron Disorders. Biomedicines 2025, 13, 1045. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Beaino, W.; Janssen, B.; Kooij, G.; van der Pol, S.M.A.; van Het Hof, B.; van Horssen, J.; Windhorst, A.D.; de Vries, H.E. Purinergic receptors P2Y12R and P2X7R: Potential targets for PET imaging of microglia phenotypes in multiple sclerosis. J. Neuroinflamm. 2017, 14, 259. [Google Scholar] [CrossRef]
- Zhou, R.; Ji, B.; Kong, Y.; Qin, L.; Ren, W.; Guan, Y.; Ni, R. PET Imaging of Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2021, 12, 739130. [Google Scholar] [CrossRef]
- Parker, C.A.; Nutt, D.J.; Tyacke, R.J. Imidazoline-I2 PET Tracers in Neuroimaging. Int. J. Mol. Sci. 2023, 24, 9787. [Google Scholar] [CrossRef] [PubMed]
- Zaharchuk, G. Next generation research applications for hybrid PET/MR and PET/CT imaging using deep learning. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Aiello, M.; Cavaliere, C.; Fiorenza, D.; Duggento, A.; Passamonti, L.; Toschi, N. Neuroinflammation in Neurodegenerative Diseases: Current Multi-modal Imaging Studies and Future Opportunities for Hybrid PET/MRI. Neuroscience 2019, 403, 125–135. [Google Scholar] [CrossRef]
- Chiu, F.Y.; Yen, Y. Imaging biomarkers for clinical applications in neuro-oncology: Current status and future perspectives. Biomark. Res. 2023, 11, 35. [Google Scholar] [CrossRef]
- Tanaka, M. Special Issue “Translating Molecular Psychiatry: From Biomarkers to Personalized Therapies”. Int. J. Mol. Sci. 2025, 26, 10238. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, V.; Brummer, T.; Muthuraman, M.; Steffen, F.; Heldt, M.; Protopapa, M.; Schraad, M.; Gonzalez-Escamilla, G.; Groppa, S.; Bittner, S.; et al. Biomarker combinations from different modalities predict early disability accumulation in multiple sclerosis. Front. Immunol. 2025, 16, 1532660. [Google Scholar] [CrossRef]
- Vassal, M.; Martins, F.; Monteiro, B.; Tambaro, S.; Martinez-Murillo, R.; Rebelo, S. Emerging Pro-neurogenic Therapeutic Strategies for Neurodegenerative Diseases: A Review of Pre-clinical and Clinical Research. Mol. Neurobiol. 2025, 62, 46–76. [Google Scholar] [CrossRef]
- Praça, C.; Rai, A.; Santos, T.; Cristovão, A.C.; Pinho, S.L.; Cecchelli, R.; Dehouck, M.P.; Bernardino, L.; Ferreira, L.S. A nanoformulation for the preferential accumulation in adult neurogenic niches. J. Control Release 2018, 284, 57–72. [Google Scholar] [CrossRef]
- Tanaka, M.; Tuka, B.; Vécsei, L. Navigating the Neurobiology of Migraine: From Pathways to Potential Therapies. Cells 2024, 13, 1098. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zhang, Q.; Sun, X.; Zhang, M.; Wang, G. Inhibition of adult hippocampal neurogenesis induced by postoperative CD8 + T-cell infiltration is associated with cognitive decline later following surgery in adult mice. J. Neuroinflamm. 2023, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Brocke, S.; Piercy, C.; Steinman, L.; Weissman, I.L.; Veromaa, T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc. Natl. Acad. Sci. USA 1999, 96, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tang, X.; Deng, P.; Hui, H.; Chen, B.; An, J.; Zhang, G.; Shi, K.; Wang, J.; He, Y.; et al. Interleukin-4 from curcumin-activated OECs emerges as a central modulator for increasing M2 polarization of microglia/macrophage in OEC anti-inflammatory activity for functional repair of spinal cord injury. Cell Commun. Signal 2024, 22, 162. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B.; Tirolo, C.; L’Episcopo, F.; Caniglia, S.; Testa, N.; Smith, J.A.; Pluchino, S.; Serapide, M.F. Parkinson’s disease, aging and adult neurogenesis: Wnt/β-catenin signalling as the key to unlock the mystery of endogenous brain repair. Aging Cell 2020, 19, e13101. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, X.; Zhu, Y.; Wang, Z.; He, X.; Wu, Z.; Xue, L.; Fan, W.; Huang, R.; Xu, Z.; et al. Immunomodulatory Layered Double Hydroxide Nanoparticles Enable Neurogenesis by Targeting Transforming Growth Factor-β Receptor 2. ACS Nano 2021, 15, 2812–2830. [Google Scholar] [CrossRef]
- Xu, L.; Ramirez-Matias, J.; Hauptschein, M.; Sun, E.D.; Lunger, J.C.; Buckley, M.T.; Brunet, A. Restoration of neuronal progenitors by partial reprogramming in the aged neurogenic niche. Nat. Aging 2024, 4, 546–567. [Google Scholar] [CrossRef]
- Dubey, S.; Heinen, S.; Krantic, S.; McLaurin, J.; Branch, D.R.; Hynynen, K.; Aubert, I. Clinically approved IVIg delivered to the hippocampus with focused ultrasound promotes neurogenesis in a model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 32691–32700. [Google Scholar] [CrossRef]
- Bonetto, V.; Grilli, M. Neural stem cell-derived extracellular vesicles: Mini players with key roles in neurogenesis, immunomodulation, neuroprotection and aging. Front. Mol. Biosci. 2023, 10, 1187263. [Google Scholar] [CrossRef]
