Modulation of Metabotropic Glutamate Receptors as a Strategy to Improve the Efficacy and Safety of Ketamine as an Antidepressant
Abstract
1. Introduction
2. The Involvement of the mGlu2 Receptor in the Effects of Ketamine
3. Synergistic Effects of Ketamine and mGlu2/3 Receptor Antagonists
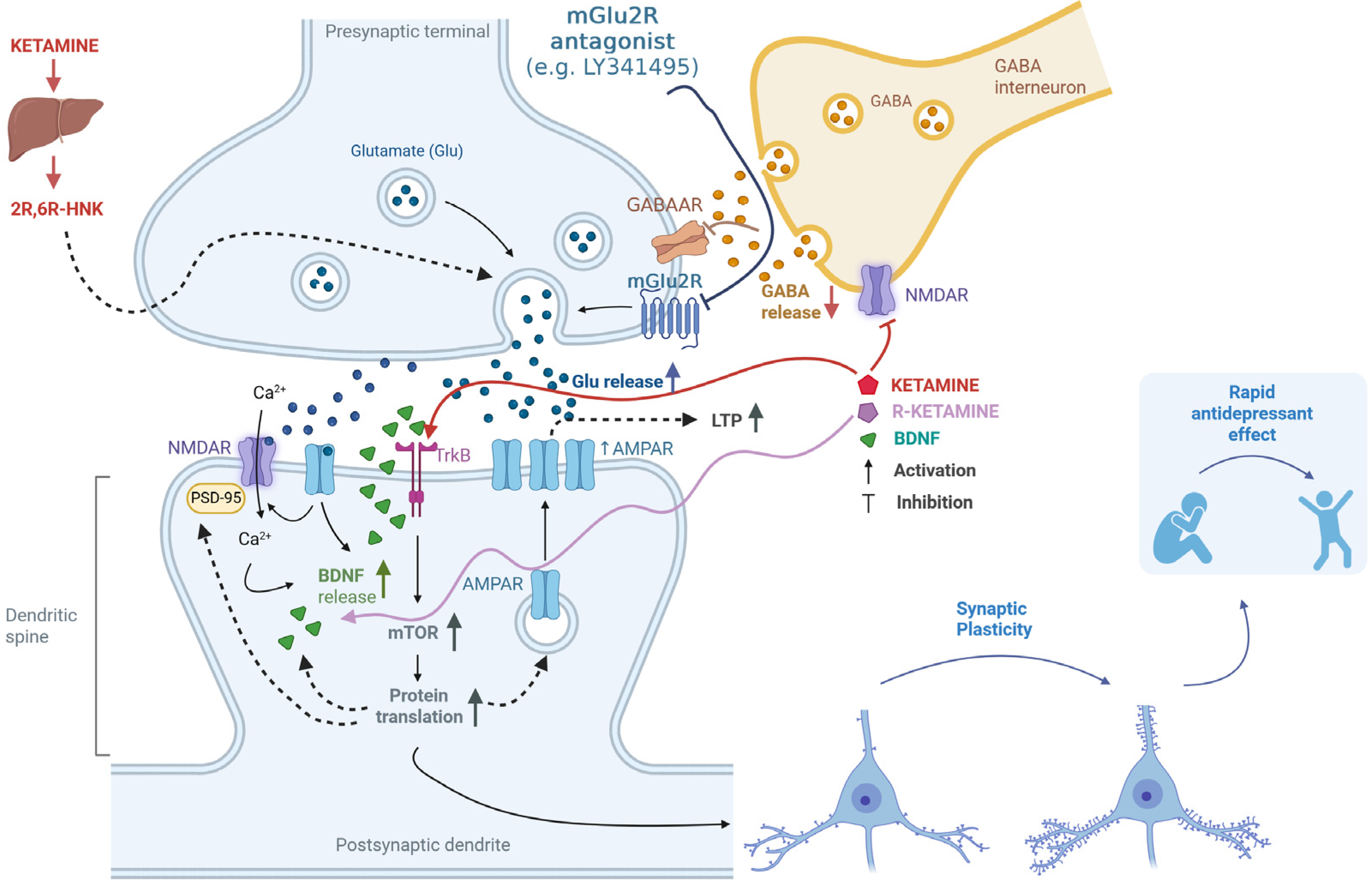
4. Possible Mechanism for the Enhancement of Ketamine’s Antidepressant Effects Through Antagonism of the mGlu2 Receptor
5. The Involvement of the mGlu5 Receptor in the Antidepressant Action of Ketamine
6. Synergistic Effects of Ketamine and mGlu5 Receptor Antagonists and NAMs
7. Putative Mechanism of Potentiation of Ketamine’s Antidepressant Effect by mGlu5 Receptor Antagonism
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPA | alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| BDNF | brain-derived neurotrophic factor |
| BOLD | blood oxygenation level-dependent |
| CUMS | chronic unpredictable mild stress |
| eEF2 | eukaryotic elongation factor 2 |
| FST | forced swim test |
| GABA | gamma-aminobutyric acid |
| HNK | hydroxynorketamine |
| LTP | long-term potentiation |
| mGlu receptor | metabotropic glutamate receptor |
| M-5MPEP | 2-(2-(3-methoxyphenyl)ethynyl)-5-methylpyridine |
| mPFC | medial prefrontal cortex |
| mTOR | mammalian target of rapamycin |
| NAM | negative allosteric modulator |
| NMDA | N-methyl-D-aspartic acid |
| NOR | novel object recognition |
| PAM | positive allosteric modulator |
| PCP | phencyclidine |
| PET | positron emission tomography |
| PFC | prefrontal cortex |
| phMRI | pharmacologic magnetic resonance imaging |
| PSD-95 | postsynaptic density 95 |
| qEEG | quantitative electroencephalography |
| RAAD | rapid-acting antidepressant drug |
| SDS | social defeat stress |
| SSRI | selective serotonin reuptake inhibitor |
| TRD | treatment-resistant depression |
| TrkB | tropomyosin receptor kinase B |
| TST | tail suspension test |
References
- Mahase, E. Esketamine is approved in Europe for treating resistant major depressive disorder. BMJ 2019, 367, l7069. [Google Scholar] [CrossRef] [PubMed]
- Bobo, W.V.; Vande Voort, J.L.; Croarkin, P.E.; Leung, J.G.; Tye, S.J.; Frye, M.A. Ketamine for treatment-resistant unipolar and bipolar major depression: Critical review and implications for clinical practice. Depress. Anxiety 2016, 33, 698–710. [Google Scholar] [CrossRef]
- Lapidus, K.A.; Levitch, C.F.; Perez, A.M.; Brallier, J.W.; Parides, M.K.; Soleimani, L.; Feder, A.; Iosifescu, D.V.; Charney, D.S.; Murrough, J.W. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol. Psychiatry 2014, 76, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A., Jr.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- aan het Rot, M.; Collins, K.A.; Murrough, J.W.; Perez, A.M.; Reich, D.L.; Charney, D.S.; Mathew, S.J. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry 2010, 67, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B., Jr.; Charney, D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 1994, 51, 199–214. [Google Scholar] [CrossRef]
- Li, L.; Vlisides, P.E. Ketamine: 50 years of modulating the mind. Front. Hum. Neurosci. 2016, 10, 612. [Google Scholar] [CrossRef]
