Foretinib Alleviates Osteoblast Senescence and Protects Against Bone Loss in Ovariectomized Mice by Promoting Osteoblast Differentiation
Abstract
1. Introduction
2. Materials and Methods
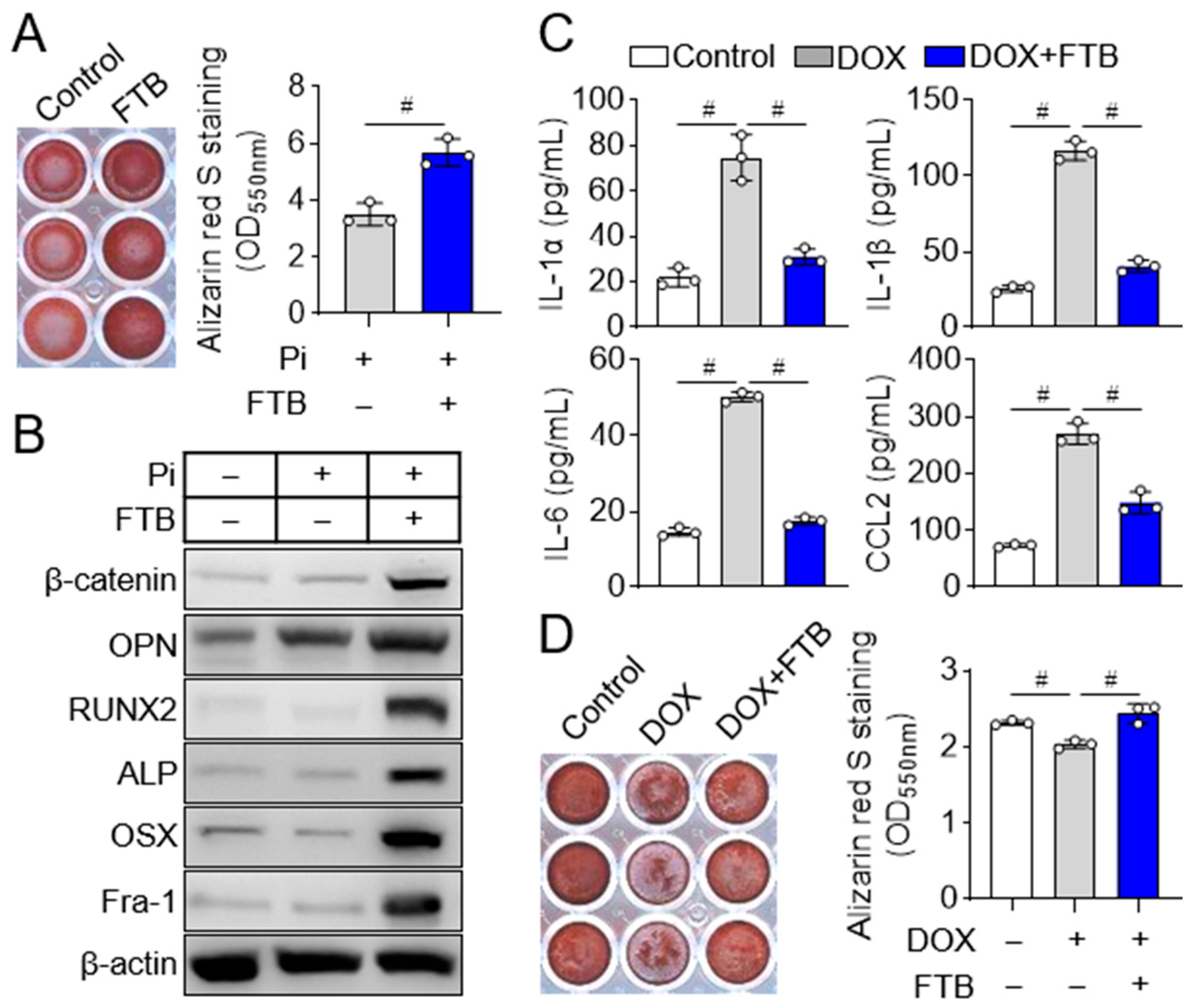
2.1. Cell Culture and Osteoblast Differentiation
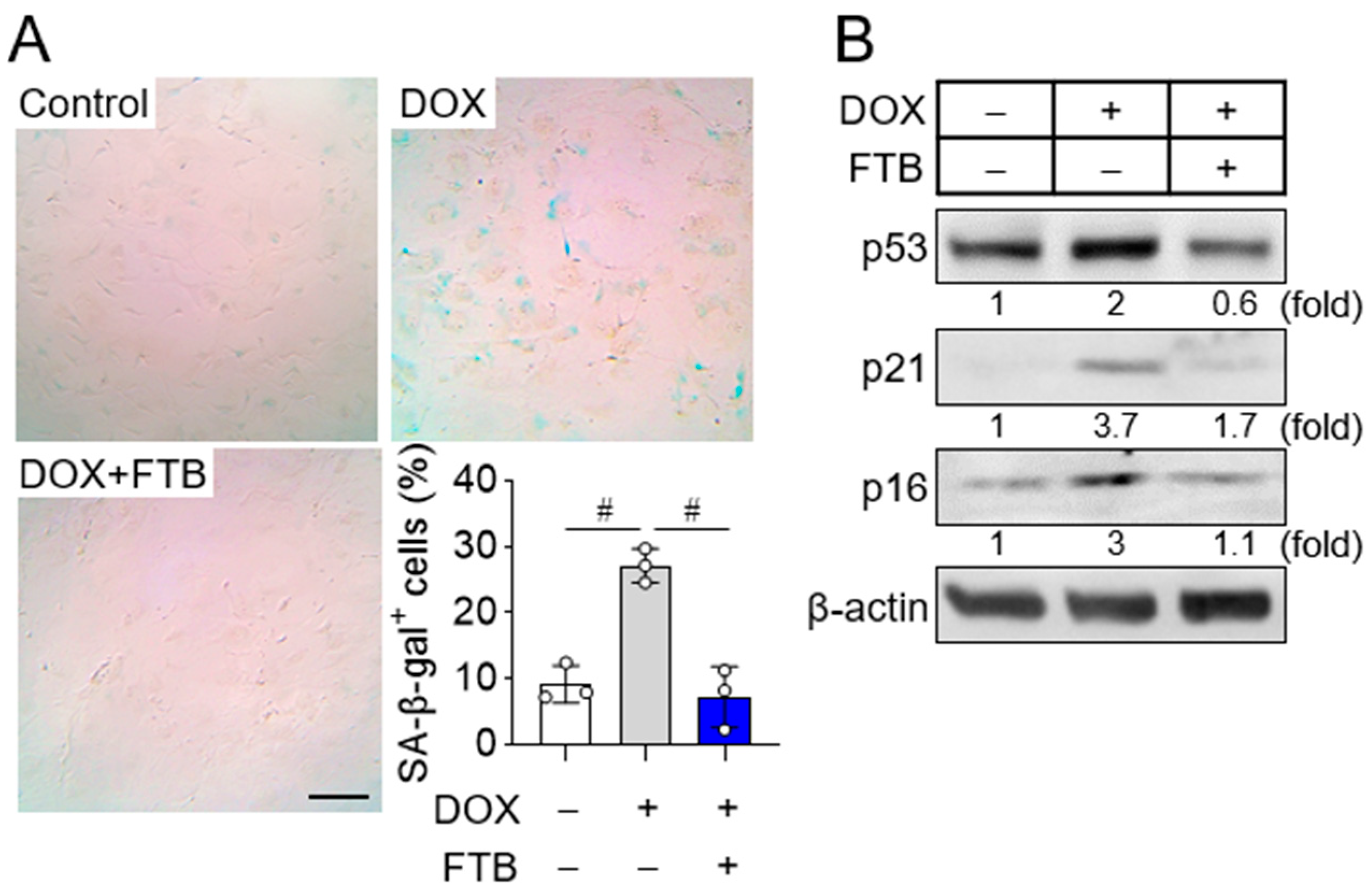
2.2. Induction of Cellular Senescence and SA-β-Gal Staining
2.3. Western Blot Analysis
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Conditioned Media Assay
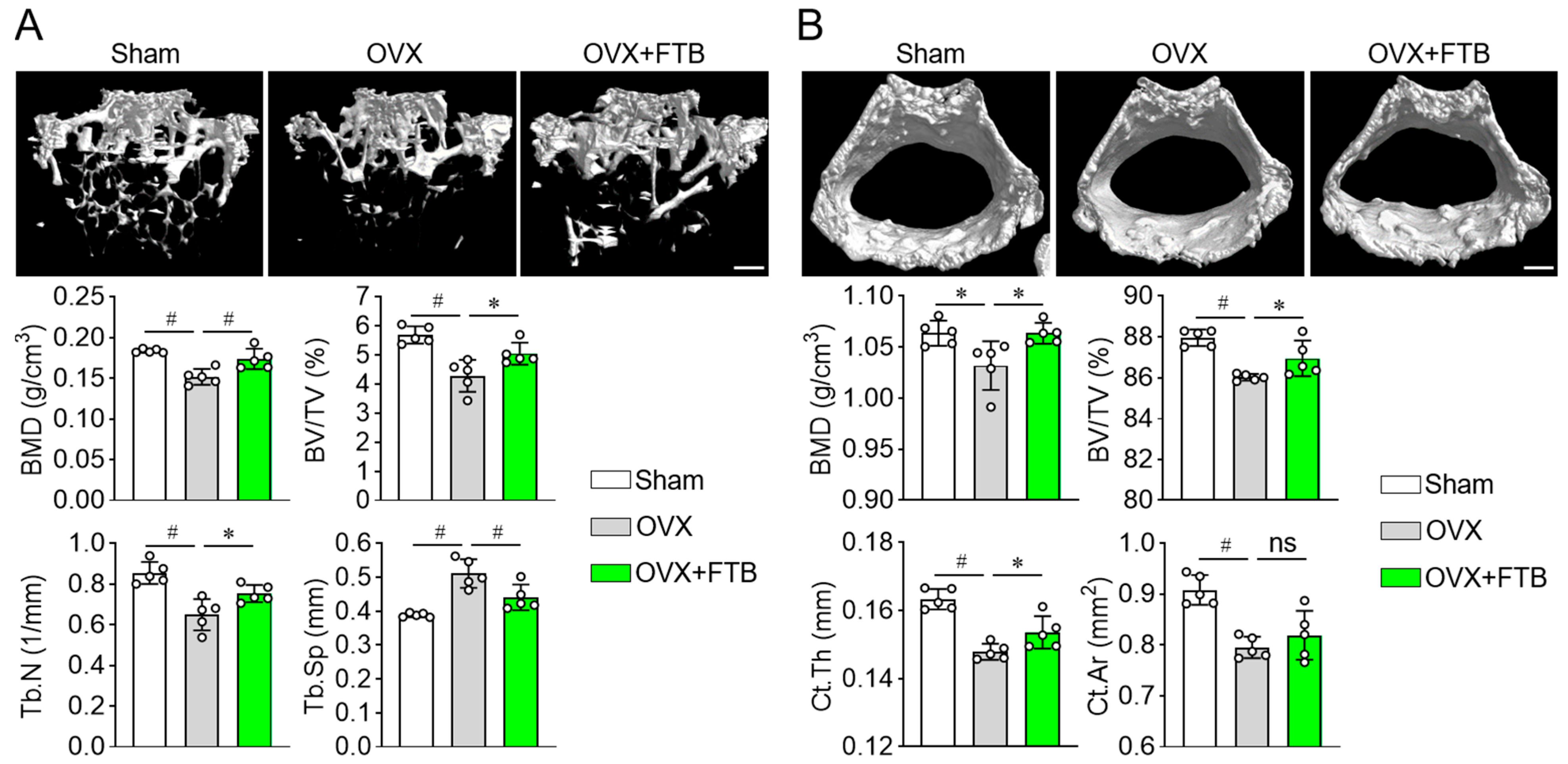
2.6. Ovariectomy and Therapeutic Foretinib Dosing
2.7. Dual-Energy X-Ray Absorptiometry (DXA) and Micro-Computed Tomography (μCT) Analysis
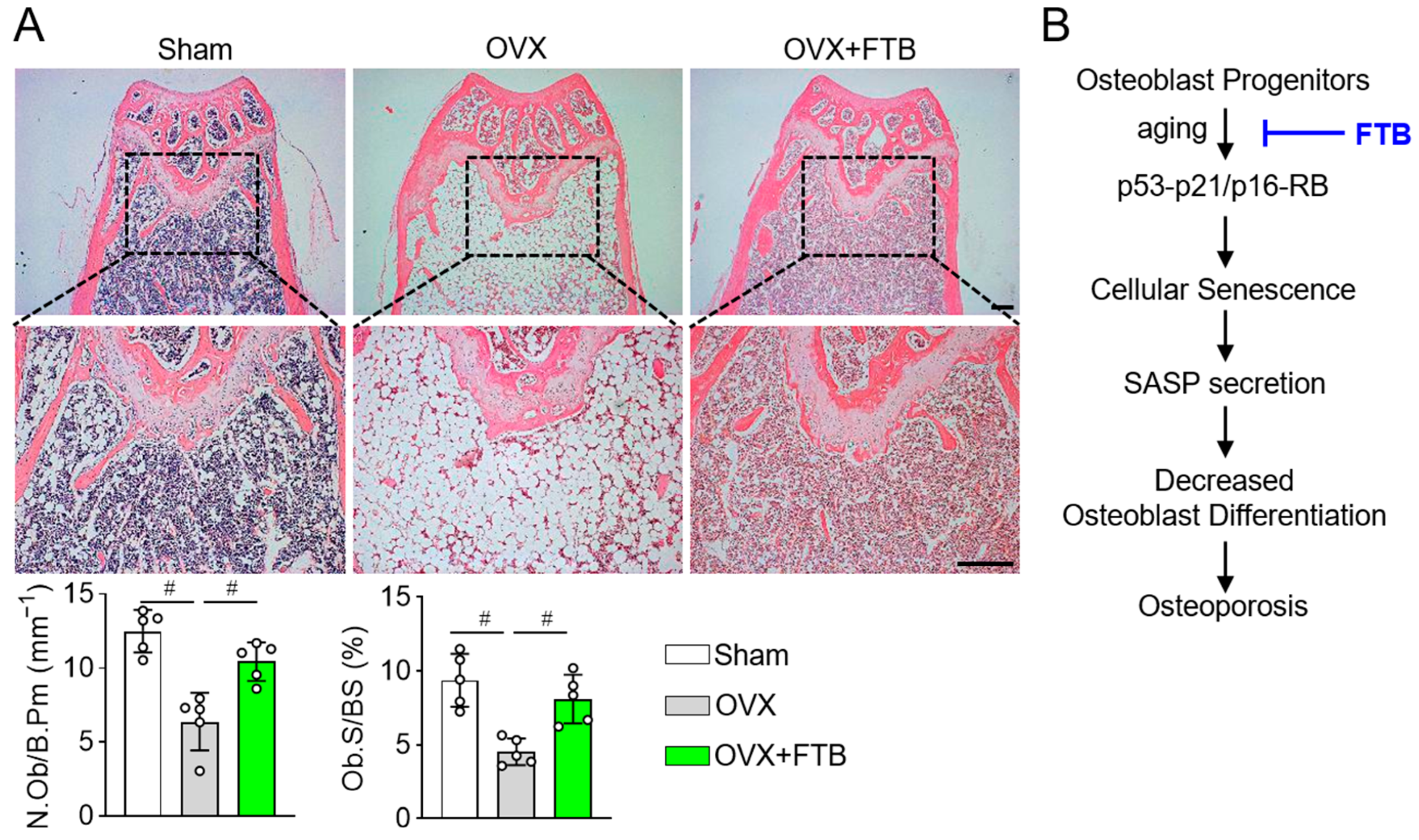
2.8. Histomorphometry Analysis
2.9. Statistical Analysis
3. Results
3.1. Foretinib Suppresses Osteoblast Progenitor Senescence
3.2. Foretinib Promotes Osteoblast Differentiation by Reducing Osteogenic Inhibitor SASP Factors
3.3. Foretinib Recovers Bone Loss in Ovariectomized Mice by Enhancing Osteoblast Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Campisi, J.; di Fagagna, F.D. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- Mi, B.; Xiong, Y.; Knoedler, S.; Alfertshofer, M.; Panayi, A.C.; Wang, H.; Lin, S.; Li, G.; Liu, G. Ageing-related bone and immunity changes: Insights into the complex interplay between the skeleton and the immune system. Bone Res. 2024, 12, 42. [Google Scholar] [CrossRef]