- Li, H.; Chen, G. In Vivo Reprogramming for CNS Repair: Regenerating Neurons from Endogenous Glial Cells. Neuron 2016, 91, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, Y.; Shibata, K.; Yoshida, K.; Shigetomi, E.; Gachet, C.; Ikenaka, K.; Tanaka, K.F.; Koizumi, S. Transformation of Astrocytes to a Neuroprotective Phenotype by Microglia via P2Y(1) Receptor Downregulation. Cell Rep. 2017, 19, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rong, P.; Zhang, L.; He, H.; Zhou, T.; Fan, Y.; Mo, L.; Zhao, Q.; Han, Y.; Li, S.; et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci. Adv. 2021, 7, eabb9888. [Google Scholar] [CrossRef]
- Cai, B.; Seong, K.J.; Bae, S.W.; Kook, M.S.; Chun, C.; Lee, J.H.; Choi, W.S.; Jung, J.Y.; Kim, W.J. Water-Soluble Arginyl-Diosgenin Analog Attenuates Hippocampal Neurogenesis Impairment Through Blocking Microglial Activation Underlying NF-κB and JNK MAPK Signaling in Adult Mice Challenged by LPS. Mol. Neurobiol. 2019, 56, 6218–6238. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Lai, X.; Xu, W.; Qin, Q.; Liang, X.; Xie, M.; Chen, L. The Mechanisms and Application Prospects of Astrocyte Reprogramming into Neurons in Central Nervous System Diseases. Curr. Neuropharmacol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, J.C.; Yeh, H.; Ma, N.X.; Lee, G.; Chen, X.A.; Wang, Y.; Lin, L.; Chen, L.; Jin, P.; et al. Small Molecules Efficiently Reprogram Human Astroglial Cells into Functional Neurons. Cell Stem Cell 2015, 17, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xie, H.; Du, X.; Wang, L.; Jin, X.; Zhang, Q.; Han, Y.; Sun, S.; Wang, L.; Li, X.; et al. In vivo chemical reprogramming of astrocytes into neurons. Cell Discov. 2021, 7, 12. [Google Scholar] [CrossRef]
- Huang, L.; Lai, X.; Liang, X.; Chen, J.; Yang, Y.; Xu, W.; Qin, Q.; Qin, R.; Huang, X.; Xie, M.; et al. A promise for neuronal repair: Reprogramming astrocytes into neurons in vivo. Biosci. Rep. 2024, 44, BSR20231717. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, M.; Urrutia, J.; Villarroel, A.; Casis, O. Microglia-Mediated Inflammation and Neural Stem Cell Differentiation in Alzheimer’s Disease: Possible Therapeutic Role of K(V)1.3 Channel Blockade. Front. Cell Neurosci. 2022, 16, 868842. [Google Scholar] [CrossRef]
- Greșiță, A.; Hermann, D.M.; Boboc, I.K.S.; Doeppner, T.R.; Petcu, E.; Semida, G.F.; Popa-Wagner, A. Glial Cell Reprogramming in Ischemic Stroke: A Review of Recent Advancements and Translational Challenges. Transl. Stroke Res. 2025, 16, 1811–1835. [Google Scholar] [CrossRef]
- Feng, X.; Li, Z.; Liu, Y.; Chen, D.; Zhou, Z. CRISPR/Cas9 technology for advancements in cancer immunotherapy: From uncovering regulatory mechanisms to therapeutic applications. Exp. Hematol. Oncol. 2024, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Xu, X.M.; Zhang, C.L. Regeneration Through in vivo Cell Fate Reprogramming for Neural Repair. Front. Cell Neurosci. 2020, 14, 107. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Wang, Y.; Moriarty, N.; Ahmed, N.Y.; Dehorter, N.; Lisowski, L.; Harvey, A.R.; Parish, C.L.; Williams, R.J.; Nisbet, D.R. Neuronal Replenishment via Hydrogel-Rationed Delivery of Reprogramming Factors. ACS Nano 2024, 18, 3597–3613. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Zhou, R.; Li, Y.; Gao, Y.; Tu, D.; Wilson, B.; Song, S.; Feng, J.; Hong, J.S.; et al. A novel role of NLRP3-generated IL-1β in the acute-chronic transition of peripheral lipopolysaccharide-elicited neuroinflammation: Implications for sepsis-associated neurodegeneration. J. Neuroinflamm. 2020, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Kuwar, R.; Rolfe, A.; Di, L.; Blevins, H.; Xu, Y.; Sun, X.; Bloom, G.S.; Zhang, S.; Sun, D. A Novel Inhibitor Targeting NLRP3 Inflammasome Reduces Neuropathology and Improves Cognitive Function in Alzheimer’s Disease Transgenic Mice. J. Alzheimers Dis. 2021, 82, 1769–1783. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E.; et al. AdipoRon’s Impact on Alzheimer’s Disease-A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 484. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, Y.; Chen, X.; Jiang, D.; Zhang, F.; Guo, Y.; Hu, B.; Xu, G.; Peng, S.; Wu, L.; et al. NLRP3 inflammasome in cognitive impairment and pharmacological properties of its inhibitors. Transl. Neurodegener. 2023, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- McManus, R.M.; Latz, E. NLRP3 inflammasome signalling in Alzheimer’s disease. Neuropharmacology 2024, 252, 109941. [Google Scholar] [CrossRef]
- Stonesifer, C.; Corey, S.; Ghanekar, S.; Diamandis, Z.; Acosta, S.A.; Borlongan, C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017, 158, 94–131. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, S.; He, Q.; Zhang, D.; Chang, J. The Role of Immune Cells in Post-Stroke Angiogenesis and Neuronal Remodeling: The Known and the Unknown. Front. Immunol. 2021, 12, 784098. [Google Scholar] [CrossRef]