- Zanos, P.; Brown, K.A.; Georgiou, P.; Yuan, P.; Zarate, C.A., Jr.; Thompson, S.M.; Gould, T.D. NMDA receptor activation-dependent antidepressant-relevant behavioral and synaptic actions of ketamine. J. Neurosci. 2023, 43, 1038–1050. [Google Scholar] [CrossRef]
- Moghaddam, B.; Adams, B.; Verma, A.; Daly, D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997, 17, 2921–2927. [Google Scholar] [CrossRef]
- Kim, J.W.; Suzuki, K.; Kavalali, E.T.; Monteggia, L.M. Bridging rapid and sustained antidepressant effects of ketamine. Trends Mol. Med. 2023, 29, 364–375. [Google Scholar] [CrossRef]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef]
- Wray, N.H.; Schappi, J.M.; Singh, H.; Senese, N.B.; Rasenick, M.M. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol. Psychiatry 2019, 24, 1833–1843. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef]
- Newport, D.J.; Carpenter, L.L.; McDonald, W.M.; Potash, J.B.; Tohen, M.; Nemeroff, C.B. APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 2015, 172, 950–966. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef]
- Levinstein, M.R.; Carlton, M.L.; Di Ianni, T.; Ventriglia, E.N.; Rizzo, A.; Gomez, J.L.; Budinich, R.C.; Shaham, Y.; Airan, R.D.; Zarate, C.A., Jr.; et al. Mu opioid receptor activation mediates (S)-ketamine reinforcement in rats: Implications for abuse liability. Biol. Psychiatry 2022, 93, 1118–1126. [Google Scholar] [CrossRef]
- Shafique, H.; Demers, J.C.; Biesiada, J.; Golani, L.K.; Cerne, R.; Smith, J.L.; Szostak, M.; Witkin, J.M. (R)-(-)-ketamine: The promise of a novel treatment for psychiatric and neurological disorders. Int. J. Mol. Sci. 2024, 25, 6804. [Google Scholar] [CrossRef] [PubMed]
- Chaki, S.; Ago, Y.; Palucha-Paniewiera, A.; Matrisciano, F.; Pilc, A. mGlu2/3 and mGlu5 receptors: Potential targets for novel antidepressants. Neuropharmacology 2013, 66, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Pałucha-Poniewiera, A.; Rafało-Ulińska, A.; Santocki, M.; Babii, Y.; Kaczorowska, K. Partial mGlu5 receptor NAM, M-5MPEP, induces rapid and sustained antidepressant-like effects in the BDNF-dependent mechanism and enhances (R)-ketamine action in mice. Pharmacol. Rep. 2024, 76, 504–518. [Google Scholar] [CrossRef]
- Witkin, J.M. mGlu2/3 receptor antagonism: A mechanism to induce rapid antidepressant effects without ketamine-associated side-effects. Pharmacol. Biochem. Behav. 2020, 190, 172854. [Google Scholar] [CrossRef]
- Dwyer, J.M.; Lepack, A.E.; Duman, R.S. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int. J. Neuropsychopharmacol. 2012, 15, 429–434. [Google Scholar] [CrossRef]
- Fukumoto, K.; Iijima, M.; Funakoshi, T.; Chaki, S. 5-HT(1A) receptor stimulation in the medial prefrontal cortex mediates the antidepressant effects of mGlu2/3 receptor antagonist in mice. Neuropharmacology 2018, 137, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Iijima, M.; Chaki, S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 2011, 61, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Lepack, A.E.; Bang, E.; Lee, B.; Dwyer, J.M.; Duman, R.S. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 2016, 111, 242–252. [Google Scholar] [CrossRef]
- Fukumoto, K.; Iijima, M.; Chaki, S. Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology 2014, 231, 2291–2298. [Google Scholar] [CrossRef]