- Lee, J.; Oh, J.; Kim, J.R.; Ha, H.; Kim, T.; Jeong, D. Cabozantinib, an Anti-Aging Agent, Prevents Bone Loss in Estrogen-Deficient Mice by Suppressing Senescence-Associated Secretory Phenotype Factors. Int. J. Mol. Sci. 2025, 26, 7123. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef]
- Li, J.; Karim, M.A.; Che, H.; Geng, Q.; Miao, D. Deletion of p16 prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Am. J. Transl. Res. 2020, 12, 672–683. [Google Scholar]
- Pignolo, R.J.; Law, S.F.; Chandra, A. Bone Aging, Cellular Senescence, and Osteoporosis. J. Bone Miner. Res. Plus 2021, 5, e10488. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhou, Y.M.; Zhang, L.L.; Chen, X.J.; Yang, Y.Y.; Zhang, D.; Zhu, K.C.; Kong, X.K.; Sun, L.H.; Tao, B.; et al. BMP9 reduces age-related bone loss in mice by inhibiting osteoblast senescence through Smad1-Stat1-P21 axis. Cell Death Discov. 2022, 8, 254. [Google Scholar] [CrossRef]
- Samakkarnthai, P.; Saul, D.; Zhang, L.; Aversa, Z.; Doolittle, M.L.; Sfeir, J.G.; Kaur, J.; Atkinson, E.J.; Edwards, J.R.; Russell, G.G.; et al. In vitro and in vivo effects of zoledronic acid on senescence and senescence-associated secretory phenotype markers. Aging 2023, 15, 3331–3355. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Sambrook, P.; Cooper, C. Osteoporosis. Lancet 2006, 367, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Maran, A.; Lotinun, S.; Hefferan, T.; Evans, G.L.; Zhang, M.; Sibonga, J.D. Animal models for osteoporosis. Rev. Endocr. Metab. Disord. 