- Nakagomi, T.; Takagi, T.; Beppu, M.; Yoshimura, S.; Matsuyama, T. Neural regeneration by regionally induced stem cells within post-stroke brains: Novel therapy perspectives for stroke patients. World J. Stem Cells 2019, 11, 452–463. [Google Scholar] [CrossRef]
- Ismael, S.; Zhao, L.; Nasoohi, S.; Ishrat, T. Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci. Rep. 2018, 8, 5971. [Google Scholar] [CrossRef]
- Bellut, M.; Bieber, M.; Kraft, P.; Weber, A.N.R.; Stoll, G.; Schuhmann, M.K. Delayed NLRP3 inflammasome inhibition ameliorates subacute stroke progression in mice. J. Neuroinflamm. 2023, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Patnala, R.; Arumugam, T.V.; Gupta, N.; Dheen, S.T. HDAC Inhibitor Sodium Butyrate-Mediated Epigenetic Regulation Enhances Neuroprotective Function of Microglia During Ischemic Stroke. Mol. Neurobiol. 2017, 54, 6391–6411. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, K.; Kumar, R.; Shyamasundar, S.; Arumugam, T.V.; Polepalli, J.S.; Dheen, S.T. Spatial Transcriptomic Analysis Reveals HDAC Inhibition Modulates Microglial Dynamics to Protect Against Ischemic Stroke in Mice. Glia 2025, 73, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Vande Walle, L.; Lamkanfi, M. Drugging the NLRP3 inflammasome: From signalling mechanisms to therapeutic targets. Nat. Rev. Drug Discov. 2024, 23, 43–66. [Google Scholar] [CrossRef]
- Barczuk, J.; Siwecka, N.; Lusa, W.; Rozpędek-Kamińska, W.; Kucharska, E.; Majsterek, I. Targeting NLRP3-Mediated Neuroinflammation in Alzheimer’s Disease Treatment. Int. J. Mol. Sci. 2022, 23, 8979. [Google Scholar] [CrossRef]
- Pattali, R.K.; Ornelas, I.J.; Nguyen, C.D.; Xu, D.; Divekar, N.S.; Nuñez, J.K. CRISPRoff epigenetic editing for programmable gene silencing in human cells without DNA breaks. bioRxiv 2024. [Google Scholar] [CrossRef]
- Yim, Y.Y.; Teague, C.D.; Nestler, E.J. In vivo locus-specific editing of the neuroepigenome. Nat. Rev. Neurosci. 2020, 21, 471–484. [Google Scholar] [CrossRef]
- Xiong, K.; Wang, X.; Feng, C.; Zhang, K.; Chen, D.; Yang, S. Vectors in CRISPR Gene Editing for Neurological Disorders: Challenges and Opportunities. Adv. Biol. 2025, 9, e2400374. [Google Scholar] [CrossRef]
- Shang, J.; Song, F.; Zhang, Z.; Chen, D.; Yang, S. Application of novel CRISPR tools in brain therapy. Life Sci. 2024, 352, 122855. [Google Scholar] [CrossRef]
- NodThera’s NLRP3 Inhibitor NT-0796 Reverses Neuroinflammation in Parkinson’s Disease Phase Ib/IIa Trial. Available online: https://www.nodthera.com/news/nodtheras-nlrp3-inhibitor-nt-0796-reverses-neuroinflammation-in-parkinsons-disease-phase-ib-iia-trial/#:~:text=NodThera%27s%20NLRP3%20Inhibitor%20NT,biomarkers%20in%20Parkinson%27s%20disease%20patients (accessed on 18 November 2025).
- Zhao, R.; Tian, X.; Xu, H.; Wang, Y.; Lin, J.; Wang, B. Aerobic Exercise Restores Hippocampal Neurogenesis and Cognitive Function by Decreasing Microglia Inflammasome Formation Through Irisin/NLRP3 Pathway. Aging Cell 2025, 24, e70061. [Google Scholar] [CrossRef]
- Yang, L.; Han, B.; Zhang, Z.; Wang, S.; Bai, Y.; Zhang, Y.; Tang, Y.; Du, L.; Xu, L.; Wu, F.; et al. Extracellular Vesicle-Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation 2020, 142, 556–574. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Terreros-Roncal, J.; Flor-García, M.; Rábano, A.; Llorens-Martín, M. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 2021, 41, 2541–2553. [Google Scholar] [CrossRef]
- Olah, M.; Patrick, E.; Villani, A.C.; Xu, J.; White, C.C.; Ryan, K.J.; Piehowski, P.; Kapasi, A.; Nejad, P.; Cimpean, M.; et al. A transcriptomic atlas of aged human microglia. Nat. Commun. 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Arellano, J.I.; Rakic, P. Modelling adult neurogenesis in the aging rodent hippocampus: A midlife crisis. Front. Neurosci. 2024, 18, 1416460. [Google Scholar] [CrossRef] [PubMed]
- Darsalia, V.; Heldmann, U.; Lindvall, O.; Kokaia, Z. Stroke-induced neurogenesis in aged brain. Stroke 2005, 36, 1790–1795. [Google Scholar] [CrossRef]
- Simard, S.; Matosin, N.; Mechawar, N. Adult Hippocampal Neurogenesis in the Human Brain: Updates, Challenges, and Perspectives. Neuroscientist 2025, 31, 141–158. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Toda, T.; Gage, F.H. Adult Hippocampal Neurogenesis: A Coming-of-Age Story. J. Neurosci. 2018, 38, 10401–10410. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Holtman, I.R.; Raj, D.D.; Miller, J.A.; Schaafsma, W.; Yin, Z.; Brouwer, N.; Wes, P.D.; Möller, T.; Orre, M.; Kamphuis, W.; et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: A co-expression meta-analysis. Acta Neuropathol. Commun. 2015, 3, 31. [Google Scholar] [CrossRef]
- Flowers, A.; Bell-Temin, H.; Jalloh, A.; Stevens, S.M., Jr.; Bickford, P.C. Proteomic anaysis of aged microglia: Shifts in transcription, bioenergetics, and nutrient response. J. Neuroinflamm. 2017, 14, 96. [Google Scholar] [CrossRef]