- Fukumoto, K.; Iijima, M.; Chaki, S. The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology 2016, 41, 1046–1056. [Google Scholar] [CrossRef]
- Koike, H.; Chaki, S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav. Brain Res. 2014, 271, 111–115. [Google Scholar] [CrossRef]
- Witkin, J.M.; Mitchell, S.N.; Wafford, K.A.; Carter, G.; Gilmour, G.; Li, J.; Eastwood, B.J.; Overshiner, C.; Li, X.; Rorick-Kehn, L.; et al. Comparative effects of LY3020371, a potent and selective metabotropic glutamate (mGlu) 2/3 receptor antagonist, and ketamine, a noncompetitive N-methyl-d-aspartate receptor antagonist in rodents: Evidence supporting the use of mGlu2/3 antagonists, for the treatment of depression. J. Pharmacol. Exp. Ther. 2017, 361, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Fukumoto, K.; Iijima, M.; Chaki, S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav. Brain Res. 2013, 238, 48–52. [Google Scholar] [CrossRef]
- Witkin, J.M.; Monn, J.A.; Schoepp, D.D.; Li, X.; Overshiner, C.; Mitchell, S.N.; Carter, G.; Johnson, B.; Rasmussen, K.; Rorick-Kehn, L.M. The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J. Pharmacol. Exp. Ther. 2016, 358, 71–82. [Google Scholar] [CrossRef]
- Chou, D.; Peng, H.Y.; Lin, T.B.; Lai, C.Y.; Hsieh, M.C.; Wen, Y.C.; Lee, A.S.; Wang, H.H.; Yang, P.S.; Chen, G.D.; et al. (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 2018, 139, 1–12. [Google Scholar] [CrossRef]
- Dong, C.; Tian, Z.; Fujita, Y.; Fujita, A.; Hino, N.; Iijima, M.; Hashimoto, K. Antidepressant-like actions of the mGlu2/3 receptor antagonist TP0178894 in the chronic social defeat stress model: Comparison with escitalopram. Pharmacol. Biochem. Behav. 2022, 212, 173316. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, J.C.; Yao, W.; Ren, Q.; Ma, M.; Yang, C.; Chaki, S.; Hashimoto, K. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: Comparison with ketamine. Int. J. Neuropsychopharmacol. 2017, 20, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.M.; Lepack, A.E.; Duman, R.S. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J. Mol. Psychiatry 2013, 1, 15. [Google Scholar] [CrossRef]
- Pałucha-Poniewiera, A.; Bobula, B.; Rafało-Ulińska, A.; Kaczorowska, K. Depression-like effects induced by chronic unpredictable mild stress in mice are rapidly reversed by a partial negative allosteric modulator of mGlu(5) receptor, M-5MPEP. Psychopharmacology 2024, 242, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Pałucha-Poniewiera, A.; Podkowa, K.; Rafało-Ulińska, A. The group II mGlu receptor antagonist LY341495 induces a rapid antidepressant-like effect and enhances the effect of ketamine in the chronic unpredictable mild stress model of depression in C57BL/6J mice. Prog. NeuroPsychopharmacol. Biol. Psychiatry 2021, 109, 110239. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.M.; Monn, J.A.; Li, J.; Johnson, B.; McKinzie, D.L.; Wang, X.S.; Heinz, B.A.; Li, R.; Ornstein, P.L.; Smith, S.C.; et al. Preclinical predictors that the orthosteric mGlu2/3 receptor antagonist LY3020371 will not engender ketamine-associated neurotoxic, motor, cognitive, subjective, or abuse-liability-related effects. Pharmacol. Biochem. Behav. 2017, 155, 43–55. [Google Scholar] [CrossRef]