2001, 2, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Vaishampayan, U.; Rosenberg, J.E.; Logan, T.F.; Harzstark, A.L.; Bukowski, R.M.; Rini, B.I.; Srinivas, S.; Stein, M.N.; Adams, L.M.; et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J. Clin. Oncol. 2013, 31, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Tsao, M.S.; Liu, G.; Tu, D.; Ho, C.; Shepherd, F.A.; Murray, N.; Goffin, J.R.; Nicholas, G.; Sakashita, S.; et al. A phase I study of foretinib plus erlotinib in patients with previously treated advanced non-small cell lung cancer: Canadian cancer trials group IND.196. Oncotarget 2017, 8, 69651–69662. [Google Scholar] [CrossRef]
- Lee, K.; Seo, I.; Choi, M.H.; Jeong, D. Roles of Mitogen-Activated Protein Kinases in Osteoclast Biology. Int. J. Mol. Sci. 2018, 19, 3004. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Kim, J.M.; Kim, M.Y.; Kim, J.R.; Lee, H.W.; Chung, Y.W.; Shin, H.I.; Kim, T.; Park, E.S.; et al. Selenoprotein W ensures physiological bone remodeling by preventing hyperactivity of osteoclasts. Nat. Commun. 2021, 12, 2258. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, K.; Shin, H.I.; Lee, K.J.; Jeong, D. Metabolic activities affect femur and lumbar vertebrae remodeling, and anti-resorptive risedronate disturbs femoral cortical bone remodeling. Exp. Mol. Med. 2021, 53, 103–114. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, S.Y.; Park, J.N.; Suh, J.H.; Choi, H.S. Doxorubicin Induces Bone Loss by Increasing Autophagy through a Mitochondrial ROS/TRPML1/TFEB Axis in Osteoclasts. Antioxidants 2022, 11, 1476. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Engst, S.; Yamaguchi, K.; Yu, P.; Won, K.A.; Mock, L.; Lou, T.; Tan, J.; Li, C.; Tam, D.; et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res. 2009, 69, 8009–8016. [Google Scholar] [CrossRef]
- Martorana, A.; La Monica, G.; Lauria, A. Quinoline-Based Molecules Targeting c-Met, EGF, and VEGF Receptors and the Proteins Involved in Related Carcinogenic Pathways. Molecules 2020, 25, 4279. [Google Scholar] [CrossRef]
- de Mera-Rodríguez, J.A.; Alvarez-Hernán, G.; Gañán, Y.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Is Senescence-Associated β-Galactosidase a Reliable Marker of Cellular Senescence During Embryonic Development? Front. Cell Dev. Biol. 2021, 9, 623175. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef]
- Ahadzadeh Ardebili, A.; Fu, T.; Dunnewold, N.; Aghajafari, F.; Billington, E.O. Bisphosphonates Preserve Bone Mineral Density and Suppress Bone Turnover Markers in Early Menopausal Women: A Systematic Review and Meta-Analysis of Randomized Trials. J. Bone Miner. Res. Plus 2023, 7, e10748. [Google Scholar] [CrossRef] [PubMed]