- Galatro, T.F.; Holtman, I.R.; Lerario, A.M.; Vainchtein, I.D.; Brouwer, N.; Sola, P.R.; Veras, M.M.; Pereira, T.F.; Leite, R.E.P.; Möller, T.; et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 2017, 20, 1162–1171. [Google Scholar] [CrossRef]
- He, R.; Zhang, Q.; Wang, L.; Hu, Y.; Qiu, Y.; Liu, J.; You, D.; Cheng, J.; Cao, X. Exploring the feasibility of using mice as a substitute model for investigating microglia in aging and Alzheimer’s disease though single cell analysis. PLoS ONE 2024, 19, e0311374. [Google Scholar] [CrossRef]
- Qi, C.; Yan, Y.; Cao, Q.; Zou, L.; Li, S.; Yang, Q.; Deng, Q.; Wu, B.; Song, B. Elucidating the mechanisms underlying astrocyte-microglia crosstalk in hippocampal neuroinflammation induced by acute diquat exposure. Environ. Sci. Pollut. Res. Int. 2024, 31, 15746–15758. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, Y.; Liang, P.; He, Y.; Peng, B.; Liu, W.; Han, S.; Yin, J.; He, X. Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis. 2020, 11, 377. [Google Scholar] [CrossRef]
- Anderson, F.L.; Biggs, K.E.; Rankin, B.E.; Havrda, M.C. NLRP3 inflammasome in neurodegenerative disease. Transl. Res. 2023, 252, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, S.; Dale, G.; de Rivero Vaccari, J.P.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: Clinical article. J. Neurosurg. 2012, 117, 1119–1125. [Google Scholar] [CrossRef]
- Chou, V.; Pearse, R.V., 2nd; Aylward, A.J.; Ashour, N.; Taga, M.; Terzioglu, G.; Fujita, M.; Fancher, S.B.; Sigalov, A.; Benoit, C.R.; et al. INPP5D regulates inflammasome activation in human microglia. Nat. Commun. 2023, 14, 7552. [Google Scholar] [CrossRef]
- Ma, C.L.; Ma, X.T.; Wang, J.J.; Liu, H.; Chen, Y.F.; Yang, Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res. 2017, 317, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13, 1129. [Google Scholar] [CrossRef]
- Mohd Sahini, S.N.; Mohd Nor Hazalin, N.A.; Srikumar, B.N.; Jayasingh Chellammal, H.S.; Surindar Singh, G.K. Environmental enrichment improves cognitive function, learning, memory and anxiety-related behaviours in rodent models of dementia: Implications for future study. Neurobiol. Learn. Mem. 2024, 208, 107880. [Google Scholar] [CrossRef] [PubMed]
- Methi, A.; Islam, M.R.; Kaurani, L.; Sakib, M.S.; Krüger, D.M.; Pena, T.; Burkhardt, S.; Liebetanz, D.; Fischer, A. A Single-Cell Transcriptomic Analysis of the Mouse Hippocampus After Voluntary Exercise. Mol. Neurobiol. 2024, 61, 5628–5645. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef]
- Asthana, A.; Tripathi, S.; Agarwal, R. Role of Nonsteroidal Anti-Inflammatory Drugs as a Protective Factor in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neurol. India 2024, 72, 1144–1151. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-inflammatories in Alzheimer’s disease-potential therapy or spurious correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.; Komai, S.; Eliava, M.; Seeburg, P.H.; Osten, P. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 2006, 1, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Kalincik, T.; Roos, I.; Sharmin, S. Observational studies of treatment effectiveness in neurology. Brain 2023, 146, 4799–4808. [Google Scholar] [CrossRef] [PubMed]
- Uff, C.E.G.; Patel, K.; Yeung, C.; Yip, P.K. Advances in Visualizing Microglial Cells in Human Central Nervous System Tissue. Biomolecules 2022, 12, 603. [Google Scholar] [CrossRef]
- Barnhart, A.J.; Dierickx, K. A Tale of Two Chimeras: Applying the Six Principles to Human Brain Organoid Xenotransplantation. Camb. Q. Healthc. Ethics 2023, 32, 555–571. [Google Scholar] [CrossRef]
- Erler, A. Human brain organoid transplantation: Testing the foundations of animal research ethics. Neuroethics 2024, 17, 20. [Google Scholar] [CrossRef]
- Neziri, S.; Köseoğlu, A.E.; Deniz Köseoğlu, G.; Özgültekin, B.; Özgentürk, N. Animal models in neuroscience with alternative approaches: Evolutionary, biomedical, and ethical perspectives. Animal Model. Exp. Med. 2024, 7, 868–880. [Google Scholar] [CrossRef]
- Mrza, M.A.; He, J.; Wang, Y. Integration of iPSC-Derived Microglia into Brain Organoids for Neurological Research. Int. J. Mol. Sci. 2024, 25, 3148. [Google Scholar] [CrossRef]
- Schafer, S.T.; Mansour, A.A.; Schlachetzki, J.C.M.; Pena, M.; Ghassemzadeh, S.; Mitchell, L.; Mar, A.; Quang, D.; Stumpf, S.; Ortiz, I.S.; et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 2023, 186, 2111–2126.e2120. [Google Scholar] [CrossRef]
- Ao, Z.; Cai, H.; Wu, Z.; Song, S.; Karahan, H.; Kim, B.; Lu, H.C.; Kim, J.; Mackie, K.; Guo, F. Tubular human brain organoids to model microglia-mediated neuroinflammation. Lab. Chip 2021, 21, 2751–2762. [Google Scholar] [CrossRef]
- Pagotto, G.L.O.; Santos, L.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.; Goulart, R.A.; Catharin, V.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L., Jr.; Kraig, E.; Orr, M.E. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Investig. 2022, 132, e158453. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J. Neural Transm. 2024, 131, 1367–1387. [Google Scholar] [CrossRef]