- Lorrain, D.S.; Baccei, C.S.; Bristow, L.J.; Anderson, J.J.; Varney, M.A. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: Modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 2003, 117, 697–706. [Google Scholar] [CrossRef]
- Lorrain, D.S.; Schaffhauser, H.; Campbell, U.C.; Baccei, C.S.; Correa, L.D.; Rowe, B.; Rodriguez, D.E.; Anderson, J.J.; Varney, M.A.; Pinkerton, A.B.; et al. Group II mGlu receptor activation suppresses norepinephrine release in the ventral hippocampus and locomotor responses to acute ketamine challenge. Neuropsychopharmacology 2003, 28, 1622–1632. [Google Scholar] [CrossRef]
- Cartmell, J.; Schoepp, D.D. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000, 75, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, H.; Neki, A.; Mizuno, N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: An immunohistochemical study with a monoclonal antibody. Neurosci. Res. 1998, 30, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto, R.; Kinoshita, A.; Wada, E.; Nomura, S.; Ohishi, H.; Takada, M.; Flor, P.J.; Neki, A.; Abe, T.; Nakanishi, S.; et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997, 17, 7503–7522. [Google Scholar] [CrossRef]
- Chin, C.L.; Upadhyay, J.; Marek, G.J.; Baker, S.J.; Zhang, M.; Mezler, M.; Fox, G.B.; Day, M. Awake rat pharmacological magnetic resonance imaging as a translational pharmacodynamic biomarker: Metabotropic glutamate 2/3 agonist modulation of ketamine-induced blood oxygenation level dependence signals. J. Pharmacol. Exp. Ther. 2011, 336, 709–715. [Google Scholar] [CrossRef]
- Mehta, M.A.; Schmechtig, A.; Kotoula, V.; McColm, J.; Jackson, K.; Brittain, C.; Tauscher-Wisniewski, S.; Kinon, B.J.; Morrison, P.D.; Pollak, T.; et al. Group II metabotropic glutamate receptor agonist prodrugs LY2979165 and LY2140023 attenuate the functional imaging response to ketamine in healthy subjects. Psychopharmacology 2018, 235, 1875–1886. [Google Scholar] [CrossRef]
- Fujáková, M.; Páleníček, T.; Brunovský, M.; Gorman, I.; Tylš, F.; Kubešová, A.; Řípová, D.; Krajča, V.; Horáček, J. The effect of ((-)-2-oxa-4-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY379268), an mGlu2/3 receptor agonist, on EEG power spectra and coherence in ketamine model of psychosis. Pharmacol. Biochem. Behav. 2014, 122, 212–221. [Google Scholar] [CrossRef]
- Hiyoshi, T.; Hikichi, H.; Karasawa, J.; Chaki, S. Metabotropic glutamate receptors regulate cortical gamma hyperactivities elicited by ketamine in rats. Neurosci. Lett. 2014, 567, 30–34. [Google Scholar] [CrossRef]
- Jones, N.C.; Reddy, M.; Anderson, P.; Salzberg, M.R.; O’Brien, T.J.; Pinault, D. Acute administration of typical and atypical antipsychotics reduces EEG γ power, but only the preclinical compound LY379268 reduces the ketamine-induced rise in γ power. Int. J. Neuropsychopharmacol. 2012, 15, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, T.; Marumo, T.; Hikichi, H.; Tomishima, Y.; Urabe, H.; Tamita, T.; Iida, I.; Yasuhara, A.; Karasawa, J.; Chaki, S. Neurophysiologic and antipsychotic profiles of TASP0433864, a novel positive allosteric modulator of metabotropic glutamate 2 receptor. J. Pharmacol. Exp. Ther. 2014, 351, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Highland, J.N.; Stewart, B.W.; Georgiou, P.; Jenne, C.E.; Lovett, J.; Morris, P.J.; Thomas, C.J.; Moaddel, R.; Zarate, C.A., Jr.; et al. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc. Natl. Acad. Sci. USA 2019, 116, 6441–6450. [Google Scholar] [CrossRef]