- Waltman, N.; Kupzyk, K.A.; Flores, L.E.; Mack, L.R.; Lappe, J.M.; Bilek, L.D. Bone-loading exercises versus risedronate for the prevention of osteoporosis in postmenopausal women with low bone mass: A randomized controlled trial. Osteoporos. Int. 2022, 33, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Moshi, M.R.; Nicolopoulos, K.; Stringer, D.; Ma, N.; Jenal, M.; Vreugdenburg, T. The Clinical Effectiveness of Denosumab (Prolia(R)) for the Treatment of Osteoporosis in Postmenopausal Women, Compared to Bisphosphonates, Selective Estrogen Receptor Modulators (SERM), and Placebo: A Systematic Review and Network Meta-Analysis. Calcif. Tissue Int. 2023, 112, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Cipriani, C.; Palermo, A.; Viapiana, O.; Zavatta, G.; Mazziotti, G. A practical approach for anabolic treatment of bone fragility with romosozumab. J. Endocrinol. Investig. 2024, 47, 2649–2662. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lim, J.Y.; Kim, T.K.; Cho, B.W.; Kwon, H.M.; Park, K.K.; Lee, W.S. Short-term anabolic agent and sequential therapy to improve bone mineral density and bone turnover markers in patients with osteoporotic hip fractures. J. Orthop. Surg. Res. 2025, 20, 662. [Google Scholar] [CrossRef] [PubMed]
- Ebina, K.; Etani, Y.; Noguchi, T.; Nakata, K.; Okada, S. Clinical effects of teriparatide, abaloparatide, and romosozumab in postmenopausal osteoporosis. J. Bone Miner. Metab. 2025, 43, 3–9. [Google Scholar] [CrossRef]