- Rao, R.V.; Subramaniam, K.G.; Gregory, J.; Bredesen, A.L.; Coward, C.; Okada, S.; Kelly, L.; Bredesen, D.E. Rationale for a Multi-Factorial Approach for the Reversal of Cognitive Decline in Alzheimer’s Disease and MCI: A Review. Int. J. Mol. Sci. 2023, 24, 1659. [Google Scholar] [CrossRef] [PubMed]
- Fekonja, L.S.; Forkel, S.J.; Aydogan, D.B.; Lioumis, P.; Cacciola, A.; Lucas, C.W.; Tournier, J.D.; Vergani, F.; Ritter, P.; Schenk, R.; et al. Translational network neuroscience: Nine roadblocks and possible solutions. Netw. Neurosci. 2025, 9, 352–370. [Google Scholar] [CrossRef]
- Barron, H.C.; Mars, R.B.; Dupret, D.; Lerch, J.P.; Sampaio-Baptista, C. Cross-species neuroscience: Closing the explanatory gap. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20190633. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, I.; Garcia-Martin, J.; Marongiu, R. Editorial: Animal models of Alzheimer’s disease and other dementias: Past, present, and future. Front. Aging Neurosci. 2024, 16, 1539837. [Google Scholar] [CrossRef]
- Vitek, M.P.; Araujo, J.A.; Fossel, M.; Greenberg, B.D.; Howell, G.R.; Rizzo, S.J.S.; Seyfried, N.T.; Tenner, A.J.; Territo, P.R.; Windisch, M.; et al. Translational animal models for Alzheimer’s disease: An Alzheimer’s Association Business Consortium Think Tank. Alzheimers Dement. 2020, 6, e12114. [Google Scholar] [CrossRef]
- Sun, N.; Victor, M.B.; Park, Y.P.; Xiong, X.; Scannail, A.N.; Leary, N.; Prosper, S.; Viswanathan, S.; Luna, X.; Boix, C.A.; et al. Human microglial state dynamics in Alzheimer’s disease progression. Cell 2023, 186, 4386–4403.e4329. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J.; et al. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knockout Mice: A Novel Model for Despair-Based Depression and Post-Traumatic Stress Disorder. Front. Biosci. 2025, 30, 25706. [Google Scholar] [CrossRef]
- Pluvinage, J.V.; Wyss-Coray, T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat. Rev. Neurosci. 2020, 21, 93–102. [Google Scholar] [CrossRef]
- Szabó, Á.; Galla, Z.; Spekker, E.; Martos, D.; Szűcs, M.; Fejes-Szabó, A.; Fehér, Á.; Takeda, K.; Ozaki, K.; Inoue, H.; et al. Behavioral Balance in Tryptophan Turmoil: Regional Metabolic Rewiring in Kynurenine Aminotransferase II Knockout Mice. Cells 2025, 14, 1711. [Google Scholar] [CrossRef] [PubMed]
- Higgins-Chen, A.T.; Thrush, K.L.; Levine, M.E. Aging biomarkers and the brain. Semin. Cell Dev. Biol. 2021, 116, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Ju, X.-C.; Li, Y.; Zeng, P.-M.; Wu, J.; Zhou, Y.-Y.; Shen, L.-B.; Dong, J.; Chen, Y.-J.; Luo, Z.-G. Generation of vascularized brain organoids to study neurovascular interactions. elife 2022, 11, e76707. [Google Scholar] [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. Decoupling Behavioral Domains via Kynurenic Acid Analog Optimization: Implications for Schizophrenia and Parkinson’s Disease Therapeutics. Cells 2025, 14, 973. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, X.; Fu, G.; Luo, S.; Zhao, Z.; Deng, S.; Li, C.; Cui, Z.; Cao, J.; Chen, P.; et al. Advances in human cellular mechanistic understanding and drug discovery of brain organoids for neurodegenerative diseases. Ageing Res. Rev. 2024, 102, 102517. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S.; Giménez-Llort, L.; Chen, C.; Hepsomali, P.; Avenanti, A.; Vécsei, L. Innovation at the Intersection: Emerging Translational Research in Neurology and Psychiatry. Cells 2024, 13, 790. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Korkki, S.; Solomon, A.; Coley, N.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Lindström, J.; Laatikainen, T.; et al. The effect of adherence on cognition in a multidomain lifestyle intervention (FINGER). Alzheimers Dement. 2022, 18, 1325–1334. [Google Scholar] [CrossRef]
- Polis, B.; Samson, A.O. Addressing the Discrepancies Between Animal Models and Human Alzheimer’s Disease Pathology: Implications for Translational Research. J. Alzheimers Dis. 2024, 98, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Young, P.N.E.; Estarellas, M.; Coomans, E.; Srikrishna, M.; Beaumont, H.; Maass, A.; Venkataraman, A.V.; Lissaman, R.; Jiménez, D.; Betts, M.J.; et al. Imaging biomarkers in neurodegeneration: Current and future practices. Alzheimers Res. Ther. 2020, 12, 49. [Google Scholar] [CrossRef]
- Wagatsuma, K.; Miwa, K.; Akamatsu, G.; Yamao, T.; Kamitaka, Y.; Sakurai, M.; Fujita, N.; Hanaoka, K.; Matsuda, H.; Ishii, K. Toward standardization of tau PET imaging corresponding to various tau PET tracers: A multicenter phantom study. Ann. Nucl. Med. 2023, 37, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Jüttner, J.; Szabo, A.; Gross-Scherf, B.; Morikawa, R.K.; Rompani, S.B.; Hantz, P.; Szikra, T.; Esposti, F.; Cowan, C.S.; Bharioke, A.; et al. Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat. Neurosci. 2019, 22, 1345–1356. [Google Scholar] [CrossRef]
- Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals 2025, 18, 607. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Prakash, R.; Kumari, N.; Ali Khan, M.; Quaiyoom Khan, A.; Uddin, S.; Verma, S.; Ab Robertson, A.; Boltze, J.; Shadab Raza, S. MCC950 reduces autophagy and improves cognitive function by inhibiting NLRP3-dependent neuroinflammation in a rat model of Alzheimer’s disease. Brain Behav. Immun. 2024, 116, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, V.; Peviani, M.; Luciani, M.; Zambonini, G.; Gritti, A. Delivery Platforms for CRISPR/Cas9 Genome Editing of Glial Cells in the Central Nervous System. Front. Genome Ed. 2021, 3, 644319. [Google Scholar] [CrossRef]
- Shi, L.; Li, S.; Zhu, R.; Lu, C.; Xu, X.; Li, C.; Huang, X.; Zhao, X.; Mao, F.; Li, K. CRISPRepi: A multi-omic atlas for CRISPR-based epigenome editing. Nucleic Acids Res. 2025, 53, D901–D913. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Guerrero, A.; Pallàs, M. Advancing personalized medicine in neurodegenerative diseases: The role of epigenetics and pharmacoepigenomics in pharmacotherapy. Pharmacol. Res. 2024, 205, 107247. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Zeyaullah, M.; Khan, M.S.; AlShahrani, A.M.; Altijani, A.A.G.; Ali, H.; Dawria, A.; Mohieldin, A.; Alam, M.S.; Mohamed, A.O.A. Pharmacogenomics for neurodegenerative disorders—A focused review. Front. Pharmacol. 2024, 15, 1478964. [Google Scholar] [CrossRef]
- Deng, S.; Xie, H.; Xie, B. Cell-based regenerative and rejuvenation strategies for treating neurodegenerative diseases. Stem Cell Res. Ther. 2025, 16, 167. [Google Scholar] [CrossRef]
- Ueda, J.; Yamazaki, T.; Funakoshi, H. Toward the Development of Epigenome Editing-Based Therapeutics: Potentials and Challenges. Int. J. Mol. Sci. 2023, 24, 4778. [Google Scholar] [CrossRef]
- Khoshandam, M.; Soltaninejad, H.; Mousazadeh, M.; Hamidieh, A.A.; Hosseinkhani, S. Clinical applications of the CRISPR/Cas9 genome-editing system: Delivery options and challenges in precision medicine. Genes. Dis. 2024, 11, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.D.; Zhang, Y.; Yin, T.L.; Yu, Y. Epigenome editing by CRISPR/Cas9 in clinical settings: Possibilities and challenges. Brief. Funct. Genom. 2020, 19, 215–228. [Google Scholar] [CrossRef]
- Tremblay, F.; Xiong, Q.; Shah, S.S.; Ko, C.W.; Kelly, K.; Morrison, M.S.; Giancarlo, C.; Ramirez, R.N.; Hildebrand, E.M.; Voytek, S.B.; et al. A potent epigenetic editor targeting human PCSK9 for durable reduction of low-density lipoprotein cholesterol levels. Nat. Med. 2025, 31, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Gemberling, M.P.; Siklenka, K.; Rodriguez, E.; Tonn-Eisinger, K.R.; Barrera, A.; Liu, F.; Kantor, A.; Li, L.; Cigliola, V.; Hazlett, M.F.; et al. Transgenic mice for in vivo epigenome editing with CRISPR-based systems. Nat. Methods 2021, 18, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cheng, L.; Zhang, X. Reprogramming Glial Cells into Functional Neurons for Neuro-regeneration: Challenges and Promise. Neurosci. Bull. 2021, 37, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Lentini, C.; d’Orange, M.; Marichal, N.; Trottmann, M.M.; Vignoles, R.; Foucault, L.; Verrier, C.; Massera, C.; Raineteau, O.; Conzelmann, K.K.; et al. Reprogramming reactive glia into interneurons reduces chronic seizure activity in a mouse model of mesial temporal lobe epilepsy. Cell Stem Cell 2021, 28, 2104–2121.e2110. [Google Scholar] [CrossRef]
- Matt, S.M.; Johnson, R.W. Neuro-immune dysfunction during brain aging: New insights in microglial cell regulation. Curr. Opin. Pharmacol. 2016, 26, 96–101. [Google Scholar] [CrossRef]
- Filgueira, L.; Larionov, A.; Lannes, N. The Influence of Virus Infection on Microglia and Accelerated Brain Aging. Cells 2021, 10, 1836. [Google Scholar] [CrossRef]
- Flick, C.; Zamani, E.D.; Stahl, B.C.; Brem, A. The future of ICT for health and ageing: Unveiling ethical and social issues through horizon scanning foresight. Technol. Forecast. Soc. Change 2020, 155, 119995. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Husain, M. Smarter adaptive platform clinical trials in neurology. Brain 2022, 145, 409–410. [Google Scholar] [CrossRef]
- Grill, J.D.; Karlawish, J. Implications of FDA Approval of a First Disease-Modifying Therapy for a Neurodegenerative Disease on the Design of Subsequent Clinical Trials. Neurology 2021, 97, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Lombardi, M.; Gressens, P.; Verderio, C. How to reprogram microglia toward beneficial functions. Glia 2018, 66, 2531–2549. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zhang, Y.; Wu, S.; Chen, Q.; Wang, L. The Role of NLRP3 Inflammasome in Alzheimer’s Disease and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 845185. [Google Scholar] [CrossRef]