- Bonaventura, J.; Gomez, J.L.; Carlton, M.L.; Lam, S.; Sanchez-Soto, M.; Morris, P.J.; Moaddel, R.; Kang, H.J.; Zanos, P.; Gould, T.D.; et al. Target deconvolution studies of (2R,6R)-hydroxynorketamine: An elusive search. Mol. Psychiatry 2022, 27, 4144–4156. [Google Scholar] [CrossRef]
- Podkowa, K.; Pochwat, B.; Brański, P.; Pilc, A.; Pałucha-Poniewiera, A. Group II mGlu receptor antagonist LY341495 enhances the antidepressant-like effects of ketamine in the forced swim test in rats. Psychopharmacology 2016, 233, 2901–2914. [Google Scholar] [CrossRef]
- Rafało-Ulińska, A.; Brański, P.; Pałucha-Poniewiera, A. Combined administration of (R)-ketamine and the mGlu2/3 receptor antagonist LY341495 induces rapid and sustained effects in the CUMS model of depression via a TrkB/BDNF-dependent mechanism. Pharmaceuticals 2022, 15, 125. [Google Scholar] [CrossRef]
- Chen, X.; Nelson, C.D.; Li, X.; Winters, C.A.; Azzam, R.; Sousa, A.A.; Leapman, R.D.; Gainer, H.; Sheng, M.; Reese, T.S. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J. Neurosci. 2011, 31, 6329–6338. [Google Scholar] [CrossRef]
- Pałucha-Poniewiera, A.; Podkowa, K.; Pilc, A. Role of AMPA receptor stimulation and TrkB signaling in the antidepressant-like effect of ketamine co-administered with a group II mGlu receptor antagonist, LY341495, in the forced swim test in rats. Behav. Pharmacol. 2019, 30, 471–477. [Google Scholar] [CrossRef]
- Seo, M.K.; Hien, L.T.; Park, M.K.; Choi, A.J.; Seog, D.H.; Kim, S.H.; Park, S.W.; Lee, J.G. AMPA receptor-mTORC1 signaling activation is required for neuroplastic effects of LY341495 in rat hippocampal neurons. Sci. Rep. 2020, 10, 993. [Google Scholar] [CrossRef]
- Brown, K.A.; Ajibola, M.I.; Gould, T.D. Rapid hippocampal synaptic potentiation induced by ketamine metabolite (2R,6R)-hydroxynorketamine persistently primes synaptic plasticity. Neuropsychopharmacology 2025, 50, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Pałucha-Poniewiera, A.; Bobula, B.; Rafało-Ulińska, A. The antidepressant-like activity and cognitive enhancing effects of the combined administration of (R)-ketamine and LY341495 in the CUMS model of depression in mice are related to the modulation of excitatory synaptic transmission and LTP in the PFC. Pharmaceuticals 2023, 16, 288. [Google Scholar] [CrossRef]
- Karimi, S.A.; Komaki, A.; Salehi, I.; Sarihi, A.; Shahidi, S. Role of group II metabotropic glutamate receptors (mGluR2/3) blockade on long-term potentiation in the dentate gyrus region of hippocampus in rats fed with high-fat diet. Neurochem. Res. 2015, 40, 811–817. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, C.; DellaGioia, N.; Bloch, M.; Sanacora, G.; Nabulsi, N.; Abdallah, C.; Yang, J.; Wen, R.; Mann, J.J.; Krystal, J.H.; et al. In vivo ketamine-induced changes in [11C]ABP688 binding to metabotropic glutamate receptor subtype 5. Biol. Psychiatry 2015, 77, 266–275. [Google Scholar] [CrossRef]