- Seiwert, T.; Sarantopoulos, J.; Kallender, H.; McCallum, S.; Keer, H.N.; Blumenschein, G., Jr. Phase II trial of single-agent foretinib (GSK1363089) in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Investig. New Drugs 2013, 31, 417–424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Lee, J.; Kim, E.-C.; Kim, J.-R.; Ha, H.; Kim, T.; Lee, K.; Jeong, D. Foretinib Alleviates Osteoblast Senescence and Protects Against Bone Loss in Ovariectomized Mice by Promoting Osteoblast Differentiation. Cells 2025, 14, 1945. https://doi.org/10.3390/cells14241945
Oh J, Lee J, Kim E-C, Kim J-R, Ha H, Kim T, Lee K, Jeong D. Foretinib Alleviates Osteoblast Senescence and Protects Against Bone Loss in Ovariectomized Mice by Promoting Osteoblast Differentiation. Cells. 2025; 14(24):1945. https://doi.org/10.3390/cells14241945
Chicago/Turabian StyleOh, Jiin, Jueun Lee, Eok-Cheon Kim, Jae-Ryoung Kim, Hyunil Ha, Taesoo Kim, Kyunghee Lee, and Daewon Jeong. 2025. "Foretinib Alleviates Osteoblast Senescence and Protects Against Bone Loss in Ovariectomized Mice by Promoting Osteoblast Differentiation" Cells 14, no. 24: 1945. https://doi.org/10.3390/cells14241945
APA StyleOh, J., Lee, J., Kim, E.-C., Kim, J.-R., Ha, H., Kim, T., Lee, K., & Jeong, D. (2025). Foretinib Alleviates Osteoblast Senescence and Protects Against Bone Loss in Ovariectomized Mice by Promoting Osteoblast Differentiation. Cells, 14(24), 1945. https://doi.org/10.3390/cells14241945