| Molecule/Pathway | Source/Cell Type | Effect on Neurogenesis | Relevance in Aging | Targeted by | References |
|---|---|---|---|---|---|
| IL-1β | Activated microglia | Inhibits NSC proliferation and newborn neuron survival; blocks maturation | Chronically elevated with NF-κB/NLRP3 activation; contributes to hostile niche | NLRP3 inhibitors (MCC950, NT-0796), anti-IL-1 drugs | [24,29,30] |
| TNF-α | Activated microglia | Suppresses progenitor proliferation and neuronal differentiation | Increased in microglial ‘primed’ states during inflammaging | TNF pathway blockers | [24,29] |
| IL-6 | Activated microglia/astrocytes | Reduces NSC proliferation; impairs plasticity | Elevated with chronic NF-κB/NLRP3 signaling | Anti-IL-6 agents (exploratory) | [24,29] |
| IFN-γ | Infiltrating CD8+ T cells; activated microglia | Suppresses NSC proliferation; antineurogenic bias | T-cell accumulation in aged niches; drives microglial priming | JAK/STAT inhibitors | [30] |
| NLRP3 inflammasome | Microglia | Sustains IL-1β/IL-18; locks antineurogenic programs | Persistently activated in aging; imprints epigenetic ‘scars’ | Brain-penetrant NLRP3 inhibitors | [29,151] |
| NF-κB | Microglia/astrocytes | Pro-inflammatory transcription; suppresses neurogenesis | Chronically active with oxidative stress; feeds cytokine loop | Pathway modulators (research) | [29,151] |
| Complement (C1q/C3) | Microglia/astrocytes | Accelerated pruning; survival loss of newborns | Heightened with chronic inflammatory tone | Complement inhibitors | [31,153] |
| CX3CL1–CX3CR1 | Neurons → microglia | Maintains microglial quiescence; supports maturation/integration | Protective tone wanes with age; disruption impairs neurogenesis | CX3CR1/CX3CL1 agonists | [28,152,154,158] |
| IGF-1 | Microglia, niche cells | Promotes NSC proliferation and survival | Declines with aging; part of youthful pro-neurogenic secretome | IGF-1 delivery/mimetics | [26,27] |
| BDNF/TrkB | Microglia, neurons | Enhances proliferation, maturation, survival; plasticity | Reduced availability under chronic inflammation | TrkB agonists; BDNF delivery | [26,27] |
| TGF-β | Microglia/astrocytes, niche | Context-dependent; supports homeostasis in youth | Elevated tonic signaling with age constrains neurogenesis | TGF-β tuning (local) | [26,27] |
| IL-10 | Microglia/astrocytes | Pro-neurogenic, supports integration | Protective signals decline with age | Cytokine augmentation | [35] |
| PI3K–Akt/ERK/Wnt–β-catenin | NSCs; microglia-modulated | Downstream pro-neurogenic cascades | Suppressed under inflammatory milieu | Small-molecule activators | [26,149] |
| CD8+ T-cell entry | Peripheral T cells | IFN-γ-mediated suppression of NSCs | Accumulate in aged SGZ/SVZ; feed-forward loop | Blockade of entry/adhesion | [30] |
| Gap | Description of Unknown | Why It Matters/Consequences | Suggested Approaches | References |
|---|---|---|---|---|
| 1. Regional Microglial Diversity | Limited understanding of how microglial phenotypes differ across brain regions and influence neurogenesis | Regional vulnerabilities exist (hippocampus vs. olfactory bulb); lack of clarity hampers targeted interventions | Single-cell RNA-seq, region-specific lineage tracing, conditional microglial manipulation | [132,133,134] |
| 2. Inflammasome Dynamics in Aging | Unresolved timeline of NLRP3/other inflammasome activation in aged niches | Unclear when inflammasome priming becomes irreversible; timing critical for therapeutic window | Longitudinal transcriptomics, in vivo biosensors, inducible knockout models | [130,131,160] |
| 3. Crosstalk Between Peripheral and CNS Immunity | Mechanisms of how peripheral T cells and cytokines reshape neurogenic niches remain obscure | Infiltrating T cells alter NSC fate; missing mechanistic detail limits translation to systemic therapies | Fate-mapping of immune infiltration, parabiosis, targeted blockade of adhesion molecules | [132,133,134] |
| 4. Beneficial vs. Detrimental Microglial States | Poorly defined markers distinguishing pro-neurogenic vs. antineurogenic microglial states | Current therapies risk indiscriminate immunosuppression; need precision immunomodulation | Multi-omics integration (proteome, epigenome), machine-learning-based state classification, microglia-specific drug screens | [5,6,7] |
| 5. Non-coding RNA & Extracellular Vesicle Signaling | Roles of EV cargo (miRNAs, lncRNAs) in regulating neurogenesis under inflammation are underexplored | Missed therapeutic opportunities; EVs may carry both detrimental and reparative signals | High-resolution EV profiling, CRISPR-based RNA manipulation, engineered EV delivery systems | [161,162,163] |
| Strategy | Examples/Tools | Goal/Effect | Stage of Development | References |
|---|---|---|---|---|