- Esterlis, I.; DellaGioia, N.; Pietrzak, R.H.; Matuskey, D.; Nabulsi, N.; Abdallah, C.G.; Yang, J.; Pittenger, C.; Sanacora, G.; Krystal, J.H.; et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: An [11C]ABP688 and PET imaging study in depression. Mol. Psychiatry 2018, 23, 824–832. [Google Scholar] [CrossRef]
- Wang, Y.; He, W.; Zhang, H.; Yao, Z.; Che, F.; Cao, Y.; Sun, H. mGluR5 mediates ketamine antidepressant response in susceptible rats exposed to prenatal stress. J. Affect. Disord. 2020, 272, 398–408. [Google Scholar] [CrossRef]
- Pałucha-Poniewiera, A.; Rafało-Ulińska, A.; Faron-Górecka, A.; Pabian, P.; Kaczorowska, K. (R)-ketamine induces mGlu5 receptor-dependent antidepressant-like effects in the chronic unpredictable mild stress model of depression in mice. Psychopharmacology 2025, 242, 2401–2415. [Google Scholar] [CrossRef]
- Ango, F.; Prézeau, L.; Muller, T.; Tu, J.C.; Xiao, B.; Worley, P.F.; Pin, J.P.; Bockaert, J.; Fagni, L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 2001, 411, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Borroto-Escuela, D.O. Basimglurant for treatment of major depressive disorder: A novel negative allosteric modulator of metabotropic glutamate receptor 5. Expert. Opin. Investig. Drugs 2015, 24, 1247–1260. [Google Scholar] [CrossRef]
- Kato, T.; Takata, M.; Kitaichi, M.; Kassai, M.; Inoue, M.; Ishikawa, C.; Hirose, W.; Yoshida, K.; Shimizu, I. DSR-98776, a novel selective mGlu5 receptor negative allosteric modulator with potent antidepressant and antimanic activity. Eur. J. Pharmacol. 2015, 757, 11–20. [Google Scholar] [CrossRef]
- Pałucha, A.; Brański, P.; Szewczyk, B.; Wierońska, J.M.; Kłak, K.; Pilc, A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol. Biochem. Behav. 2005, 81, 901–906. [Google Scholar] [CrossRef]
- Gokalp, D.; Unal, G. The role of mGluR5 on the therapeutic effects of ketamine in Wistar rats. Psychopharmacology 2024, 241, 1399–1415. [Google Scholar] [CrossRef] [PubMed]
- Sou, J.-H.; Chan, M.-H.; Chen, H.-H. Ketamine, but not propofol, anaesthesia is regulated by metabotropic glutamate 5 receptors. Br. J. Anaesth. 2006, 96, 597–601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chan, M.-H.; Chiu, P.-H.; Sou, J.-H.; Chen, H.-H. Attenuation of ketamine-evoked behavioral responses by mGluR5 positive modulators in mice. Psychopharmacology 2008, 198, 141–148. [Google Scholar] [CrossRef]
- Gould, R.W.; Amato, R.J.; Bubser, M.; Joffe, M.E.; Nedelcovych, M.T.; Thompson, A.D.; Nickols, H.H.; Yuh, J.P.; Zhan, X.; Felts, A.S.; et al. Partial mGlu5 negative allosteric modulators attenuate cocaine-mediated behaviors and lack psy-chotomimetic-like effects. Neuropsychopharmacology 2016, 41, 1166–1178. [Google Scholar] [CrossRef]
- Homayoun, H.; Stefani, M.R.; Adams, B.W.; Tamagan, G.D.; Moghaddam, B. Functional interaction between NMDA and mGlu5 receptors: Effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology 2004, 29, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Kinney, G.G.; Burno, M.; Campbell, U.C.; Hernandez, L.M.; Rodriguez, D.; Bristow, L.J.; Conn, P.J. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J. Pharmacol. Exp. Ther. 2003, 306, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Holter, K.M.; Lekander, A.D.; LaValley, C.M.; Bedingham, E.G.; Pierce, B.E.; Sands, L.P., 3rd; Lindsley, C.W.; Jones, C.K.; Gould, R.W. Partial mGlu5 negative allosteric modulator M-5MPEP demonstrates antidepressant-like effects on sleep without affecting cognition or quantitative EEG. Front. Neurosci. 2021, 15, 700822. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Hubert, G.W.; Smith, Y.; Levey, A.I.; Conn, P.J. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J. Neurosci. 2000, 20, 7871–7879. [Google Scholar] [CrossRef]
- Chen, H.-H.; Liao, P.-F.; Chan, M.-H. mGluR5 positive modulators both potentiate activation and restore inhibition in NMDA receptors by PKC dependent pathway. J. Biomed. Sci. 2011, 18, 19. [Google Scholar] [CrossRef]
- Jin, D.; Guo, M.; Xue, B.; Mao, L.; Wang, J.Q. Differential regulation of CaMKIIα interactions with mGluR5 and NMDA receptors by Ca2+ in neurons. J. Neurochem. 2013, 127, 620–631. [Google Scholar] [CrossRef]
- Kotecha, S.A.; Jackson, M.F.; Al-Mahrouki, A.; Roder, J.C.; Orser, B.A.; MacDonald, J.F. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J. Biol. Chem. 2003, 278, 27742–27749. [Google Scholar] [CrossRef]
- Rosenbrock, H.; Kramer, G.; Hobson, S.; Koros, E.; Grundl, M.; Grauert, M.; Reymann, K.G.; Schröder, U.H. Functional interaction of metabotropic glutamate receptor 5 and NMDA-receptor by a metabotropic glutamate receptor 5 positive allosteric modulator. Eur. J. Pharmacol. 2010, 639, 40–46. [Google Scholar] [CrossRef]
- Perroy, J.; Raynaud, F.; Homburger, V.; Rousset, M.C.; Telley, L.; Bockaert, J.; Fagni, L. Direct interaction enables cross-talk between ionotropic and group I metabotropic glutamate receptors. J. Biol. Chem. 2008, 283, 6799–6805. [Google Scholar] [CrossRef]
- Tu, J.C.; Xiao, B.; Naisbitt, S.; Yuan, J.P.; Petralia, R.S.; Brakeman, P.; Doan, A.; Aakalu, V.K.; Lanahan, A.A.; Sheng, M.; et al. Coupling of mGluR/Homer and PSD-95 complexes by the shank family of postsynaptic density proteins. Neuron 1999, 23, 583–592. [Google Scholar] [CrossRef]
- Bockaert, J.; Perroy, J.; Ango, F. The complex formed by group I Metabotropic Glutamate Receptor (mGluR) and Homer1a plays a central role in metaplasticity and homeostatic synaptic scaling. J. Neurosci. 2021, 41, 5567–5578. [Google Scholar] [CrossRef]
- Clifton, N.E.; Trent, S.; Thomas, K.L.; Hall, J. Regulation and function of activity-dependent homer in synaptic plasticity. Mol. Neuropsychiatry 2019, 5, 147–161. [Google Scholar] [CrossRef]
- Kammermeier, P.J.; Worley, P.F. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc. Natl. Acad. Sci. USA 2007, 104, 6055–6060. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Sarappa, C.; Buonaguro, E.F.; Marmo, F.; Eramo, A.; Tomasetti, C.; Iasevoli, F. Different effects of the NMDA receptor antagonists ketamine, MK-801, and memantine on postsynaptic density transcripts and their topography: Role of Homer signaling, and implications for novel antipsychotic and pro-cognitive targets in psychosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Serchov, T.; Clement, H.W.; Schwarz, M.K.; Iasevoli, F.; Tosh, D.K.; Idzko, M.; Jacobson, K.A.; de Bartolomeis, A.; Normann, C.; Biber, K.; et al. Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron 2015, 87, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Serchov, T.; Heumann, R.; van Calker, D.; Biber, K. Signaling pathways regulating Homer1a expression: Implications for antidepressant therapy. Biol. Chem. 2016, 397, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, E.; Le Corf, K.; Dupuis, J.; Zhang, P.; Ginger, M.; Labrousse, V.; Spatuzza, M.; Haberl, M.G.; Costa, L.; Shigemoto, R.; et al. Altered surface mGluR5 dynamics provoke synaptic NMDAR dysfunction and cognitive defects in Fmr1 knockout mice. Nat. Commun. 2017, 8, 1103. [Google Scholar] [CrossRef]
- Elmeseiny, O.S.A.; Müller, H.K. A molecular perspective on mGluR5 regulation in the antidepressant effect of ketamine. Pharmacol. Res. 2024, 200, 107081. [Google Scholar] [CrossRef]


| Treatment | Species/Behavioral Model/Test | Effect | Reference |
|---|---|---|---|
| (R,S)-ketamine (3 mg/kg) +mGlu2R antagonist (LY341495) (0.3 mg/kg) | mouse/CUMS/Splash | Anti-apathetic | [38] |
| mouse/CUMS/SPT | Anti-anhedonic | ||
| mouse/CUMS/TST | AD-like | ||
| mouse/CUMS/FST | No effect | ||
| (R,S)-ketamine (3 mg/kg) +mGlu2R antagonist (LY341495) (0.3 mg/kg) | rat/FST | AD-like | [53] |
| (R,S)-ketamine (3 mg/kg) +mGlu2R antagonist (LY341495) (0.1 mg/kg) | rat/FST | AD-like | [56] |
| (R,S)-ketamine (1 mg/kg) +mGlu2R antagonist (LY341495) (0.1 mg/kg) | mouse/FST | AD-like | [51] |
| (2R,6R)-HNK (1 mg/kg) +mGlu2R antagonist (LY341495) (0.1 mg/kg) | mouse/FST | AD-like | [51] |
| (R)-ketamine (1 mg/kg) +mGlu2R antagonist (LY341495) (0.3 mg/kg) | mouse/TST | AD-like | [54] |
| mouse/CUMS/Splash | Anti-apathetic | [54] | |
| mouse/CUMS/SPT | Anti-anhedonic | [54] | |
| mouse/CUMS/TST | AD-like | [54] | |
| mouse/CUMS/FST | AD-like | [59] | |
| (S)-ketamine (1 mg/kg) +mGlu2R antagonist (LY341495) (0.3 mg/kg) | mouse/TST | AD-like | [54] |
| mouse/CUMS/Splash | No effect | ||
| mouse/CUMS/SPT | No effect | ||
| mouse/CUMS/TST | No effect | ||
| (R,S)-ketamine (1 mg/kg) +mGlu5R antagonist (MTEP) (1.25 mg/kg) | rat/FST | AD-like | [69] |
| (R)-ketamine (1 mg/kg) +mGlu5R NAM (M-5MPEP) (3 mg/kg) | mouse/TST | AD-like | [21] |
| mouse/CUMS/Splash | Anti-apathetic | [64] | |
| mouse/CUMS/SPT | Anti-anhedonic | [64] | |
| mouse/CUMS/TST | AD-like | [64] | |
| (S)-ketamine (1 mg/kg) +mGlu5R NAM (M-5MPEP) (3 mg/kg) | mouse/TST | No effect | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pałucha-Poniewiera, A. Modulation of Metabotropic Glutamate Receptors as a Strategy to Improve the Efficacy and Safety of Ketamine as an Antidepressant. Cells 2025, 14, 1967. https://doi.org/10.3390/cells14241967
Pałucha-Poniewiera A. Modulation of Metabotropic Glutamate Receptors as a Strategy to Improve the Efficacy and Safety of Ketamine as an Antidepressant. Cells. 2025; 14(24):1967. https://doi.org/10.3390/cells14241967
Chicago/Turabian StylePałucha-Poniewiera, Agnieszka. 2025. "Modulation of Metabotropic Glutamate Receptors as a Strategy to Improve the Efficacy and Safety of Ketamine as an Antidepressant" Cells 14, no. 24: 1967. https://doi.org/10.3390/cells14241967
APA StylePałucha-Poniewiera, A. (2025). Modulation of Metabotropic Glutamate Receptors as a Strategy to Improve the Efficacy and Safety of Ketamine as an Antidepressant. Cells, 14(24), 1967. https://doi.org/10.3390/cells14241967