| Longitudinal Imaging | [^18F]FLT-PET for neurogenesis, TSPO-PET for microglial activation | Enables in vivo monitoring of neurogenesis and neuroinflammation across lifespan | Preclinical for neurogenesis tracers; TSPO-PET in human use | [216,217,218,245,246] |
| Brain-Penetrant NLRP3 Inhibitors | MCC950, NT-0796, BGE-102 | Reduce chronic IL-1β release, restore neurogenic potential | Preclinical to Phase 1 clinical trials | [221,222,223,224,225,226] |
| Glial Reprogramming | AAV-NeuroD1, SOX2-based astrocyte-to-neuron conversion | Replace lost neurons; rejuvenate circuits | Proof-of-concept in rodents | [192,219,273] |
| CRISPR Epigenetic Editing | CRISPR-dCas9 targeting IL-1β/NLRP3 loci; enhancer repression | Long-term silencing of pro-inflammatory genes without DNA cleavage | Lab-stage; in vitro and early in vivo | [227,228,229,230,231,232] |
| Niche Immunomodulation | Anti-IL-1β, anti-TNF, IL-6R antibodies; microglia-specific modulators | Dampens chronic inflammation in neurogenic niches | Several agents in AD, MCI, depression trials | [294,295,301] |
| Extracellular Vesicle (EV) Therapeutics | Engineered EVs carrying miRNAs, BDNF, or IGF-1 cargo | Deliver pro-neurogenic and anti-inflammatory signals | Preclinical; first-in-human safety studies emerging | [161,163,302] |
| Lifestyle & Activity-Based Interventions | Exercise, enriched environment, caloric modulation | Boost endogenous IGF-1/BDNF, reduce inflammatory priming | Multiple human cohort studies and ongoing clinical trials | [249,301] |
| Small-Molecule Neurotrophic Enhancers | TrkB agonists, phosphodiesterase inhibitors | Enhance BDNF signaling, promote synaptic/neurogenic resilience | Early-stage clinical testing, mixed outcomes | [216,248] |
| Microglial State Modulation | CSF1R inhibitors, TREM2 agonists | Shift microglia from pro-inflammatory to reparative states | Preclinical; TREM2 antibodies in Phase 2 AD trials | [217,250] |
| Combinatorial Approaches | NLRP3 inhibitor + exercise; anti-TNF + BDNF mimetics | Target multiple axes (inflammatory and trophic) simultaneously | Conceptual and early preclinical testing | [221,301] |
| Aspect | Rodents (Murine) | Humans | References |
|---|---|---|---|
| Adult hippocampal neurogenesis (baseline) | Thousands of new neurons per day in young adult hippocampus; robust measurable pools | Far fewer (hundreds/day in young adults by some estimates); highly variable depending on methodology | [1,2,3] |
| Age of significant decline in neurogenesis | Detectable decline starting mid-life (12–18 months); still measurable in aged animals | Steep decline reported from middle age; ongoing debate whether residual neurogenesis persists in elderly | [1,2,3] |
| Microglial density and activation state in aging | Well-characterized shift to ‘primed’ phenotype with pro-inflammatory gene expression and reduced phagocytic resolution | Less comprehensive; aged human microglia show pro-inflammatory signatures, distinct subsets identified via single-cell transcriptomics | [116,165] |
| Peripheral immune cell involvement in CNS with age | Increased infiltration of T cells (especially CD8+) into hippocampus and SVZ with aging; enhances IFN-γ tone | Limited but growing evidence; T-cell presence in human hippocampus in aging and neurodegeneration; mechanisms less defined | [132,133] |
| Evidence for exercise or enrichment effects | Exercise and enriched environments robustly increase neurogenesis and improve cognition in mice | Human studies show hippocampal volume increases and cognitive benefits; direct evidence for neurogenesis boost is indirect (MRI, blood biomarkers) | [216,248,301] |
| Inflammasome/NLRP3 activation with age | Strong evidence for NLRP3-driven IL-1β increase in aged rodent hippocampus, reducing neurogenesis | Human post-mortem and transcriptomic studies support NLRP3 upregulation in aging brain; functional causality harder to confirm | [221,222,301] |
| Translational caveats | High plasticity, short lifespan, and controlled environments amplify experimental effects | Human variability, long lifespan, and heterogeneous exposures complicate translation; methodological debates on detecting neurogenesis | [1,2,3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tanaka, M. Neurogenesis and Neuroinflammation in Dialogue: Mapping Gaps, Modulating Microglia, Rewiring Aging. Cells 2026, 15, 78. https://doi.org/10.3390/cells15010078
Tanaka M. Neurogenesis and Neuroinflammation in Dialogue: Mapping Gaps, Modulating Microglia, Rewiring Aging. Cells. 2026; 15(1):78. https://doi.org/10.3390/cells15010078
Chicago/Turabian StyleTanaka, Masaru. 2026. "Neurogenesis and Neuroinflammation in Dialogue: Mapping Gaps, Modulating Microglia, Rewiring Aging" Cells 15, no. 1: 78. https://doi.org/10.3390/cells15010078
APA StyleTanaka, M. (2026). Neurogenesis and Neuroinflammation in Dialogue: Mapping Gaps, Modulating Microglia, Rewiring Aging. Cells, 15(1), 78. https://doi.org/10.3390/cells15010078






